Enhancing Phytochemical Compounds, Functional Properties, and Volatile Flavor Profiles of Pomelo (Citrus grandis (L.) Osbeck) Juices from Different Cultivars through Fermentation with Lacticaseibacillus paracasei
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials, Bacteria, and Chemicals
2.2. Fermentation of Pomelo Juices
2.3. Determination of pH and Color Properties
2.4. Total Sugar and Reducing Sugar Content Analysis
2.5. Analysis of Organic Acid
2.6. Enumeration of Viable Lactobacilli
2.7. Determination of Phytochemical Compounds and Antioxidant Activity
2.8. Quantification of Major Flavonoid Constituents
2.9. Bile Acid Binding, Cholesterol Micellization, and Pancreatic Lipase Inhibition
2.10. Analysis of Volatile Compounds by Gas Chromatography
2.11. Organoleptic Test
2.12. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Fermented Pomelo Juices
3.2. Viable Lactobacilli Counts of Fermented Pomelo Juices
3.3. Phytochemicals and Biological Activities of Fermented Pomelo Juices
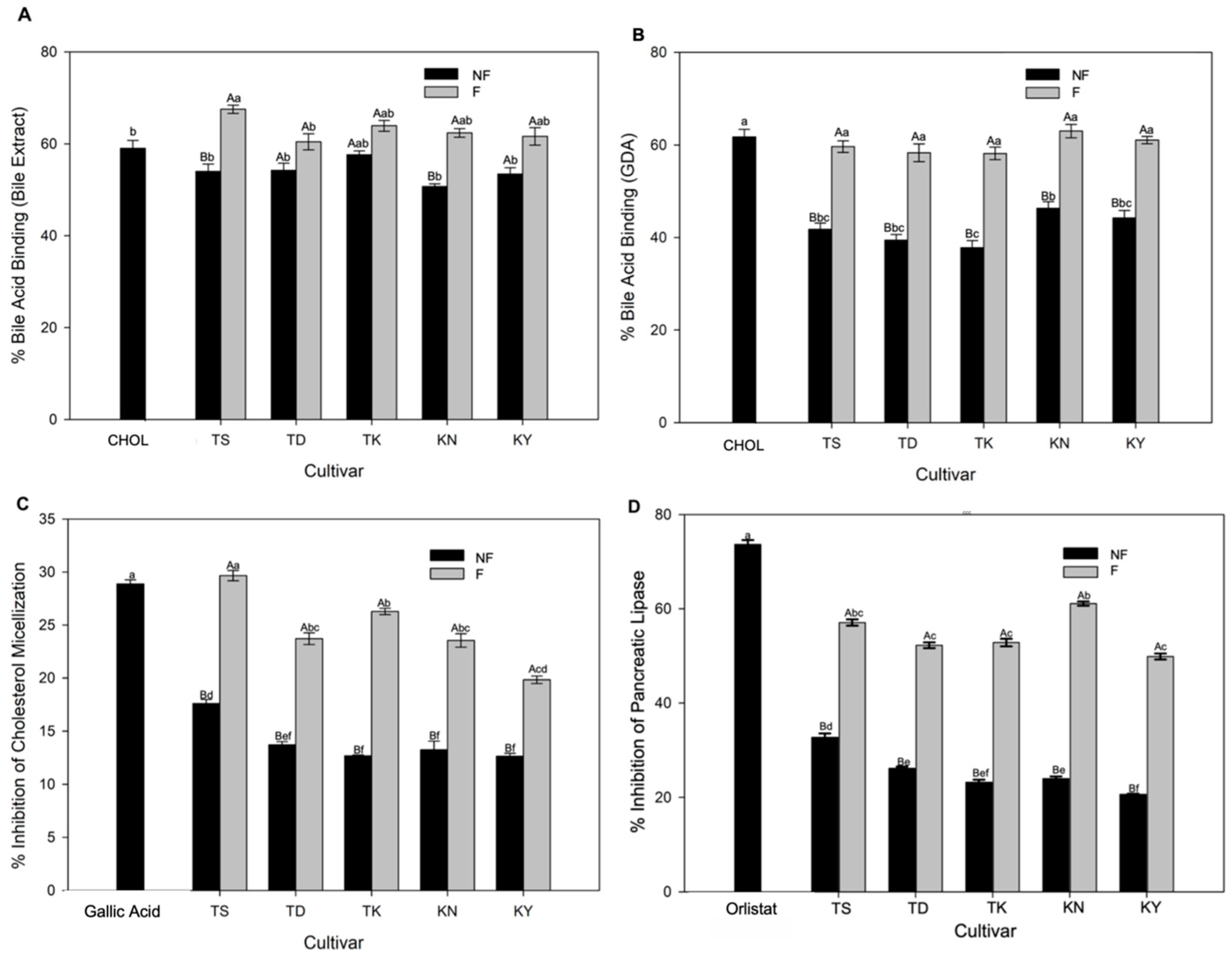
3.4. Functional Properties of Fermented Pomelo Juices
3.5. Volatile Compounds of Fermented Pomelo Juices
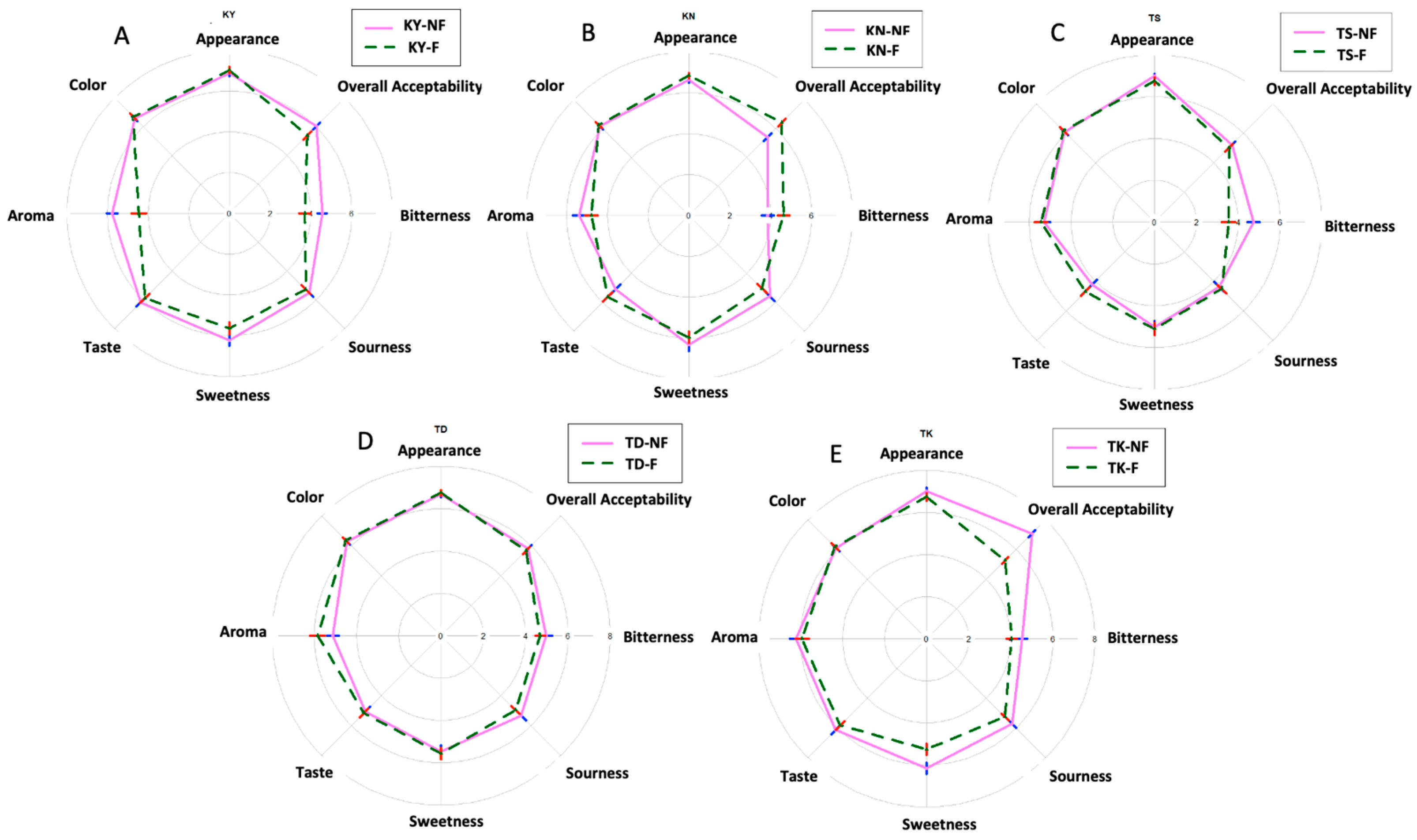
3.6. Organoleptic Acceptability Scores of Fermented Pomelo Juices
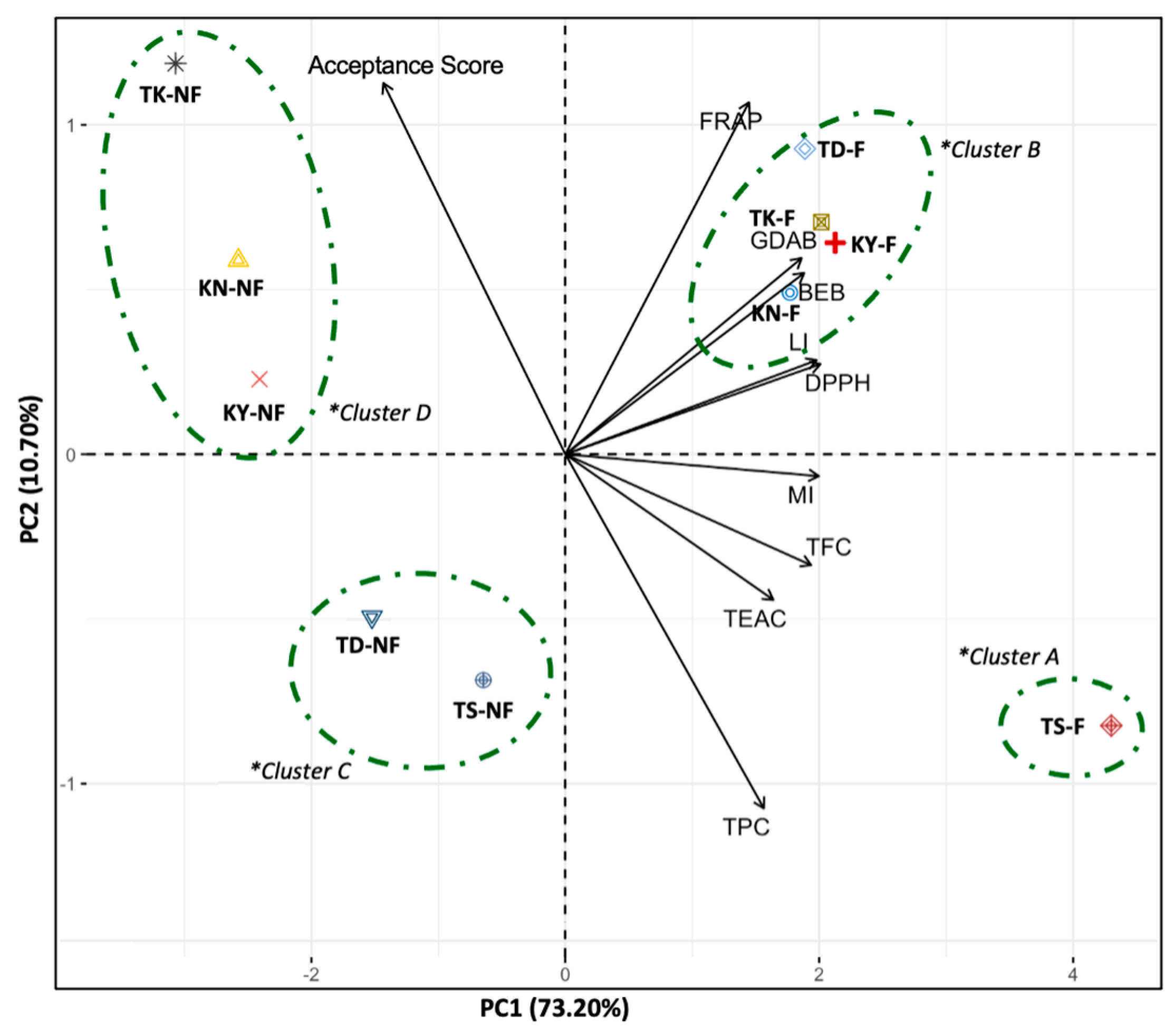
3.7. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols-a non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Begum, P.; Madhavi, G.; Rajagopal, S.; Viswanath, B.; Razak, M.; Venkataratnamma, V. Probiotics as functional foods: Potential effects on human health and its impact on neurological diseases. Int. J. Nutr. Pharmacol. Neurol. Dis. 2017, 7, 23–33. [Google Scholar]
- Abraham, B.P.; Quigley, E.M.M. Probiotics in inflammatory bowel disease. Gastroenterol. Clin. N. Am. 2017, 46, 769–782. [Google Scholar] [CrossRef]
- Son, S.H.; Dae-Kyung, K.; Eun, B.; Hye-Lin, J.; Hyun-Dong, P.; Na-Kyoung, L.; Young-Seo, P. Potential probiotic Lactobacillus plantarum Ln4 from kimchi: Evaluation of β-galactosidase and antioxidant activities. LWT-Food Sci. Technol. 2017, 85, 181–186. [Google Scholar] [CrossRef]
- Zoumpopoulou, G.; Pot, B.; Tsakalidou, E.; Papadimitriou, K. Dairy probiotics: Beyond the role of promoting gut and immune health. Int. Dairy J. 2016, 67, 46–60. [Google Scholar] [CrossRef]
- Pothuraju, R.; Hussain, S.A. Probiotics: An important player in the obesity management alone? Obes. Med. 2017, 8, 13–14. [Google Scholar] [CrossRef]
