Preharvest Methyl Jasmonate Treatment Affects the Mineral Profile, Metabolites, and Antioxidant Capacity of Radish Microgreens Produced without Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
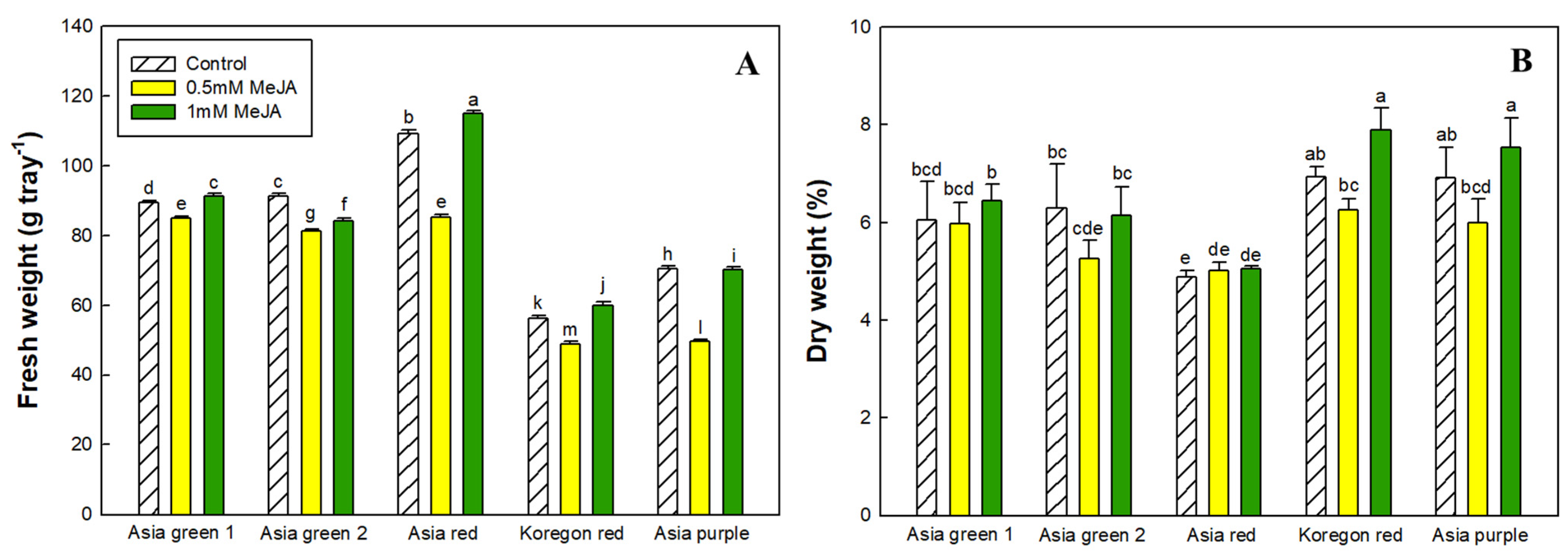
2.2. Fresh Weight and Dry Matter
2.3. Mineral Contents
2.4. Measurement of Amino Acids
2.5. Secondary Metabolites
2.5.1. Chlorophylls
- Chl a (mg g−1 dry weight) = [(12.7 × A663) − (2.69 × A645)] × (V/1000 × W)
- Chl b (mg g−1 dry weight) = [(22.9 × A645) − (4.68 × A663)] × (V/1000 × W)
- Total Chls = Chl a + Chl b
2.5.2. Anthocyanin Content
2.5.3. Vitamin C Content
2.5.4. Total Phenolics and Flavonoids
2.5.5. Glucosinolates Analysis
2.6. Antioxidant Capacity
2.7. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Fresh Weight, Dry Matter and Mineral Contents
3.2. Amino Acids
3.3. Secondary Metabolites
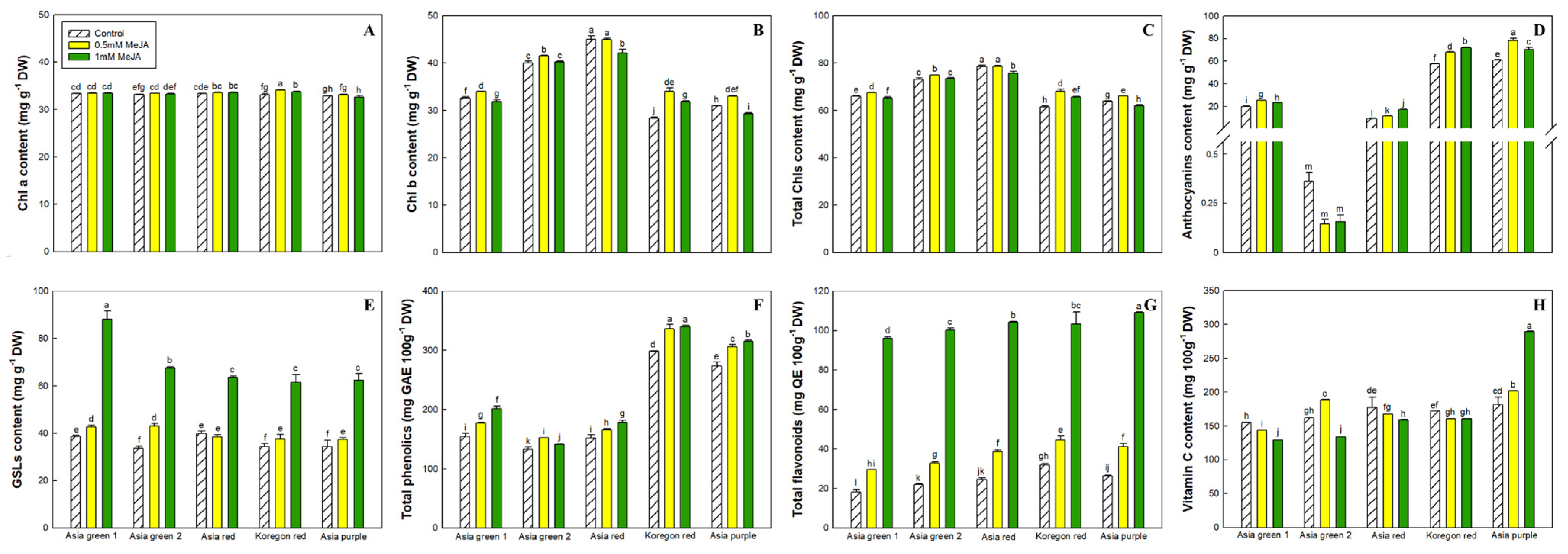
3.3.1. Chlorophylls and Anthocyanins
3.3.2. Glucosinolates
3.3.3. Total Phenolics and Flavonoids Content
3.3.4. Vitamin C Content
3.4. Antioxidant Capacity
3.5. Principal Component and Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramirez, D.; Abellán-Victorio, A.; Beretta, V.; Camargo, A.; Moreno, D.A. Functional Ingredients from Brassicaceae Species: Overview and Perspectives. Int. J. Mol. Sci. 2020, 21, 1998. [Google Scholar] [CrossRef]
- Baek, M.W.; Choi, H.R.; Lee, H.C.; Lee, J.H.; Lee, O.H.; Hong, J.S.; Jeong, C.S.; Tilahun, S. Preharvest Methyl Jasmonate and Salicylic Acid Treatments Improve the Nutritional Qualities and Postharvest Storability of Tomato. Sci. Hortic. 2023, 321, 112332. [Google Scholar] [CrossRef]
- Podsedek, A. Natural Antioxidants and Antioxidant Capacity of Brassica Vegetables: A Review. LWT—Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Hanlon, P.R.; Barnes, D.M. Phytochemical Composition and Biological Activity of 8 Varieties of Radish (Raphanus Sativus L.) Sprouts and Mature Taproots. J. Food Sci. 2011, 76, 185–192. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Rani, S.; Singh, N.; Maurya, S. The Comparative Nutrients Assessment of Spicer Salad: Radish Microgreens. Curr. Adv. Agric. Sci. 2018, 10, 107–111. [Google Scholar] [CrossRef]
- Teng, J.; Liao, P.; Wang, M. The Role of Emerging Micro-Scale Vegetables in Human Diet and Health Benefits—An Updated Review Based on Microgreens. Food Funct. 2021, 12, 1914–1932. [Google Scholar] [CrossRef]
- Teng, Z.; Luo, Y.; Pearlstein, D.J.; Wheeler, R.M.; Johnson, C.M.; Wang, Q.; Fonseca, J. Microgreens for Home, Commercial, and Space Farming: A Comprehensive Update of the Most Recent Developments. Annu. Rev. Food Sci. Technol. 2023, 14, 539–562. [Google Scholar] [CrossRef]
- Wang, J.; Mao, S.; Liang, M.; Zhang, W.; Chen, F.; Huang, K.; Wu, Q. Preharvest Methyl Jasmonate Treatment Increased Glucosinolate Biosynthesis, Sulforaphane Accumulation, and Antioxidant Activity of Broccoli. Antioxidants 2022, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Huey, R.B.; Carlson, M.; Crozier, L.; Frazier, M.; Hamilton, H.; Harley, C.; Hoang, A.; Kingsolver, J.G. Plants versus Animals: Do They Deal with Stress in Different Ways? Integr. Comp. Biol. 2002, 42, 415–423. [Google Scholar] [CrossRef]
- Wang, S.; Shi, X.; Liu, F.; Laborda, P. Effects of Exogenous Methyl Jasmonate on Quality and Preservation of Postharvest Fruits: A Review. Food Chem. 2021, 353, 129482. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y. Short- and Long-Distance Signaling in Plant Defense. Plant J. 2021, 105, 505–517. [Google Scholar] [CrossRef]
