The Effects of Sunflower and Maize Crop Residue Extracts as a New Ingredient on the Quality Properties of Pork Liver Pâtés
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Crop Residue Extracts
2.3. Evaluation of Total Phenol Content
2.4. Evaluation of Total Flavonoid Content
2.5. LC-MS Analysis of Ethanol Extracts Obtained from Corn and Sunflower Crop Residues
2.6. Evaluation of Antioxidant Activity
2.7. Production Process of Pork Liver Pâtés
2.8. Determination of Pork Liver Pâtés’ Chemical Composition
2.9. Determination of Pork Liver Pâtés’ Physicochemical Properties
2.10. Determination of Pork Liver Pâtés’ Color
2.11. Determination of Pork Liver Pâtés’ Fatty Acid Profile
2.12. Determination of Pork Liver Pâtés’ Lipid Oxidative Stability
2.13. Microbiological Analysis of Pork Liver Pâtés
2.14. Sensory Analysis of Pork Liver Pâtés
2.15. Statistical Analysis
3. Results and Discussion
3.1. TPC, TFC, Antioxidant Activity, and Phenolic Compounds of Sunflower and Maize Stalk Residue Extracts
3.2. Proximate Composition of Pork Liver Pâtés
3.3. Fatty Acid Composition of Pork Liver Pâtés
3.4. Water Activity (aw) Values of Pork Liver Pâtés
3.5. pH of Pork Liver Pâtés
3.6. Color of Pork Liver Pâtés
3.7. TBARs and Peroxide Value (POV)
3.8. Microbiological Analyses
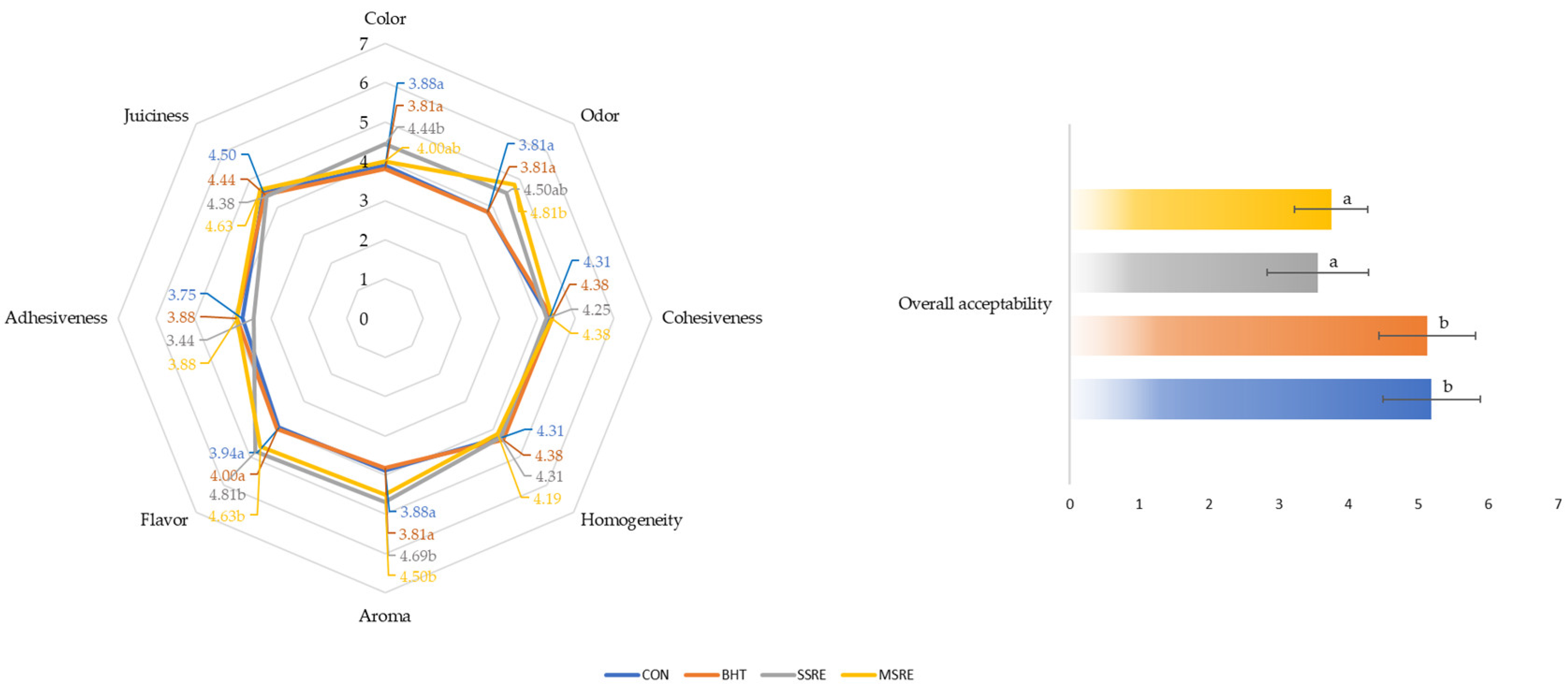
3.9. Sensory Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estévez, M.; Ventanas, J.; Cava, R.; Puolanne, E. Characterisation of a traditional Finnish liver sausage and different types of Spanish liver pâtés: A comparative study. Meat Sci. 2005, 71, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Terrasa, A.M.; Staffolo, M.D.; Tomás, M.C. Nutritional improvement and physicochemical evaluation of liver pâté formulations. LWT 2016, 66, 678–684. [Google Scholar] [CrossRef]
- Lucas-González, R.; Pellegrini, M.; Viuda-Martos, M.; Pérez-Álvarez, J.Á.; Fernández-López, J. Persimmon (Diospyros kaki Thunb.) coproducts as a new ingredient in pork liver pâté: Influence on quality properties. Int. J. Food Sci. Technol. 2019, 54, 1232–1239. [Google Scholar] [CrossRef]
- Estévez, M.; Cava, R. Lipid and protein oxidation, release of iron from heme molecule and colour deterioration during refrigerated storage of liver pâté. Meat Sci. 2004, 68, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Ventanas, S.; Cava, R. Effect of natural and synthetic antioxidants on protein oxidation and colour and texture changes in refrigerated stored porcine liver pâté. Meat Sci. 2006, 74, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.; Ramírez, R.; Ventanas, S.; Cava, R. Sage and rosemary essential oils versus BHT for the inhibition of lipid oxidative reactions in liver pâté. LWT-Food Sci. Technol. 2007, 40, 58–65. [Google Scholar] [CrossRef]
- World Health Organization. The Global Prevalence of Anaemia in 2011; World Health Organization: Geneva, Switzerland, 2015; Available online: https://iris.who.int/bitstream/handle/10665/177094/9789241564960_eng.pdf?sequence=1 (accessed on 12 January 2024).
