The Flavor Characteristics, Antioxidant Capability, and Storage Year Discrimination Based on Backpropagation Neural Network of Organic Green Tea (Camellia sinensis) during Long-Term Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Tea Samples and Chemicals
2.2. Determinations of Main Quality Components in Organic Green Tea
2.3. Intelligent Sensory Evaluation
2.3.1. Electronic Tongue Measurement
2.3.2. Colorimeter Measurement
2.4. Determination of In Vitro Antioxidant Activities
2.5. The Construction of a Classification Prediction Model Based on BPNN
2.6. Statistical Analysis
3. Results and Discussion
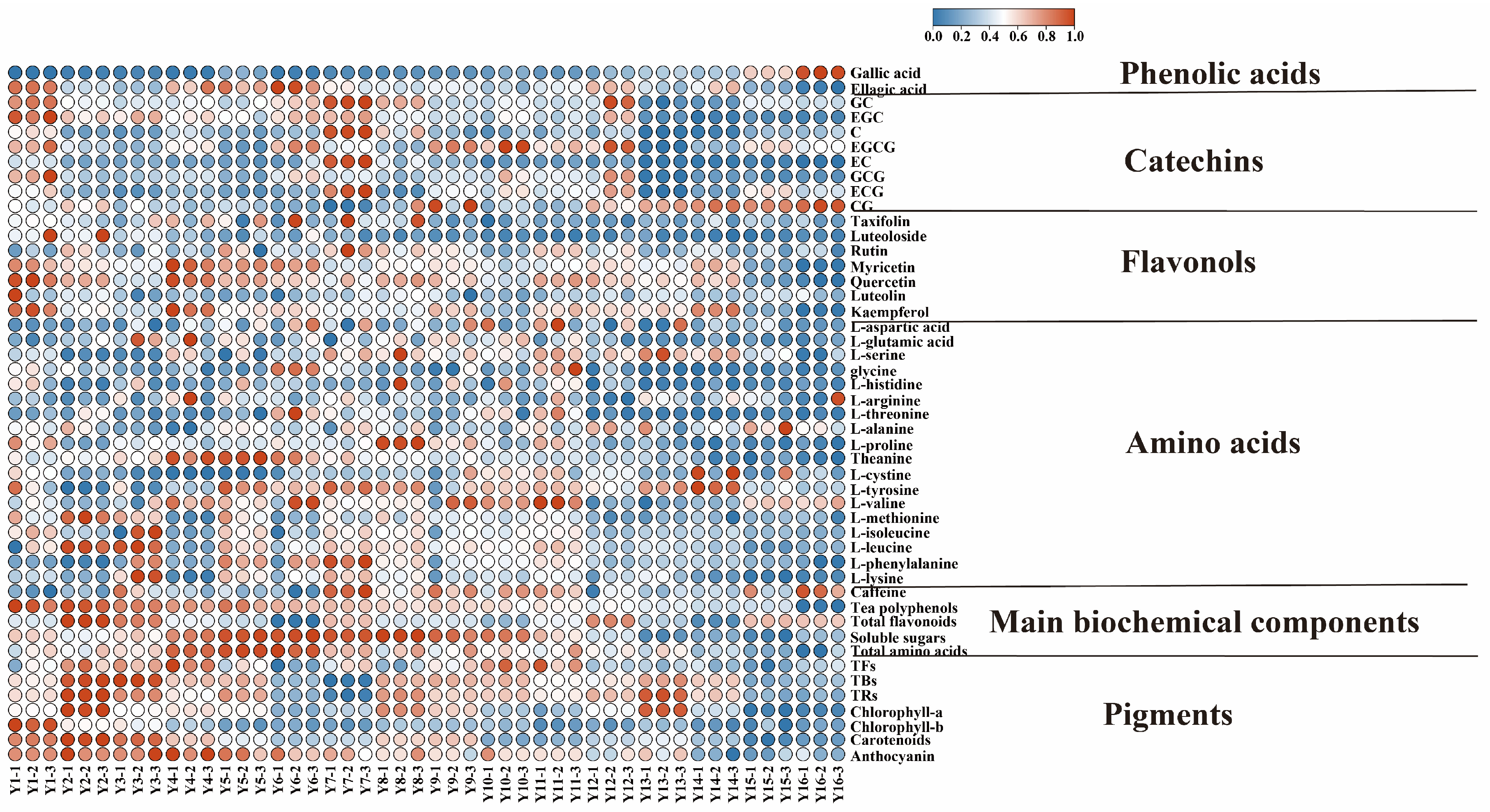
3.1. Dynamic Changes in Functional Components of Organic Green Tea during Storage
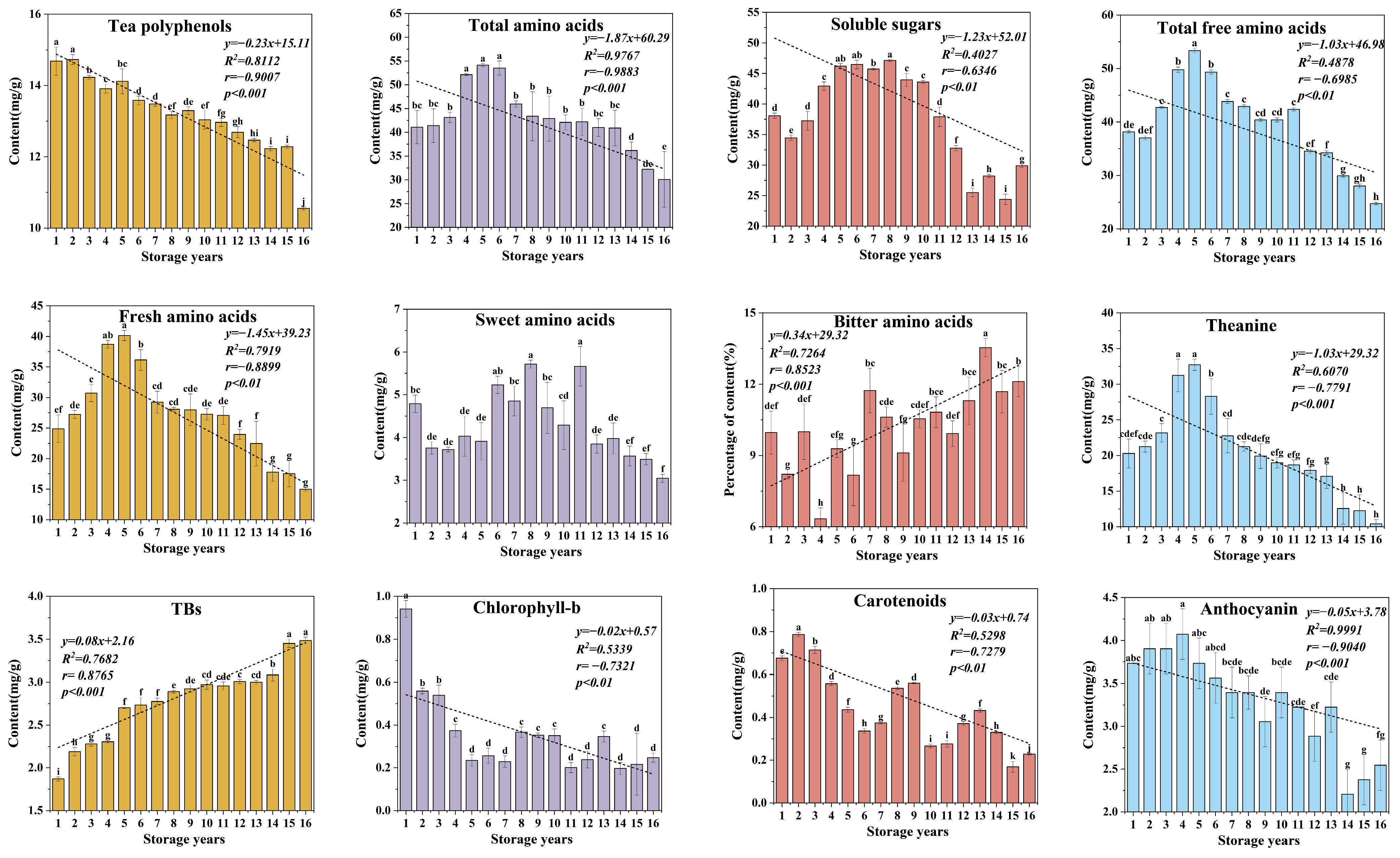
3.1.1. Comparative Analysis for the Contents of Biochemical Components of Organic Green Tea during Storage
3.1.2. Changes in Catechins and Gallic Acid of Organic Green Tea during Storage
3.1.3. Changes in Flavonols and Ellagic Acid of Organic Green Tea during Storage
3.1.4. Changes in Free Amino Acids of Organic Green Tea during Storage
3.1.5. Change in Pigments of Organic Green Tea during Storage
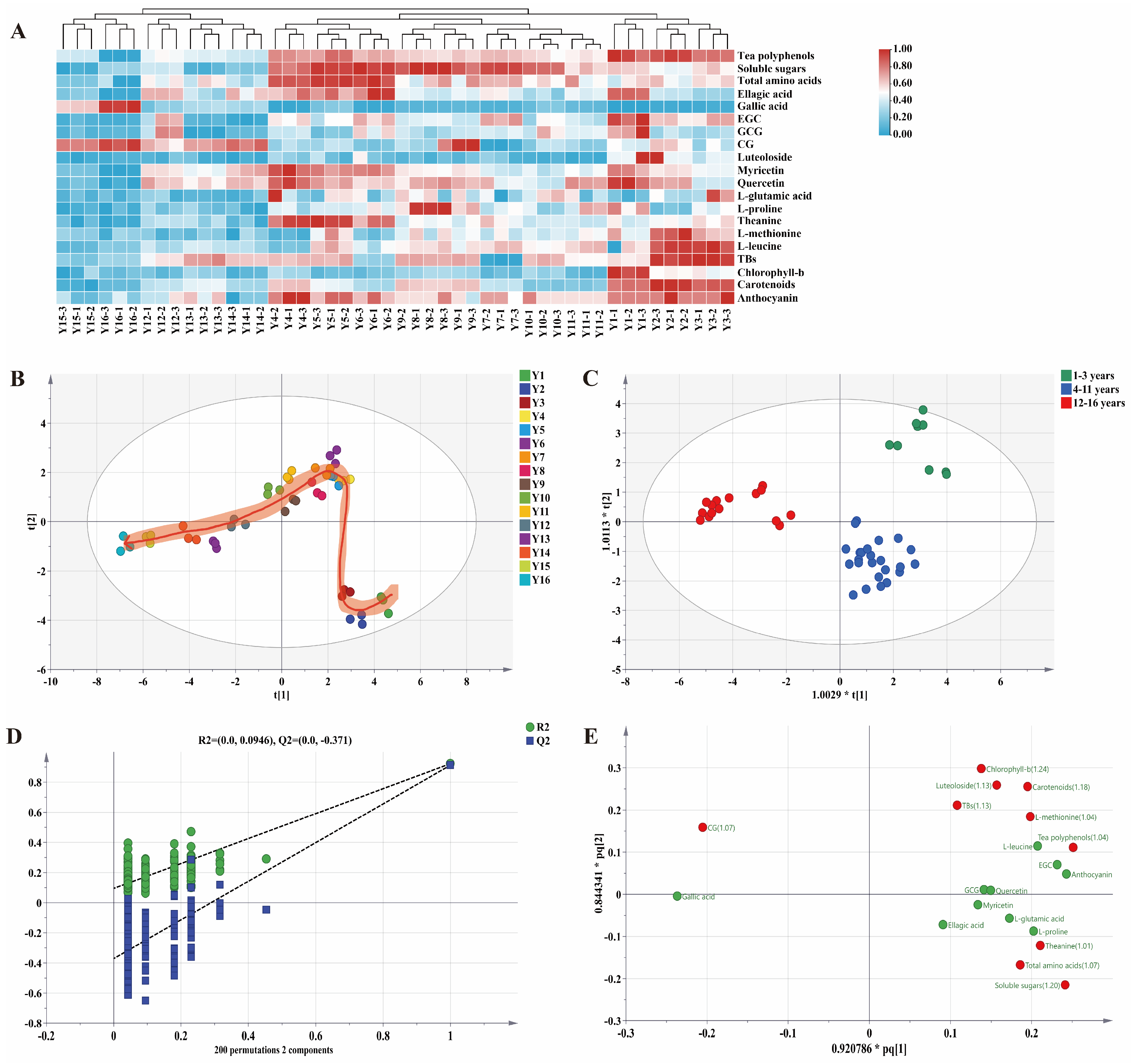
3.2. The Multivariate Statistical Analysis Results of Functional Components of Organic Green Tea during Storage
