The Effect of Co-Culture with Different Pichia kluyveri and Saccharomyces cerevisiae on Volatile Compound and Characteristic Fingerprints of Mulberry Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Mulberry Juice Fermentation
2.3. Methods
2.4. Statistical Analysis
3. Results and Discussion
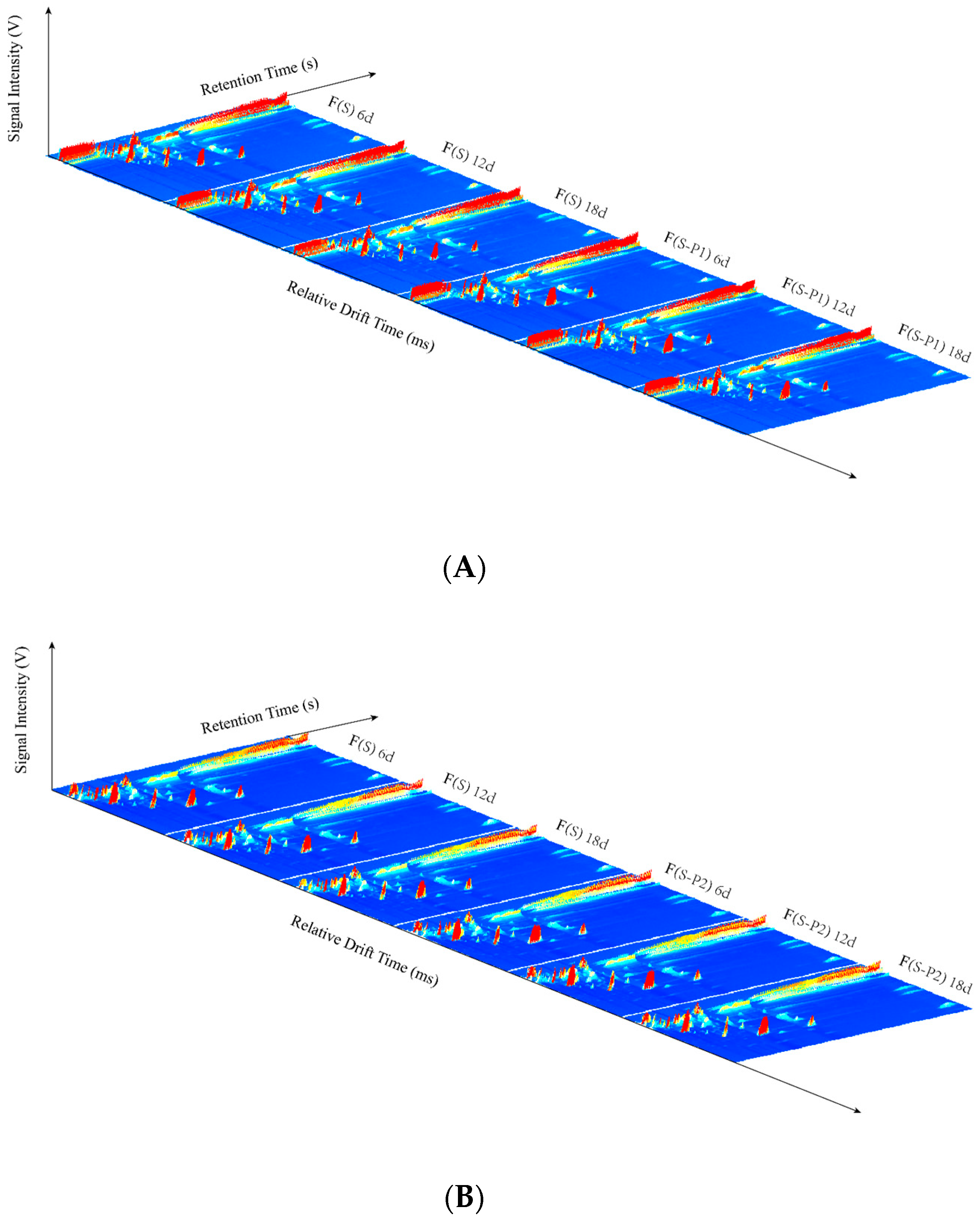
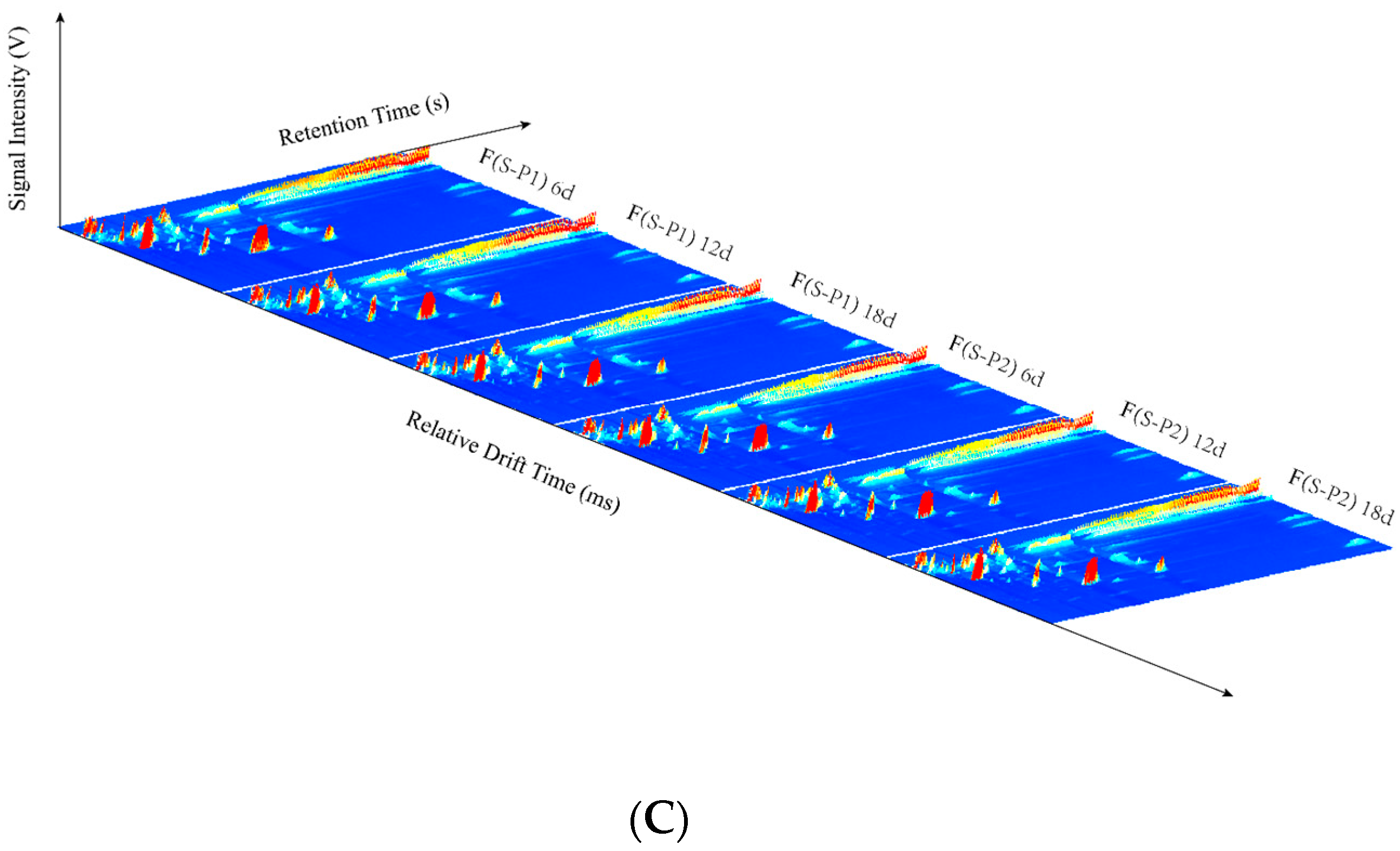
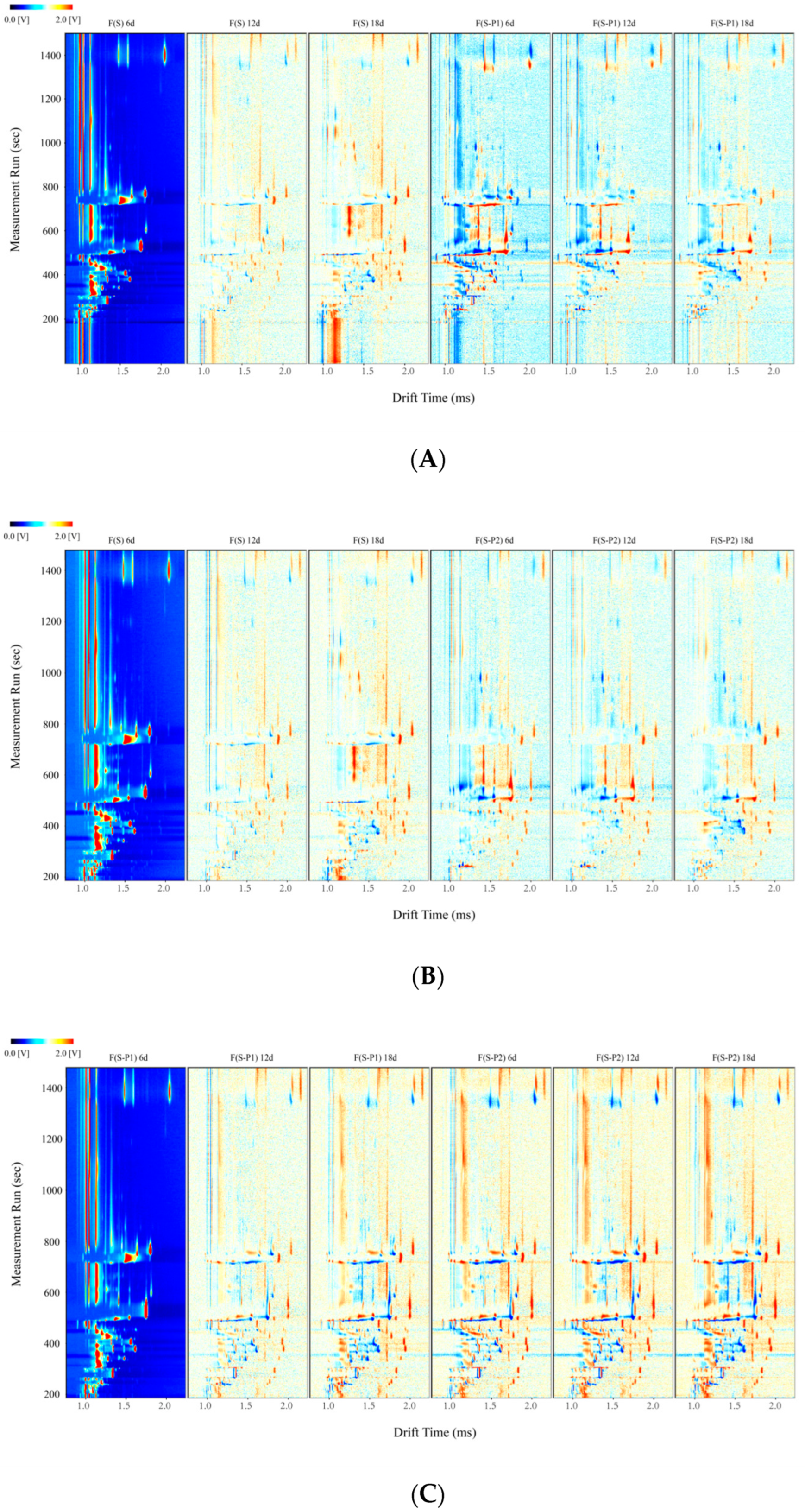
3.1. GC–IMS Topographic Plots in Mulberry Wine during Fermentation
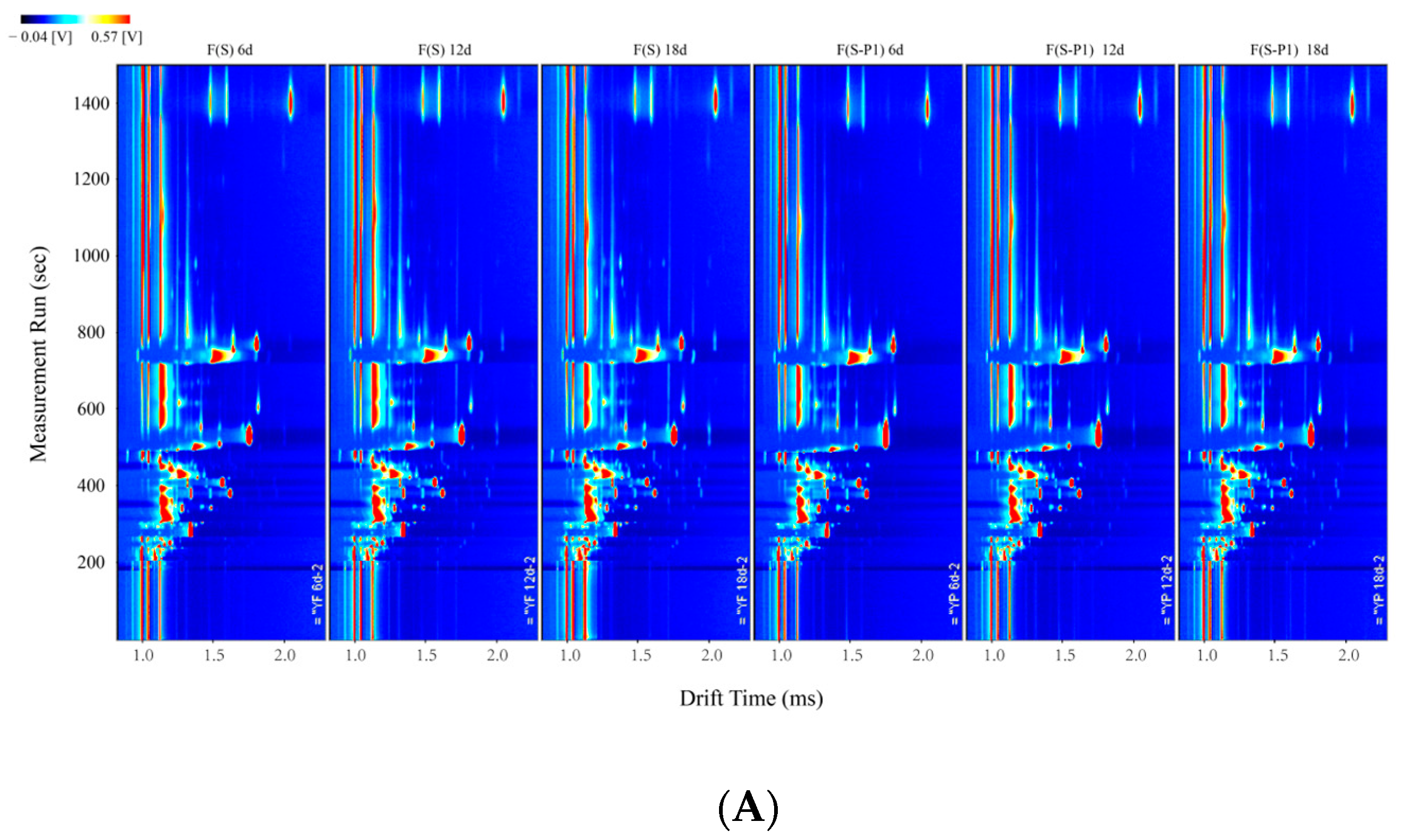
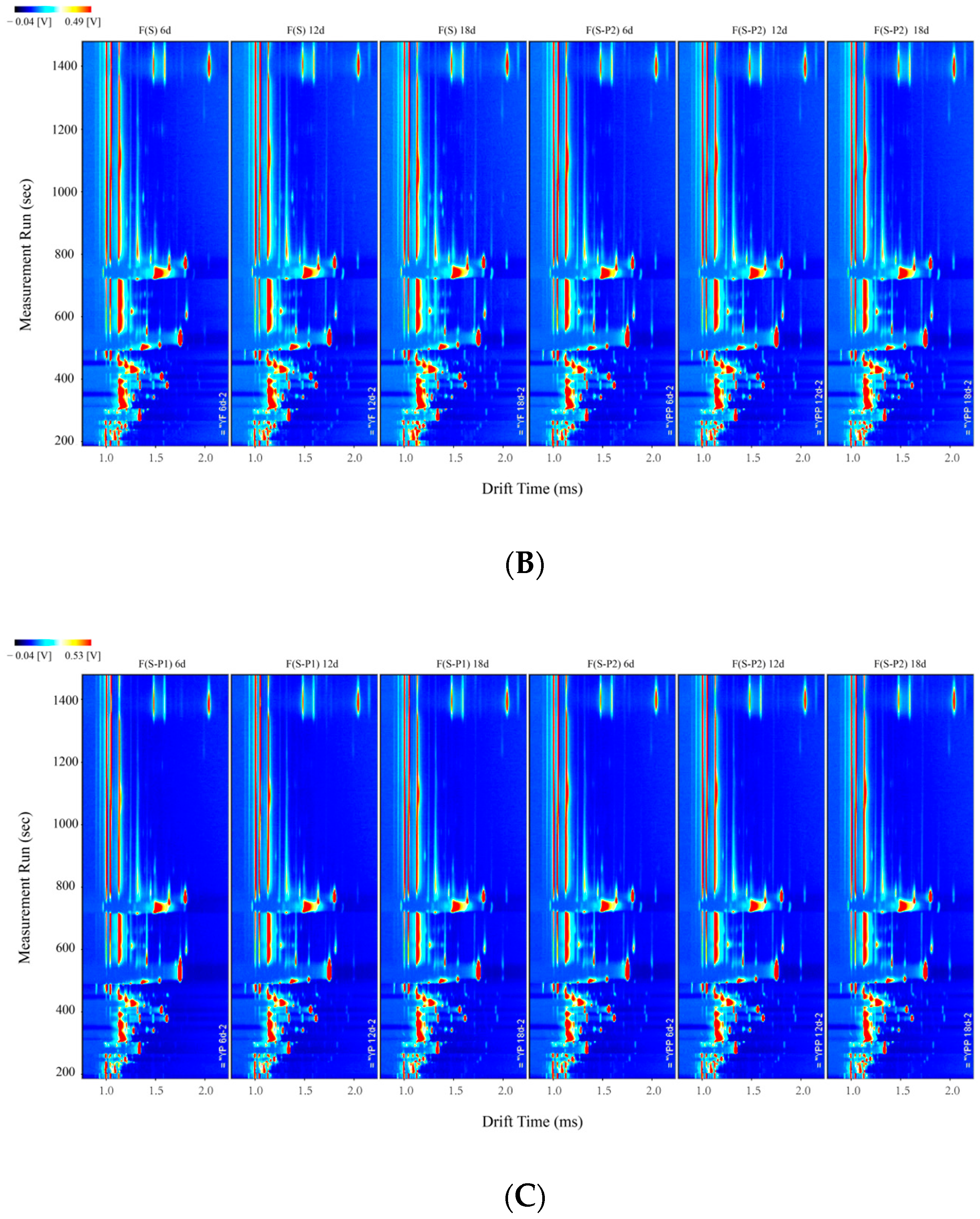
3.2. Fingerprint Analysis of Volatile Compounds during the Fermentation Process of Mulberry Wine with Different Strains
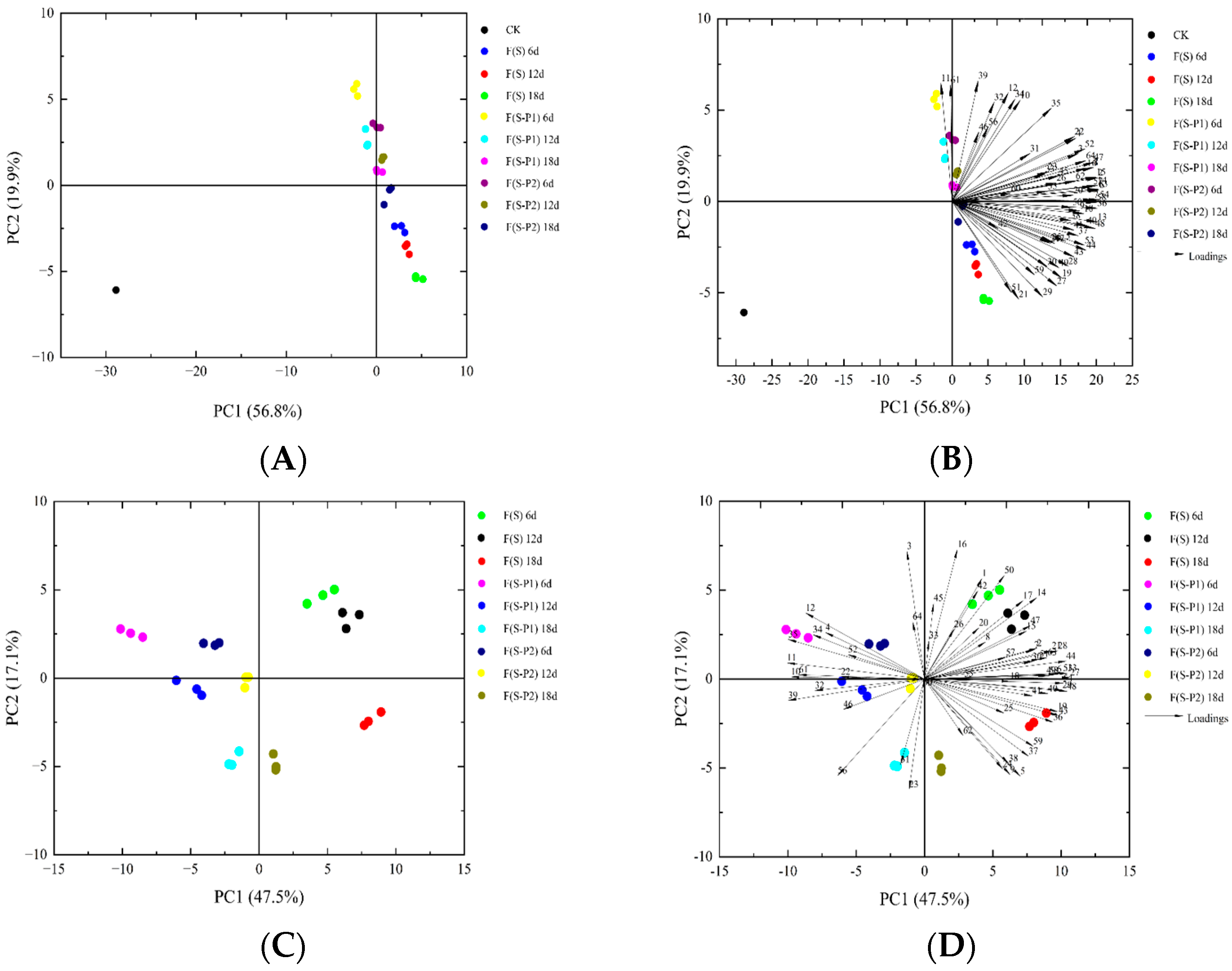
3.3. PCA Analysis of Different Mulberry Wine during Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.X.; Ma, Z.F.; Luo, X.Q.; Li, X.L. Effects of Mulberry Fruit (Morus alba L.) Consumption on Health Outcomes: A Mini-Review. Antioxidants 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Sangteerakij, D.; Rodyu, S.; Junden, S. Nutrition composition and anthocyanin content of mulberry fruits (Mores alba L.) by different ripening stage grown in the south of Thailand, applicants in sorbet and sherbet ice-cream and analysis of physical, chemical and evaluation. J. Fac. Sci. 2023, 35, 130–138. [Google Scholar]
- Yuan, Q.X.; Zhao, L.Y. The Mulberry (Morus alba L.) Fruit-A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, D.; Kostic, D.; Paunovic, D.; Mitic, M.; Krstic, J.; Misic, I.R.; Arsic, B. Antimicrobial Activity and The Quantitative Analyses of Phenolic Compounds and Heavy Metals of Red Mulberry Extracts (Morus rubra L.) From Serbia. Stud. Univ. Babes-Bolyai Chem. 2022, 67, 195–207. [Google Scholar] [CrossRef]
- Gahukar, R.T. Plant-derived products in crop protection: Effects of various application methods on pests and diseases. Phytoparasitica 2016, 44, 379–391. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Y.L.; Yang, J.; Wang, Q.; Jiang, N.; Chu, D.T.; Han, Y.B.; Zhou, J.Z. Chemical composition and sensory profiles of mulberry wines as fermented with different Saccharomyces cerevisiae strains. Int. J. Food Prop. 2017, 20, 2006–2021. [Google Scholar]
