Efficient Production of High-Quality Infrared-Assisted Spouted Bed-Dried Areca taro Based on the Drying Temperature and Cutting Size Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
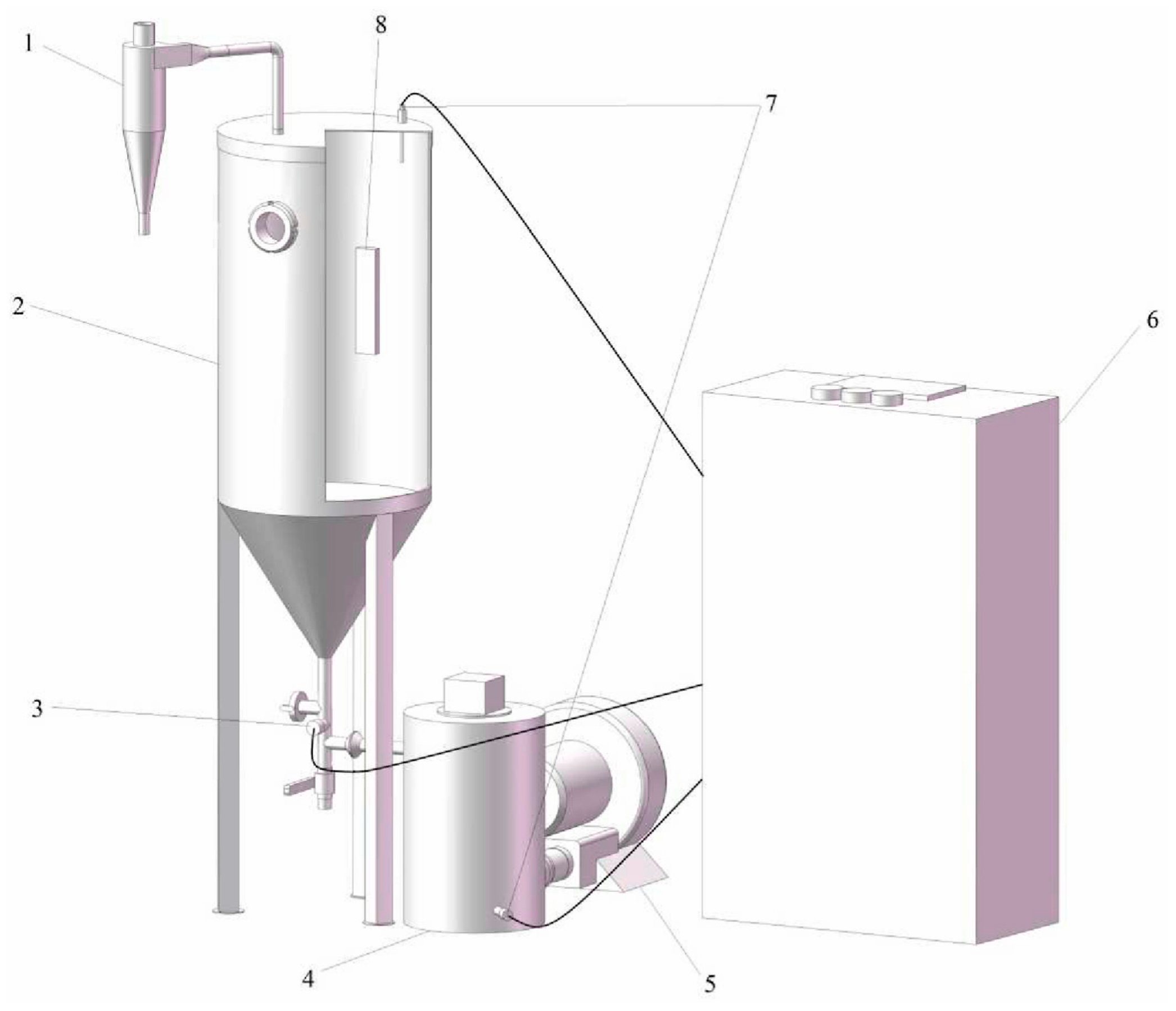
2.2. Experimental Equipment
2.3. Drying Experiments
2.4. Determination of Moisture Content on a Dry Basis
2.5. Temperature Uniformity Evaluation
2.6. Colour Analysis
2.7. Determination of Rehydration Ratio
2.8. Determination of Total Phenol Content (TPC)
2.9. Determination of Total Flavonoid Content (TFC)
2.10. Determination of Antioxidant Activity
2.11. Determination of the Total Energy Consumption
2.12. Statistical Analysis
3. Results
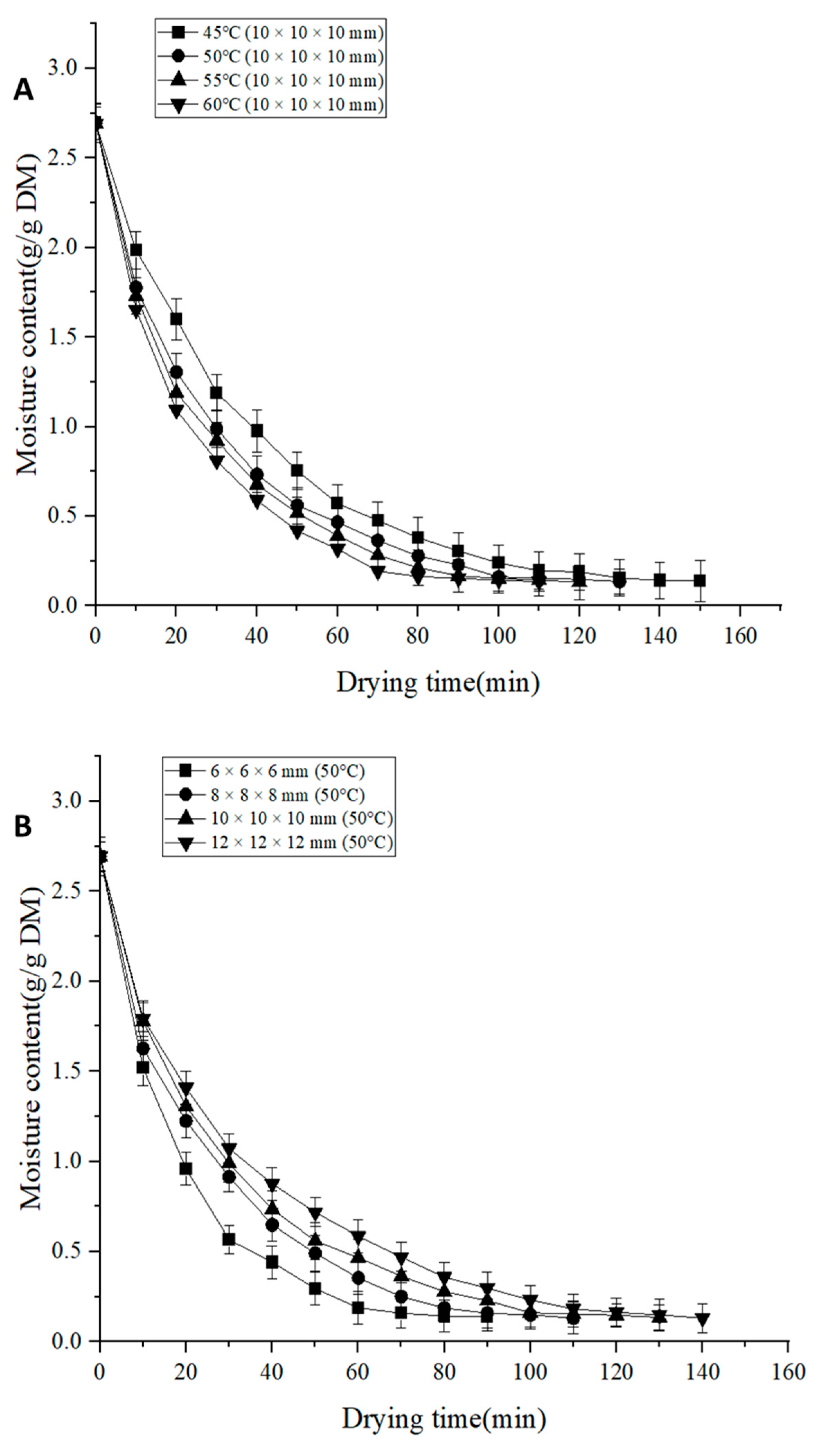
3.1. Drying Characteristics
3.2. Drying Uniformity
3.3. Colour Analysis
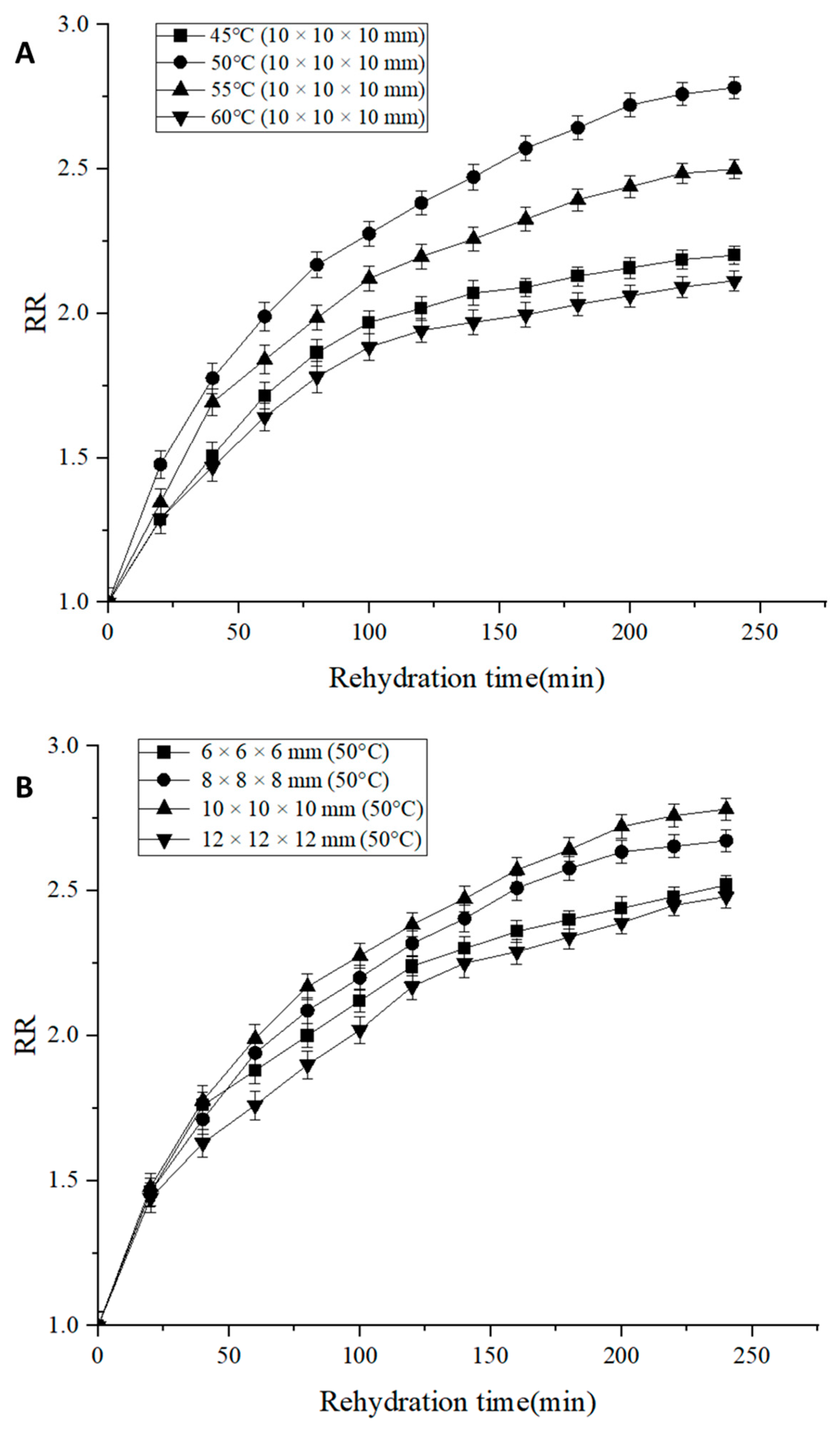
3.4. Rehydration Ratio
3.5. Bioactive Compounds
3.6. Antioxidant Activity
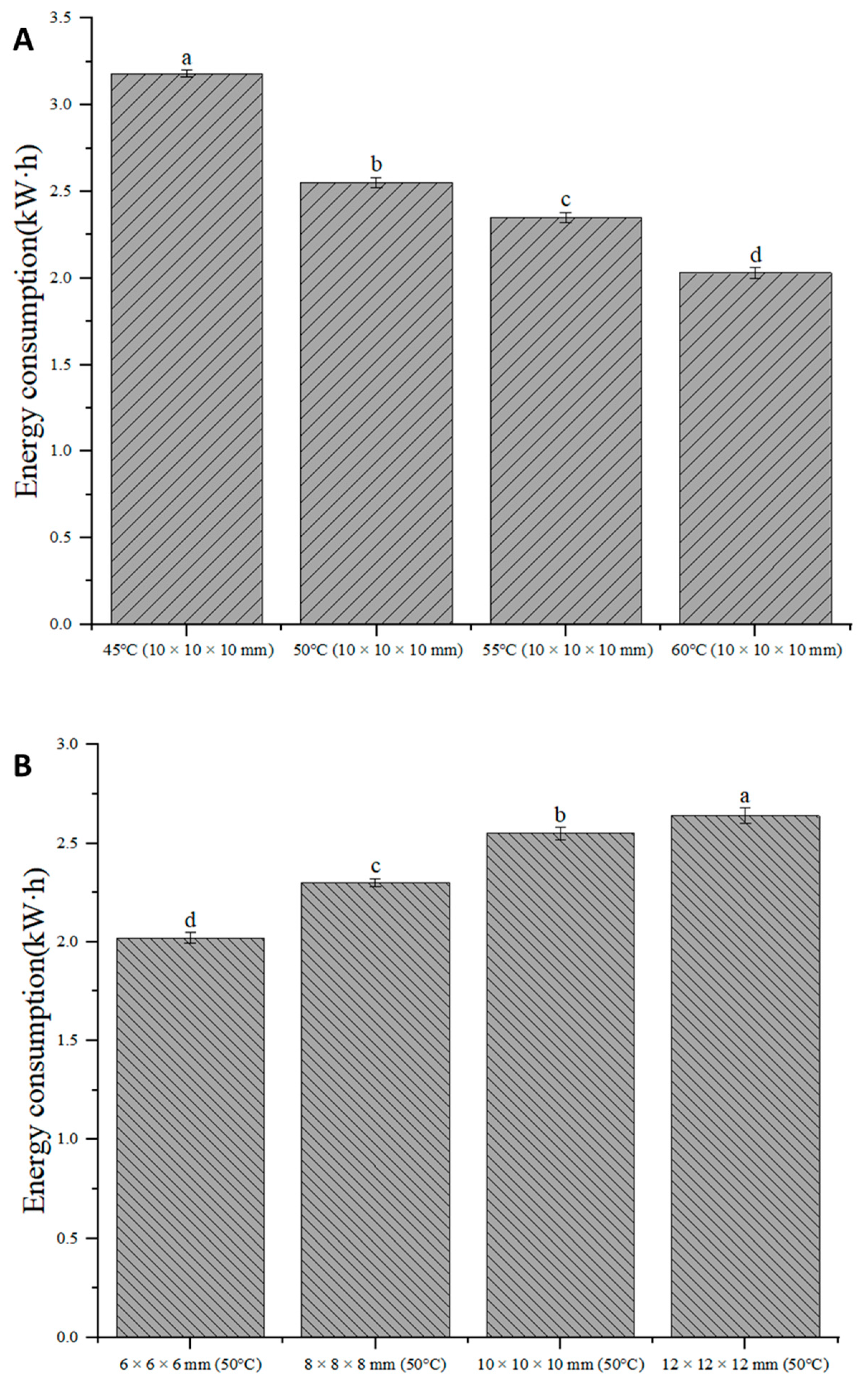