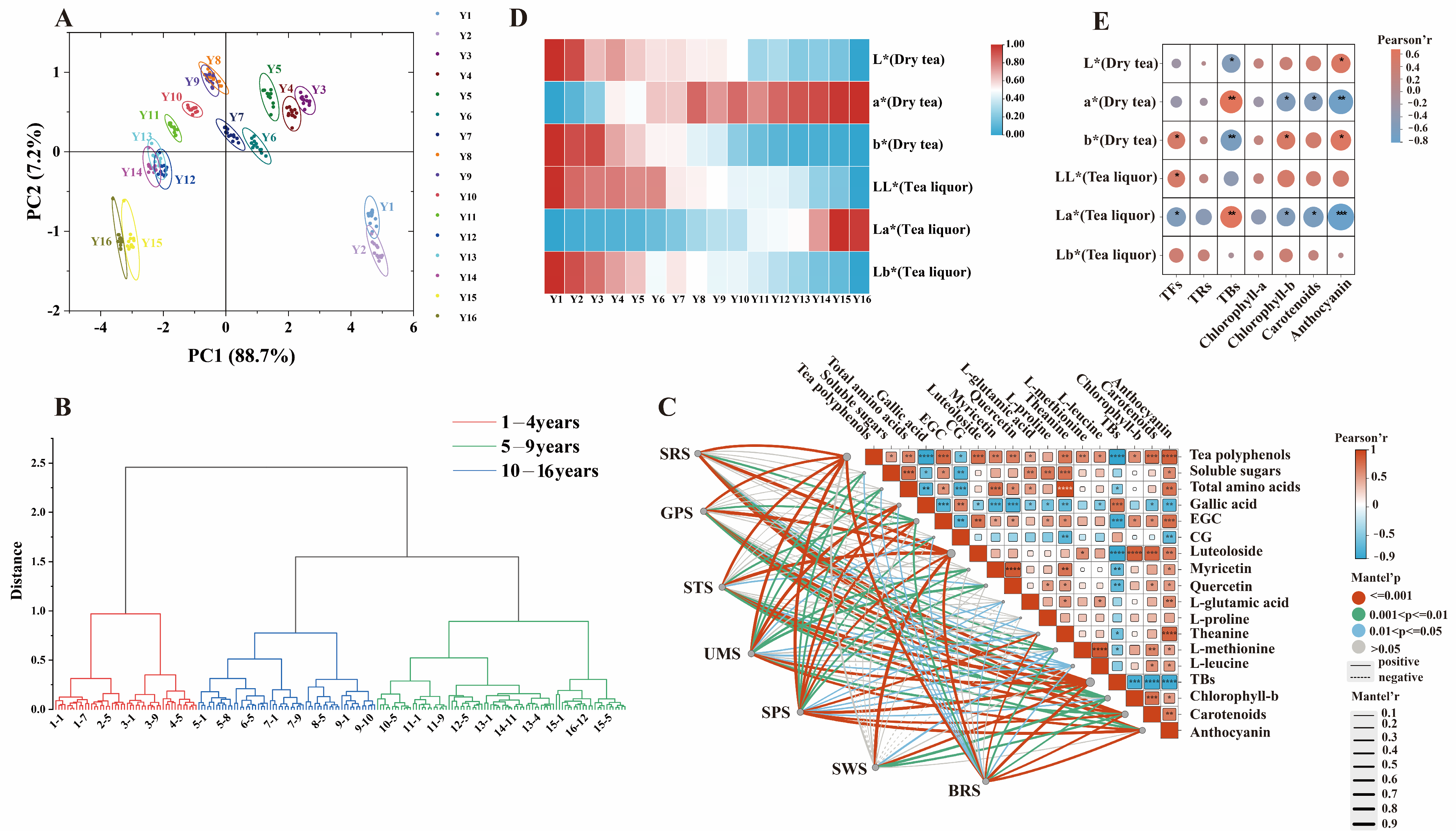
3.3. The Taste Analysis of Organic Green Tea during Storage Based on Electronic Tongue
3.4. The Chromatic Analysis of Organic Green Tea during Storage Based on Colorimeter
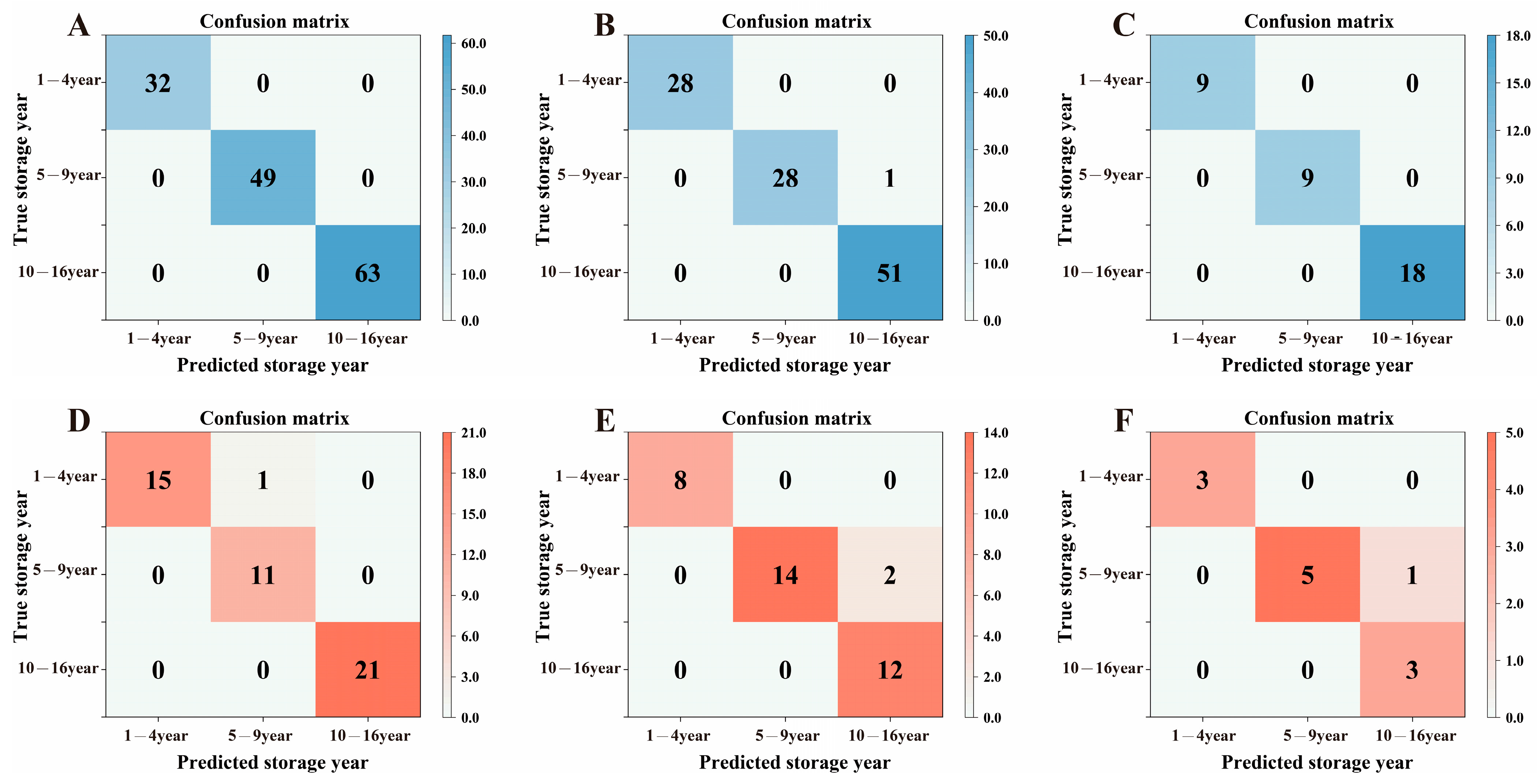
3.5. The Classification Predication Analysis of the Storage Years of Organic Green Tea Based on BPNN
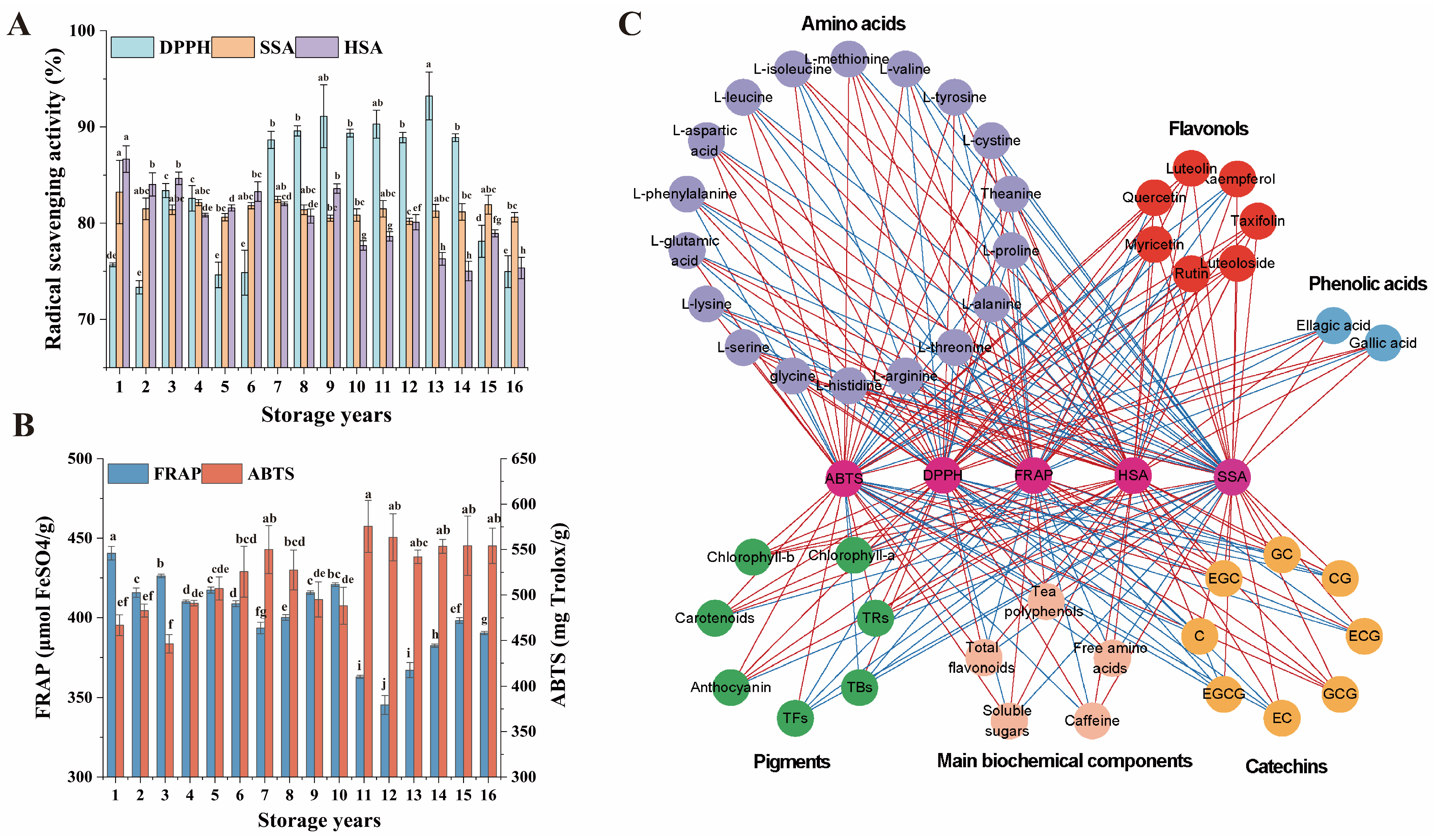
3.6. Comparative Analysis of the In Vitro Antioxidant Capacity among Organic Green Teas during Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engelhardt, U.H. Tea chemistry—What do and what don’t we know?—A micro review. Food Res. Int. 2020, 132, 109120. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Zhang, L.; Ho, C.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and biological activities of processed Camellia sinensis teas: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef]
- Luo, Q.; Luo, L.; Zhao, J.; Wang, Y.; Luo, H. Biological potential and mechanisms of Tea’s bioactive compounds: An Updated review. J. Adv. Res. 2023; in press. [Google Scholar] [CrossRef]
- Li, J.; Han, S.; Mei, X.; Wang, M.; Han, B. Changes in profiles of volatile compounds and prediction of the storage year of organic green tea during the long-term storage. Food Chem. 2024, 437, 137831. [Google Scholar] [CrossRef]