- Martín-Sánchez, A.M.; Ciro-Gómez, G.; Sayas, E.; Vilella-Esplá, J.; Ben-Abda, J.; Pérez-Álvarez, J.Á. Date palm by-products as a new ingredient for the meat industry: Application to pork liver pâté. Meat Sci. 2013, 93, 880–887. [Google Scholar] [CrossRef]
- Pateiro, M.; Lorenzo, J.M.; Vázquez, J.A.; Franco, D. Oxidation stability of pig liver pâté with increasing levels of natural antioxidants (grape and tea). Antioxidants 2015, 4, 102–123. [Google Scholar] [CrossRef]
- Vijayalaxmi, S.; Jayalakshmi, S.K.; Sreeramulu, K. Polyphenols from different agricultural residues: Extraction, identification and their antioxidant properties. J. Food Sci. Technol. 2015, 52, 2761–2769. [Google Scholar] [CrossRef]
- European Commission, Directorate-General for Research and Innovation. A Sustainable Bioeconomy for Europe—Strengthening the Connection between Economy, Society and the Environment—Updated Bioeconomy Strategy; Publications Office of the European Union: Luxembourg, 2018; Available online: https://data.europa.eu/doi/10.2777/792130 (accessed on 12 January 2024).
- United States Department of Agriculture (USDA); The Foreign Agricultural Service (FAS). World Agricultural Production. 2023. Available online: https://ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=2224000 (accessed on 28 January 2024).
- Santana-Méridas, O.; González-Coloma, A.; Sánchez-Vioque, R. Agricultural residues as a source of bioactive natural products. Phytochem. Rev. 2012, 11, 447–466. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-food byproducts as a new source of natural food additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Lizárraga-Velázquez, C.E.; Hernández, C.; Sánchez-Gutiérrez, E.Y. Exploitation of agro-industrial waste as potential source of bioactive compounds for aquaculture. Foods 2020, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Glišić, M.; Bošković Cabrol, M.; Čobanović, N.; Baltić, M.Ž.; Vranešević, J.; Samardžić, S.; Maksimović, Z. Antimicrobial activity of ethanolic extracts from wheat, sunflower and maize crop residues. Arch. Vet. Sci. 2023, 16, 53–67. [Google Scholar] [CrossRef]
- Ahmad, I.; Yanuar, A.; Mulia, K.; Mun’im, A. Application of ionic liquid as a green solvent for polyphenolics content extraction of Peperomia pellucida (L) Kunth Herb. J. Young Pharm. 2017, 9, 486–490. [Google Scholar] [CrossRef]
- Chatatikun, M.; Chiabchalard, A. Phytochemical screening and free radical scavenging activities of orange baby carrot and carrot (Daucus carota Linn.) root crude extracts. J. Chem. Pharm. Res. 2013, 5, 97–102. [Google Scholar]
- Sembring Novia, E.; Elya, B.; Sauriasari, R. Phytochemical screening, total flavonoid and total phenolic content and antioxidant activity of different parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn. J. 2018, 10, 123–127. [Google Scholar] [CrossRef]
- Prieto, J.M. Procedure: Preparation of DPPH Radical, and Antioxidant Scavenging Assay. Dr. Prieto’s DPPH Microplate Protocol. 2012, pp. 7–9. Available online: https://www.researchgate.net/file.PostFileLoader.html?id=503cd1c9e39d5ead11000043&assetKey=AS%3A271744332435456%401441800305338 (accessed on 21 February 2024).
- Xiao, F.; Tao, X.; Baiyi, L.; Ruihai, L. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Benzie, I.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 77–106. [Google Scholar] [CrossRef]
- Delgado-Pando, G.; Cofrades, S.; Rodríguez-Salas, L.; Jiménez-Colmenero, F. A healthier oil combination and konjac gel as functional ingredients in low-fat pork liver pâté. Meat Sci. 2011, 88, 241–248. [Google Scholar] [CrossRef]
- Serbian Regulation 53/2018-22. Regulation on the Food Additives. Official Gazette of the Republic of Serbia. 2018. Available online: https://www.pravno-informacioni-sistem.rs/SlGlasnikPortal/eli/rep/sgrs/ministarstva/pravilnik/2018/53/1/reg (accessed on 12 January 2024).
- ISO 1444:1996; Meat and Meat Products—Determination of Free Fat Content. International Organization for Standardization: Geneva, Switzerland, 1996.
- ISO 1442:1997; Meat and Meat Products—Determination of Moisture Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 1997.
- ISO 937:1978; Meat and Meat Products—Determination of Nitrogen Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 1978.
- ISO 936:1998; Meat and Meat Products—Determination of Total Ash. International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO 2918:1975; Meat and Meat Products—Determination of Nitrite Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 1975.
- ISO 1841-1:1996; Meat and Meat Products—Determination of Chloride Content—Part 1: Volhard Method. International Organization for Standardization: Geneva, Switzerland, 1996.
- ISO 23776:2021; Meat and Meat Products—Determination of Total Phosphorous Content. International Organization for Standardization: Geneva, Switzerland, 2021.