- Zhang, S.R.; Xing, X.; Chu, Q.; Sun, S.Y.; Wang, P. Impact of co-culture of Lactobacillus plantarum and Oenococcus oeni at different ratios on malolactic fermentation, volatile and sensory characteristics of mulberry wine. LWT—Food Sci. Technol. 2022, 169, 113995. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tasting the terroir of wine yeast innovation. FEMS Yeast Res. 2020, 20, foz084. [Google Scholar] [CrossRef]
- van Wyk, N.; Grossmann, M.; Wendland, J.; von Wallbrunn, C.; Pretorius, I.S. The Whiff of Wine Yeast Innovation: Strategies for Enhancing Aroma Production by Yeast during Wine Fermentation. J. Agric. Food Chem. 2019, 67, 13496–13505. [Google Scholar] [CrossRef]
- Gonzalez, R.; Morales, P. Truth in wine yeast. Microb. Biotechnol. 2021, 15, 1339–1356. [Google Scholar] [CrossRef]
- Wei, J.P.; Zhang, Y.X.; Wang, Y.W.; Ju, H.M.; Niu, C.; Song, Z.H.; Yuan, Y.H.; Yue, T.L. Assessment of chemical composition and sensorial properties of ciders fermented with different non-Saccharomyces yeasts in pure and mixed fermentations. Int. J. Food Microbiol. 2020, 318, 108471. [Google Scholar] [CrossRef]
- Gao, M.M.; Hu, J.N.; Wang, X.J.; Zhang, H.Y.; Du, Z.P.; Ma, L.J.; Du, L.P.; Zhang, H.L.; Tian, X.J.; Yang, W.M. Effects of Pichia kluyveri on the flavor characteristics of wine by co-fermentation with Saccharomyces cerevisiae. Eur. Food Res. Technol. 2023, 249, 1449–1460. [Google Scholar] [CrossRef]
- Dutraive, O.; Benito, S.; Fritsch, S.; Beisert, B.; Patz, C.D.; Rauhut, D. Effect of Sequential Inoculation with Non-Saccharomyces and Saccharomyces Yeasts on Riesling Wine Chemical Composition. Fermentation 2019, 5, 79. [Google Scholar] [CrossRef]