- Han, W.; Wang, D.; Fu, S.; Ahmed, S. Tea from organic production has higher functional quality characteristics compared with tea from conventional management systems in China. Biol. Agric. Hortic. 2018, 34, 120–131. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, Y.; Gao, C.; Wu, W.; Kong, J.; Zhou, Z.; Wang, Z.; Huang, Y.; Sun, W. The flavor characteristics and antioxidant capability of aged Jinhua white tea and the mechanisms of its dynamic evolution during long-term aging. Food Chem. 2024, 436, 137705. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Wen, S.; Sun, L.; Chen, R.; Zhang, Z.; Cao, J.; Lai, Z.; Li, Z.; Lai, X.; et al. Metabolomics reveals the effects of different storage times on the acidity quality and metabolites of large-leaf black tea. Food Chem. 2023, 426, 136601. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, S.; Li, Q.; Yuan, E.; Chen, R.; Yan, F.; Lai, X.; Zhang, Z.; Chen, Z.; Li, Q.; et al. Metabolomics and electronic tongue reveal the effects of different storage years on metabolites and taste quality of Oolong Tea. Food Control 2023, 152, 109847. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Zhang, J.; Zhu, J.; Liu, P.; Xu, L.; Wei, K.; Zhou, H.; Peng, L.; Zhang, J.; et al. Dynamic changes of metabolic profile and taste quality during the long-term aging of Qingzhuan Tea: The impact of storage age. Food Chem. 2021, 359, 129953. [Google Scholar] [CrossRef]
- Liu, H.; Zhuang, S.; Gu, Y.; Shen, Y.; Zhang, W.; Ma, L.; Xiao, G.; Wang, Q.; Zhong, Y. Effect of storage time on the volatile compounds and taste quality of Meixian green tea. LWT 2023, 173, 114320. [Google Scholar] [CrossRef]
- Dai, W.; Lou, N.; Xie, D.; Hu, Z.; Song, H.; Lu, M.; Shang, D.; Wu, W.; Peng, J.; Yin, P.; et al. N-ethyl-2-pyrrolidinone-substituted flavan-3-ols with anti-inflammatory activity in lipopolysaccharide-stimulated macrophages are storage-related marker compounds for green tea. J. Agric. Food Chem. 2020, 68, 12164–12172. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, Z.; Jia, Z.; Hou, M.; Yang, X.; Ying, X.; Ji, Z. Artificial neural network-based shelf life prediction approach in the food storage process: A review. Crit. Rev. Food Sci. Nutr. 2023; in press. [Google Scholar] [CrossRef]
- Xie, D.; Dai, W.; Lu, M.; Tan, J.; Zhang, Y.; Chen, M.; Lin, Z. Nontargeted metabolomics predicts the storage duration of white teas with 8-C N-ethyl-2-pyrrolidinone-substituted flavan-3-ols as marker compounds. Food Res. Int. 2019, 125, 108635. [Google Scholar] [CrossRef]