- Silva, A.R.A.; Silva, M.M.N.; Ribeiro, B.D. Health issues and technological aspects of plant-based alternative milk. Food Res. Int. 2020, 131, 108972. [Google Scholar] [CrossRef]
- Leonard, W.; Liang, A.; Ranadheera, C.S.; Fang, Z.; Zhang, P. Fruit juices as a carrier of probiotics to modulate gut phenolics and microbiota. Food Funct. 2022, 13, 10333–10346. [Google Scholar] [CrossRef]
- Quan, Q.; Liu, W.; Guo, J.; Ye, M.; Zhang, J. Effect of six lactic acid bacteria strains on physicochemical characteristics, antioxidant activities and sensory properties of fermented orange juices. Foods 2022, 11, 1920. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Xiao, L. Effect of lactic acid fermentation on the phytochemical, volatile profile and sensory attributes of mulberry juice. J. Food Nutr. Res. 2017, 56, 305–317. [Google Scholar]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Toh, J.; Khoo, H.; Azrina, A. Comparison of antioxidant properties of pomelo [Citrus grandis (L) Osbeck] varieties. Int. Food Res. J. 2013, 20, 1661–1668. [Google Scholar]
- Mäkynen, K.; Jitsaardkul, S.; Tachasamran, P.; Sakai, N.; Puranachoti, S.; Nirojsinlapachai, N.; Chattapat, V.; Caengprasath, N.; Ngamukote, S.; Adisakwattana, S. Cultivar variations in antioxidant and antihyperlipidemic properties of pomelo pulp (Citrus grandis [L.] Osbeck) in Thailand. Food Chem. 2013, 139, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Marnpae, M.; Chusak, C.; Balmori, V.; Kamonsuwan, K.; Dahlan, W.; Nhujak, T.; Hamid, N.; Adisakwattana, S. Probiotic Gac fruit beverage fermented with Lactobacillus paracasei: Physiochemical properties, phytochemicals, antioxidant activities, functional properties, and volatile flavor compounds. LWT-Food Sci. Technol. 2022, 169, 113986. [Google Scholar] [CrossRef]
- Nielsen, S.S. Phenol-Sulfuric Acid Method for Total Carbohydrates. In Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; pp. 47–53. [Google Scholar]
- Jain, A.; Jain, R.; Jain, S. Quantitative analysis of reducing sugars by 3,5-dinitrosalicylic acid (DNSA method). In Basic Techniques in Biochemistry, Microbiology and Molecular Biology: Principles and Techniques; Springer: New York, NY, USA, 2020; pp. 181–183. [Google Scholar]
- Balmori, V.; Dizon, E.; Elegado, F. Effect of adjunct inoculation of Lactobacillus plantarum BS and Pediococcus acidilactici 3G3 on the microbiological, physicochemical, and sensory properties of fermented carabeef (Pindang damulag). Food Res. 2019, 3, 70–78. [Google Scholar] [CrossRef]