- Liu, Y.J.; Lu, Y.Y.; Liu, S.Q. The potential of spent coffee grounds hydrolysates fermented with Torulaspora delbrueckii and Pichia kluyveri for developing an alcoholic beverage: The yeasts growth and chemical compounds modulation by yeast extracts. Curr. Res. Food Sci. 2021, 4, 489–498. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Li, Y.Q.; Liu, L.X.; Zheng, M.N.; Feng, Z.J.; Hu, K.; Tao, Y.S. Effects of inoculation timing and mixed fermentation with Pichia fermentans on Oenococcus oeni viability, fermentation duration and aroma production during wine malolactic fermentation. Food Res. Int. 2022, 159, 111604. [Google Scholar] [CrossRef]
- Cuadros-Inostroza, A.; Giavalisco, P.; Hummel, J.; Eckardt, A.; Willmitzer, L.; Peña-Cortés, H. Discrimination of Wine Attributes by Metabolome Analysis. Anal. Chem. 2010, 82, 3573–3580. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Bindon, K.; Gawel, R.; Smith, P.; Vasilev, K.; Butt, H.J.; Koynov, K. Fluorescence correlation spectroscopy to unravel the interactions between macromolecules in wine. Food Chem. 2021, 352, 129343. [Google Scholar] [CrossRef]
- Yang, X.L.; Zhang, T.X.; Yang, D.D.; Xie, J.C. Application of gas chromatography-ion mobility spectrometry in the analysis of food volatile components. Acta Chromatogr. 2023, 35, 35–45. [Google Scholar] [CrossRef]
- Shvartsburg, A.A.; Tang, K.Q.; Smith, R.D. Differential Ion Mobility Separations of Peptides with Resolving Power Exceeding 50. Anal. Chem. 2010, 82, 32–35. [Google Scholar] [CrossRef]
- Rodríguez-Maecker, R.; Vyhmeister, E.; Meisen, S.; Martinez, A.R.; Kuklya, A.; Telgheder, U. Identification of terpenes and essential oils by means of static headspace gas chromatography-ion mobility spectrometry. Anal. Bioanal. Chem. 2017, 409, 6595–6603. [Google Scholar] [CrossRef] [PubMed]
- Perl, T.; Jünger, M.; Vautz, W.; Nolte, J.; Kuhns, M.; Zepelin, M.B.V.; Quintel, M. Detection of characteristic metabolites of Aspergillus fumigatus and Candida species using ion mobility spectrometry–metabolic profiling by volatile organic compounds. Mycoses 2011, 54, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.; Goggin, K.A.; Tahir, N.I.; Brodrick, E.; Singh, R.; Sambanthamurthi, R.; Parveez, G.K.A.; Davies, A.N.; Murad, A.J.; Muhammad, N.H.; et al. Use of headspace-gas chromatography-ion mobility spectrometry to detect volatile fingerprints of palm