- Yudd, D.B.; Wyszecki, G. Color in Business, Science and Industry, 3rd ed.; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Spiric, A.; Trbovic, D.; Vranic, D.; Djinovic, J.; Petronijevic, R.; Matekalo-Sverak, V. Statistical evaluation of fatty acid profile and cholesterol content in fish (common carp) lipids obtained by different sample preparation procedures. Anal. Chim. Acta 2010, 672, 66–71. [Google Scholar] [CrossRef]
- Glisic, M.; Baltic, M.; Glisic, M.; Trbovic, D.; Jokanovic, M.; Parunovic, N.; Suvajdzic, B.; Boskovic, M.; Vasilev, D. Inulin-based emulsion-filled gel as a fat replacer in prebiotic-and PUFA-enriched dry fermented sausages. Int. J. Food Sci. Technol. 2019, 54, 787–797. [Google Scholar] [CrossRef]
- ISO 5509:2000; Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2000.
- Fuchs, R.H.B.; Ribeiro, R.P.; Matsushita, M.; Tanamati, A.A.C.; Bona, E.; de Souza, A.H.P. Enhancement of the nutritional status of Nile tilapia (Oreochromis niloticus) croquettes by adding flaxseed flour. LWT 2013, 54, 440–446. [Google Scholar] [CrossRef]
- Tarladgis, B.G.; Pearson, A.M.; Dugan, L.R. Chemistry of the 2-thibarbituric acid test for determination oxidative rancidity in foods. II Formation of the TBA malonaldehyde complex without acid-heat treatment. J. Sci. Food Agric. 1964, 15, 602–607. [Google Scholar] [CrossRef]
- Holland, C.D. Determination of malonaldehyde as an index of rancidity in nut meats. J. AOAC 1971, 54, 1024–1026. [Google Scholar] [CrossRef]
- ISO 3960:2017; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. International Organization for Standardization: Geneva, Switzerland, 2017.
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Nutritional quality of some wild and cultivated seaweeds: Nutrient composition, total phenolic content and in vitro digestibility. J. Appl. Phycol. 2016, 28, 3575–3585. [Google Scholar] [CrossRef]
- Pateiro, M.; Lorenzo, J.M.; Amado, I.R.; Franco, D. Effect of addition of green tea, chestnut and grape extract on the shelf-life of pig liver pâté. Food Chem. 2014, 147, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Van Cuong, T.; Chin, K.B. Effects of annatto (Bixa orellana L.) seeds powder on physicochemical properties, antioxidant and antimicrobial activities of pork patties during refrigerated storage. Korean J. Food Sci. Anim. Resour. 2016, 36, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Domínguez, R.; Campagnol, P.C.B.; Franco, D.; Trindade, M.A.; Lorenzo, J.M. Effect of natural antioxidants on physicochemical properties and lipid stability of pork liver pâté manufactured with healthy oils during refrigerated storage. J. Food Sci. Technol. 2017, 54, 4324–4334. [Google Scholar] [CrossRef]
- Lucas-González, R.; Pérez-Álvarez, J.Á.; Viuda-Martos, M.; Fernández-López, J. Pork liver pâté enriched with persimmon coproducts: Effect of in vitro gastrointestinal digestion on its fatty acid and polyphenol profile stability. Nutrients 2021, 13, 1332. [Google Scholar] [CrossRef]
- Martín-Sánchez, A.M.; Ciro-Gómez, G.L.; Zapata-Montoya, J.E.; Vilella-Esplá, J.; Pérez-Álvarez, J.A.; Sayas-Barberá, E. Effect of date palm coproducts and annatto extract on lipid oxidation and microbial quality in a pork liver pâté. J. Food Sci. 2014, 79, M2301–M2307. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef]
- Kamal, J. Quantification of alkaloids, phenols and flavonoids in sunflower (Helianthus annuus L.). Afr. J. Biotechnol. 2011, 10, 3149–3151. [Google Scholar]
- Vazquez-Olivo, G.; López-Martínez, L.X.; Contreras-Angulo, L.; Heredia, J.B. Antioxidant capacity of lignin and phenolic compounds from corn stover. Waste Biomass Valor. 2019, 10, 95–102. [Google Scholar] [CrossRef]
- Gai, F.; Karamać, M.; Janiak, M.A.; Amarowicz, R.; Peiretti, P.G. Sunflower (Helianthus annuus L.) plants at various growth stages subjected to extraction-Comparison of the antioxidant activity and phenolic profile. Antioxidants 2020, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Adekunle, A.E.; Rabeya, T.; Jehadin, F.; Asad, M.A.; Ayodele, O.O.; Islam, M.S. Compositional and microstructural changes in compressed hot water pretreated corn stalk. Curr. Res. Green. Sustain. Chem. 2021, 4, 100057. [Google Scholar] [CrossRef]
- Weisz, G.M.; Kammerer, D.R.; Carle, R. Identification and quantification of phenolic compounds from sunflower (Helianthus annuus L.) kernels and shells by HPLC-DAD/ESI-MSn. Food Chem. 2009, 115, 758–765. [Google Scholar] [CrossRef]
- Skałecki, I.; de Espíndola Sobczyk, A.; Marczak, L.D.F.; Sarkis, J. Optimization of ultrasound assisted extraction of phenolic compounds from sunflower seed cake using response surface methodology. Waste Biomass Valor. 2019, 10, 33–44. [Google Scholar] [CrossRef]
- Alexandrino, T.D.; da Silva, M.G.; Ferrari, R.A.; Ruiz, A.L.T.G.; Duarte, R.M.T.; Simabuco, F.M.; Bezzera, R.M.N.; Pacheco, M.T.B. Evaluation of some in vitro bioactivities of sunflower phenolic compounds. Curr. Res. Food Sci. 2021, 4, 662–669. [Google Scholar] [CrossRef]
- Karamać, M.; Kosińska, A.; Estrella, I.; Hernández, T.; Duenas, M. Antioxidant activity of phenolic compounds identified in sunflower seeds. Eur. Food Res. Technol. 2012, 235, 221–230. [Google Scholar] [CrossRef]
- Abbasi-Parizad, P.; De Nisi, P.; Scaglia, B.; Scarafoni, A.; Pilu, S.; Adani, F. Recovery of phenolic compounds from agro-industrial by-products: Evaluating antiradical activities and immunomodulatory properties. Food Bioprod. Process. 2021, 127, 338–348. [Google Scholar] [CrossRef]
- Galeana-López, J.A.; Lizárraga-Velázquez, C.E.; Hernández, C.; Leyva-López, N.; Heredia, J.B. Corn husk phenolics modulate hepatic antioxidant response in Nile Tilapia (Oreochromis niloticus) exposed to hypoxia. Molecules 2021, 26, 6161. [Google Scholar] [CrossRef]
- Boz, H. p-Coumaric acid in cereals: Presence, antioxidant and antimicrobial effects. Int. J. Food Sci. Technol. 2015, 50, 2323–2328. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Halpani, C.G.; Mishra, S. Design, synthesis, characterization of ferulic acid and p-coumaric acid amide derivatives as an antibacterial/antioxidant agent. Pharm. Sci. Adv. 2024, 2, 100023. [Google Scholar] [CrossRef]
- Serbian Regulations 94/2015, 104/2015, 19/2017. Regulation on the Quality of Ground Meat, Meat Preparations and Meat Products. Official Gazette of the Republic of Serbia. 2017. Available online: https://www.pravno-informacioni-sistem.rs/SlGlasnikPortal/eli/rep/sgrs/ministarstva/pravilnik/2015/94/2 (accessed on 12 January 2024).