- Chusak, C.; Chanbunyawat, P.; Chumnumduang, P.; Chantarasinlapin, P.; Suantawee, T.; Adisakwattana, S. Effect of gac fruit (Momordica cochinchinensis) powder on in vitro starch digestibility, nutritional quality, textural and sensory characteristics of pasta. LWT-Food Sci. Technol. 2020, 118, 108856. [Google Scholar] [CrossRef]
- Chayaratanasin, P.; Barbieri, M.A.; Suanpairintr, N.; Adisakwattana, S. Inhibitory effect of Clitoria ternatea flower petal extract on fructose-induced protein glycation and oxidation-dependent damages to albumin in vitro. BMC Complement. Altern. Med. 2015, 15, 27. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Ruengsamran, T.; Kampa, P.; Sompong, W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complement. Altern. Med. 2012, 12, 110. [Google Scholar] [CrossRef]
- Mesquita, E.; Monteiro, M. Simultaneous HPLC determination of flavonoids and phenolic acids profile in Pêra-Rio orange juice. Food Res. Int. 2018, 106, 54–63. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Chen, J.; Luo, W.; Cheng, L.; Wu, J.; Yu, Y.; Li, L.; Xu, Y. Influence of Cultivar and Turbidity on Physicochemical Properties, Functional Characteristics and Volatile Flavor Substances of Pomelo Juices. Foods 2023, 12, 1028. [Google Scholar] [CrossRef]
- Makkumrai, W.; Huang, Y.; Qiang, X. Comparison of pomelo (Citrus maxima) grown in China and Thailand. Front. Agric. Sci. Eng. 2021, 8, 335–352. [Google Scholar]
- Pinto, T.; Vilela, A.; Cosme, F. Chemical and sensory characteristics of fruit juice and fruit fermented beverages and their consumer acceptance. Beverages 2022, 8, 33. [Google Scholar] [CrossRef]
- Fernández-Vázquez, R.; Hewson, L.; Fisk, I.; Vila, D.H.; Mira, F.J.H.; Vicario, I.M.; Hort, J. Colour influences sensory perception and liking of orange juice. Flavour 2014, 3, 1. [Google Scholar] [CrossRef]
- Minaker, S.; Mason, R.; Chow, D. Optimizing color performance of the ngenuity 3-dimensional visualization system. Ophthalmol. Sci. 2021, 1, 100054. [Google Scholar] [CrossRef] [PubMed]
- Anh, T.T.M.; Nguyen, T.B.; Duc, V.N.; Bujna, E.; Mai, D.; Nguyen, Q.D. Changes in bitterness, antioxidant activity and total phenolic content of grapefruit juice fermented by Lactobacillus and Bifidobacterium strains. Acta Aliment. 2020, 49, 103–110. [Google Scholar]
- Zhao, D.; Shah, N.P. Changes in antioxidant capacity, isoflavone profile, phenolic and vitamin contents in soymilk during extended fermentation. LWT-Food Sci. Technol. 2014, 58, 454–462. [Google Scholar] [CrossRef]
- Wu, C.; Li, T.; Qi, J.; Jiang, T.; Xu, H.; Lei, H. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. LWT-Food Sci. Technol. 2020, 122, 109064. [Google Scholar] [CrossRef]