fibre oil and sludge palm oil in samples of crude palm oil. BMC Res. Notes 2019, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; Martín-Gómez, A.; Jurado-Campos, N.; Garrido-Delgado, R.; Arce, C.; Arce, L. Target vs spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace–gas chromatography-ion mobility spectrometry. Food Chem. 2018, 246, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Chen, W.; Wang, Z.H.; Wang, J. Rapid determination of potential aflatoxigenic fungi contamination on peanut kernels during storage by data fusion of HS-GC-IMS and fluorescence spectroscopy. Postharvest Biol. Technol. 2020, 171, 111361. [Google Scholar] [CrossRef]
- Jia, S.L.; Li, Y.; Zhuang, S.; Sun, X.H.; Zhang, L.T.; Shi, J.; Hong, H.; Luo, Y.K. Biochemical changes induced by dominant bacteria in chill-stored silver carp (Hypophthalmichthys molitrix) and GC-IMS identification of volatile organic compounds. Food Microbiol. 2019, 84, 103248. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, J.; Arce, C.; Jordano, R.; Arce, L.; Medina, L.M. Target identification of volatile metabolites to allow the differentiation of lactic acid bacteria by gas chromatography-ion mobility spectrometry. Food Chem. 2017, 220, 362–370. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Liao, L.Y.; Qin, Y.Y.; Jiang, L.W.; Liu, Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2021, 361, 130055. [Google Scholar] [CrossRef]
- Feng, T.; Sun, J.Q.; Song, S.Q.; Wang, H.T.; Yao, L.Y.; Sun, M.; Wang, K.; Chen, D. Geographical differentiation of Molixiang table grapes grown in China based on volatile compounds analysis by HS-GC-IMS coupled with PCA and sensory evaluation of the grapes. Food Chem. X 2022, 15, 100423. [Google Scholar] [CrossRef]
- López-Feria, S.; Cárdenas, S.; Valcárcel, M. Simplifying chromatographic analysis of the volatile fraction of foods. TrAC Trends Anal. Chem. 2008, 27, 794–803. [Google Scholar] [CrossRef]
- He, G.Q.; Huang, J.; Liang, R.; Wu, C.D.; Zhou, R.Q. Comparing the differences of characteristic flavour between natural maturation and starter culture for Mucor-type Douchi. Int. J. Food Sci. Technol. 2016, 51, 1252–1259. [Google Scholar] [CrossRef]