- Munekata, P.E.S.; Fernandes, R.D.P.P.; de Melo, M.P.; Trindade, M.A.; Lorenzo, J.M. Influence of peanut skin extract on shelf-life of sheep patties. Asian Pac. J. Trop. Biomed. 2016, 6, 586–596. [Google Scholar] [CrossRef]
- Echarte, M.; Conchillo, A.; Ansorena, D.; Astiasarán, I. Evaluation of the nutritional aspects and cholesterol oxidation products of pork liver and fish patés. Food Chem. 2004, 86, 47–53. [Google Scholar] [CrossRef]
- Agregán, R.; Franco, D.; Carballo, J.; Tomasevic, I.; Barba, F.J.; Gómez, B.; Muchenje, V.; Lorenzo, J.M. Shelf life study of healthy pork liver pâté with added seaweed extracts from Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Food Res. Int. 2018, 112, 400–411. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Estévez, M.; Morcuende, D.; Ramírez, R.; Ventanas, J.; Cava, R. Extensively reared Iberian pigs versus intensively reared white pigs for the manufacture of liver pâté. Meat Sci. 2004, 67, 453–461. [Google Scholar] [CrossRef]
- D’arrigo, M.; Hoz, L.; Cambero, I.; Lopez-Bote, C.J.; Pin, C.; Ordóñez, J.A. Production of n-3 fatty acid enriched pork liver pâté. LWT-Food Sci. Technol. 2004, 37, 585–591. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Fat and fatty acid requirements for adults. In Fats and Fatty Acids in Human Nutrition—Report of an Expert Consultation; FAO (Food and Agriculture Organization of the United Nations): Rome, Italy, 2010; pp. 55–62. [Google Scholar]
- Vlaicu, P.A.; Panaite, T.D.; Turcu, R.P. Enriching laying hens eggs by feeding diets with different fatty acid composition and antioxidants. Sci. Rep. 2021, 11, 20707. [Google Scholar] [CrossRef]
- Amaral, D.S.; Silva, F.A.P.; Bezerra, T.K.A.; Arcanjo, N.M.O.; Guerra, I.C.D.; Dalmás, P.S.; Madruga, M.S. Effect of storage time and packaging on the quality of lamb pâté prepared with ‘variety meat’. Food Packag. Shelf Life 2015, 3, 39–46. [Google Scholar] [CrossRef]
- Lazárková, Z.; Kratochvílová, A.; Salek, R.N.; Polášek, Z.; Šiška, L.; Pětová, M.; Buňka, F. Influence of heat treatment on the chemical, physical, microbiological and sensorial properties of pork liver pâté as affected by fat content. Foods 2023, 12, 2423. [Google Scholar] [CrossRef]
- Francis, F.J.; Clydesdale, F.M. Food Colorimetry: Theory and Applications; AVI Publishing Co., Inc.: Westport, CT, USA, 1975. [Google Scholar]
- Fernández-López, J.; Pérez-Alvarez, J.A.; Aranda-Catalá, V. Effect of mincing degree on colour properties in pork meat. Color. Res. Appl. 2000, 25, 376–380. [Google Scholar] [CrossRef]
- Fernández-López, J.; Sayas-Barberá, E.; Sendra, E.; Pérez-Alvarez, J.A. Quality characteristics of ostrich liver pâté. J. Food Sci. 2004, 69, snq85–snq91. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations; World Health Organization. Codex-Standard 211-1999. Codex Standard for Named Animal Fats. Section 3. Codex Standard for Fats and Oils from Animal Sources. The Codex Alimentarius. Fats, Oils and Related Products, 2nd ed.; Food and Agriculture Organization of the United Nations; World Health Organization: Rome, Italy, 2001; Volume 8, pp. 1–7. [Google Scholar]
- Munekata, P.E.S.; Calomeni, A.D.V.; Rodrigues, C.E.D.C.; Fávaro-Trindade, C.S.; Alencar, S.M.D.; Trindade, M.A. Peanut skin extract reduces lipid oxidation in cooked chicken patties. Poult. Sci. 2015, 94, 442–446. [Google Scholar] [CrossRef]
- Cerón-Guevara, M.I.; Santos, E.M.; Lorenzo, J.M.; Pateiro, M.; Bermúdez-Piedra, R.; Rodríguez, J.A.; Rosas, J.C.; Rangel-Vargas, E. Partial replacement of fat and salt in liver pâté by addition of Agaricus bisporus and Pleurotus ostreatus flour. Int. J. Food Sci. Technol. 2021, 56, 6171–6181. [Google Scholar] [CrossRef]