3.7. Energy Consumption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; An, F.; He, H.; He, D.; Wang, Y.; Song, H. Pickering emulsions stabilized by compound modified areca taro (Colocasia esculenta (L.) Schott) starch with ball-milling and OSA. Colloids Surf. 2018, 556, 185–194. [Google Scholar] [CrossRef]
- Mitharwal, S.; Kumar, A.; Chauhan, K.; Taneja, N.K. Nutritional, phytochemical composition and potential health benefits of taro (Colocasia esculenta L.) leaves: A review. Food Chem. 2022, 383, 132406. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liang, D.; Wang, X.Q.; Li, F.; Fan, X.Y.; Liu, Y.H. Effects of contact ultrasound & far-infrared radiation strengthening drying on water migration and quality characteristics of taro slices. J. Food Process. Preserv. 2022, 46, e17030. [Google Scholar]
- Wei, Q.Y.; Huang, J.P.; Zhang, Z.Y.; Lia, D.J.; Liu, C.Q.; Xiao, Y.D.; Lagnika, C.; Zhang, M. Effects of different combined drying methods on drying uniformity and quality of dried taro slices. Dry. Technol. 2019, 37, 322–330. [Google Scholar] [CrossRef]
- Hnin, K.K.; Zhang, M.; Li, Z.; Wang, B. Comparison of quality aspects and energy consumption of restructured taro and potato chips under three drying methods. J. Food Process Eng. 2019, 42, e13249. [Google Scholar] [CrossRef]