- Ali, M.S.; Baek, K.H. Jasmonic Acid Signaling Pathway in Response to Abiotic Stresses in Plants. Int. J. Mol. Sci. 2020, 21, 621. [Google Scholar] [CrossRef]
- Björkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.A.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E. Phytochemicals of Brassicaceae in Plant Protection and Human Health–Influences of Climate, Environment and Agronomic Practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Methyl Jasmonate and Salinity Increase Anthocyanin Accumulation in Radish Sprouts. Horticulturae 2019, 5, 62. [Google Scholar] [CrossRef]
- Park, W.T.; Kim, Y.B.; Seo, J.M.; Kim, S.J.; Chung, E.; Lee, J.H.; Park, S.U. Accumulation of Anthocyanin and Associated Gene Expression in Radish Sprouts Exposed to Light and Methyl Jasmonate. J. Agric. Food Chem. 2013, 61, 4127–4132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Q.; Li, W.; Zhang, S.; Xi, W. Identification of Key Genes and Regulators Associated with Carotenoid Metabolism in Apricot (Prunus Armeniaca) Fruit Using Weighted Gene Coexpression Network Analysis. BMC Genom. 2019, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.W.; Choi, H.R.; Solomon, T.; Jeong, C.S.; Lee, O.-H.; Tilahun, S. Preharvest Methyl Jasmonate Treatment Increased the Antioxidant Activity and Glucosinolate Contents of Hydroponically Grown Pak Choi. Antioxidants 2021, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, S.; Baek, M.W.; An, K.-S.; Choi, H.R.; Lee, J.H.; Hong, J.S.; Jeong, C.S. Radish Microgreens Produced without Substrate in a Vertical Multi-Layered Growing Unit Are Rich in Nutritional Metabolites. Front. Plant Sci. 2023, 14, 1236055. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.M.; Juvik, J.A. Environmental Stress and Methyl Jasmonate-Mediated Changes in Flavonoid Concentrations and Antioxidant Activity in Broccoli Florets and Kale Leaf Tissues. HortScience 2013, 48, 996–1002. [Google Scholar] [CrossRef]
- Vinklárková, B.; Chromý, V.; Šprongl, L.; Bittová, M.; Rikanová, M.; Ohnútková, I.; Žaludová, L. The Kjeldahl Method as a Primary Reference Procedure for Total Protein in Certified Reference Materials Used in Clinical Chemistry. II. Selection of Direct Kjeldahl Analysis and Its Preliminary Performance Parameters. Crit. Rev. Anal. Chem. 2015, 45, 112–118. [Google Scholar] [CrossRef]
- EPA. Method 3051A Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils. Z. Für Anal. Chem 2007, 111, 362–366. [Google Scholar]
- Henderson, J.W.; Ricker, R.D.; Bidlingmeyer, B.A.; Woodward, C. Rapid, Accurate, Sensitive, and Reproducible HPLC Analysis of Amino Acids. Amino Acids 2000, 1100, 1–10. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Tian, Z.; Pan, Z.; Feng, X. Identification and Quantification of Anthocyanins in Different Coloured Cultivars of Ornamental Kale (Brassica Oleracea L. Var. Acephala DC). J. Hortic. Sci. Biotechnol. 2018, 93, 466–473. [Google Scholar] [CrossRef]
- Kim, H.S.; Jung, J.Y.; Kim, H.K.; Ku, K.M.; Suh, J.K.; Park, Y.; Kang, Y.H. Influences of Meteorological Conditions of Harvest Time on Water-Soluble Vitamin Contents and Quality Attributes of Oriental Melon. J. Bio-Environ. Control 2011, 20, 290–296. [Google Scholar]
- Tilahun, S.; Jeong, M.J.; Choi, H.R.; Baek, M.W.; Hong, J.S.; Jeong, C.S. Prestorage High CO2 and 1-MCP Treatment Reduce Chilling Injury, Prolong Storability, and Maintain Sensory Qualities and Antioxidant Activities of “Madoka” Peach Fruit. Front. Nutr. 2022, 9, 903352. [Google Scholar] [CrossRef]
