The Addition of Resveratrol-Loaded Emulsions to Yogurts: Physicochemical Characterization, In Vitro Bioaccessibility and NMR-Based Nutritional Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Resveratrol-Loaded Emulsion
2.3. Preparation of Yogurts
2.4. In Vitro Gastrointestinal Digestion
2.5. Sample Preparation for NMR Analysis
2.6. Determination of pH and Acidity of Yogurt Samples
2.7. Determination of Water-Holding Capacity (WHC) of Yogurt Samples
2.8. Texture Analysis of Yogurt Samples
2.9. Determination of Resveratrol Retention Rate
2.10. The Bioaccessibility of Resveratrol
2.11. 1H NMR Spectra Acquisition of Yogurt
2.12. Data Analysis
3. Results
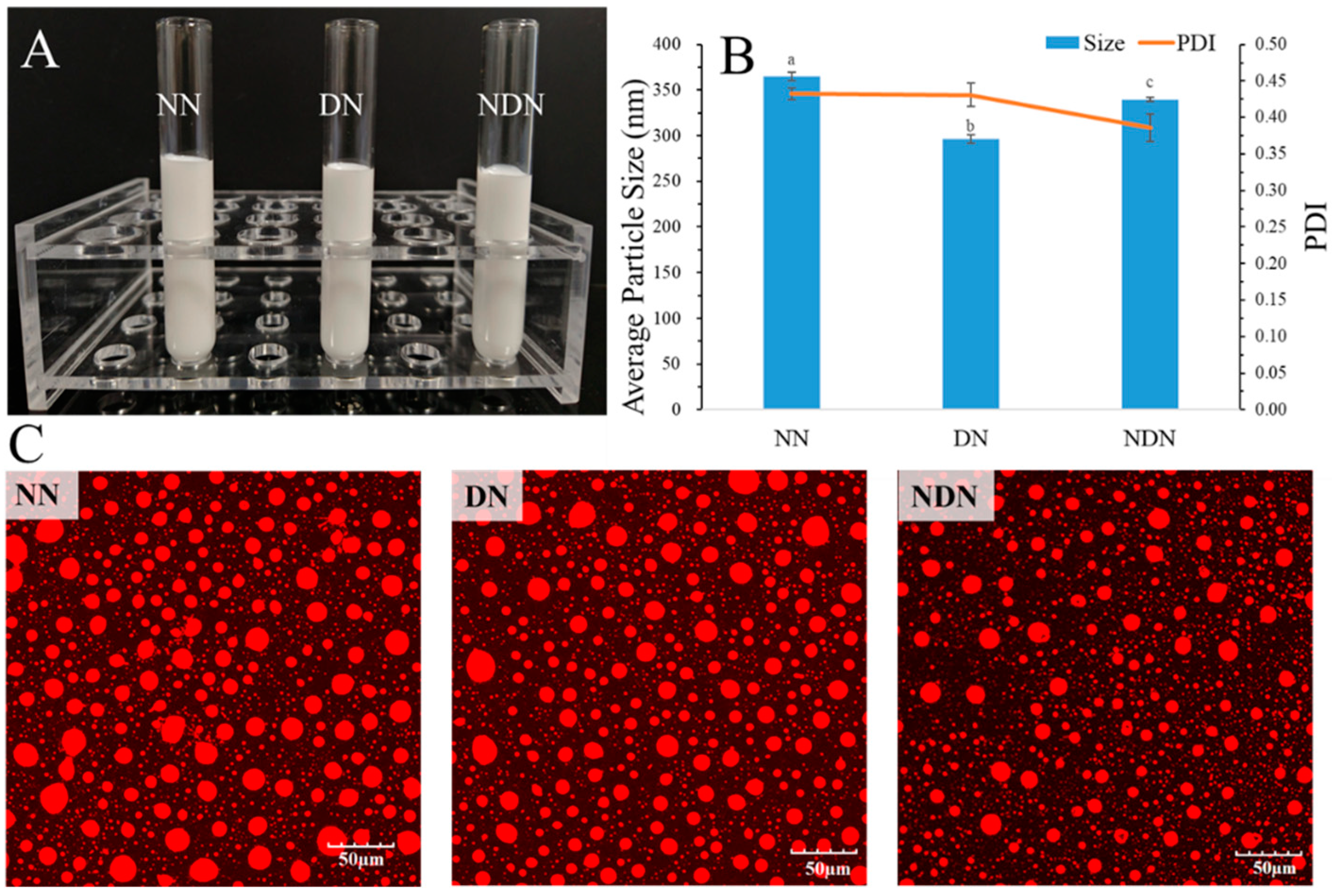
3.1. The Appearance and Particle Size of Emulsion Stablized by NaCas(NN), DGMO (DN), and NaCas/DGMO Particles (NDN)
3.2. The Physicochemical Properties of Yogurts
3.3. Retention of Resveratrol in Yogurt Samples
3.4. Bioaccessibility of Resveratrol in Yogurt Samples
3.5. Change in Yogurt Nutritional Profile before and after In Vitro Digestion
600 MHz 1H NMR Spectra of Yogurts
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farhan, M.; Rizvi, A. The Pharmacological Properties of Red Grape Polyphenol Resveratrol: Clinical Trials and Obstacles in Drug Development. Nutrients 2023, 15, 4486. [Google Scholar] [CrossRef]
- Focaccetti, C.; Palumbo, C.; Benvenuto, M.; Carrano, R.; Melaiu, O.; Nardozi, D.; Angiolini, V.; Lucarini, V.; Kërpi, B.; Masuelli, L.; et al. The Combination of Bioavailable Concentrations of Curcumin and Resveratrol Shapes Immune Responses While Retaining the Ability to Reduce Cancer Cell Survival. Int. J. Mol. Sci. 2024, 25, 232. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg. 2013, 19, 29–43. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Perez-Ciordia, S.; Marin-Arroyo, M.R.; McClements, D.J. Improving resveratrol bioaccessibility using biopolymer nanoparticles and complexes: Impact of protein-carbohydrate maillard conjugation. J. Agric. Food. Chem. 2015, 63, 3915–3923. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.P.; Sanguansri, L.; Augustin, M.A. Binding of resveratrol with sodium caseinate in aqueous solutions. Food Chem. 2013, 141, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Balakrishnan, A.; Shanmughan, P.; Maliakel, B.; Illathu Madhavamenon, K. Micelle/Hydrogel Composite as a “Natural Self-Emulsifying Reversible Hybrid Hydrogel (N’SERH)” Enhances the Oral Bioavailability of Free (Unconjugated) Resveratrol. ACS Omega 2022, 7, 12835–12845. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Chen, B.; Hu, C.Y.; Guo, Y.R.; Shen, Y.H.; Meng, Y.H. Resveratrol inhibits lipid and protein co-oxidation in sodium caseinate-walnut oil emulsions by reinforcing oil-water interface. Food Res. Int. 2022, 158, 111541. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, Q.; Rehman, A.; Jafari, S.M.; Zuo, M.; Khan, M.A.; Ali, A.; Khan, S.; Karim, A.; Usman, M.; Hussain, A.; et al. Improving the oxidative stability of fish oil emulsions by co-encapsulation with curcumin and resveratrol. Colloids Surf. B Biointerfaces 2021, 199, 111481. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Li, M.; Zhu, N.; Yao, L.; Fang, W.; Wu, Y.; Xu, H.; Hu, Z.; Xu, X. Unsaturated guluronate oligosaccharide used as a stabilizer of oil-in-water emulsions loaded with bioactive nutrients. Food Chem. X 2022, 16, 100469. [Google Scholar] [CrossRef] [PubMed]
- Martínez, N.N.; Hernández, J.T.; Morales, J.O. Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach. Nanotechnol. Rev. 2023, 12, 20220548. [Google Scholar] [CrossRef]
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017, 216, 146–152. [Google Scholar] [CrossRef]
- De Campo, C.; Assis, R.Q.; da Silva, M.M.; Costa, T.M.H.; Paese, K.; Guterres, S.S.; Rios, A.D.; Flôresa, S.H. Incorporation of zeaxanthin nanoparticles in yogurt: Influence on physicochemical properties, carotenoid stability and sensory analysis. Food Chem. 2019, 301, 125230. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Solomakou, N.; Kokkinomagoulos, E.; Kandylis, P. Yogurts Supplemented with Juices from Grapes and Berries. Foods 2020, 9, 1158. [Google Scholar] [CrossRef]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.J.; Frutos, M.-J. Microencapsulated saffron floral waste extracts as functional ingredients for antioxidant fortification of yogurt: Stability during the storage. LWT 2023, 184, 114976. [Google Scholar] [CrossRef]
- Chen, X.; He, Z.; He, L.; Li, C.; Tao, H.; Wang, X.; Liu, L.; Zeng, X.; Ran, G. Effects of perilla seed oil addition on the physicochemical properties, sensory, and volatile compounds of potato blueberry flavored yogurt and its shelf-life prediction. LWT 2023, 173, 114383. [Google Scholar] [CrossRef]
- Medina, L.; Aleman, R.S.; Cedillos, R.; Aryana, K.; Olson, D.W.; Marcia, J.; Boeneke, C. Effects of carao (Cassia grandis L.) on physico-chemical, microbiological and rheological characteristics of yogurt. LWT 2023, 183, 114891. [Google Scholar] [CrossRef]
- Qin, X.; Yang, C.; Si, J.; Chen, Y.; Xie, J.; Tang, J.; Dong, X.; Cheng, Y.; Hu, X.; Yu, Q. Fortified yogurt with high-quality dietary fiber prepared from the by-products of grapefruit by superfine grinding combined with fermentation treatment. LWT 2023, 188, 115396. [Google Scholar] [CrossRef]