- Shen, S.; Huang, J.; Li, T.; Wei, Y.; Xu, S.; Wang, Y.; Ning, J. Untargeted and targeted metabolomics reveals potential marker compounds of an tea during storage. LWT 2022, 154, 112791. [Google Scholar] [CrossRef]
- Huang, A.; Jiang, Z.; Tao, M.; Wen, M.; Xiao, Z.; Zhang, L.; Zha, M.; Chen, J.; Liu, Z.; Zhang, L. Targeted and nontargeted metabolomics analysis for determining the effect of storage time on the metabolites and taste quality of keemun black tea. Food Chem. 2021, 359, 129950. [Google Scholar] [CrossRef]
- Jiang, L.; Zheng, K. Towards the intelligent antioxidant activity evaluation of green tea products during storage: A joint cyclic voltammetry and machine learning study. Food Control 2023, 148, 109660. [Google Scholar] [CrossRef]
- Li, L.; Huang, J.; Wang, Y.; Jin, S.; Li, M.; Sun, Y.; Ning, J.; Chen, Q.; Zhang, Z. Intelligent evaluation of storage period of green tea based on VNIR hyperspectral imaging combined with chemometric analysis. Infrared Phys. Technol. 2020, 110, 103450. [Google Scholar] [CrossRef]
- GB/T 8313; Determination of Total Polyphenols and Catechins Content in Tea. [NSPRC] National Standards of the People’s Republic of China: Beijing, China, 2018.