- Ku, K.M.; Jeffery, E.H.; Juvik, J.A. Exogenous Methyl Jasmonate Treatment Increases Glucosinolate Biosynthesis and Quinone Reductase Activity in Kale Leaf Tissue. PLoS ONE 2014, 9, e103407. [Google Scholar] [CrossRef]
- Han, N.; Ku, K.M.; Kim, J. Postharvest Variation of Major Glucosinolate and Their Hydrolytic Products in Brassicoraphanus ‘BB1’. Postharvest Biol. Technol. 2019, 154, 70–78. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Singh, N.; Aditika, S.; Rani, S.; Chaurasia, O.P. Vegetable microgreens farming in high-altitude region of Trans-Himalayas to maintain nutritional diet of Indian troops. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 743–752. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and Their in Vitro Bioactive Properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Almeida, A.A.; Aguiar, A.A.; Ferreira, I.M.P.L.V.O. Comparison between the Mineral Profile and Nitrate Content of Microgreens and Mature Lettuces. J. Food Compos. Anal. 2015, 37, 38–43. [Google Scholar] [CrossRef]
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, Microgreens and “Baby Leaf” Vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Springer: Berlin/Heidelberg, Germany, 2017; pp. 403–432. [Google Scholar]
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; Volume 916, ISBN 924120916X. [Google Scholar]
- Wu, G. Functional Amino Acids in Growth. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Benevenuto, R.F.; Seldal, T.; Hegland, S.J.; Rodriguez-Saona, C.; Kawash, J.; Polashock, J. Transcriptional Profiling of Methyl Jasmonate-Induced Defense Responses in Bilberry (Vaccinium Myrtillus L.). BMC Plant Biol. 2019, 19, 70. [Google Scholar] [CrossRef]
- Choi, H.R.; Baek, M.W.; Cheol, L.H.; Jeong, C.S.; Tilahun, S. Changes in Metabolites and Antioxidant Activities of Green ‘Hayward’ and Gold ‘Haegeum’ Kiwifruits during Ripening with Ethylene Treatment. Food Chem. 2022, 384, 132490. [Google Scholar] [CrossRef]
- Baek, M.W.; Choi, H.R.; Jae, L.Y.; Kang, H.M.; Lee, O.H.; Jeong, C.S.; Tilahun, S. Preharvest Treatment of Methyl Jasmonate and Salicylic Acid Increase the Yield, Antioxidant Activity and Gaba Content of Tomato. Agronomy 2021, 11, 2293. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments: A Review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, S.; Choi, H.R.; Baek, M.W.; Cheol, L.H.; Kwak, K.W.; Park, D.S.; Solomon, T.; Jeong, C.S. Antioxidant Properties, γ-Aminobutyric Acid (GABA) Content, and Physicochemical Characteristics of Tomato Cultivars. Agronomy 2021, 11, 1204. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant Activity of Chlorophylls and Their Derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Garcia, C.; Blesso, C.N. Antioxidant Properties of Anthocyanins and Their Mechanism of Action in Atherosclerosis. Free Radic. Biol. Med. 2021, 172, 152–166. [Google Scholar] [CrossRef]
- Demir, K.; Sarıkamış, G.; Çakırer Seyrek, G. Effect of LED Lights on the Growth, Nutritional Quality and Glucosinolate Content of Broccoli, Cabbage and Radish Microgreens. Food Chem. 2023, 401, 134088. [Google Scholar] [CrossRef] [PubMed]
- Mlinarić, S.; Piškor, A.; Melnjak, A.; Mikuška, A.; Šrajer Gajdošik, M.; Begović, L. Antioxidant Capacity and Shelf Life of Radish Microgreens Affected by Growth Light and Cultivars. Horticulturae 2023, 9, 76. [Google Scholar] [CrossRef]