- Estévez, M.; Cava, R. Effectiveness of rosemary essential oil as an inhibitor of lipid and protein oxidation: Contradictory effects in different types of frankfurters. Meat Sci. 2006, 72, 348–355. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, M.; Zhou, G.; Zou, Y.; Xu, X. Prooxidant effects of the combination of green tea extract and sodium nitrite for accelerating lipolysis and lipid oxidation in pepperoni during storage. J. Food Sci. 2011, 76, C694–C700. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y.; Peng, H.H. Antioxidant and pro-oxidant effects of various tea extracts. J. Agric. Food Chem. 1997, 45, 30–34. [Google Scholar] [CrossRef]
- Pegg, R.B.; Shahidi, F. Nitrite Curing of Meat, the N-Nitrosamine Problem and Nitrite Alternatives; Food & Nutrition Press, Inc.: Trumbull, CT, USA, 2000; pp. 23–104. [Google Scholar]
- Radi, R.; Denicola, A.; Alvarez, B.; Ferrer-Sueta, G.; Rubbo, H. The biological chemistry of peroxynitrite. In Nitric Oxide, Biology and Pathobiology; Ignarro, L., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 57–82. [Google Scholar]
- Tsai, P.J.; Tsai, T.H.; Yu, C.H.; Ho, S.C. Comparison of NO-scavenging and NO-suppressing activities of different herbal teas with those of green tea. Food Chem. 2007, 103, 181–187. [Google Scholar] [CrossRef]
- Ghiretti, G.P.; Zanardi, E.; Novelli, E.; Campanini, G.; Dazzi, G.; Madarena, G.; Chizzolini, R. Comparative evaluation of some antioxidants in salame Milano and mortadella production. Meat Sci. 1997, 47, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.Y.; Yun, I.R.; Kim, G.D.; Jung, E.Y.; Joo, S.T.; Yang, H.S. Effect of puerariae radix extracts on pH, color, 2-thiobarbituric acid reactive substances (TBARS) and reduced nitrite content of emulsion-type pork sausage during storage. In Proceedings of the 56th International Congress of Meat Science and Technology (ICoMST), Jeju, Republic of Korea, 15–20 August 2010. [Google Scholar]
- Wójciak, K.M.; Dolatowski, Z.J. Oxidative stability of fermented meat products. Acta Sci. Pol. Technol. Aliment. 2012, 11, 99–109. [Google Scholar]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Centre for Food Safety. Microbiological Guidelines for Food (For Ready-to-Eat Food in General and Specific Food Items); Food and Environmental Hygiene Department: Hong Kong, China, 2014.
- Sun-Waterhouse, D.; Wadhwa, S.S. Industry-relevant approaches for minimising the bitterness of bioactive compounds in functional foods: A review. Food Bioprocess Technol. 2013, 6, 607–627. [Google Scholar] [CrossRef]
- Colindres, P.; Susan Brewer, M. Oxidative stability of cooked, frozen, reheated beef patties: Effect of antioxidants. J. Sci. Food Agric. 2011, 91, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Boles, J.A.; Pegg, R. Meat Color; Montana State University and Saskatchewan Food Product Innovation Program; University of Saskatchewan: Saskatoon, SK, Canada, 2010. [Google Scholar]
| Ingredients | Batches | |||
|---|---|---|---|---|
| CON | BHT | SSRE | MSRE | |
| Liver | 330 | 330 | 330 | 330 |
| Subcutaneous fat | 300 | 300 | 300 | 300 |
| Lean meat | 207 | 207 | 207 | 207 |
| Water | 125 | 125 | 125 | 125 |
| Soy protein isolate | 15 | 15 | 15 | 15 |
| Curing salt (0.6% nitrite in NaCl) | 15 | 15 | 15 | 15 |
| Sodium tripolyphosphate | 5 | 5 | 5 | 5 |
| Mix of spices | 3 | 3 | 3 | 3 |
| BHT | - | 0.2 | - | - |
| Sunflower stalk residues extract | - | - | 10 | - |
| Maize stalk residues extract | - | - | - | 10 |
| SSRE | MSRE | |
|---|---|---|
| TPC (mg GAE/g) 1 | 15.83 ± 0.30 a | 20.44 ± 0.23 b |