- GB/T 8314; Tea—Determination of Free Amino Acids Content. [NSPRC] National Standards of the People’s Republic of China: Beijing, China, 2013.
- Zhu, J.; Wang, J.; Yuan, H.; Ouyang, W.; Li, J.; Hua, J.; Jiang, Y. Effects of fermentation temperature and time on the color attributes and tea pigments of Yunnan Congou black tea. Foods 2022, 11, 1845. [Google Scholar] [CrossRef]
- Amulya, P.R.; ul Islam, R. Optimization of enzyme-assisted extraction of anthocyanins from eggplant (Solanum melongena L.) peel. Food Chem. X 2023, 18, 100643. [Google Scholar] [CrossRef]
- Xie, J.; Yao, S.; Ming, J.; Deng, L.; Zeng, K. Variations in chlorophyll and carotenoid contents and expression of genes involved in pigment metabolism response to oleocellosis in citrus fruits. Food Chem. 2019, 272, 49–57. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, M.; Cui, L.; Han, S.J.; Yu, P.; Han, B. Resistance of tea cultivars to the tea green leafhopper analyzed by EPG technique and their resistance-related substances. Acta Ecol. Sin. 2017, 37, 8015–8028. [Google Scholar]
- Wang, M.; Bo, X.; Han, S.; Wang, J.; Han, B. Difference of taste among streaming tea soup from tea plantation under different measures against frost based on electronic tongue. Trans. Chin. Soc. Agric. Eng. 2016, 32, 300–306. [Google Scholar]
- Nian, B.; Chen, L.; Yi, C.; Shi, X.; Jiang, B.; Jiao, W.; Liu, Q.; Lv, C.; Ma, Y.; Zhao, M. A high performance liquid chromatography method for simultaneous detection of 20 bioactive components in tea extracts. Electrophoresis 2019, 40, 2837–2844. [Google Scholar] [CrossRef]
- Ren, G.; Li, T.; Wei, Y.; Ning, J.; Zhang, Z. Estimation of Congou black tea quality by an electronic tongue technology combined with multivariate analysis. Microchem. J. 2021, 163, 105899. [Google Scholar] [CrossRef]
- GB/T 23776; Methodology for Sensory Evaluation of Tea. [NSPRC] National Standards of the People’s Republic of China: Beijing, China, 2013.
- Ma, B.; Wang, J.; Xu, C.; Wang, Z.; Yin, D.; Zhou, B.; Ma, C. Interrelation analysis between phenolic compounds and in vitro antioxidant activities in Pu-erh tea. LWT 2022, 158, 113117. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Adhikari, B.; Devahastin, S.; Wang, H. Double-layer indicator films aided by BP-ANN-enabled freshness detection on packaged meat products. Food Packag. Shelf Life 2022, 31, 100808. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Shi, W.; Wang, X. Comparison of Arrhenius model and artificial neuronal network for predicting quality changes of frozen tilapia (Oreochromis niloticus). Food Chem. 2022, 372, 131268. [Google Scholar] [CrossRef]
- Wan, X. Biochemistry of Tea, 3rd ed.; China Agriculture Press: Beijing, China, 2003. [Google Scholar]
- Zhao, F.; Chen, M.; Jin, S.; Wang, S.; Yue, W.; Zhang, L.; Ye, N. Macro-composition quantification combined with metabolomics analysis uncovered key dynamic chemical changes of aging white tea. Food Chem. 2022, 366, 130593. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gupta, K.; Sharma, A.; Das, M.; Ansari, I.A.; Dwivedi, P.D. Maillard reaction in food allergy: Pros and cons. Crit. Rev. Food Sci. Nutr. 2018, 58, 208–226. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, R.; Chen, Y.; Ho, C.T.; Hou, A.; Zhang, X.; Zhu, M.; Zhang, C.; Wang, Y.; Liu, Z.; et al. Dynamics changes in volatile profile, non-volatile metabolites and antioxidant activities of dark tea infusion during submerged fermentation with Eurotium cristatum. Food Biosci. 2023, 55, 102966. [Google Scholar] [CrossRef]
- Ye, L.; Wang, X.; Wang, R.; He, Y.; Zhou, L. Effects of amino acid foliar fertilizer on growth and tea quality of Lingyun Pekoe tea under organic planting conditions. J. Tea Commun. 2023, 50, 184–190. [Google Scholar]
- Xie, J. Studies on the Quality Chemical Composition of Wuyi Rock Tea under Different Storage Time; Fujian Agriculture and Forestry University: Fuzhou, China, 2018. [Google Scholar]
- Lv, H.; Feng, X.; Song, H.; Ma, S.; Hao, Z.; Hu, H.; Yang, Y.; Pan, Y.; Zhou, S.; Fan, F.; et al. Tea storage: A not thoroughly recognized and precisely designed process. Trends Food Sci. Technol. 2023, 140, 104172. [Google Scholar] [CrossRef]