- Yadav, L.P.; Koley, T.K.; Tripathi, A.; Singh, S. Antioxidant Potentiality and Mineral Content of Summer Season Leafy Greens: Comparison at Mature and Microgreen Stages Using Chemometric. Agric. Res. 2019, 8, 165–175. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Lv, J.; Li, J.; Gao, Y.; Patience, B.E.; Niu, T.; Yu, J.; Xie, J. Effect of Methyl Jasmonate Treatment on Primary and Secondary Metabolites and Antioxidant Capacity of the Substrate and Hydroponically Grown Chinese Chives. Front. Nutr. 2022, 9, 859035. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.J.; Choi, Y. Do Methyl Jasmonate as a Vital Substance in Plants. Trends Genet. 2003, 19, 409–413. [Google Scholar] [CrossRef]
- Reyes-Díaz, M.; Lobos, T.; Cardemil, L.; Nunes-Nesi, A.; Retamales, J.; Jaakola, L.; Alberdi, M.; Ribera-Fonseca, A. Methyl Jasmonate: An Alternative for Improving the Quality and Health Properties of Fresh Fruits. Molecules 2016, 21, 567. [Google Scholar] [CrossRef]
- Choi, H.R.; Baek, M.W.; Tilahun, S.; Jeong, C.S. Long-Term Cold Storage Affects Metabolites, Antioxidant Activities, and Ripening and Stress-Related Genes of Kiwifruit Cultivars. Postharvest Biol. Technol. 2022, 189, 111912. [Google Scholar] [CrossRef]
| Minerals (mg kg−1) DW | Asia Green 1 | Asia Green 2 | Asia Red | Koregon Red | Asia Purple | Significance Level | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.5 mM MeJA | 1.0 mM MeJA | Control | 0.5 mM MeJA | 1.0 mM MeJA | Control | 0.5 mM MeJA | 1.0 mM MeJA | Control | 0.5 mM MeJA | 1.0 mM MeJA | Control | 0.5 mM MeJA | 1.0 mM MeJA | Cultivr (A) | Treatment (B) | A × B | |
| N | 55,770 ± 40 e | 55,170 ± 53 h | 55,080 ± 97 i | 47,410 ± 83 n | 50,775 ± 80 l | 48,675 ± 93 m | 46,840 ± 78 o | 56,490 ± 62 d | 58,685 ± 43 b | 65,685 ± 63 a | 57,690 ± 63 c | 55,355 ± 39 f | 53,875 ± 65 k | 54,285 ± 49 j | 55,295 ± 43 g | *** | *** | *** |
| P | 9459 ± 8 o | 9722 ± 67 n | 9781 ± 48 m | 10,575 ± 67 j | 11,519 ± 7 c | 11,787 ± 43 a | 10,251 ± 72 k | 10,877 ± 22 g | 10,183 ± 5 l | 10,935 ± 5 f | 11,594 ± 28 b | 11,164 ± 66 e | 10,806 ± 13 h | 11,174 ± 7 d | 10,740 ± 61 i | *** | *** | *** |
| K | 7890 ± 4 i | 7148 ± 68 m | 6883 ± 31 n | 9568 ± 55 d | 7231 ± 3 k | 8120 ± 35 h | 10,372 ± 59 a | 9906 ± 53 b | 9706 ± 4 c | 7221 ± 4 l | 8404 ± 51 g | 7353 ± 62 j | 8526 ± 65 f | 8720 ± 5 e | 6650 ± 59 o | *** | *** | *** |
| Ca | 4040 ± 12 h | 4244 ± 41 g | 3997 ± 10 i | 3547 ± 63 n | 3552 ± 21 m | 3658 ± 12 k | 3536 ± 64 o | 3648 ± 38 l | 3692 ± 2 j | 4698 ± 3 f | 5282 ± 87 b | 5141 ± 12 c | 5034 ± 42 d | 5395 ± 13 a | 4822 ± 63 e | *** | *** | *** |
| Mg | 3474 ± 21 k | 3615 ± 12 d | 3600 ± 29 e | 3548 ± 23 h | 3873 ± 16 a | 3872.34 a | 3511 ± 25 j | 3817 ± 38 b | 3532.91 i | 3404 ± 20 m | 3577 ± 36 f | 3629.89 c | 3433 ± 14 l | 3558 ± 32 g | 3509.50 j | *** | *** | *** |
| Na | 1414 ± 3.2 b | 1412 ± 2.9 b | 1083 ± 3.4 i | 1208 ± 7.1 e | 915 ± 2.2 m | 9880 ± 5.6 k | 1121 ± 6.8 h | 956 ± 1.8 l | 1015 ± 2.4 j | 1179 ± 3.7 g | 1304 ± 1.9 d | 1460 ± 6.9 a | 1194 ± 3.1 f | 1313 ± 1.3 c | 1196 ± 7.3 f | *** | *** | *** |
| Mn | 23.6 ± 0.1 l | 24.4 ± 4.1 k | 24.9 ± 0.1 j | 30.9 ± 2.4 c | 35.6 ± 0.1 a | 35.0 ± 0.1 b | 18.6 ± 2.5 o | 21.4 ± 2.3 m | 20.7 ± 0.1 n | 25.6 ± 0.1 h | 27.1 ± 2.3 d | 26.5 ± 0.3 f | 25.3 ± 4.1 i | 26.1 ± 0.1 g | 26.7 ± 0.3 e | *** | *** | *** |
| Fe | 67.67 ± 0.3 k | 73.59 ± 1.0 f | 71.27 ± 3.1 g | 59.99 ± 0.3 m | 69.53 ± 0.1 g | 64.21 ± 3.1 l | 57.00 ± 0.3 o | 68.13 ± 0.7 j | 58.96 ± 0.1 n | 77.83 ± 0.1 b | 77.16 ± 0.7 c | 74.49 ± 1.6 e | 68.52 ± 1.03 i | 74.98 ± 0.1 d | 79.16 ± 1.7 a | *** | *** | *** |
| Cu | 3.37 ± 0.1 b | 3.40 ± 0.3 b | 3.38 ± 0.34 b | 2.35 ± 0.3 f | 2.27 ± 0.1 g | 2.33 ± 0.3 f | 3.25 ± 0.3 d | 3.33 ± 0.1 c | 3.85 ± 0.1 a | 2.48 ± 0.1 e | 1.70 ± 0.3 i | 1.54 ± 0.6 j | 1.56 ± 0.1 j | 1.35 ± 0.1 k | 1.78 ± 0.6 h | *** | *** | *** |