- Shaheryar, M.; Afzaal, M.; Nosheen, F.; Imran, A.; Islam, F.; Noreen, R.; Shehzadi, U.; Shah, M.A.; Rasool, A. Functional exploration of taro starch (Colocasia esculenta) supplemented yogurt. Food Sci. Nutr. 2023, 11, 2697–2707. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhong, Q.L.; Guo, Y.H.; Xie, Y.F.; Cheng, Y.L.; Yao, W.R. Potential of resveratrol in mitigating advanced glycation end-products formed in baked milk and baked yogurt. Food Res. Int. 2020, 133, 109191. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.C.; Wang, Y.; Ren, Y.Y.; Ai, T.Y.; Zhou, P.Y.; Hu, L.; Wang, L.; Li, J.; Li, B. gastric emptying characteristics of konjac glucomannan with different viscosity and its effects on appetite regulation. Food Funct. 2020, 11, 7596–7610. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.L.; Yang, Z.X.; Chen, Y.F.; Yang, H.S. Konjac glucomannan decreases metabolite release of a plant-based fishball analogue during digestion by affecting amino acid and carbohydrate metabolic pathways. Food Hydrocoll. 2022, 129, 107623. [Google Scholar] [CrossRef]
- Li, Q.Q.; Lu, J.; Chang, Y.J.; Shen, G.P.; Feng, J.H. Effect of different cooking methods on nutritional intake and different storage treatments on nutritional losses of abalone. Food Chem. 2022, 377, 132047. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lim, S.Y.; Lau, H.; Ni, W.Z.; Li, S.F.Y. Effect of glycinebetaine on metabolite profiles of cold-stored strawberry revealed by 1H NMR-based metabolomics. Food Chem. 2022, 393, 133452. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, M.; He, J.; Lin, H.; Hu, Z.; Qi, Y.; Zhang, W. Preparation of decaglycerol monooleate-sodium caseinate complex and stabilization for citral. Mod. Food Sci. Technol. 2022, 38, 74–83. [Google Scholar]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Balandrano, D.D.; Beta, T.; Chai, Z.; Zhang, X.; Li, Y.; Huang, W. Effect of in vitro gastro-intestinal digestion on the phenolic composition and antioxidant capacity of Burdock roots at different harvest time. Food Chem. 2021, 358, 129897. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Coscia, A.; Orru, S.; Di Nicola, P.; Giuliani, F.; Varalda, A.; Peila, C.; Fabris, C.; Conti, A.; Bertino, E. Detection of cow’s milk proteins and minor components in human milk using proteomics techniques. J. Matern. Fetal Neonatal Med. 2012, 25, 57–59. [Google Scholar] [CrossRef]
- Ahrens, B.; de Oliveira, L.C.L.; Grabenhenrich, L.; Schulz, G.; Niggemann, B.; Wahn, U.; Beyer, K. Individual cow’s milk allergens as prognostic markers for tolerance development? Clin. Exp. Allergy 2012, 42, 1630–1637. [Google Scholar] [CrossRef]