- Šalić, A.; Šamec, D. Changes in the content of glucosinolates, polyphenols and carotenoids during lactic-acid fermentation of cruciferous vegetables: A mini review. Food Chem. X 2022, 16, 100457. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Walkowiak, J.; Pietrzak, R.; Bazan-Woźniak, A.; Cielecka-Piontek, J. Bioavailability of hesperidin and its aglycone hesperetin-compounds found in citrus fruits as a parameter conditioning the pro-health potential (neuroprotective and antidiabetic activity)-mini-review. Nutrients 2022, 14, 2647. [Google Scholar] [CrossRef]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An overview of bioactive flavonoids from citrus fruits. Appl. Sci. 2022, 12, 29. [Google Scholar] [CrossRef]
- Rosales, C.; Suwonsichon, S. Sensory lexicon of pomelo fruit over various cultivars and fresh-cut storage. J. Sens. Stud. 2015, 30, 12133. [Google Scholar] [CrossRef]
- Naumann, S.; Haller, D.; Eisner, P.; Schweiggert-Weisz, U. Mechanisms of interactions between bile acids and plant compounds—A review. Int. J. Mol. Sci. 2020, 21, 6495. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Bello, F.; Ademosun, A. Hypocholesterolemic properties of grapefruit (Citrus paradisii) and shaddock (Citrus maxima) juices and inhibition of angiotensin-1-converting enzyme activity. J. Food Drug Anal. 2014, 4, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Tomaro-Duchesneau, C.; Jones, M.L.; Shah, D.; Jain, P.; Saha, S.; Prakash, S. Cholesterol assimilation by Lactobacillus probiotic bacteria: An in vitro investigation. BioMed Res. Int. 2014, 2014, 380316. [Google Scholar] [CrossRef]
- Buchholz, T.; Melzig, M.F. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med. 2015, 81, 771–783. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, Á. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Cheong, M.; Shao, Q.L.; Weibiao, Z.; Philip, C.; Bin, Y. Chemical Composition and Sensory Profile of Pomelo (Citrus grandis (L.) Osbeck) Juice. Food Chem. 2012, 135, 2505–2513. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; Alamar, M.C.; Gutiérrez, A.; Granell, A. Comparative Analysis of the Volatile Fraction of Fruit Juice from Different Citrus Species. PLoS ONE 2011, 6, e22016. [Google Scholar] [CrossRef]
- Mandha, J.; Shumoy, H.; Devaere, J.; Schouteten, J.J.; Gellynck, X.; De Winne, A.; Matemu, A.O.; Raes, K. Effect of lactic acid fermentation on volatile compounds and sensory characteristics of mango (Mangifera indica) juices. Foods 2022, 11, 383. [Google Scholar] [CrossRef]
