Assessment of CPME as Sustainable Low VOC Alternative to Hexane: Optimization of Extraction Efficiency and Bioactive Compound Yield from Fenugreek Seed Oil Using Computational and Experimental Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solvents
2.2. Plant Material
2.3. Computational Methods
2.3.1. Hansen Solubility Parameters (HSPs)
2.3.2. COSMO-RS Prediction
2.4. Solvent Extraction of Fenugreek Seed Oil
2.5. Determination of Physicochemical Properties
2.6. Oxidative Stability
2.7. Fatty Acid Composition Analysis
2.8. Micronutrient Analysis
2.8.1. Tocopherol and Sterol Determination
2.8.2. Total Phenolic Content
2.9. In Vitro Antioxidant Activity
2.9.1. Total Antioxidant Capacity (TAC)
2.9.2. DPPH Free Radical Scavenging Activity
2.9.3. Reducing Power Assay
2.10. Antimicrobial Activity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Computational Methods
3.2. HSPs
3.3. COSMO-RS Prediction
3.4. The Effect of CPME on the Extraction of Fenugreek Oil
3.4.1. Oil Yield
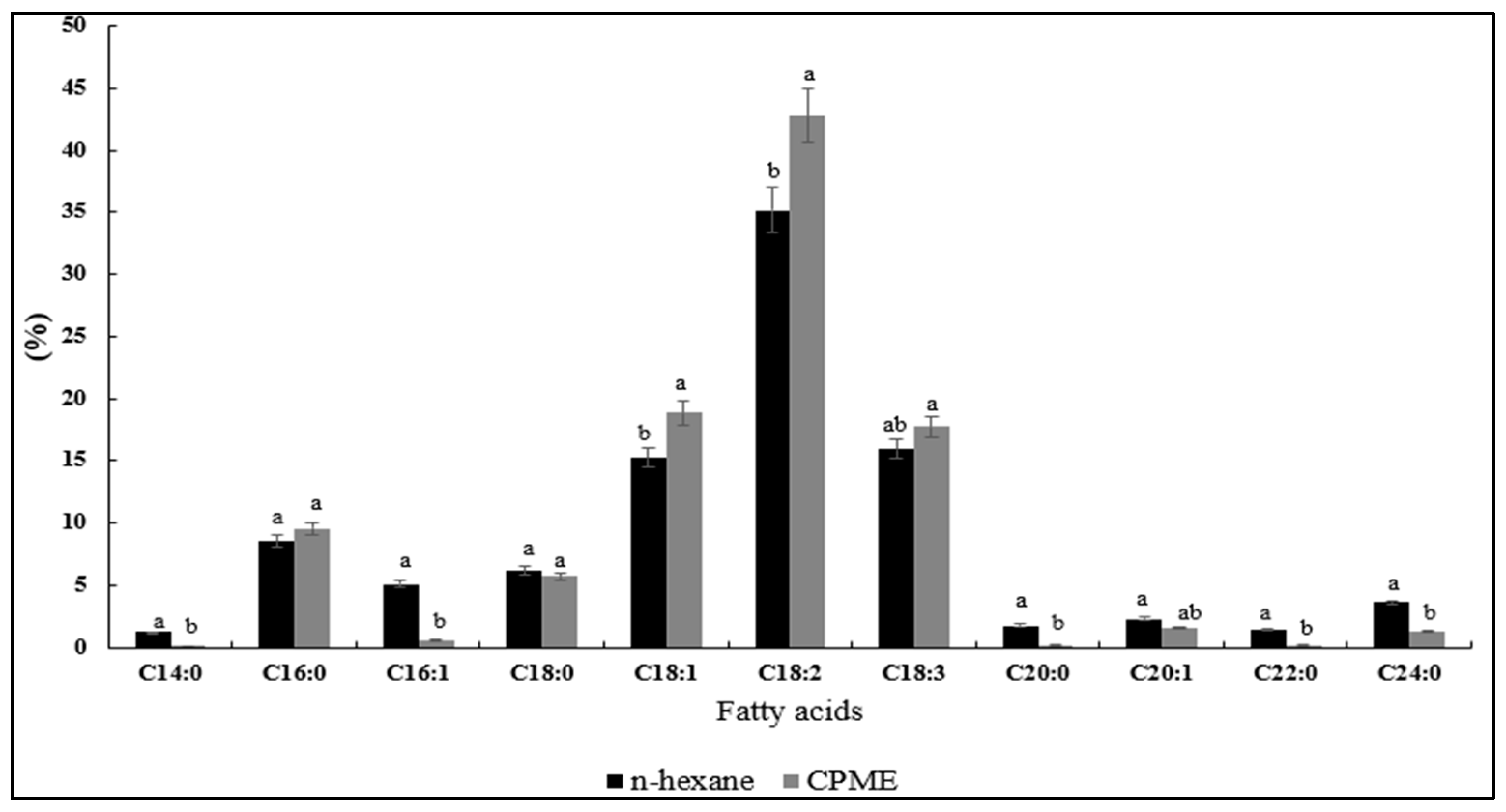
3.4.2. Fatty Acid Composition
3.5. Analysis of Fenugreek Oil Extracted by Alternative Solvent
3.5.1. Physiochemical Properties
3.5.2. Micronutrient Analysis
3.5.3. Phenolic Content
3.5.4. In Vitro Antioxidant Activity
3.5.5. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boulard, C.; Bardet, M.; Chardot, T.; Dubreucq, B.; Gromova, M.; Guillermo, A.; Miquel, M.; Nesi, N.; Yen-Nicoley, S.; Jolivet, P. The structural organization of seed oil bodies could explain the contrasted oil extractability observed in two rapeseed genotypes. Planta 2015, 242, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, C.; Claux, O.; Bartier, M.; Fabiano-Tixier, A.S.; Tabasso, S. Leading edge technologies and perspectives in industrial oilseed extraction. Foods 2023, 28, 5973. [Google Scholar] [CrossRef] [PubMed]
- Kashif, A.; Young-Min, K.; Mian Anjum, M.; Nasir, M.A.; Khan, F.A.; Khan, M.A.; Mahmood, S.; Abid, M. Extraction of oil from oilseeds. In Extraction Processes in the Food Industry; Woodhead Publishing: Cambridge, UK, 2024; pp. 149–175. [Google Scholar]