- Xu, E.B.; Long, J.; Wu, Z.Z.; Li, H.Y.; Wang, F.; Xu, X.M.; Jin, Z.Y.; Jiao, A.Q. Characterization of Volatile Flavor Compounds in Chinese Rice Wine Fermented from Enzymatic Extruded Rice. J. Food Sci. 2015, 80, C1476–C1489. [Google Scholar] [CrossRef] [PubMed]
- García-Carpintero, E.G.; Sánchez-Palomo, E.; Gallego, M.A.G.; González-Viñas, M.A. Characterization of impact odorants and sensory profile of Bobal red wines from Spain’s La Mancha region. Flavour Fragr. J. 2012, 27, 60–68. [Google Scholar] [CrossRef]
- Gracia-Moreno, E.; Lopez, R.; Ferreira, V. Quantitative determination of five hydroxy acids, precursors of relevant wine aroma compounds in wine and other alcoholic beverages. Anal. Bioanal. Chem. 2015, 407, 7925–7934. [Google Scholar] [CrossRef]
- Ugliano, M.; Dieval, J.B.; Siebert, T.E.; Kwiatkowski, M.; Aagaard, O.; Vidal, S.; Waters, E.J. Oxygen Consumption and Development of Volatile Sulfur Compounds during Bottle Aging of Two Shiraz Wines. Influence of Pre- and Postbottling Controlled Oxygen Exposure. J. Agric. Food Chem. 2012, 60, 8561–8570. [Google Scholar] [CrossRef]
- Fedrizzi, B.; Magno, F.; Badocco, D.; Nicolini, G.; Versini, G. Aging effects and grape variety dependence on the content of sulfur volatiles in wine. J. Agric. Food Chem. 2007, 55, 10880–10887. [Google Scholar] [CrossRef]
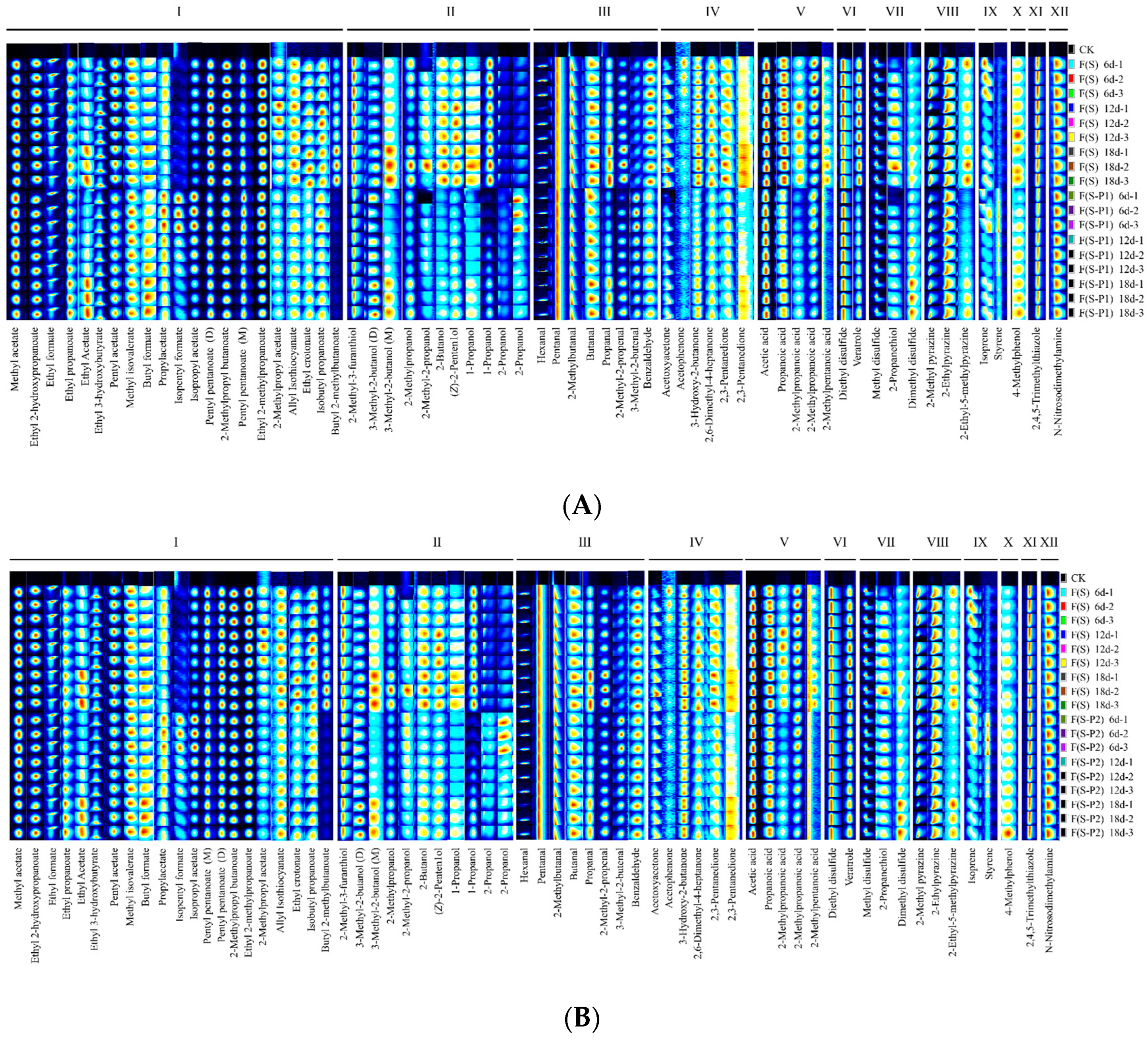
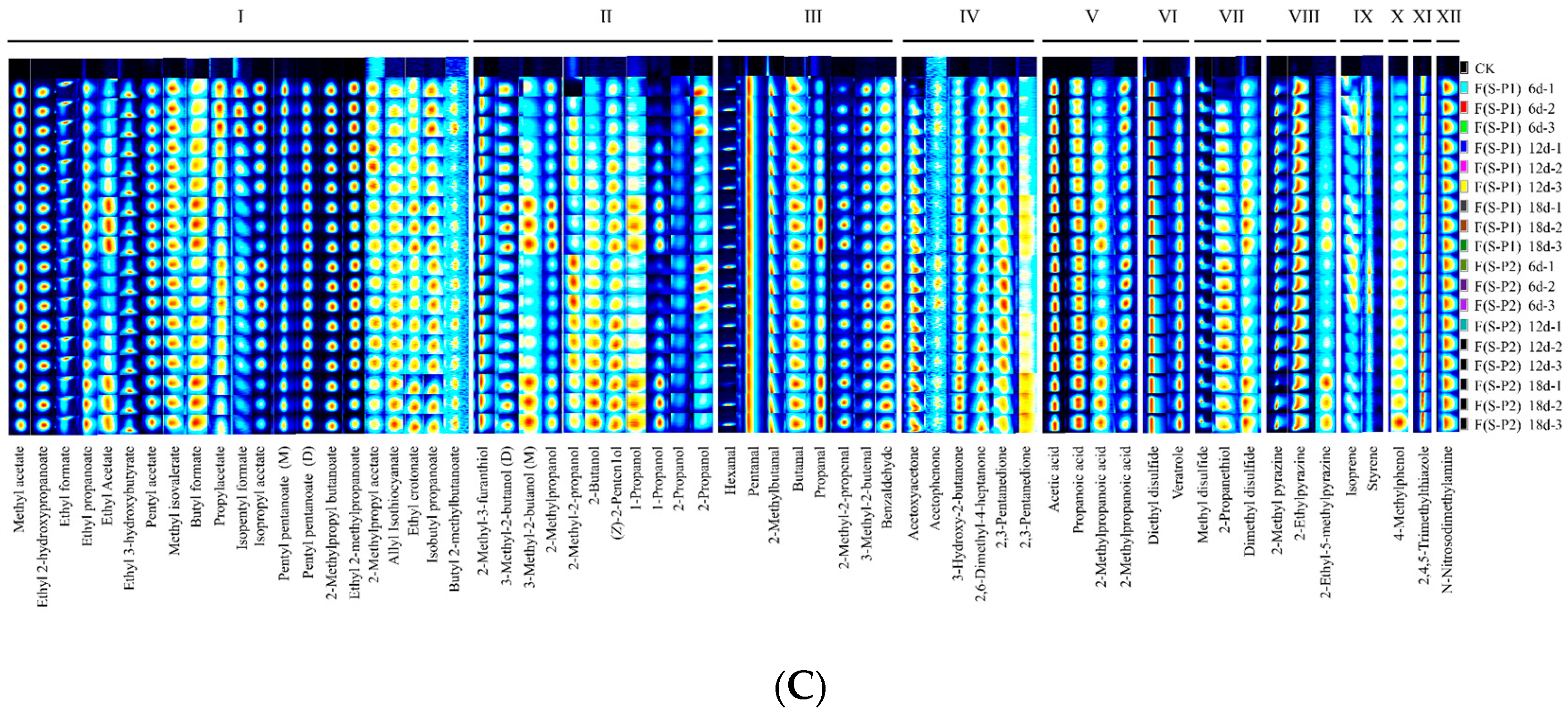
| No. | Compound | CAS | Molecule Formula | MW a | RI b | RT c [s] | DT d [ms] | Comment |
|---|---|---|---|---|---|---|---|---|
| Ⅰ Esters | ||||||||
| 1 | Methyl acetate | 79-20-9 | C3H6O2 | 74.1 | 545 | 250.986 | 1.2007 | |
| 2 | Ethyl 2-hydroxYPPropanoate | 97-64-3 | C5H10O3 | 118.1 | 811.2 | 505.891 | 1.552 | |
| 3 | Ethyl formate | 109-94-4 | C3H6O2 | 74.1 | 622.9 | 303.678 | 1.2087 | |
| 4 | Ethyl propanoate | 105-37-3 | C5H10O2 | 102.1 | 715.7 | 387.553 | 1.1441 | |
| 5 | Ethyl Acetate | 141-78-6 | C4H8O2 | 88.1 | 562 | 261.299 | 1.0895 | |