| Zn | 31.12 ± 0.1 i | 41.30 ± 0.1 a | 32.41 ± 0.3 g | 27.89 ± 1.1 m | 36.29 ± 0.1 b | 34.09 ± 0.1 d | 27.32 ± 1.1 o | 35.42 ± 0.1 c | 33.71 ± 0.1 e | 27.65 ± 0.1 n | 29.66 ± 0.1 j | 32.90 ± 0.4 f | 28.55 ± 0.23 l | 31.35 ± 0.1 h | 28.86 ± 0.4 k | *** | *** | *** |
| Mo | 1.51 ± 0.1 a | 1.44 ± 0.2 b | 1.41 ± 0.1 c | 0.26 ± 0.4 g | 0.26 ± 0.1 g | 0.27 ± 0.1 g | 0.78 ± 0.4 e | 0.81 ± 0.2 d | 0.74 ± 0.1 f | 0.08 ± 0.1 i | 0.07 ± 0.2 i | 0.02 ± 0.4 j | 0.12 ± 0.2 h | 0.02 ± 0.1 j | 0.02 ± 0.4 j | *** | *** | *** |
| Total | 82,174 ± 59 i | 81,455 ± 125 j | 80,558 ± 347 k | 75,979 ± 212 n | 78,009 ± 96 l | 77,238 ± 342 m | 75,739 ± 207 o | 85,825 ± 577 d | 86,932 ± 59 c | 93,256 ± 76 a | 87,988 ± 584 b | 84,239 ± 56 f | 82,994 ± 16 g | 84,581 ± 66 e | 82,351 ± 52 h | *** | *** | *** |
| Amino Acids (mg kg−1) DW | Asia Green 1 | Asia Green 2 | Asia Red | Koregon Red | Asia Purple | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.5 mM MeJA | 1.0 mM MeJA | Control | 0.5 mM MeJA | 1.0 mM MeJA | Control | 0.5 mM MeJA | 1.0 mM MeJA | Control | 0.5 mM MeJA | 1.0 mM MeJA | Control | 0.5 mM MeJA | 1.0 mM MeJA | |
| Aspartic acid | 605 ± 7.4 g | 668 ± 4.7 e | 680 ± 8.9 e | 567 ± 10 i | 590 ± 4.1 gh | 575 ± 2.6 hi | 665 ± 1.7 e | 707 ± 3.1 d | 680 ± 2.3 e | 635 ± 0.6 f | 843 ± 4.6 a | 747.23 ± 6.9 c | 724 ± 4.4 d | 846 ± 0.6 a | 776 ± 13 b |
| Glutamic acid | 2545 ± 12 de | 2443 ± 25 gh | 2628 ± 25 c | 2114 ± 18 i | 2016 ± 13 j | 2017 ± 4.6 j | 2523 ± 5.9 ef | 2426 ± 11 h | 2486 ± 16 fg | 2580 ± 8.6 cd | 2619 ± 15 c | 2683 ± 15 b | 2705 ± 8.2 ab | 2736 ± 3.2 a | 2456 ± 36 gh |
| Asparagine | 4479 ± 11 e | 4465 ± 31 e | 3681 ± 9.3 h | 2862 ± 44 j | 2380 ± 14 k | 2453 ± 1.1 k | 5012 ± 16 c | 4736 ± 21 d | 4668 ± 21 d | 5530 ± 20 a | 4443 ± 13 e | 5116 ± 15 b | 4007 ± 11 f | 3785 ± 10 g | 2950 ± 35 i |
| Serine | 1400 ± 2.8 d | 1343 ± 10 e | 1547 ± 2.9 c | 1313 ± 19 f | 1060 ± 7.9 hi | 1061 ± 1.1 hi | 1125 ± 1.3 g | 1004 ± 3.6 j | 899 ± 3.9 k | 1079 ± 11 h | 864 ± 7.7 l | 1041 ± 3.5 i | 1731 ± 1.7 a | 1669 ± 1.6 b | 1544 ± 8.9 c |
| Glutamine | 34,484 ± 11 b | 28,867 ± 23 g | 30,138 ± 69 f | 29,830 ± 31 f | 22,449 ± 14 l | 24,024 ± 78 k | 33,311 ± 4.1 c | 27,077 ± 89 h | 26,712 ± 91 h | 37,506 ± 19 a | 25,241 ± 94 j | 31,029 ± 79 e | 32,835 ± 19 d | 26,077 ± 44 i | 21,472 ± 66 m |
| Histidine (E) | 5108 ± 12 ef | 5273 ± 46 bc | 5141 ± 6.4 de | 4942 ± 5.8 fg | 5059 ± 62 ef | 4798 ± 28 g | 5175 ± 39 cd | 5326 ± 39 ab | 4535 ± 23 h | 5483 ± 36 a | 5279 ± 44 bc | 4773 ± 18 g | 4956 ± 39 fg | 5478 ± 37 a | 4945 ± 19 fg |
| Glycine | 450 ± 1.6 a | 390 ± 1.2 c | 413 ± 17 b | 286 ± 8.1 d | 285 ± 5.9 d | 259 ± 5.8 e | 266 ± 8.5 de | 201 ± 1.6 f | 179 ± 0.9 g | 281 ± 8.1 d | 188 ± 6.1 fg | 205 ± 3.6 f | 467 ± 3.7 a | 458 ± 1.7 a | 393 ± 0.5 c |
| Threonine (E) | 2241 ± 1.8 d | 2133 ± 8.2 e | 2287 ± 28 d | 2011 ± 27 f | 1872 ± 14 h | 1897 ± 5.5 h | 1960 ± 25 g | 1545 ± 6.2 k | 1598 ± 26 j | 2094 ± 13 e | 1354 ± 9.4 l | 1782 ± 5.6 i | 2820 ± 11 a | 2570 ± 5.3 b | 2389 ± 3.3 c |
| Citrulline | 80 ± 0.3 d | 58 ± 1.3 g | 72 ± 3.2 e | 57 ± 0.53 g | 45 ± 2.8 h | 87 ± 0.4 c | 72 ± 1.5 e | 66 ± 2.5 f | 82 ± 1.1 cd | 81 ± 0.7 d | 74 ± 3.1 e | 93 ± 1.9 b | 110 ± 2.2 a | 85 ± 2.6 cd | 108 ± 0.6 a |
| Arginine | 1203 ± 6.6 h | 851 ± 11 i | 704 ± 4.6 k | 786 ± 9.1 j | 544 ± 4.9 l | 484 ± 6.7 m | 3356 ± 4.6 b | 2686 ± 12 d | 2219 ± 19 g | 2960 ± 27 c | 2262 ± 21 f | 2668 ± 21 d | 3849 ± 9.2 a | 2950 ± 4.1 c | 2371 ± 13 e |