- Kumar, S.P.J.; Prasad, S.R.; Banerjee, R.; Agarwal, D.K.; Kulkarni, K.S.; Ramesh, K.V. Green solvents and technologies for oil extraction from oilseeds. Chem. Cent. J. 2017, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Kumar, A.; Panghal, A.; Kumar, S.V.; Kumar, G.M. Novel oil extraction technologies: Process conditions, quality parameters, and optimization. Compr. Rev. Food Sci. Food Saf. 2019, 19, 3–20. [Google Scholar] [CrossRef]
- Hadi, S.T.; Abed, M.M.; Fadhil, N.J. Chemical composition of Trigonella foenum-graecum seeds and inhibitory activity of their seeds oil against some microbes. Inter. J. Life Sci. Biotech. 2018, 1, 75–83. [Google Scholar]
- Prasad, R. Identification of High Seed Yielding and Stable Fenugreek Mutants. Master’s Thesis, University of Lethbridge, Lethbridge, AB, Canada, 2014. [Google Scholar]
- Al-Oqail, M.M.; Farshori, N.N.; Al-Sheddi, E.S.; Musarrat, J.; Al-Khedhairy, A.A.; Siddiqui, M.A. In vitro cytotoxic activity of seed oil of fenugreek against various cancer cell lines. Asian Pac. J. Cancer Prev. 2013, 14, 1829–1832. [Google Scholar] [CrossRef]
- Bienkowski, T.; Zuk-Golaszewska, K.; Kaliniewicz, J.; Golaszewski, J. Content of biogenic elements and fatty acid composition of fenugreek seeds cultivated under different conditions. Chil. J. Agric. Res. 2017, 77, 134–141. [Google Scholar] [CrossRef]
- Aljuhaimi, F.; Şimşek, Ş.; Özcan, M.M.; Ghafoor, K.; Babiker, E.E. Effect of location on chemical properties, amino acid and fatty acid compositions of fenugreek (Trigonella foenum-graecum L.) seed and oils. J. Food Process. Pres. 2018, 42, e13569. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Younes, R.M.; Alara, O.R.; Abayoumi, O.O. Extraction, characterization and antioxidant activity of fenugreek (Trigonella foenum-graecum) seed oil. Mater. Sci. Energy Technol. 2019, 2, 349–355. [Google Scholar] [CrossRef]
- Gu, L.B.; Liu, X.N.; Liu, H.M.; Pang, H.L.; Qin, G.Y. Extraction of fenugreek (Trigonella foenum-graecum L.) seed oil using subcritical butane: Characterization and process optimization. Molecules 2017, 22, 228. [Google Scholar] [CrossRef]