- Park, S.K.; Jo, D.M.; Yu, D.; Khan, F.; Lee, Y.B.; Kim, Y.M. Reduction of trimethylamine off-odor by lactic acid bacteria isolated from korean traditional fermented food and their in-situ application. J. Microbiol. Biotechnol. 2020, 30, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Strani, L.; D’Alessandro, A.; Ballestrieri, D.; Durante, C.; Cocchi, M. Fast Gc E-Nose and Chemometrics for the Rapid Assessment of Basil Aroma. Chemosensors 2022, 10, 105. [Google Scholar] [CrossRef]
- Wu, X.; Fauconnier, M.L.; Bi, J. Characterization and Discrimination of Apples by Flash Gc E-Nose: Geographical Regions and Botanical Origins Studies in China. Foods 2022, 11, 1631. [Google Scholar] [CrossRef] [PubMed]
| Samples | pH | Organic Acids (mg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Tartaric Acid | Ascorbic Acid | Citric Acid | Lactic Acid | Acetic Acid | Total Organic Acids | |||
| KN | NF | 4.56 ± 0.12 abA | 0.03 ± 0.00 deB | 0.10 ± 0.01 bcA | 1.50 ± 0.03 cdA | 0.0 cB | 0.0 eB | 1.40 ± 0.03 cB |
| F | 4.49 ± 0.18 aA | 0.06 ± 0.02 bcA | 0.10 ± 0.01 bcA | 1.27 ± 0.21 bcA | 2.77 ± 0.26 bA | 0.43 ± 0.04 aA | 4.90 ± 0.85 aA | |
| KY | NF | 4.53 ± 0.16 abA | 0.03 ± 0.00 deB | 0.08 ± 0.00 cdA | 1.22 ± 0.19 cdA | 0.0 cB | 0.0 eB | 1.69 ± 0.19 bB |
| F | 4.37 ± 0.01 abA | 0.08 ± 0.01 abA | 0.10 ± 0.01 bcA | 1.15 ± 0.16 cdA | 3.65 ± 0.33 aA | 0.19 ± 0.04 cA | 5.12 ± 0.29 aA | |
| TK | NF | 4.57 ± 0.03 bA | 0.08 ± 0.01 abA | 0.06 ± 0.01 dB | 2.13 ± 0.16 aA | 0.0 cB | 0.0 eB | 2.63 ± 0.24 bB |
| F | 4.44 ± 0.02 aA | 0.02 ± 0.01 dfB | 0.08 ± 0.01 cdA | 1.28 ± 0.06 cdB | 2.89 ± 0.21 bA | 0.29 ± 0.06 bA | 4.31 ± 0.69 aA | |
| TD | NF | 4.46 ± 0.11 bA | 0.09 ± 0.01 aA | 0.13 ± 0.02 abA | 1.15 ± 0.15 cdA | 0.0 cB | 0.0 eB | 1.50 ± 0.17 bcB |
| F | 4.27 ± 0.17 aA | 0.09 ± 0.01 aA | 0.10 ± 0.01 bcA | 1.01 ± 0.04 dA | 2.85 ± 0.21 bA | 0.17 ± 0.05 cA | 5.39 ± 0.31 aA | |
| TS | NF | 4.49 ± 0.05 aA | 0.05 ± 0.01 cdA | 0.17 ± 0.00 aA | 1.81 ± 0.04 abA | 0.0 cB | 0.0 eB | 2.12 ± 0.01 bB |
| F | 4.26 ± 0.15 aA | 0.06 ± 0.01 bcA | 0.14 ± 0.01 aA | 0.98 ± 0.03 dB | 3.13 ± 0.26 abA | 0.09 ± 0.02 deA | 4.40 ± 0.29 aA | |
| Samples | Total Sugar (mg/mL) | Reducing Sugar (mg/mL) | Color Values | Lactic Acid Bacteria Count (log cfu/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ∆E | |||||
| KN | NF | 109.04 ± 1.56 abA | 13.54 ± 0.02 abA | 27.79 ± 0.47 bB | −3.15 ± 0.15 cA | −2.67 ± 0.29 cdA | 4.53 ± 0.41 | 7.09 ± 0.50 aB |