| Alanine | 1196 ± 4.7 b | 864 ± 5.2 h | 1036 ± 11 e | 1152 ± 13 c | 699 ± 5.1 k | 824 ± 2.2 i | 1191 ± 0.5 b | 823 ± 2.5 i | 695 ± 4.3 k | 1091 ± 6.1 d | 773 ± 5.6 j | 769 ± 2.7 j | 1405 ± 2.9 a | 984 ± 0.9 f | 907 ± 7.1 g |
| GABA | 159 ± 0.9 e | 149 ± 0.5 fg | 138 ± 3.1 h | 172 ± 3.3 d | 138 ± 1.3 h | 159 ± 2.9 e | 148 ± 2.4 fg | 153 ± 0.5 ef | 108 ± 1.1 i | 173 ± 4.1 d | 144 ± 1.4 gh | 110 ± 1.7 i | 218 ± 0.6 c | 262 ± 1.4 a | 244 ± 2.9 b |
| Tyrosine | 302 ± 1.1 j | 257 ± 2.8 l | 276 ± 2.9 k | 368 ± 3.4 i | 281 ± 2.5 k | 278 ± 0.3 k | 671 ± 0.4 cd | 449 ± 1.8 f | 393 ± 3.5 g | 678 ± 5.4 c | 377 ± 6.1 h | 514 ± 1.3 e | 884 ± 1.7 a | 748 ± 0.7 b | 668 ± 3.1 d |
| Valine (E) | 1425 ± 3.3 h | 1406 ± 13 h | 1611 ± 21 f | 1297 ± 9.8 j | 1029 ± 6.1 m | 1095 ± 1.2 l | 1844 ± 4.9 d | 1346 ± 4.1 i | 1253 ± 6.8 k | 1792 ± 8.6 e | 1097 ± 4.6 l | 1534 ± 11 g | 2392 ± 6.2 a | 2111 ± 1.4 b | 2039 ± 13 c |
| Methionine (E) | 120 ± 1.4 b | 100 ± 1.4 e | 111 ± 3.1 cd | 77 ± 1.1 h | 73 ± 0.8 hi | 70 ± 0.1 i | 93 ± 1.3 f | 77 ± 0.1 h | 75 ± 0.2 h | 108 ± 1.1 d | 87 ± 0.6 g | 76 ± 0.3 h | 135 ± 1.1 a | 120 ± 0.7 b | 113 ± 2.2 c |
| Tryptophane (E) | 358 ± 2.2 i | 358 ± 3.3 i | 281 ± 8.5 k | 303 ± 3.3 j | 270 ± 1.2 k | 249 ± 3.2 l | 621 ± 7.3 b | 416 ± 2.7 f | 392 ± 1.6 gh | 748 ± 1.1 a | 384 ± 1.4 h | 445 ± 5.9 e | 575 ± 3.2 c | 494 ± 0.9 d | 402 ± 7.6 g |
| Phenylalanine (E) | 707 ± 2.4 e | 844 ± 11 d | 626 ± 5.1 f | 527 ± 2.2 h | 573 ± 5.5 g | 584 ± 0.9 g | 469 ± 2.4 i | 385 ± 1.9 k | 372 ± 2.9 k | 530 ± 8.7 h | 427 ± 7.8 j | 385 ± 9.3 k | 1154 ± 0.8 b | 1230 ± 1.6 a | 931 ± 3.1 c |
| Isoleucine (E) | 2673 ± 10 j | 2834 ± 34 i | 3175 ± 45 h | 2837 ± 48 i | 3438 ± 31 g | 2913 ± 40 i | 4470 ± 44 f | 5209 ± 27 c | 5527 ± 54 b | 4690 ± 76 e | 5150 ± 44 c | 5714 ± 31 a | 4481 ± 17 f | 5697 ± 22 a | 4868 ± 19 d |
| Ornitnine | 321 ± 1.4 cd | 337 ± 4.5 bc | 351 ± 19 b | 310 ± 4.4 d | 340 ± 1.2 b | 273 ± 4.5 e | 310 ± 6.5 d | 312 ± 1.2 d | 274 ± 4.1 e | 302 ± 2.8 d | 389 ± 2.5 a | 314 ± 4.6 d | 273 ± 5.3 e | 350 ± 0.6 b | 303 ± 1.8 d |
| Leucine (E) | 154 ± 1.8 f | 132 ± 1.9 h | 143 ± 1.5 g | 152 ± 1.4 f | 100 ± 0.2 j | 89 ± 0.3 k | 239 ± 0.8 b | 152 ± 3.1 f | 145 ± 1.3 g | 238 ± 1.3 b | 118 ± 0.6 i | 196 ± 1.3 e | 283 ± 1.4 a | 206 ± 0.2 c | 201 ± 1.2 d |
| Lysine (E) | 332 ± 3.6 gh | 254 ± 8.6 ij | 253 ± 14 ij | 356 ± 8.3 gh | 306 ± 7.1 hi | 229 ± 12 j | 930 ± 17 a | 637 ± 9.8 c | 426 ± 16 ef | 750 ± 15 b | 471 ± 8.8 d | 461 ± 18 de | 674 ± 13 c | 486 ± 4.2 d | 389 ± 5.5 fg |
| Proline | 269 ± 9.6 cde | 242 ± 0.7 e | 250 ± 9.9 de | 225 ± 6.9 ef | 177 ± 6.7 f | 237 ± 8.7 e | 341 ± 6.4 ab | 184 ± 8.1 f | 267 ± 2.9 cde | 322 ± 6.8 ab | 366 ± 6.9 a | 222 ± 2.1 ef | 298 ± 0.7 bcd | 250 ± 0.1 de | 307 ± 5.8 bc |
| Total E | 13,122 ± 11 h | 13,339 ± 56 gh | 13,631 ± 58 g | 12,506 ± 40 i | 12,724 ± 47 i | 11,928 ± 18 j | 15,804 ± 12 d | 15,098 ± 66 e | 14,327 ± 29 f | 16,438 ± 60 c | 14,372 ± 78 f | 15,370 ± 19 e | 17,476 ± 36 b | 18,395 ± 64 a | 16,279 ± 83 c |
| Total amino acids | 60,622 ± 84 d | 54,280 ± 79 g | 55,553 ± 58 f | 52,554 ± 78 h | 43,734 ± 31 k | 44,667 ± 10 j | 64,802 ± 5 c | 55,927 ± 25 f | 53,994 ± 35 g | 69,663 ± 61 a | 52,964 ± 55 h | 60,886 ± 68 d | 66,988 ± 74 b | 59,600 ± 68 e | 50,785 ± 56 i |
| Cultivar (A) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Treatment (B) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| AXB | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Glucosinolates (mg g−1 DW) | Asia Green 1 | Asia Green 2 | Asia Red | Koregon Red | Asia Purple | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.5 mM MeJA | 1.0 mM MeJA | Control | 0.5 mM MeJA | 1.0 mM MeJA | Control | 0.5 mM MeJA | 1.0 mM MeJA | Control | 0.5 mM MeJA | 1.0 mM MeJA | Control | 0.5 mM MeJA | 1.0 mM MeJA | |