- Probst, K.V.; Wales, M.D.; Rezac, M.E.; Vadlani, P.V. Evaluation of green solvents: Oil extraction from oleaginous yeast Lipomyces starkeyi using cyclopentyl methyl ether (CPME). Biotechnol. Prog. 2017, 33, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Zhang, Z.; Wang, Y.; Li, Y. The effect of alternative solvents to n-hexane on the green extraction of Litsea cubeba kernel oils as new oil sources. Ind. Crops Prod. 2018, 126, 340–346. [Google Scholar] [CrossRef]
- Chaabani, E.; Abert Vian, M.; Bott, R.; Ginies, C.; Defoort, C.; Ksouri, R.; Chemat, F. Extraction of aromas from Pistacia lentiscus L. leaves using alternative solvents: COSMO-RS-assisted solvent screening and GC-MS metabolites profiling. Sep. Sci. Technol. 2020, 55, 716–727. [Google Scholar] [CrossRef]
- Joorasty, M.; Rahbar-Kelishami, A.; Hemmati, A. A performance comparison of cyclopentyl methyl ether (CPME) and n-hexane solvents in oil extraction from sewage sludge for biodiesel production; RSM optimization. J. Mol. Liq. 2022, 368, 120573. [Google Scholar] [CrossRef]
- Trad, S.; Chaabani, E.; Aidi Wannes, W.; Dakhlaoui, S.; Nait Mohamed, S.; Khammessi, S.; Hammami, M.; Bourgou, S.; Saidani Tounsi, M.; Fabiano-Tixier, A.S.; et al. Quality of edible sesame oil as obtained by green solvents: In silico versus experimental screening approaches. Foods 2023, 12, 3263. [Google Scholar] [CrossRef] [PubMed]
- Tabee, E.; Azadmard-Damirchi, S.; Jägerstad, M.; Dutta, P.C. Lipids and phytosterol oxidation in commercial French fries commonly consumed in Sweden. J. Food Comp. Anal. 2008, 21, 169–177. [Google Scholar] [CrossRef]
- ISO 9936:2016; Animal and Vegetable Fats and Oils—Determination of Tocopherol and Tocotrienol Contents by High-Performance Liquid Chromatography. International Organization for Standardization (ISO): Geneva, Switzerland, 2016.
- ISO 12228-1:2014; Determination of Individual and Total Sterols Contents—Gas Chromatographic Method. International Organization for Standardization (ISO): Geneva, Switzerland, 2014.