| 6 | Ethyl 3-hydroxybutanoate | 5405-41-4 | C6H12O3 | 132.2 | 950.1 | 762.614 | 1.6458 | |
| 7 | Pentyl acetate | 628-63-7 | C7H14O2 | 130.2 | 932.1 | 722.363 | 1.3195 | |
| 8 | Methyl isovalerate | 556-24-1 | C6H12O2 | 116.2 | 767 | 446.34 | 1.2081 | |
| 9 | Butyl formate | 592-84-7 | C5H10O2 | 102.1 | 746.3 | 421.426 | 1.2121 | |
| 10 | Propylacetate | 109-60-4 | C5H10O2 | 102.1 | 717.3 | 389.246 | 1.4832 | |
| 11 | Isopentyl formate | 110-45-2 | C6H12O2 | 116.2 | 775.9 | 457.729 | 1.6333 | |
| 12 | Isopropyl acetate | 108-21-4 | C5H10O2 | 102.1 | 670.6 | 343.527 | 1.489 | |
| 13 | Pentyl pentanoate | 2173-56-0 | C10H20O2 | 172.3 | 1146.9 | 1407.006 | 1.4827 | Monomer |
| 14 | Pentyl pentanoate | 2173-56-0 | C10H20O2 | 172.3 | 1145.7 | 1401.398 | 2.0471 | Dimer |
| 15 | 2-methylpropyl butanoate | 539-90-2 | C8H16O2 | 144.2 | 954.5 | 773.001 | 1.8113 | |
| 16 | Ethyl 2-methylpropanoate | 97-62-1 | C6H12O2 | 116.2 | 736.4 | 410.037 | 1.5711 | |
| 17 | 2-Methylpropyl acetate | 110-19-0 | C6H12O2 | 116.2 | 745.4 | 420.316 | 1.6156 | |
| 18 | Allyl Isothiocyanate | 1957/6/7 | C4H5NS | 99.2 | 878.7 | 615.893 | 1.3861 | |
| 19 | Ethyl crotonate | 623-70-1 | C6H10O2 | 114.1 | 846.4 | 560.062 | 1.5493 | |
| 20 | Isobutyl propanoate | 540-42-1 | C7H14O2 | 130.2 | 842.2 | 553.34 | 1.7146 | |
| 21 | butyl 2-methylbutanoate | 15706-73-7 | C9H18O2 | 158.2 | 1032 | 980.844 | 1.385 | |
| Ⅱ Alcohols | ||||||||
| 22 | 2-Methyl-3-furanthiol | 28588-74-1 | C5H6OS | 114.2 | 861.1 | 584.731 | 1.1402 | |
| 23 | 3-Methyl-2-butanol | 598-75-4 | C5H12O | 88.1 | 677.8 | 350.056 | 1.238 | Monomer |
| 24 | 3-Methyl-2-butanol | 598-75-4 | C5H12O | 88.1 | 673.3 | 345.996 | 1.4334 | Dimer |
| 25 | 2-methylpropanol | 78-83-1 | C4H10O | 74.1 | 616 | 298.43 | 1.3799 | |
| 26 | 2-Methyl-2-propanol | 75-65-0 | C4H10O | 74.1 | 509.6 | 231.177 | 1.1317 | |
| 27 | 2-Butanol | 78-92-2 | C4H10O | 74.1 | 610.1 | 294.048 | 1.1509 | |
| 28 | (Z)-2-Penten1ol | 1576-95-0 | C5H10O | 86.1 | 751.2 | 427.121 | 1.4368 | |
| 29 | 1-Propanol | 71-23-8 | C3H8O | 60.1 | 531.3 | 243.032 | 1.1178 | |
| 30 | 1-Propanol | 71-23-8 | C3H8O | 60.1 | 558.9 | 259.421 | 1.1108 | |