| Glucoiberin | 0.26 ± 0.04 c | 0.04 ± 0.01 c | 23.35 ± 0.48 a | 0.32 ± 0.06 c | 0.04 ± 0.01 c | 23.44 ± 0.44 a | 0.22 ± 0.10 c | 0.04 ± 0.01 c | 13.18 ± 0.61 b | 0.25 ± 0.11 c | 0.03 ± 0.01 c | 20.37 ± 0.33 a | 0.27 ± 0.03 c | 0.04 ± 0.01 c | 21.93 ± 0.15 a |
| Glucoraphenin | 3.87 ± 0.16 de | 4.64 ± 0.25 d | 1.03 ± 0.01 g | 1.86 ± 0.13 fg | 3.81 ± 0.23 de | 1.09 ± 0.01 fg | 1.37 ± 0.19 fg | 3.93 ± 0.15 de | 2.69 ± 0.16 ef | 14.05 ± 0.65 ab | 11.78 ± 0.63 c | 2.56 ± 0.10 fg | 12.72 ± 0.82 bc | 14.64 ± 0.54 a | 2.42 ± 0.12 fg |
| Gluconapin | 0.01 ± 0.00 ab | 0.02 ± 0.01 a | 0.01 ± 0.00 ab | 0.00 ± 0.00 b | 0.01 ± 0.0 ab | 0.00 ± 0.0 b | 0.00 ± 0.0 b | 0.00 ± 0.0 b | 0.01 ± 0.0 ab | 0.00 ± 0.0 b | 0.00 ± 0.0 b | 0.01 ± 0.0 ab | 0.00 ± 0.0 b | 0.00 ± 0.0 b | 0.01 ± 0.01 ab |
| 4-Hydroxyglucobrassicin | 0.60 ± 0.03 c | 1.12 ± 0.04 ab | 0.13 ± 0.01 d | 0.65 ± 0.03 c | 1.25 ± 0.04 a | 0.13 ± 0.02 d | 0.64 ± 0.02 c | 1.36 ± 0.05 a | 0.73 ± 0.03 bc | 0.78 ± 0.03 bc | 0.99 ± 0.05 abc | 0.13 ± 0.01 d | 0.93 ± 0.03 abc | 0.81 ± 0.01 bc | 0.16 ± 0.03 d |
| Glucoerucin | 0.67 ± 0.02 de | 1.17 ± 0.05 b | 1.64 ± 0.02 a | 0.67 ± 0.12 d | 1.18 ± 0.05 b | 1.15 ± 0.05 bc | 1.08 ± 0.02 bc | 1.08 ± 0.03 bc | 1.16 ± 0.04 b | 0.48 ± 0.02 e | 0.68 ± 0.00 d | 1.07 ± 0.01 bc | 0.53 ± 0.02 de | 0.63 ± 0.00 de | 0.97 ± 0.01 c |
| Glucoraphastin | 30.77 ± 0.26 bc | 31.92 ± 0.68 bc | 60.19 ± 1.42 a | 27.70 ± 0.45 c | 31.42 ± 0.55 bc | 34.50 ± 1.76 b | 34.74 ± 0.73 b | 28.31 ± 0.61 c | 34.70 ± 1.59 b | 16.89 ± 0.08 d | 20.11 ± 0.48 d | 31.08 ± 0.80 bc | 17.92 ± 0.67 d | 17.46 ± 0.21 d | 28.75 ± 1.41 c |
| Glucobrassicin | 0.34 ± 0.12 g | 1.13 ± 0.08 ef | 1.01 ± 0.01 ef | 0.60 ± 0.05 fg | 2.42 ± 0.11 b | 1.75 ± 0.22 cd | 0.29 ± 0.01 g | 1.72 ± 0.11 cd | 1.53 ± 0.21 de | 0.32 ± 0.03 g | 1.99 ± 0.05 cd | 3.40 ± 0.29 a | 0.38 ± 0.03 g | 1.90 ± 0.04 cd | 2.06 ± 0.07 bc |
| 4-Methoxyglucobrassicin | 2.06 ± 0.05 b | 2.65 ± 0.14 a | 0.87 ± 0.01 g | 1.74 ± 0.13 def | 2.81 ± 0.11 a | 0.81 ± 0.08 g | 1.45 ± 0.04 f | 2.02 ± 0.11 bc | 0.87 ± 0.33 g | 1.58 ± 0.01 def | 1.89 ± 0.06 bcd | 0.71 ± 0.04 g | 1.50 ± 0.06 ef | 1.81 ± 0.02 cde | 0.72 ± 0.03 g |
| TGSLs | 38.57 ± 0.24 cde | 42.69 ± 0.36 cd | 88.22 ± 1.86 a | 33.54 ± 0.59 e | 42.94 ± 0.64 c | 62.87 ± 2.65 b | 39.80 ± 0.60 cde | 38.47 ± 0.47 cde | 54.86 ± 3.63 b | 34.35 ± 0.74 de | 37.47 ± 1.10 cde | 59.33 ± 2.84 b | 34.26 ± 1.54 de | 37.30 ± 0.49 cde | 57.01 ± 4.12 b |
| Cultivar (A) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Treatment (B) | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| AXB | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Parameters/Sample Concentration | Treatments | Cultivars | ||||
|---|---|---|---|---|---|---|
| Asia Green 1 | Asia Green 2 | Asia Red | Koregon Red | Asia Purple | ||
| DPPH 1.0 mg mL−1 | Control | 107.9 ± 3.8 def | 83.6 ± 5.7 g | 95.9 ± 6.2 fg | 139.8 ± 3.1 c | 148.0 ± 2.3 bc |
| 0.5 MeJA | 109.5 ± 1.4 de | 96.5 ± 3.0 f | 98.0 ± 1.6 ef | 149.8 ± 2.7 bc | 155.2 ± 9.4 ab | |
| 1 MeJA | 111.9 ± 0.6 d | 99.5 ± 4.9 ef | 105.4 ± 3.7 def | 156.8 ± 3.3 ab | 165.0 ± 2.8 a | |
| DPPH 2.5 mg mL−1 | Control | 142.5 ± 4.3 ef | 107.2 ± 3.8 h | 124.1 ± 1.1 g | 204.4 ± 0.9 c | 210.0 ± 2.0 c |
| 0.5 MeJA | 151.9 ± 3.0 d | 125.6 ± 3.3 g | 135.1 ± 1.7 f | 226.1 ± 0.3 b | 238.7 ± 0.3 a | |
| 1 MeJA | 155.1 ± 3.6 d | 127.0 ± 4.1 g | 143.3 ± 3.8 b | 224.4 ± 2.0 b | 244.4 ± 1.4 a | |