| F | 98.58 ± 3.39 bB | 10.83 ± 0.79 bB | 32.29 ± 0.76 bA | −2.78 ± 0.06 cA | −3.09 ± 0.02 dA | 8.86 ± 0.48 abA | ||
| KY | NF | 124.5 ± 3.50 aA | 14.33 ± 0.09 aA | 29.33 ± 0.30 abB | 1.67 ± 0.13 bB | −3.71 ± 0.24 dA | 4.53 ± 0.01 | 7.08 ± 0.01 aB |
| F | 120.40 ± 9.9 aB | 8.50 ± 0.43 cB | 33.77 ± 0.66 abA | 2.45 ± 0.06 bA | −4.15 ± 0.16 eA | 9.12 ± 0.51 aA | ||
| TK | NF | 96.88 ± 6.88 bA | 12.46 ± 0.05 bA | 27.40 ± 0.79 bB | −3.41 ± 0.13 cA | −1.67 ± 0.19 bcA | 4.84 ± 0.23 | 7.06 ± 0.05 aB |
| F | 92.45 ± 4.16 bB | 9.76 ± 0.09 bcB | 32.21 ± 0.48 bA | −3.04 ± 0.07 cA | −2.02 ± 0.33 cA | 8.80 ± 0.52 bA | ||
| TD | NF | 101.87 ± 0.39 abA | 12.27 ± 0.13 bA | 28.52 ± 1.10 abB | 1.52 ± 0.10 bB | −1.65 ± 0.52 bA | 5.26 ± 0.16 | 7.08 ± 0.15 aB |
| F | 92.55 ± 3.33 abB | 11.02 ± 0.07 bcB | 33.73 ± 0.29 abA | 2.25 ± 0.25 bA | −1.82 ± 0.57 bA | 9.01 ± 0.17 abA | ||
| TS | NF | 126.19 ± 6.29 aA | 14.41 ± 0.09 aA | 32.53 ± 1.35 aB | 4.25 ± 0.10 aB | 1.54 ± 0.22 aA | 4.31 ± 0.13 | 7.09 ± 0.02 aB |
| F | 112.8 ± 4.71 abB | 8.44 ± 0.34 cB | 36.71 ± 0.84 aA | 5.29 ± 0.07 aA | 1.67 ± 0.04 aA | 9.28 ± 0.30 aA | ||
| Samples | TPC (μg GAE/mL) | TFC (μg QE/mL) | FRAP (mmol FeSO4/mL) | TEAC (mg Trolox Eq/mL) | DPPH (mg AAE/mL) | |
|---|---|---|---|---|---|---|
| KN | NF | 910.76 ± 2.06 bcB | 25.21 ± 0.09 gB | 8.71 ± 0.18 cB | 1.70 ± 0.08 bB | 0.29 ± 0.06 cB |
| F | 963.89 ± 3.16 bcA | 54.00 ± 0.26 dA | 10.56 ± 0.08 bcA | 1.94 ± 0.03 abA | 0.82 ± 0.02 abA | |
| KY | NF | 913.43 ± 1.07 bcB | 31.87 ± 0.27 fB | 9.81 ± 0.28 bcB | 1.56 ± 0.04 bcB | 0.26 ± 0.02 bB |
| F | 1053 ± 6.09 aA | 56.92 ± 0.46 cA | 10.96 ± 0.29 abA | 1.76 ± 0.01 bA | 0.64 ± 0.12 bA | |
| TK | NF | 893.16 ± 3.07 cB | 31.43 ± 0.26 fB | 8.46 ± 0.11 cB | 1.42 ± 0.06 cB | 0.23 ± 0.01 cB |
| F | 962.78 ± 2.09 bcA | 65.22 ± 0.17 bA | 9.45 ± 0.19 bcA | 1.73 ± 0.02 bA | 0.93 ± 0.05 aA | |
| TD | NF | 957.45 ± 2.01 bcB | 34.50 ± 0.18 eB | 8.82 ± 0.24 cB | 1.75 ± 0.02 bB | 0.27 ± 0.02 cB |
| F | 1037.2 ± 3.02 abA | 54.45 ± 0.74 dA | 11.57 ± 0.31 abA | 1.85 ± 0.02 bA | 0.92 ± 0.03 aA | |
| TS | NF | 1063.14 ± 1.06 abB | 81.71 ± 0.16 bB | 11.85 ± 0.42 abB | 2.21 ± 0.03 aB | 0.34 ± 0.03 cB |
| F | 1288.67 ± 3.05 aA | 118.99 ± 0.16 aA | 13.06 ± 0.28 aA | 2.56 ± 0.08 aA | 0.98 ± 0.06 aA | |
| Samples | Naringin (μg/g) | Hesperidin (μg/g) | Neohesperidin (μg/g) | Naringenin (μg/g) | Hesperetin (μg/g) | |
|---|---|---|---|---|---|---|
| KN | NF | 442.80 ± 7.59 cB | 77.57 ± 2.34 bA | 41.21 ± 11.66 bA | 1.86 ± 0.23 bcB | 0.51 ± 0.03 deB |