- Guo, C.F.; Xin, L.; Dong, Y.H.; Zhang, X.Y.; Wang, X.J.; Fu, H.F.; Wang, Y.Y. Dielectric Properties of Yogurt for Online Monitoring of Fermentation Process. Food Bioprocess Technol. 2018, 11, 1096–1100. [Google Scholar] [CrossRef]
- Celik, O.F.; Temiz, H. Lactobacilli isolates as potential aroma producer starter cultures: Effects on the chemical, physical, microbial, and sensory properties of yogurt. Food Biosci. 2022, 48, 101802. [Google Scholar] [CrossRef]
- Le Bras, G.; Garel, J.R. Properties of D-lactate dehydrogenase from Lactobacillus bulgaricus: A possible different evolutionary origin for the D- and L-lactate dehydrogenases. FEMS Microbiol. Lett. 1991, 63, 89–93. [Google Scholar] [CrossRef]
- Wang, X.H.; Ren, H.Y.; Liu, D.Y.; Wang, B.; Zhu, W.Y.; Wang, W. H+-ATPase-Defective Variants of Lactobacillus delbrueckii subsp. bulgaricus Contribute to Inhibition of Postacidification of Yogurt during Chilled Storage. J. Food Sci. 2013, 78, M297–M302. [Google Scholar] [CrossRef] [PubMed]
- Settachaimongkon, S.; Nout, M.J.R.; Fernandes, E.C.A.; van Hooijdonk, T.C.M.; Zwietering, M.H.; Smid, E.J.; van Valenberg, H.J.F. The impact of selected strains of probiotic bacteria on metabolite formation in set yoghurt. Int. Dairy J. 2014, 38, 1–10. [Google Scholar] [CrossRef]
- Lucey, J.A. ADSA Foundation Scholar Award. Formation and physical properties of milk protein gels. J. Dairy Sci. 2002, 85, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Alu’datt, M.H.; Alli, I.; Nagadi, M. Preparation, characterization and properties of whey-soy proteins co-precipitates. Food Chem. 2012, 134, 294–300. [Google Scholar] [CrossRef]
- Isanga, J.; Zhang, G. Production and evaluation of some physicochemical parameters of peanut milk yoghurt. LWT-Food Sci. Technol. 2009, 42, 1132–1138. [Google Scholar] [CrossRef]
- Amar-Yuli, I.; Garti, N. Transitions induced by solubilized fat into reverse hexagonal mesophases. Colloids Surf. B Biointerfaces 2005, 43, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-H.; Hanley, T.; Porter, C.J.H.; Larson, I.; Boyd, B.J. Phytantriol and glyceryl monooleate cubic liquid crystalline phases as sustained-release oral drug delivery systems for poorly water soluble drugs I. Phase behaviour in physiologically-relevant media. J. Pharm. Pharmacol. 2010, 62, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Raigond, P.; Singh, Y.; Mishra, T.; Singh, B.; Lal, M.K.; Dutt, S. Recent updates on bioaccessibility of phytonutrients. Trends Food Sci. Technol. 2020, 97, 366–380. [Google Scholar] [CrossRef]