| TFC (mg QE/g) 2 | 8.98 ± 0.34 | 9.37 ± 0.83 |
| Tests | Extract/Positive Control | |||
|---|---|---|---|---|
| SSRE | MSRE | BHT | L-AA | |
| DPPH IC50 (mg/mL) | 1.02 ± 0.01 a | 0.41 ± 0.02 b | 0.038 ± 0.001 c | 0.0057 ± 0.0003 d |
| ABTS IC50 (mg/mL) | 4.53 ± 1.20 a | 1.72 ± 0.10 b | 0.04 ± 0.002 c | 0.0316 ± 0.0003 d |
| FRAP (µmol Fe2+/g) | 0.14 ± 0.01 a | 0.25 ± 0.01 b | 0.41 ± 0.03 c | 1.618 ± 0.004 d |
| Item | Batches | p Value | |||
|---|---|---|---|---|---|
| CON | BHT | SSRE | MSRE | ||
| Moisture (%) | 54.77 ± 0.31 | 54.67 ± 0.24 | 54.50 ± 0.71 | 54.44 ± 0.69 | 0.6875 |
| Protein (%) | 14.39 ± 0.04 | 14.50 ± 0.17 | 14.40 ± 0.14 | 14.42 ± 0.12 | 0.3777 |
| Fat (%) | 27.95 ± 0.31 | 27.97 ± 0.21 | 28.32 ± 0.53 | 28.29 ± 0.71 | 0.3941 |
| Ash (%) | 2.86 ± 0.02 | 2.81 ± 0.01 | 2.86 ± 0.01 | 2.83 ± 0.07 | 0.0970 |
| NaCl (g/100 g) | 1.75 ± 0.13 | 1.61 ± 0.02 | 1.59 ± 0.08 | 1.78 ± 0.19 | 0.0564 |
| NaNO2 (mg/kg) | 26.11 ± 2.92 | 27.64 ± 1.94 | 29.35 ± 1.48 | 27.40 ± 1.95 | 0.1057 |
| P2O5 (g/kg) | 6.70 ± 0.16 | 6.64 ± 0.11 | 6.69 ± 0.03 | 6.76 ± 0.05 | 0.2727 |
| Energy value (Kcal/100 g) | 309.10 ± 2.75 | 309.70 ± 1.94 | 312.50 ± 5.16 | 312.30 ± 6.26 | 0.4455 |
| Fatty Acid | Batches | p Value | |||
|---|---|---|---|---|---|
| CON | BHT | SSRE | MSRE | ||
| C14:0 | 1107 ± 19.04 | 1113 ± 31.41 | 1110 ± 14.14 | 1095 ± 18.71 | 0.5042 |
| C15:0 | 35.00 ± 10.49 | 32.67 ± 3.27 | 35.50 ± 5.01 | 30.00 ± 2.76 | 0.4166 |
| C16:0 | 27,397 ± 138.70 | 27,349 ± 80.52 | 27,380 ± 33.47 | 27,265 ± 97.31 | 0.1120 |
| C16:1 | 1380 ± 48.58 | 1384 ± 32.00 | 1370 ± 23.66 | 1365 ± 24.29 | 0.7457 |
| C17:0 | 266.7 ± 15.06 | 260.0 ± 38.99 | 265.0 ± 22.58 | 265.0 ± 18.71 | 0.9718 |
| C18:0 | 15,093 ± 158.80 | 14,982 ± 33.12 | 15,085 ± 69.50 | 15,092 ± 74.94 | 0.1583 |
| C18:1cis-9 | 43,542 ± 237.90 | 43,627 ± 159.00 | 43,510 ± 137.00 | 43,598 ± 158.20 | 0.6631 |
| C18:2n-6 | 10,105 ± 122.60 a | 10,192 ± 68.82 ab | 10,275 ± 86.89 b | 10,292 ± 59.47b | 0.0053 |
| C18:3n-3 | 368.3 ± 19.41 | 380.0 ± 14.14 | 375.0 ± 28.11 | 375.0 ± 30.82 | 0.8692 |
| C20:0 | 213.3 ± 20.66 | 208.3 ± 14.38 | 205.0 ± 18.71 | 225.0 ± 27.39 | 0.3878 |
| C20:2 | 366.7 ± 13.66 | 365.0 ± 17.61 | 345.0 ± 18.71 | 365.0 ± 24.29 | 0.1843 |
| C20:3n-3 | 35.83 ± 4.36 | 39.17 ± 2.64 | 39.67 ± 2.87 | 40.00 ± 2.45 | 0.1197 |
| SFA | 44,112 ± 282.00 | 43,945 ± 83.34 | 44,081 ± 60.51 | 43,972 ± 129.60 | 0.2478 |
| MUFA | 44,972 ± 198.10 | 45,086 ± 130.90 | 44,880 ± 131.30 | 44,963 ± 166.80 | 0.2009 |
| PUFA | 10,876 ± 126.20 a | 10,976 ± 60.62 ab | 11,035 ± 100.50 b | 11,072 ± 73.23 b | 0.0096 |
| n-6 | 10,105 ± 122.60 a | 10,192 ± 68.82 ab | 10,275 ± 86.89 b | 10,292 ± 59.47 b | 0.0053 |
| n-3 | 404.2 ± 17.75 | 419.2 ± 15.84 | 414.7 ± 28.18 | 415.0 ± 32.19 | 0.7481 |
| n-6/n-3 | 25.05 ± 1.27 | 24.35 ± 1.00 | 24.87 ± 1.54 | 24.93 ± 1.97 | 0.8525 |
| PUFA/SFA | 0.2450 ± 0.005 a | 0.2498 ± 0.001 ab | 0.2503 ± 0.002 b | 0.2518 ± 0.001 b | 0.0064 |
| AI | 0.5737 ± 0.005 | 0.5710 ± 0.003 | 0.5726 ± 0.001 | 0.5684 ± 0.004 | 0.1291 |
| TI | 1.516 ± 0.016 | 1.503 ± 0.005 | 1.512 ± 0.005 | 1.505 ± 0.006 | 0.0841 |
| HH | 1.897 ± 0.019 | 1.907 ± 0.008 | 1.901 ± 0.004 | 1.913 ± 0.011 | 0.1115 |
| aw | Batches | Group p Value | |||
|---|---|---|---|---|---|
| CON | BHT | SSRE | MSRE | ||
| 0 day | 0.946 ± 0.001 aA | 0.946 ± 0.001 aA | 0.941 ± 0.001 bA | 0.947 ± 0.0001 aA | <0.0001 |
| 20 day | 0.956 ± 0.003 B | 0.959 ± 0.001 B | 0.957 ± 0.005 B | 0.958 ± 0.005 B | 0.4577 |
| 40 day | 0.935 ± 0.001 aC | 0.950 ± 0.010 bA | 0.951 ± 0.007 bcB | 0.962 ± 0.005 cB | <0.0001 |
| 60 day | 0.921 ± 0.001 aD | 0.926 ± 0.001 bC | 0.923 ± 0.001 aC | 0.926 ± 0.001 bC | <0.0001 |