- Šilarová, P.; Česlová, L.; Meloun, M. Fast gradient HPLC/MS separation of phenolics in green tea to monitor their degradation. Food Chem. 2017, 237, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Dai, W.; Lu, M.; Yan, Y.; Zhang, Y.; Chen, D.; Wu, W.; Gao, J.; Dong, M.; Lin, Z. New insights into the influences of baking and storage on the nonvolatile compounds in oolong tea: A nontargeted and targeted metabolomics study. Food Chem. 2022, 375, 131872. [Google Scholar] [CrossRef]
- El-Shahawy, A.A.G.; Dief, E.M.; El-Dek, S.I.; Farghali, A.A.; Abo El-Ela, F.I. Nickel-gallate metal–organic framework as an efficient antimicrobial and anticancer agent: In Vitro Study. Cancer Nanotechnol. 2023, 14, 60. [Google Scholar] [CrossRef]
- Li, Z.Q.; Yin, X.L.; Gu, H.W.; Zou, D.; Ding, B.; Li, Z.; Chen, Y.; Long, W.; Fu, H.; She, Y. Revealing the chemical differences and their application in the storage year prediction of Qingzhuan tea by SWATH-MS based metabolomics analysis. Food Res. Int. 2023, 173, 113238. [Google Scholar] [CrossRef]
- Li, Q.; Jin, Y.; Jiang, R.; Xu, Y.; Zhang, Y.; Luo, Y.; Huang, J.; Wang, K.; Liu, Z. Dynamic changes in the metabolite profile and taste characteristics of Fu brick tea during the manufacturing process. Food Chem. 2021, 344, 128576. [Google Scholar] [CrossRef]
- Ning, J.M.; Ding, D.; Song, Y.S.; Zhang, Z.Z.; Luo, X.; Wan, X.C. Chemical constituents analysis of white tea of different qualities and different storage times. Eur. Food Res. Technol. 2016, 242, 2093–2104. [Google Scholar] [CrossRef]
- Tao, M.; Xiao, Z.; Huang, A.; Chen, J.; Yin, T.; Liu, Z. Effect of 1–20 years storage on volatiles and aroma of Keemun congou black tea by solvent extraction-solid phase extraction-gas chromatography-mass spectrometry. LWT 2021, 136, 110278. [Google Scholar] [CrossRef]
- Zou, C.; Li, R.Y.; Chen, J.X.; Wang, F.; Gao, Y.; Fu, Y.Q.; Xu, Y.Q.; Yin, J.F. Zijuan tea-based kombucha: Physicochemical, sensorial, and antioxidant profile. Food Chem. 2021, 363, 130322. [Google Scholar] [CrossRef]
- Fan, F.Y.; Huang, C.S.; Tong, Y.L.; Guo, H.W.; Zhou, S.J.; Ye, J.H.; Gong, S.Y. Widely targeted metabolomics analysis of white peony teas with different storage time and association with sensory attributes. Food Chem. 2021, 362, 130257. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Mujumdar, A.S.; Yu, D. Pulse-spouted microwave freeze drying of raspberry: Control of moisture using ANN model aided by LF-NMR. J. Food Eng. 2021, 292, 110354. [Google Scholar] [CrossRef]
- Yang, C.; Wang, M.; Soung, H.; Tseng, H.; Lin, F.; Chang, K.; Tsai, C. Through its powerful antioxidative properties, l-theanine ameliorates vincristine-induced neuropathy in rats. Antioxidants 2023, 12, 803. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Yang, X.; Zhu, F.; Wu, D.; Li, H.; Gan, R. Green extraction, chemical composition, and in vitro antioxidant activity of theabrownins from Kangzhuan dark tea. Curr. Res. Food Sci. 2022, 5, 1944–1954. [Google Scholar] [CrossRef]
| Dry Tea | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y1 | Y2 | Y3 | Y4 | Y5 | Y6 | Y7 | Y8 | Y9 | Y10 | Y11 | Y12 | Y13 | Y14 | Y15 | Y16 | |
| Y1 | 0.00 | |||||||||||||||
| Y2 | 1.68 | 0.00 | ||||||||||||||
| Y3 | 6.45 | 4.99 | 0.00 | |||||||||||||
| Y4 | 6.13 | 4.47 | 3.04 | 0.00 | ||||||||||||
| Y5 | 8.39 | 6.72 | 3.76 | 2.32 | 0.00 | |||||||||||
| Y6 | 9.84 | 8.16 | 5.03 | 3.72 | 1.48 | 0.00 | ||||||||||
| Y7 | 9.70 | 8.02 | 4.97 | 3.58 | 1.36 | 0.17 | 0.00 | |||||||||
| Y8 | 10.92 | 9.25 | 6.24 | 4.80 | 2.68 | 1.24 | 1.34 | 0.00 | ||||||||
| Y9 | 11.09 | 9.42 | 6.53 | 5.00 | 2.89 | 1.49 | 1.57 | 0.46 | 0.00 | |||||||
| Y10 | 12.88 | 11.24 | 8.59 | 6.90 | 4.92 | 3.56 | 3.63 | 2.40 | 2.07 | 0.00 | ||||||
| Y11 | 16.69 | 15.02 | 11.48 | 10.60 | 8.34 | 6.88 | 7.02 | 5.86 | 5.67 | 4.30 | 0.00 | |||||
| Y12 | 16.60 | 14.94 | 11.60 | 10.52 | 8.32 | 6.85 | 6.98 | 5.76 | 5.54 | 3.95 | 0.76 | 0.00 | ||||
| Y13 | 18.20 | 16.53 | 12.96 | 12.11 | 9.86 | 8.39 | 8.53 | 7.35 | 7.16 | 5.68 | 1.53 | 1.74 | 0.00 | |||