- Xu, D.; Yuan, F.; Gao, Y.; Panya, A.; McClements, D.J.; Decker, E.A. Influence of whey protein-beet pectin conjugate on the properties and digestibility of beta-carotene emulsion during in vitro digestion. Food Chem. 2014, 156, 374–379. [Google Scholar] [CrossRef]
- Torcello-Gomez, A.; Maldonado-Valderrama, J.; Martin-Rodriguez, A.; McClements, D.J. Physicochemical properties and digestibility of emulsified lipids in simulated intestinal fluids: Influence of interfacial characteristics. Soft Matter 2011, 7, 6167–6177. [Google Scholar] [CrossRef]
- Chu, B.-S.; Rich, G.T.; Ridout, M.J.; Faulks, R.M.; Wickham, M.S.J.; Wilde, P.J. Modulating Pancreatic Lipase Activity with Galactolipids: Effects of Emulsion Interfacial Composition. Langmuir 2009, 25, 9352–9360. [Google Scholar] [CrossRef] [PubMed]
- Ukmar-Godec, T.; Hutten, S.; Grieshop, M.P.; Rezaei-Ghaleh, N.; Cima-Omori, M.-S.; Biernat, J.; Mandelkow, E.; Söding, J.; Dormann, D.; Zweckstetter, M. Lysine/RNA-interactions drive and regulate biomolecular condensation. Nat. Commun. 2019, 10, 2909. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, L.; Concato, M.; Giacomello, E. Resveratrol, a Multitasking Molecule That Improves Skeletal Muscle Health. Nutrients 2023, 15, 3413. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, A.; Titone, R.; Follo, C.; Castiglioni, A.; Chiorino, G.; Dhanasekaran, D.N.; Isidoro, C. The protein restriction mimetic Resveratrol is an autophagy inducer stronger than amino acid starvation in ovarian cancer cells. Mol. Carcinog. 2017, 56, 2681–2691. [Google Scholar] [CrossRef]
- Rodríguez, M.S.; Montero, M.; Staffolo, M.D.; Martino, M.; Bevilacqua, A.; Albertengo, L. Chitosan influence on glucose and calcium availability from yogurt: In vitro comparative study with plants fibre. Carbohydr. Polym. 2008, 74, 797–801. [Google Scholar] [CrossRef]
- Alves, F.M.; Caldow, M.K.; Trieu, J.; Naim, T.; Montgomery, M.K.; Watt, M.J.; Lynch, G.S.; Koopman, R. Choline administration attenuates aspects of the dystrophic pathology in mdx mice. Clin. Nutr. Exp. 2019, 24, 83–91. [Google Scholar] [CrossRef]
- Randhawa, K.; Singh, V.; Kaur, S.; Kaur, R.; Kumar, S.; Shri, R. Isolation of Pleurotus florida derived acetylcholinesterase inhibitor for the treatment of cognitive dysfunction in mice. Food Sci. Hum. Wellness 2021, 10, 490–496. [Google Scholar] [CrossRef]








| Sample | NaCas (g/100 g) | DGMO (g/100 g) | Rice Bran Oil (g/100 g) | Resveratrol (g/100 g) | Water (g/100 g) |
|---|---|---|---|---|---|
| Y-C | - | - | - | 0.0075 | 15 |
| Y-R | - | - | - | ||
| Y-NN | 0.135 | - | 1.364 | ||
| Y-DN | - | 0.135 | 1.364 | ||
| Y-NDN | 0.0675 | 0.0675 | 1.364 |
| Sensory | Days | YC | Y-R | Y-NN | Y-DN | Y-NDN |
|---|---|---|---|---|---|---|