| F | 1271.59 ± 15.34 abA | 51.81 ± 9.74 bA | 67.51 ± 13.89 bA | 3.19 ± 0.62 bA | 2.25 ± 0.08 abcA | |
| KY | NF | 208.99 ± 9.48 cB | 45.78 ± 5.81 bB | 56.83 ± 6.54 bA | 0.53 ± 0.0.06 cA | 0.33 ± 0.03 eB |
| F | 472.19 ± 5.27 cA | 71.33 ± 8.46 bA | 52.92 ± 5.45 bA | 0.81 ± 0.07 cA | 0.86 ± 0.04 bcdeA | |
| TK | NF | 1114.91 ± 32.12 bcA | 217.85 ± 15.13 abA | 99.21 ± 12.41 abB | 1.00 ± 0.03 cA | 0.38 ± 0.01 eA |
| F | 1291.22 ± 19.42 abcA | 237.79 ± 18.06 abA | 144.91 ± 23.08 aA | 1.76 ± 0.08 bcA | 0.58 ± 0.05 cdeA | |
| TD | NF | 710.77 ± 7.38 cA | 333.98 ± 7.74 aB | 73.69 ± 4.68 bB | 1.18 ± 0.05 cA | 1.66 ± 0.05 bcdeB |
| F | 717.54 ± 5.02 cA | 460.09 ± 20.30 aA | 105.68 ± 12.44 abA | 1.95 ± 0.04 cA | 2.31 ± 0.08 abA | |
| TS | NF | 2271.38 ± 43.04 abA | 403.75 ± 8.65 aA | 113.30 ± 9.13 abA | 3.48 ± 0.06 bB | 2.12 ± 0.24 abcdA |
| F | 2517.00 ± 30.51 aA | 263.97 ± 4.14 abA | 61.67 ± 5.84 bB | 6.66 ± 0.78 aA | 3.33 ± 0.15 aA | |
| Compound | Tentative Identification | Odor Description | Peak Area (×102) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KN | KY | TD | TK | TS | ||||||||
| NF | F | NF | F | NF | F | NF | F | NF | F | |||
| Alcohols | ||||||||||||
| 1 | 1-Hexanol | Green, Fruity | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | 2.84 ± 0.15 A | 1.69 ± 0.09 A | 4.08 ± 1.36 A | 1.23 ± 0.13 B |
| 2 | 1-Octen-3-ol | Earthy, Herbaceous | 1.68 ± 0.35 A | 1.05 ± 0.06 A | N.D. | N.D. | 1.17 ± 0.03 A | 1.72 ± 0.03 A | N.D. | N.D. | N.D. | N.D. |
| Aldehydes | ||||||||||||
| 3 | (Z)-3-Hexenal | Green, Leafy | N.D. | N.D. | 3.09 ± 0.16 A | 2.83 ± 0.15 A | N.D. | N.D. | 7.73 ± 1.190 | N.D. | 15.67 ± 1.99 | N.D. |
| 4 | 2,4-Decadienal, (E,E)- | Citrus, Green | 5.66 ± 0.81 A | 3.45 ± 0.5 B | 3.85 ± 0.93 A | 1.46 ± 0.81 B | 1.27 ± 0.97 | N.D. | 4.71 ± 0.52 A | 3.54 ± 0.44 A | 7.12 ± 1.92 | N.D. |
| Esters | ||||||||||||
| 5 | Ethyl acetate | Acidic, Sweet | 5.91 ± 0.59 B | 17.13 ±1.26 A | 5.74 ± 0.08 A | 5.69 ± 0.03 A | 9.07 ± 1.71 B | 30.66 ± 1.31 A | 7.86 ± 0.04 A | 7.73 ± 0.10 A | 11.10 ± 0.10 A | 11.14 ± 0.06 A |
| Hydrocarbons | ||||||||||||
| 6 | Myrcene | Lemon, Musty | 4.03 ± 0.57 A | 1.22 ± 0.26 B | N.D. | N.D. | 1.31 ± 0.43 | N.D. | 24.58 ± 2.70 A | 18.38 ± 1.80 B | N.D. | N.D. |
| 7 | (+)-alpha-Phellandrene | Citrus, Green | 8.94 ± 1.88 A | 6.48 ± 1.25 B | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 8 | L-Limonene | Citrus, Terpenic | 37.69 ±1.75 A | 27.91 ± 1.29 B | 13.78 ± 0.11 A | 4.12 ± 0.15 B | 14.60 ± 0.10 A | 2.56 ± 0.14 B | 11.74 ± 0.13 A | 1.19 ± 0.07 B | 12.94 ± 1.01 A | 9.07 ± 0.49 B |