| Y14 | 18.10 | 16.43 | 12.91 | 12.01 | 9.77 | 8.30 | 8.44 | 7.24 | 7.05 | 5.54 | 1.45 | 1.59 | 0.21 | 0.00 | ||
| Y15 | 18.88 | 17.21 | 13.59 | 12.77 | 10.52 | 9.06 | 9.20 | 8.01 | 7.84 | 6.36 | 2.21 | 2.42 | 0.69 | 0.84 | 0.00 | |
| Y16 | 21.93 | 20.26 | 16.29 | 15.85 | 13.55 | 12.13 | 12.28 | 11.17 | 11.04 | 9.72 | 5.45 | 5.81 | 4.07 | 4.24 | 3.42 | 0.00 |
| Tea Liquor | ||||||||||||||||
| Y1 | 0 | |||||||||||||||
| Y2 | 2.41 | 0.00 | ||||||||||||||
| Y3 | 3.28 | 0.92 | 0.00 | |||||||||||||
| Y4 | 3.54 | 1.44 | 0.73 | 0.00 | ||||||||||||
| Y5 | 4.37 | 2.24 | 1.38 | 0.84 | 0.00 | |||||||||||
| Y6 | 4.92 | 3.09 | 2.34 | 1.66 | 1.04 | 0.00 | ||||||||||
| Y7 | 7.72 | 5.35 | 4.45 | 4.27 | 3.52 | 3.63 | 0.00 | |||||||||
| Y8 | 8.13 | 5.78 | 4.87 | 4.65 | 3.88 | 3.89 | 0.49 | 0.00 | ||||||||
| Y9 | 8.63 | 6.29 | 5.37 | 5.14 | 4.36 | 4.29 | 1.01 | 0.52 | 0.00 | |||||||
| Y10 | 9.37 | 7.00 | 6.10 | 5.91 | 5.14 | 5.11 | 1.66 | 1.26 | 0.82 | 0.00 | ||||||
| Y11 | 9.51 | 7.17 | 6.26 | 6.03 | 5.25 | 5.15 | 1.87 | 1.41 | 0.91 | 0.43 | 0.00 | |||||
| Y12 | 9.77 | 7.44 | 6.53 | 6.26 | 5.46 | 5.29 | 2.19 | 1.70 | 1.18 | 0.81 | 0.44 | 0.00 | ||||
| Y13 | 10.65 | 8.33 | 7.41 | 7.15 | 6.34 | 6.13 | 3.05 | 2.57 | 2.06 | 1.50 | 1.21 | 0.89 | 0.00 | |||
| Y14 | 13.02 | 10.67 | 9.77 | 9.53 | 8.74 | 8.54 | 5.36 | 4.91 | 4.41 | 3.71 | 3.51 | 3.28 | 2.41 | 0.00 | ||
| Y15 | 14.11 | 11.77 | 10.87 | 10.64 | 9.85 | 9.64 | 6.49 | 6.04 | 5.54 | 4.84 | 4.64 | 4.40 | 3.55 | 1.18 | 0.00 | |
| Y16 | 17.12 | 14.75 | 13.86 | 13.65 | 12.86 | 12.67 | 9.43 | 9.01 | 8.51 | 7.77 | 7.62 | 7.40 | 6.54 | 4.13 | 3.08 | 0.00 |
| Date Sources | Hidden-Layer Neurons | MSE | R |
|---|---|---|---|
| Electronic tongue | 3 | 1.4339 × 10−6 | 1 |
| 4 | 3.7988 × 10−6 | 0.99999 | |
| 5 | 1.007 × 10−4 | 0.99984 | |
| 6 | 4.0372 × 10−7 | 1 | |
| 7 | 4.5154 × 10−4 | 0.99928 | |
| 8 | 3.4907 × 10−7 | 1 | |
| 9 | 1.7369 × 10−6 | 1 | |
| 10 | 5.0162 × 10−5 | 0.99992 | |
| 11 | 1.4991 × 10−5 | 0.99998 | |
| 12 | 9.9914 × 10−5 | 0.99984 | |
| 10 compounds (VIP ≥ 1) | 4 | 0.11556 | 0.81912 |
| 5 | 0.049652 | 0.92228 | |
| 6 | 0.0087612 | 0.98629 | |
| 7 | 0.014276 | 0.97766 | |
| 8 | 0.033338 | 0.94782 | |
| 9 | 0.0031345 | 0.99509 | |
| 10 | 0.010991 | 0.98280 | |
| 11 | 0.030782 | 0.95182 | |
| 12 | 0.0039172 | 0.99387 | |
| 13 | 0.0053279 | 0.99166 | |
| Chromatism | 3 | 0.032164 | 0.95102 |
| 4 | 0.049487 | 0.92464 | |
| 5 | 0.000813 | 0.99876 | |
| 6 | 0.001205 | 0.99816 | |
| 7 | 0.001968 | 0.99700 | |
| 8 | 0.025203 | 0.96162 | |
| 9 | 0.003561 | 0.99799 | |
| 10 | 0.001317 | 0.99799 | |
| 11 | 0.040915 | 0.93769 | |
| 12 | 0.001798 | 0.99726 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Han, S.; Wang, J.; Zhang, Y.; Tan, L.; Chen, C.; Han, B.; Wang, M. The Flavor Characteristics, Antioxidant Capability, and Storage Year Discrimination Based on Backpropagation Neural Network of Organic Green Tea (Camellia sinensis) during Long-Term Storage. Foods 2024, 13, 753. https://doi.org/10.3390/foods13050753
Wen X, Han S, Wang J, Zhang Y, Tan L, Chen C, Han B, Wang M. The Flavor Characteristics, Antioxidant Capability, and Storage Year Discrimination Based on Backpropagation Neural Network of Organic Green Tea (Camellia sinensis) during Long-Term Storage. Foods. 2024; 13(5):753. https://doi.org/10.3390/foods13050753
Chicago/Turabian StyleWen, Xiaomei, Shanjie Han, Jiahui Wang, Yanxia Zhang, Lining Tan, Chen Chen, Baoyu Han, and Mengxin Wang. 2024. "The Flavor Characteristics, Antioxidant Capability, and Storage Year Discrimination Based on Backpropagation Neural Network of Organic Green Tea (Camellia sinensis) during Long-Term Storage" Foods 13, no. 5: 753. https://doi.org/10.3390/foods13050753
APA StyleWen, X., Han, S., Wang, J., Zhang, Y., Tan, L., Chen, C., Han, B., & Wang, M. (2024). The Flavor Characteristics, Antioxidant Capability, and Storage Year Discrimination Based on Backpropagation Neural Network of Organic Green Tea (Camellia sinensis) during Long-Term Storage. Foods, 13(5), 753. https://doi.org/10.3390/foods13050753