| Hardness/gf | 0 | 1.669 ± 0.001 a | 1.597 ± 0.074 a | 2.124 ± 0.059 a | 1.645 ± 0.177 a | 1.580 ± 0.289 a |
| 5 | 2.723 ± 0.048 b | 3.475 ± 0.039 c | 2.917 ± 0.128 bc | 1.871 ± 0.005 a | 1.845 ± 0.404 a | |
| 10 | 3.160 ± 0.074 b | 4.008 ± 0.039 c | 3.404 ± 0.297 b | 1.812 ± 0.159 a | 1.734 ± 0.023 a | |
| 15 | 1.673 ± 0.17 abc | 1.973 ± 0.007 bc | 2.269 ± 0.353 c | 1.043 ± 0.455 ab | 0.875 ± 0.142 a | |
| Chewiness/gf | 0 | 1.016 ± 0.207 a | 1.414 ± 0.069 a | 2.870 ± 0.045 a | 2.412 ± 1.184 a | 1.959 ± 1.110 a |
| 5 | 0.497 ± 0.067 a | 0.709 ± 0.709 a | 0.576 ± 0.174 a | 1.700 ± 0.322 a | 0.591 ± 0.306 a | |
| 10 | 0.989 ± 0.022 a | 2.045 ± 0.093 b | 1.194 ± 0.254 a | 2.307 ± 0.351 b | 1.172 ± 0.006 a | |
| 15 | 2.324 ± 1.306 a | 2.871 ± 0.972 a | 1.222 ± 0.257 a | 2.383 ± 1.864 a | 0.319 ± 0.056 a | |
| Gumminess/gf | 0 | 1.388 ± 0.091 a | 1.424 ± 0.060 a | 2.870 ± 0.045 a | 2.412 ± 1.184 a | 2.154 ± 0.914 a |
| 5 | 2.081 ± 0.015 a | 1.565 ± 1.565 a | 2.248 ± 0.200 a | 1.856 ± 0.166 a | 2.392 ± 1.196 a | |
| 10 | 2.797 ± 0.106 b | 3.695 ± 0.195 c | 2.899 ± 0.079 b | 2.307 ± 0.351 b | 1.323 ± 0.016 a | |
| 15 | 2.403 ± 1.227 a | 2.871 ± 0.972 a | 2.811 ± 1.133 a | 2.383 ± 1.864 a | 0.442 ± 0.068 a | |
| Cohesiveness/gf | 0 | 0.832 ± 0.055 a | 0.892 ± 0.004 a | 1.353 ± 0.058 a | 1.405 ± 0.569 a | 1.301 ± 0.341 a |
| 5 | 0.765 ± 0.019 a | 0.456 ± 0.456 a | 0.775 ± 0.103 a | 0.992 ± 0.086 a | 1.213 ± 0.383 a | |
| 10 | 0.885 ± 0.013 a | 0.921 ± 0.040 a | 0.860 ± 0.098 a | 1.300 ± 0.308 a | 0.763 ± 0.001 a | |
| 15 | 1.377 ± 0.594 a | 1.454 ± 0.488 a | 1.190 ± 0.314 a | 1.860 ± 0.977 a | 0.505 ± 0.005 a | |
| Resilience/gf | 0 | 0.603 ± 0.156 a | 0.588 ± 0.072 a | 0.812 ± 0.067 a | 0.447 ± 0.236 a | 0.685 ± 0.014 a |
| 5 | 0.387 ± 0.133 a | 0.376 ± 0.035 a | 0.269 ± 0.070 a | 0.360 ± 0.123 a | 0.345 ± 0.166 a | |
| 10 | 0.308 ± 0.004 ab | 0.294 ± 0.001 a | 0.282 ± 0.023 a | 0.720 ± 0.061 c | 0.427 ± 0.040 b | |
| 15 | 0.252 ± 0.132 a | 0.509 ± 0.087 a | 0.202 ± 0.072 a | 0.589 ± 0.140 a | 1.202 ± 0.678 a |
| Number | Compound Name | Functional Group | Chemical Shift (ppm) |
|---|---|---|---|
| 1 | L-Threonine | αCH | 3.52 (d) a |
| βCH | 4.17 (m) | ||
| γCH3 | 1.30 (d) | ||
| CH2 | 4.07 (m) | ||
| 2 | Lactic acid | α-CH | 4.08 (q) |
| β-CH3 | 1.33 (d) | ||
| 3 | Citric acid | 1CH | 2.66 (d) |
| 3CH | 2.58 (d) | ||
| 4 | Lecithin | 1CH2 | 2.81 (d) |
| 2CH | 3.89 (t) | ||
| 5 | D-Maltose | 1CH2 | 3.85 (m) |
| 2CH | 3.70 (m) | ||
| 4CH | 3.68 (m) | ||
| 6CH | 5.37 (d) | ||
| 8CH | 3.94 (m) | ||
| 9CH2 | 3.46 (dd) | ||
| 10CH | 5.21 (d) | ||
| 12CH2 | 3.79 (m) | ||
| 12CH2 | 3.92 (dd) | ||
| 6 | α-D-Glucose | 2CH | 3.58 (dd) |
| 3CH | 3.80 (dd) | ||