| DPPH 5 mg mL−1 | Control | 196.2 ± 2.0 e | 147.6 ± 3.0 h | 178.8 ± 0.2 f | 286.1 ± 0.1 b | 288.8 ± 0.1 b |
| 0.5 MeJA | 209.9 ± 2.3 d | 171.0 ± 0.6 g | 195.6 ± 2.4 e | 298.4 ± 0.6 a | 301.1 ± 0.3 a | |
| 1 MeJA | 221.8 ± 1.5 c | 177.3 ± 2.4 f | 198.2 ± 3.3 e | 301.2 ± 0.2 ba | 301.3 ± 0.7 a | |
| ABTS 1 mg mL−1 | Control | 6.0 ± 0.2 ef | 5.3 ± 0.1 g | 5.2 ± 0.2 g | 7.8 ± 0.2 c | 7.7 ± 0.3 c |
| 0.5 MeJA | 7.0 ± 0.2 d | 5.6 ± 0.1 fg | 6.4 ± 0.2 e | 8.9 ± 0.2 b | 9.4 ± 0.3 ab | |
| 1 MeJA | 7.7 ± 0.2 c | 6.0 ± 0.1 ef | 6.5 ± 0.3 de | 9.4 ± 0.2 ab | 9.6 ± 0.1 a | |
| ABTS 2.5 mg mL−1 | Control | 9.2 ± 0.1 gh | 6.8 ± 0.3 j | 7.7 ± 0.3 i | 14.1 ± 0.4 c | 13.3 ± 0.2 d |
| 0.5 MeJA | 10.0 ± 0.2 f | 9.5 ± 0.1 fg | 8.0 ± 0.1 i | 15.2 ± 0.1 b | 15.5 ± 0.1 b | |
| 1 MeJA | 11.1 ± 0.1 e | 7.7 ± 0.3 i | 8.9 ± 0.2 h | 16.3 ± 0.1 a | 15.8 ± 0.2 ab | |
| ABTS 5 mg mL−1 | Control | 13.9 ± 0.1 h | 10.8 ± 0.3 i | 14.3 ± 0.2 gh | 22.4 ± 0.2 d | 22.7 ± 0.3 d |
| 0.5 MeJA | 16.2 ± 0.1 f | 16.3 ± 0.2 f | 14.8 ± 0.1 g | 26.1 ± 0.2 c | 27.2 ± 0.1 b | |
| 1 MeJA | 17.3 ± 0.2 e | 13.9 ± 0.3 h | 14.2 ± 0.1 gh | 28.0 ± 0.3 a | 26.1 ± 0.1 c | |
| FRAP 1 mg mL−1 | Control | 13.6 ± 0.1 j | 11.4 ± 0.1 k | 14.2 ± 0.1 ij | 25.9 ± 0.1 e | 27.2 ± 0.1 d |
| 0.5 MeJA | 16.1 ± 0.2 fg | 13.5 ± 0.1 j | 14.7 ± 0.1 hi | 28.4 ± 0.2 c | 29.3 ± 0.2 bc | |
| 1 MeJA | 16.7 ± 0.5 f | 13.4 ± 0.2 j | 15.4 ± 0.1 gh | 29.5 ± 0.3 b | 31.6 ± 0.9 a | |
| FRAP 2.5 mg mL−1 | Control | 26.4 ± 0.2 i | 22.6 ± 0.1 k | 25.1 ± 0.1 j | 47.6 ± 0.7 d | 49.9 ± 0.2 c |
| 0.5 MeJA | 29.7 ± 0.1 ef | 27.5 ± 0.1 h | 28.1 ± 0.4 gh | 54.5 ± 0.3 b | 58.9 ± 0.2 a | |
| 1 MeJA | 30.4 ± 0.2 e | 25.2 ± 0.2 j | 28.7 ± 0.3 fg | 58.7 ± 0.6 a | 59.4 ± 0.3 a | |
| FRAP 5 mg mL−1 | Control | 51.0 ± 0.5 j | 45.0 ± 0.1 k | 50.4 ± 0.2 j | 93.6 ± 0.8 e | 95.8 ± 0.8 d |
| 0.5 MeJA | 56.2 ± 0.4 h | 51.4 ± 0.7 j | 57.7 ± 0.3 g | 102.1 ± 0.1 c | 108.8 ± 0.6 a | |
| 1 MeJA | 61.6 ± 0.3 f | 51.5 ± 0.2 j | 54.6 ± 0.3 i | 109.6 ± 0.1 a | 106.2 ± 0.1 b | |
| Significance (p) | Cultivar (A) | *** | *** | *** | *** | *** |
| Treatment (B) | *** | *** | *** | *** | *** | |
| AXB | *** | *** | *** | *** | *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tilahun, S.; Baek, M.W.; An, K.-S.; Choi, H.R.; Lee, J.H.; Tae, S.H.; Park, D.S.; Hong, J.S.; Jeong, C.S. Preharvest Methyl Jasmonate Treatment Affects the Mineral Profile, Metabolites, and Antioxidant Capacity of Radish Microgreens Produced without Substrate. Foods 2024, 13, 789. https://doi.org/10.3390/foods13050789
Tilahun S, Baek MW, An K-S, Choi HR, Lee JH, Tae SH, Park DS, Hong JS, Jeong CS. Preharvest Methyl Jasmonate Treatment Affects the Mineral Profile, Metabolites, and Antioxidant Capacity of Radish Microgreens Produced without Substrate. Foods. 2024; 13(5):789. https://doi.org/10.3390/foods13050789
Chicago/Turabian StyleTilahun, Shimeles, Min Woo Baek, Ki-Seok An, Han Ryul Choi, Jong Hwan Lee, Su Ho Tae, Do Su Park, Jin Sung Hong, and Cheon Soon Jeong. 2024. "Preharvest Methyl Jasmonate Treatment Affects the Mineral Profile, Metabolites, and Antioxidant Capacity of Radish Microgreens Produced without Substrate" Foods 13, no. 5: 789. https://doi.org/10.3390/foods13050789
APA StyleTilahun, S., Baek, M. W., An, K.-S., Choi, H. R., Lee, J. H., Tae, S. H., Park, D. S., Hong, J. S., & Jeong, C. S. (2024). Preharvest Methyl Jasmonate Treatment Affects the Mineral Profile, Metabolites, and Antioxidant Capacity of Radish Microgreens Produced without Substrate. Foods, 13(5), 789. https://doi.org/10.3390/foods13050789