| 31 | 2-Propanol | 67-63-0 | C3H8O | 60.1 | 531.6 | 243.228 | 1.1779 | Monomer |
| 32 | 2-Propanol | 67-63-0 | C3H8O | 60.1 | 536.4 | 245.931 | 1.2186 | Dimer |
| Ⅲ Aldehydes | ||||||||
| 33 | Hexanal | 66-25-1 | C6H12O | 100.2 | 805.1 | 497.264 | 1.2544 | |
| 34 | Pentanal | 110-62-3 | C5H10O | 86.1 | 689.2 | 360.844 | 1.1745 | |
| 35 | 2-Methylbutanal | 96-17-3 | C5H10O | 86.1 | 678.4 | 350.696 | 1.4006 | |
| 36 | Butanal | 123-72-8 | C4H8O | 72.1 | 610.1 | 294.048 | 1.1133 | |
| 37 | Propanal | 123-38-6 | C3H6O | 58.1 | 468.6 | 211.079 | 1.1393 | |
| 38 | 2-Methyl-2-propenal | 78-85-3 | C4H6O | 70.1 | 572.1 | 267.793 | 1.2092 | |
| 39 | 3-Methyl-2-butenal | 107-86-8 | C5H8O | 84.1 | 775.9 | 457.729 | 1.3505 | |
| 40 | Benzaldehyde | 100-52-7 | C7H6O | 106.1 | 960.5 | 787.283 | 1.4573 | |
| Ⅳ Ketones | ||||||||
| 41 | Acetoxyacetone | 592-20-1 | C5H8O3 | 116.1 | 488.5 | 220.495 | 1.0945 | |
| 42 | Acetophenone | 98-86-2 | C8H8O | 120.2 | 1095.1 | 1195.186 | 1.5748 | |
| 43 | 3-Hydroxy-2-butanone | 513-86-0 | C4H8O2 | 88.1 | 737 | 410.749 | 1.3244 | |
| 44 | 2,6-Dimethyl-4-heptanone | 108-83-8 | C9H18O | 142.2 | 972.2 | 815.848 | 1.3218 | |
| 45 | 2,3-Pentanedione | 600-14-6 | C5H8O2 | 100.1 | 689.8 | 361.451 | 1.2115 | |
| 46 | 2,3-Pentanedione | 600-14-6 | C5H8O2 | 100.1 | 710.9 | 382.528 | 1.217 | |
| Ⅴ Acids | ||||||||
| 47 | Acetic acid | 64-19-7 | C2H4O2 | 60.1 | 600.8 | 287.345 | 1.341 | |
| 48 | Propanoic acid | 79-09-4 | C3H6O2 | 74.1 | 711.4 | 382.987 | 1.3465 | |
| 49 | 2-Methylpropanoic acid | 79-31-2 | C4H8O2 | 88.1 | 769.8 | 449.899 | 1.3746 | |
| 50 | 2-Methylpropanoic acid | 79-31-2 | C4H8O2 | 88.1 | 746.9 | 422.138 | 1.3906 | |
| 51 | 2-Methylpentanoic acid | 97-61-0 | C6H12O2 | 116.2 | 1031.6 | 979.77 | 1.2668 | |
| Ⅵ Ethers | ||||||||
| 52 | Diethyl disulfide | 110-81-6 | C4H10S2 | 122.2 | 916.8 | 689.903 | 1.1448 | |
| 53 | Veratrole | 91-16-7 | C8H10O2 | 138.2 | 1146.3 | 1404.202 | 1.5962 | |
| Ⅶ Sulpho compound | ||||||||
| 54 | Methyl disulfide | 624-92-0 | C2H6S2 | 94.2 | 771.5 | 452.035 | 1.1459 | |