| 4CH | 3.49 (dd) | ||
| 5CH | 3.91 (dt) | ||
| 6CH | 3.85 (m) | ||
| 7 | β-D-Glucose | 1CH2 | 3.76 (d) |
| 2CH | 3.49 (dt) | ||
| 3CH | 3.40 (dd) | ||
| 4CH | 3.50 (dd) | ||
| 5CH | 3.28 (dd) | ||
| 6CH | 4.61 (d) | ||
| 8 | α-D-galactose | 1CH2 | 3.77 (quint) |
| 2CH | 3.78 (q) | ||
| 9 | β-D-galactose | 1CH | 4.57 (d) |
| 2CH | 3.49 (m) | ||
| 10 | Lactose | 2CH | 5.18 (td) |
| 3CH | 5.37 (d) | ||
| 5CH | 4.12 (dd) | ||
| 6CH | 3.87 (dd) | ||
| 8CH | 3.61 (dd) | ||
| 9CH | 3.90 (dd) | ||
| 10CH | 4.02 (dd) | ||
| 11CH | 3.86 (td) | ||
| 12CH2 | 3.75 (d) | ||
| 11 | α-D-Ribose | 1CH | 5.40 (d) |
| 2CH | 4.12 (q) | ||
| 12 | N-Acetyl-D-Glucosamine | 3CH | 4.44 (dd) |
| 5CH | 3.44 (dd) | ||
| 5.22 (dd) | |||
| 13 | Choline | 1CH3 | 3.22 (s) |
| 14 | Histidine | 2CH | 3.98 (dd) |
| 15 | Glycine | 1CH2 | 3.55 (s) |
| 16 | Lysine | 5CH2 | 3.02 (t) |
| 1CH | 3.75 (t) | ||
| 17 | Glycerol | 1CH2 | 3.60 (dd) |
| 2CH | 3.84 (m) | ||
| 18 | Sorbitol | 3.82 (dd) | |
| 3CH | 3.84 (dd) | ||
| 3.94 (ddd) | |||
| 19 | Sucrose | 3.69 (s) | |
| 4.22 (d) | |||
| 5.42 (d) | |||
| 20 | Malic acid | 2.36 (dd) | |
| 1CH | 2.68 (dd) | ||
| 2CH | 4.31 (dd) | ||
| 21 | Creatine | 2CH3 | 3.02 (s) |
| 22 | N-acety-glycoproteins | CH3 | 2.05 (s) |
| Number | Metabolite | δ1H (ppm) | Correlation Coefficient (r) a |
|---|---|---|---|
| 12 | N-acetyl-D-glucosamine | 5.219 | 0.842 |
| 13 | Choline | 3.225 | 0.883 |
| Number | Metabolite | δ1H (ppm) | Correlation Coefficient (r) a |
|---|---|---|---|
| 13 | Choline | 3.225 | 0.932 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Chen, H.; He, J.; Zhang, W.; Lin, H. The Addition of Resveratrol-Loaded Emulsions to Yogurts: Physicochemical Characterization, In Vitro Bioaccessibility and NMR-Based Nutritional Profiles. Foods 2024, 13, 426. https://doi.org/10.3390/foods13030426
Shi Z, Chen H, He J, Zhang W, Lin H. The Addition of Resveratrol-Loaded Emulsions to Yogurts: Physicochemical Characterization, In Vitro Bioaccessibility and NMR-Based Nutritional Profiles. Foods. 2024; 13(3):426. https://doi.org/10.3390/foods13030426
Chicago/Turabian StyleShi, Zihui, Huan Chen, Junbo He, Weinong Zhang, and Hong Lin. 2024. "The Addition of Resveratrol-Loaded Emulsions to Yogurts: Physicochemical Characterization, In Vitro Bioaccessibility and NMR-Based Nutritional Profiles" Foods 13, no. 3: 426. https://doi.org/10.3390/foods13030426
APA StyleShi, Z., Chen, H., He, J., Zhang, W., & Lin, H. (2024). The Addition of Resveratrol-Loaded Emulsions to Yogurts: Physicochemical Characterization, In Vitro Bioaccessibility and NMR-Based Nutritional Profiles. Foods, 13(3), 426. https://doi.org/10.3390/foods13030426