- Ganesan, P.; Kumar, C.S.; Bhaskar, N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour. Technol. 2008, 99, 2717–2723. [Google Scholar] [CrossRef]
- Parry, J.; Su, L.; Luther, M.; Zhou, K.; Yurawecz, P.; Whitaker, P.; Yu, L. Fatty acid composition and anti-oxidant properties of cold-pressed marionberry, boysenberry, red raspberry, and blueberry seed oils. J. Agric. Food Chem. 2005, 53, 566–573. [Google Scholar] [CrossRef]
- Benzie, F.; Szeto, Y.T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Calhelha, R.C.; Rouphael, Y.; Petrović, J.; Soković, M.; Ferreira, I.C.F.R.; Barros, L. Antimicrobial properties, cytotoxic effects, and fatty acids composition of vegetable oils from purslane, linseed, luffa, and pumpkin seeds. App. Sci. 2021, 11, 5738. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Selka, A.; Fabiano-Tixier, A.S.; Balcells, M.; Canela-Garayoa, R.; Bily, A.; Touaibia, M.; Chemat, F. Solvent from forestry biomass: Pinane, a stable terpene derived from pine tree byproducts to substitute n-hexane for the extraction of bioactive compounds. Foods 2016, 18, 6596–6608. [Google Scholar] [CrossRef]
- Sicaire, A.G.; Vian, M.A.; Fine, F.; Carré, P.; Tostain, S.; Chemat, F. Experimental approach versus COSMO-RS assisted solvent screening for predicting the solubility of rapeseed oil. Oilseeds Crops Lipids 2015, 22, D404. [Google Scholar] [CrossRef][Green Version]
- Breil, C.; Vian, M.A.; Zemb, T.; Kunz, W.; Chemat, F. “Bligh and Dyer” and Folch methods for solid–liquid–liquid extraction of lipids from microorganisms: Comprehension of solvation mechanisms and towards substitution with alternative solvents. Int. J. Mol. Sci. 2017, 18, 708. [Google Scholar] [CrossRef] [PubMed]
- Munshi, M.; Arya, P.; Kumar, P. physico-chemical analysis and fatty acid profiling of fenugreek (Trigonella foenum-graecum) seed oil using different solvents. J. Oleo Sci. 2020, 69, 1349–1358. [Google Scholar] [CrossRef]
- Ren, X.F.; Zhu, W.J. Optimal conditions for extraction of oil from fenugreek (Trigonella foenum-graecum L.) by supercritical CO2 fluids (SFE-CO2). Adv. Mater. Res. 2011, 236, 2980–2983. [Google Scholar] [CrossRef]
- Yousefi, M.; Yousefi, M.; Hosseini, H. Evaluation of hexane content in edible vegetable oils consumed in Iran. J. Extra Corp. Technol. 2017, 1, 27–30. [Google Scholar] [CrossRef]
- Gasparetto, H.; Castilhos, F.; Salau, N.P.G. Screening, experimental data, and robust kinetic modeling of vegetable oil extraction using p-cymene as a neoteric solvent for n-hexane replacement. J. Clean. Prod. 2023, 392, 136336. [Google Scholar] [CrossRef]
- Zhang, X.P.; Zhang, Y.; Qu, Z.H.; Gao, Y.; Yu, X.Z. Research on acid value detection of edible vegetable oil. Sci. Technol. Cereals Oils Foods 2021, 29, 163–176. [Google Scholar]
- Sakaino, M.; Sano, T.; Kato, S.; Shimizu, N.; Ito, J.; Rahmania, H.; Imagi, J.; Nakagawa, K. Carboxylic acids derived from triacylglycerols that contribute to the increase in acid value during the thermal oxidation of oils. Foods 2022, 11, 12460. [Google Scholar] [CrossRef]
- Symoniuk, E.; Wroniak, M.; Napiorkowska, K.; Brzezinska, R.; Ratusz, K. Oxidative stability and antioxidant activity of selected cold-Pressed oils and oils mixtures. Foods 2022, 11, 1597. [Google Scholar] [CrossRef]