| 90 day | 0.902 ± 0.003 aE | 0.906 ± 0.001 bD | 0.910 ± 0.001 cD | 0.910 ± 0.001 cD | <0.0001 |
| Time p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Group × Time p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Days of Storage | Batches | Parameters | |||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h° | pH | ||
| 0 | |||||||
| CON | 56.05 ± 1.71 aA | 12.43 ± 0.66 a | 12.15 ± 0.75 a | 17.39 ± 0.77 aA | 44.38 ± 2.27 aAB | 6.48 ± 0.02 aAB | |
| BHT | 53.68 ± 4.45 aA | 13.24 ± 1.38 bABC | 12.56 ± 0.91 aA | 18.26 ± 1.45 bAC | 44.05 ± 1.16 aA | 6.46 ± 0.02 bA | |
| SSRE | 50.92 ± 2.86 bA | 12.89 ± 0.57 abA | 11.89 ± 0.39 aA | 17.46 ± 0.53 abA | 42.45 ± 1.46 aA | 6.42 ± 0.01 cA | |
| MSRE | 55.87 ± 2.70 aAB | 11.22 ± 0.61 cA | 14.08 ± 1.22 bA | 18.04 ± 0.83 abA | 51.36 ± 3.71 bA | 6.42 ± 0.01 cA | |
| 20 | |||||||
| CON | 59.00 ± 1.71 aB | 12.90 ± 0.34 ac | 12.31 ± 0.44 a | 17.96 ± 0.69 aAB | 43.66 ± 0.76 aA | 6.50 ± 0.01 aB | |
| BHT | 58.09 ± 1.43 aB | 12.80 ± 0.56 aAB | 11.23 ± 0.33 bB | 17.04 ± 0.41 bBC | 41.30 ± 1.73 bB | 6.53 ± 0.01 bB | |
| SSRE | 52.17 ± 3.48 bA | 13.22 ± 0.36 cA | 11.95 ± 0.52 aAC | 17.73 ± 0.74 aA | 42.10 ± 0.93 abA | 6.45 ± 0.01 cBD | |
| MSRE | 57.18 ± 2.02 aAB | 10.86 ± 0.49 bA | 14.70 ± 1.05 cA | 18.30 ± 0.84 aA | 53.46 ± 3.01 cA | 6.49 ± 0.01 dB | |
| 40 | |||||||
| CON | 54.03 ± 3.90 aA | 12.54 ± 0.70 a | 12.66 ± 0.67 a | 17.73 ± 0.74 aAB | 45.27 ± 1.77 aAB | 6.43 ± 0.01 aC | |
| BHT | 56.36 ± 3.52 abAB | 12.56 ± 0.34 aA | 11.26 ± 0.37 bB | 16.87 ± 0.41 bB | 41.88 ± 1.47 bB | 6.47 ± 0.01 bA | |
| SSRE | 57.57 ± 0.84 bB | 14.26 ± 0.49 bB | 12.84 ± 1.00 aB | 19.19 ± 1.09 cB | 41.93 ± 1.25 bA | 6.33 ± 0.06 cC | |
| MSRE | 57.91 ± 3.33 bA | 11.24 ± 0.47 cA | 14.13 ± 0.57 cA | 18.06 ± 0.57 aA | 51.48 ± 1.37 cA | 6.39 ± 0.01 dC | |
| 60 | |||||||
| CON | 59.48 ± 3.46 aB | 12.70 ± 0.52 a | 12.34 ± 0.39 a | 17.71 ± 0.48 aAB | 44.16 ± 1.58 aAB | 6.49 ± 0.04 acB | |
| BHT | 57.57 ± 2.06 acB | 13.37 ± 0.51 bBC | 11.62 ± 0.46 bB | 17.69 ± 0.55 aC | 40.99 ± 1.46 bB | 6.51 ± 0.05 aC | |
| SSRE | 54.45 ± 1.82 bC | 14.01 ± 0.58 cB | 12.49 ± 0.44 aBC | 18.72 ± 0.75 bB | 41.49 ± 1.15 bA | 6.46 ± 0.01 bD | |
| MSRE | 55.54 ± 1.82 bcB | 12.58 ± 0.50 aB | 14.29 ± 0.52 cA | 19.03 ± 0.69 bB | 48.67 ± 0.79 cB | 6.47 ± 0.01 bcD | |
| 90 | |||||||
| CON | 59.36 ± 3.57 aB | 12.53 ± 0.55 a | 12.71 ± 0.76 a | 18.08 ± 0.90 aB | 45.92 ± 3.19 aB | 6.49 ± 0.01 aB | |
| BHT | 55.06 ± 2.64 bA | 13.68 ± 0.59 bC | 13.77 ± 0.74 bC | 19.41 ± 0.82 bD | 45.18 ± 1.38 abA | 6.52 ± 0.01 bBC | |
| SSRE | 51.51 ± 1.46 cA | 14.79 ± 0.39 cC | 14.34 ± 0.37 bD | 20.60 ± 0.37 cC | 44.11 ± 1.08 bB | 6.43 ± 0.01 cAB | |
| MSRE | 55.56 ± 1.70 bB | 12.13 ± 0.41 aB | 15.87 ± 1.01 cB | 19.98 ± 0.94 bcC | 52.55 ± 1.55 cA | 6.44 ± 0.01 dE | |
| Group | p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Time | p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Group × Time | p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| ΔE 0 | ΔE 90 | ΔE* | |||||
| CON | - | - | 3.35 | ||||
| BHT | 2.54 | 4.57 | 1.89 | ||||
| SSRE | 5.16 | 8.33 | 3.15 | ||||
| MSRE | 2.29 | 4.96 | 2.03 | ||||
| Days of Storage | Batches | Parameters | |
|---|---|---|---|
| POV (mmol/kg) | TBARs (MDA mg/kg) | ||
| 0 | |||
| CON | 0.312 ± 0.053 aA | 0.088 ± 0.012 aA | |
| BHT | 0.018 ± 0.005 bA | 0.015 ± 0.004 bA | |
| SSRE | 1.442 ± 0.085 cA | 0.088 ± 0.010 aA | |
| MSRE | 1.665 ± 0.143 dA | 0.045 ± 0.010 cA | |
| 20 | |||
| CON | 0.421 ± 0.058 aA | 0.090 ± 0.007 aA | |
| BHT | 0.183 ± 0.031 bB | 0.088 ± 0.007 aB | |
| SSRE | 2.355 ± 0.119 cB | 0.195 ± 0.007 bB | |