| 55 | 2-Propanethiol | 75-33-2 | C3H8S | 76.2 | 562.5 | 261.649 | 1.1434 | |
| 56 | Dimethyl disulfide | 624-92-0 | C2H6S2 | 94.2 | 741.7 | 416.084 | 1.1397 | |
| Ⅷ Pyrazine | ||||||||
| 57 | 2-Methyl pyrazine | 109-08-0 | C5H6N2 | 94.1 | 808.2 | 501.576 | 1.3943 | |
| 58 | 2-Ethylpyrazine | 13925-00-3 | C6H8N2 | 108.1 | 938.5 | 736.295 | 1.5252 | |
| 59 | 2-Ethyl-5-methylpyrazine | 13360-64-0 | C7H10N2 | 122.2 | 1004.9 | 901.848 | 1.2119 | |
| Ⅸ Terpenes | ||||||||
| 60 | Isoprene | 78-79-5 | C5H8 | 68.1 | 524.7 | 239.328 | 1.2107 | |
| 61 | Styrene | 100-42-5 | C8H8 | 104.2 | 905.9 | 667.742 | 1.431 | |
| Ⅹ Phenol | ||||||||
| 62 | 4-Methylphenol | 106-44-5 | C7H8O | 108.1 | 1069.9 | 1104.14 | 1.142 | |
| Ⅺ Thiazole | ||||||||
| 63 | 2,4,5-Trimethylthiazole | 13623-11-5 | C6H9NS | 127.2 | 1002.6 | 895.425 | 1.1343 | |
| Ⅻ Nitrogen compound | ||||||||
| 64 | N-Nitrosodimethylamine | 62-75-9 | C2H6N2O | 74.1 | 755.5 | 432.25 | 1.2705 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, B.; Zhao, S.; Zhang, W.; Lin, Y.; Xiong, L. The Effect of Co-Culture with Different Pichia kluyveri and Saccharomyces cerevisiae on Volatile Compound and Characteristic Fingerprints of Mulberry Wine. Foods 2024, 13, 422. https://doi.org/10.3390/foods13030422
Ding B, Zhao S, Zhang W, Lin Y, Xiong L. The Effect of Co-Culture with Different Pichia kluyveri and Saccharomyces cerevisiae on Volatile Compound and Characteristic Fingerprints of Mulberry Wine. Foods. 2024; 13(3):422. https://doi.org/10.3390/foods13030422
Chicago/Turabian StyleDing, Bo, Shutian Zhao, Wenxue Zhang, Ying Lin, and Ling Xiong. 2024. "The Effect of Co-Culture with Different Pichia kluyveri and Saccharomyces cerevisiae on Volatile Compound and Characteristic Fingerprints of Mulberry Wine" Foods 13, no. 3: 422. https://doi.org/10.3390/foods13030422
APA StyleDing, B., Zhao, S., Zhang, W., Lin, Y., & Xiong, L. (2024). The Effect of Co-Culture with Different Pichia kluyveri and Saccharomyces cerevisiae on Volatile Compound and Characteristic Fingerprints of Mulberry Wine. Foods, 13(3), 422. https://doi.org/10.3390/foods13030422