- Flores, M.; Avendaño, V.; Bravo, J.; Valdés, C.; Forero-Doria, O.; Quitral, V.; Vilcanqui, Y.; Ortiz-Viedma, J. Edible oil parameters during deterioration processes. Int. J. Food Sci. 2021, 2021, 7105170. [Google Scholar] [CrossRef] [PubMed]
- Malheiro, R.; Rodrigues, N.; Manzke, G.; Bento, A.; Pereira, J.A.; Casal, S. The use of olive leaves and tea extracts as effective antioxidants against the oxidation of soybean Oil under microwave heating. Ind. Crops Prod. 2013, 44, 37–43. [Google Scholar] [CrossRef]
- Raji-Idowu, F.O.O. Antibacterial activities of fenugreek oil and seed extracts on selected pathogenic bacteria and proximate composition of fenugreek seed. Nig. J. Microbiol. 2023, 37, 6729–6735. [Google Scholar]
- Yuan, G.; Guan, Y.; Yi, H. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Sulieman, A.M.E.; Ali, A.O.; Hemavathy, J. Lipid content and fatty acid composition of fenugreek (Trigonella foenum-graecum L.) seeds grown in Sudan. Int. J. Food Sci. Tech. 2008, 43, 380–382. [Google Scholar] [CrossRef]
- Wei, M.; Wang, P.; Li, T.; Wang, Q.; Su, M.; Gu, L.; Wang, S. Antimicrobial and antibiofilm effects of essential fatty acids against clinically isolated vancomycin-resistant Enterococcus faecium. Front. Cell. Infect. Microbiol. 2023, 29, 1266674. [Google Scholar] [CrossRef]
- Arjmand, G.; Haeri, M.R. Antibacterial effect of some eukaryotic sterol biosynthesis inhibitors. Adv. Biomed. Res. 2022, 29, 11–90. [Google Scholar] [CrossRef]
- Poudel, P.; Petropoulos, S.A.; Di Gioia, F. Plant tocopherols and phytosterols and their bioactive properties. In Natural Secondary Metabolites; Carocho, M., Heleno, S.A., Barros, L., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar]
| Hexane | CPME | |
|---|---|---|
| Chemical structure | 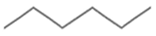 | 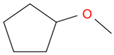 |
| Solvent properties | ||
| Mw (g.mol−1) | 86.18 | 100.2 |
| Density (g.mL−1) | 0.66 | 0.86 |
| Log P | 3.48 | 1.7 |
| R I | 1.38 | 1.41 |
| Flash point (°C) | −17 | 10 |
| Melting point | −119.8 | −122.2 |
| Energy efficiency | ||
| B.p (°C) | 68.5 | 105.3 |
| δHvap (KJ/mol) | 28.9 | 33 |
| Cp, liquid (KJ/mol*K) | 0.195 | 0.2 |
| Toxicity | ||
| CMR * | 2 | 5 |
| Itox | No | 4 |
| Resource | Petroleum | Chemical synthesis |
| CO2 Footprint (kg.kg−1) | 1.9 | 2 |
| Hexane | CPME | ||
|---|---|---|---|
| HSPs | δD | 13.9 | 16.7 |
| δP | 0.1 | 4.3 | |
| δH | 0.1 | 4.3 | |
| Hansen (RED) | C16:0 | 2.39 | 0.69 |
| C18:0 | 2.15 | 0.67 | |
| C18:1 | 2.32 | 0.62 | |
| C18:2 | 2.58 | 0.72 | |
| C18:3 | 2.59 | 0.62 | |
| COSMO-RS prediction (Log10 (X_solub)) | C16:0 | −1.27 | 0 |
| C18:0 | −1.31 | 0 | |
| C18:1 | −1.34 | 0 | |
| C18:2 | −1.76 | 0 | |
| C18:3 | −1.80 | 0 |
| Hexane | CPME | |
|---|---|---|
| Extraction yield (g/100 g DM) | 4.25 ± 0.34 b | 7.23 ± 0.05 a |
| AV (mg KOH/g oil) | 1.62 ± 0.06 a | 1.59 ± 0.04 a |
| PV (mEq O2/kg) | 0.71 ± 0.01 a | 0.92 ± 0.00 a |
| IV (g of I2/100 g) | 133.92 ± 0.20 a | 149.03 ± 0.12 ab |
| RI (20° C) | 1.30 ± 0.03 a | 1.36 ± 0.02 a |
| Oxidative stability (h) | 2.73 ± 0.01 b | 3.95 ± 0.01 a |
| Hexane | CPME | |
|---|---|---|
| Sterol Contents (mg/kg oil) | ||