| MSRE | 2.345 ± 0.051 cB | 0.145 ± 0.009 cB | |
| 40 | |||
| CON | 0.515 ± 0.093 aA | 0.135 ± 0.011 aB | |
| BHT | 0.474 ± 0.123 aC | 0.125 ± 0.004 bC | |
| SSRE | 2.660 ± 0.597 bB | 0.225 ± 0.011 cC | |
| MSRE | 2.500 ± 0.071 bC | 0.195 ± 0.012 dC | |
| 60 | |||
| CON | 2.045 ± 0.469 aB | 0.145 ± 0.004 aB | |
| BHT | 1.175 ± 0.059 bD | 0.130 ± 0.008 aC | |
| SSRE | 4.335 ± 0.607 cC | 0.275 ± 0.011 bD | |
| MSRE | 2.645 ± 0.174 dD | 0.205 ± 0.038 cC | |
| 90 | |||
| CON | 1.275 ± 0.152 aC | 0.340 ± 0.044 acC | |
| BHT | 1.142 ± 0.088 bD | 0.195 ± 0.027 bD | |
| SSRE | 2.515 ± 0.120 cB | 0.450 ± 0.021 cE | |
| MSRE | 1.195 ± 0.048 aE | 0.345 ± 0.033 aD | |
| Group | p Value | <0.0001 | <0.0001 |
| Time | p Value | <0.0001 | <0.0001 |
| Group × Time | p Value | <0.0001 | <0.0001 |
| Days of Storage | Batches | Microbial Counts | ||
|---|---|---|---|---|
| TVC | LAB | Psychrotrophic | ||
| 0 | ||||
| CON | 1.80 ± 0.19 aA | 1.53 ± 0.13 aA | 1.51 ± 0.14 aA | |
| BHT | 1.75 ± 0.21 aA | 1.60 ± 0.11 aA | 1.40 ± 0.16 abA | |
| SSRE | 1.47 ± 0.17 bA | 1.41 ± 0.13 bA | 1.30 ± 0.11 bA | |
| MSRE | 1.55 ± 0.15 bA | 1.35 ± 0.11 bA | 1.36 ± 0.13 bA | |
| 20 | ||||
| CON | 2.37 ± 0.15 aB | 2.34 ± 0.13 aB | 2.20 ± 0.14 B | |
| BHT | 2.44 ± 0.14 aB | 2.22 ± 0.13 acB | 2.24 ± 0.12 B | |
| SSRE | 2.19 ± 0.09 bB | 2.01 ± 0.27 bB | 2.15 ± 0.10 B | |
| MSRE | 2.21 ± 0.08 bB | 2.10 ± 0.28 bcB | 2.14 ± 0.14 B | |
| 40 | ||||
| CON | 3.37 ± 0.10 aC | 2.96 ± 0.44 aC | 3.18 ± 0.08 aC | |
| BHT | 3.44 ± 0.14 aC | 2.74 ± 0.32 abC | 3.10 ± 0.11 abC | |
| SSRE | 3.12 ± 0.13 bC | 2.56 ± 0.33 bC | 3.03 ± 0.09 bC | |
| MSRE | 3.20 ± 0.12 bC | 2.70 ± 0.37 abC | 3.03 ± 0.10 bC | |
| 60 | ||||
| CON | 4.52 ± 0.14 aD | 4.22 ± 0.26 aD | 4.10 ± 0.19 aD | |
| BHT | 4.41 ± 0.09 bD | 3.95 ± 0.24 bD | 4.00 ± 0.11 aD | |
| SSRE | 3.98 ± 0.14 cD | 3.75 ± 0.33 bD | 3.80 ± 0.11 bD | |
| MSRE | 3.81 ± 0.06 dD | 3.70 ± 0.32 bD | 3.83 ± 0.13 bD | |
| 90 | ||||
| CON | 5.05 ± 0.16 aE | 4.71 ± 0.19 aE | 4.60 ± 0.17 aE | |
| BHT | 5.11 ± 0.15 aE | 4.63 ± 0.22 aE | 4.49 ± 0.14 aE | |
| SSRE | 4.69 ± 0.16 bE | 4.14 ± 0.17 bE | 4.00 ± 0.14 bE | |
| MSRE | 4.73 ± 0.11 bE | 4.16 ± 0.24 bE | 4.07 ± 0.14 bE | |
| Group | p Value | <0.0001 | <0.0001 | <0.0001 |
| Time | p Value | <0.0001 | <0.0001 | <0.0001 |
| Group × Time | p Value | <0.0001 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glišić, M.; Bošković Cabrol, M.; Čobanović, N.; Starčević, M.; Samardžić, S.; Veličković, I.; Maksimović, Z. The Effects of Sunflower and Maize Crop Residue Extracts as a New Ingredient on the Quality Properties of Pork Liver Pâtés. Foods 2024, 13, 788. https://doi.org/10.3390/foods13050788
Glišić M, Bošković Cabrol M, Čobanović N, Starčević M, Samardžić S, Veličković I, Maksimović Z. The Effects of Sunflower and Maize Crop Residue Extracts as a New Ingredient on the Quality Properties of Pork Liver Pâtés. Foods. 2024; 13(5):788. https://doi.org/10.3390/foods13050788
Chicago/Turabian StyleGlišić, Milica, Marija Bošković Cabrol, Nikola Čobanović, Marija Starčević, Stevan Samardžić, Ivona Veličković, and Zoran Maksimović. 2024. "The Effects of Sunflower and Maize Crop Residue Extracts as a New Ingredient on the Quality Properties of Pork Liver Pâtés" Foods 13, no. 5: 788. https://doi.org/10.3390/foods13050788
APA StyleGlišić, M., Bošković Cabrol, M., Čobanović, N., Starčević, M., Samardžić, S., Veličković, I., & Maksimović, Z. (2024). The Effects of Sunflower and Maize Crop Residue Extracts as a New Ingredient on the Quality Properties of Pork Liver Pâtés. Foods, 13(5), 788. https://doi.org/10.3390/foods13050788