| Ketones | ||||||||||||
| 9 | Butan-2-one | Cheese, Ether-like | N.D. | 62.75 ± 2.37 | N.D. | 121.52 ± 3.46 | N.D. | 92.70 ± 1.65 | N.D. | 66.80 ± 7.60 | N.D. | 165.92 ± 9.78 |
| 10 | Acetoin | Creamy, Buttery | N.D. | 26.98 ± 1.88 | N.D. | 2.82 ± 0.50 | N.D. | 1.98 ± 0.14 | N.D. | 2.37 ± 0.64 | N.D. | 3.38 ± 0.64 |
| 11 | 3,5-Octadien-2-one | Fruity, Mushroom | N.D. | 28.02 ± 2.97 | N.D. | 23.09 ± 2.47 | N.D. | 5.04 ± 0.49 | N.D. | 21.02 ± 2.72 | N.D. | 11.47 ± 1.81 |
| Others | ||||||||||||
| 12 | Indole | Floral, Sweet | N.D. | N.D. | 2.65 ± 0.38 A | 3.87 ± 0.64 A | N.D. | N.D. | 1.60 ± 0.32 | N.D. | 1.39 ± 0.34 A | 1.34 ± 0.23 A |
| 13 | bis(2-furylmethyl) sulfide | Green, Earthy | 13.57 ± 0.36 A | 8.41 ± 0.14 B | 14.58 ± 0.44 A | 9.61 ± 1.53 B | 12.87 ± 3.64 A | 5.14 ± 0.94 B | 15.88 ± 2.52 A | 8.89 ± 0.27 B | 19.43 ± 3.83 A | 7.45 ± 1.79 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balmori, V.; Marnpae, M.; Chusak, C.; Kamonsuwan, K.; Katelakha, K.; Charoensiddhi, S.; Adisakwattana, S. Enhancing Phytochemical Compounds, Functional Properties, and Volatile Flavor Profiles of Pomelo (Citrus grandis (L.) Osbeck) Juices from Different Cultivars through Fermentation with Lacticaseibacillus paracasei. Foods 2023, 12, 4278. https://doi.org/10.3390/foods12234278
Balmori V, Marnpae M, Chusak C, Kamonsuwan K, Katelakha K, Charoensiddhi S, Adisakwattana S. Enhancing Phytochemical Compounds, Functional Properties, and Volatile Flavor Profiles of Pomelo (Citrus grandis (L.) Osbeck) Juices from Different Cultivars through Fermentation with Lacticaseibacillus paracasei. Foods. 2023; 12(23):4278. https://doi.org/10.3390/foods12234278
Chicago/Turabian StyleBalmori, Vernabelle, Marisa Marnpae, Charoonsri Chusak, Kritmongkhon Kamonsuwan, Kasinee Katelakha, Suvimol Charoensiddhi, and Sirichai Adisakwattana. 2023. "Enhancing Phytochemical Compounds, Functional Properties, and Volatile Flavor Profiles of Pomelo (Citrus grandis (L.) Osbeck) Juices from Different Cultivars through Fermentation with Lacticaseibacillus paracasei" Foods 12, no. 23: 4278. https://doi.org/10.3390/foods12234278
APA StyleBalmori, V., Marnpae, M., Chusak, C., Kamonsuwan, K., Katelakha, K., Charoensiddhi, S., & Adisakwattana, S. (2023). Enhancing Phytochemical Compounds, Functional Properties, and Volatile Flavor Profiles of Pomelo (Citrus grandis (L.) Osbeck) Juices from Different Cultivars through Fermentation with Lacticaseibacillus paracasei. Foods, 12(23), 4278. https://doi.org/10.3390/foods12234278