| Cholesterol | 3.3 ± 0.01 b | 5.17 ± 0.06 a |
| Campesterol | 22.76 ± 1.02 b | 33.10 ± 1.29 a |
| Stigmasterol | 8.09 ± 0.96 b | 12.38 ± 0.02 a |
| β-Sitosterol | 53.26 ± 1.23 b | 72.10 ± 1.04 a |
| Total | 87.41 ± 2.70 b | 122.75 ± 3.29 a |
| Tocopherol Contents (mg/kg of oil) | ||
| α-Tocopherol | 643.94 ± 0.13 ab | 703.01 ± 1.26 a |
| β-Tocopherol | 18.22 ± 3.11 ab | 22.76 ± 1.45 a |
| γ-Tocopherol | 5.30 ± 0.23 b | 7.14 ± 0.04 a |
| δ-Tocopherol | 1.94 ± 0.02 b | 3.03 ± 0.09 a |
| Total | 669.4 ± 0.56 b | 735.94 ± 0.23 a |
| TPC (mg GAE/g) | TAC (mg GAE/g) | DPPH (IC50 µg/mL) | Reducing Power (EC50 µg/mL) | |
|---|---|---|---|---|
| Hexane | 12.03 ± 1.02 b | 35.75 ± 0.63 a | 280 ± 0.92 b | 374.96 ± 2.03 b |
| CPME | 15.80 ± 2.71 a | 38.33 ± 0.04 a | 126.85 ± 1.83 a | 293.28 ± 1.93 a |
| ATCC | Strains | MIC (mg/mL) | MBC (mg/mL) | |
|---|---|---|---|---|
| Hexane | ||||
| Gram (+) | 29,212 | Entrococcus feacalis | 50 | 60 |
| Gram (+) | 25,923 | Staphylococcus aureus | 50 | 70 |
| Gram (−) | 3739 | Escherichia coli | 50 | 70 |
| Gram (−) | 14,028 | Salmonella thyphimirium | 50 | ND |
| CPME | ||||
| Gram (+) | 29,212 | Entrococcus feacalis | 50 | 60 |
| Gram (+) | 25,923 | Staphylococcus aureus | 50 | 50 |
| Gram (−) | 3739 | Escherichia coli | 50 | 60 |
| Gram (−) | 14,028 | Salmonella thyphimirium | 50 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Abdennebi, A.; Chaabani, E.; Ben Jemaa, M.; Hammami, M.; Khammassi, S.; Nait Mohamed, S.; Aidi Wannes, W.; Hamrouni Sellami, I.; Fabiano Tixier, A.-S.; Bettaieb Rebey, I. Assessment of CPME as Sustainable Low VOC Alternative to Hexane: Optimization of Extraction Efficiency and Bioactive Compound Yield from Fenugreek Seed Oil Using Computational and Experimental Methods. Foods 2024, 13, 3899. https://doi.org/10.3390/foods13233899
Ben Abdennebi A, Chaabani E, Ben Jemaa M, Hammami M, Khammassi S, Nait Mohamed S, Aidi Wannes W, Hamrouni Sellami I, Fabiano Tixier A-S, Bettaieb Rebey I. Assessment of CPME as Sustainable Low VOC Alternative to Hexane: Optimization of Extraction Efficiency and Bioactive Compound Yield from Fenugreek Seed Oil Using Computational and Experimental Methods. Foods. 2024; 13(23):3899. https://doi.org/10.3390/foods13233899
Chicago/Turabian StyleBen Abdennebi, Ameni, Emna Chaabani, Mariem Ben Jemaa, Majdi Hammami, Saber Khammassi, Salma Nait Mohamed, Wissem Aidi Wannes, Ibtissem Hamrouni Sellami, Anne-Sylvie Fabiano Tixier, and Iness Bettaieb Rebey. 2024. "Assessment of CPME as Sustainable Low VOC Alternative to Hexane: Optimization of Extraction Efficiency and Bioactive Compound Yield from Fenugreek Seed Oil Using Computational and Experimental Methods" Foods 13, no. 23: 3899. https://doi.org/10.3390/foods13233899
APA StyleBen Abdennebi, A., Chaabani, E., Ben Jemaa, M., Hammami, M., Khammassi, S., Nait Mohamed, S., Aidi Wannes, W., Hamrouni Sellami, I., Fabiano Tixier, A.-S., & Bettaieb Rebey, I. (2024). Assessment of CPME as Sustainable Low VOC Alternative to Hexane: Optimization of Extraction Efficiency and Bioactive Compound Yield from Fenugreek Seed Oil Using Computational and Experimental Methods. Foods, 13(23), 3899. https://doi.org/10.3390/foods13233899