- Walters, R.H.; Bhatnagar, B.; Tchessalov, S.; Izutsu, K.I.; Tsumoto, K.; Ohtake, S. Next Generation Drying Technologies for Pharmaceutical Applications. J. Pharm. Sci. 2014, 103, 2673–2695. [Google Scholar] [CrossRef] [PubMed]
- Riadh, M.H.; Ahmad, S.A.B.; Marhaban, M.H.; Soh, A.C. Infrared Heating in Food Drying: An Overview. Dry. Technol. 2015, 33, 322–335. [Google Scholar] [CrossRef]
- Kahyaoglu, L.N.; Sahin, S.; Sumnu, G. Spouted bed and microwave-assisted spouted bed drying of parboiled wheat. Food Bioprod. Process. 2012, 90, 301–308. [Google Scholar] [CrossRef]
- Zhu, K.; Ren, G.; Duan, X.; Qiu, C.; Li, L.; Chu, Q.; Yu, Z. Backward Propagation (BP) Neural Network-Based Prediction of Moisture Ratio of Fresh In-shell Peanut during Infrared-Assisted Spouted Bed Drying. J. Food Sci. 2022, 43, 9–18. [Google Scholar]
- Huang, D.; Huang, W.Y.; Huang, S.; Zhou, F.; Gong, G.L.; Li, L.J.; Sunden, B. Applications of spouted bed technology in the drying of food products. Lwt 2023, 182, 114880. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Li, L.L.; Ren, G.Y.; Duan, X.; Cao, W.W.; Qiu, C.X. Efficient Production of Dried Whole Peanut Fruits Based on Infrared Assisted Spouted Bed Drying. Foods 2021, 10, 2383. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Pan, H.; Chen, J.; Cao, W.; Liu, W.; Duan, X.; Ren, G. Infrared-assisted spouted bed drying of Chinese yam cubes: Effect of constant and variable temperature drying processes on drying behavior, uniformity, and quality attributes. J. Sci. Food Agric. 2023, 103, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.M.; Liu, W.C.; Li, L.L.; Cao, W.W.; Zhao, M.Y.; Dong, J.Y.; Ren, G.Y.; Bhandari, B.; Duan, X. Dynamic changes of non-volatile compounds and evaluation on umami during infrared assisted spouted bed drying of shiitake mushrooms. Food Control 2022, 142, 109245. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, J.; Zhang, Y.; Liang, J.; Zhang, L. Effects of Drying Methods on the Quality Characteristics and Microstructure of Shiitake Mushrooms (Lentinus edodes). J. Food Sci. 2020, 41, 150–156. [Google Scholar]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Gao, X.Q.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Zhao, L.J.; Liu, W.; Xiong, S.H.; Tang, J.; Lou, Z.H.; Xie, M.X.; Xia, B.H.; Lin, L.M.; Liao, D.F. Determination of Total Flavonoids Contents and Antioxidant Activity of Ginkgo biloba Leaf by Near-Infrared Reflectance Method. Int. J. Anal. Chem. 2018, 2018, 8195784. [Google Scholar] [CrossRef]
- Singh, R.P.; Murthy, K.N.C.; Jayaprakasha, G.K. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef]
- Terpinc, P.; Cigic, B.; Polak, T.; Hribar, J.; Pozrl, T. LC-MS analysis of phenolic compounds and antioxidant activity of buckwheat at different stages of malting. Food Chem. 2016, 210, 9–17. [Google Scholar] [CrossRef]
- Supeno, D.; Pratama, P.S.; Choi, W.S. Impact of Slice Size on Kinetic Behavior and Drying Time of Fresh-Cut Apple (Malus domestica). AMA Agric. Mech. Asia Afr. Lat. Am. 2020, 50, 61–64. [Google Scholar]
- Doymaz, I. Infrared drying of sweet potato (Ipomoea batatas L.) slices. J. Food Sci. Technol. 2012, 49, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, E.; Asl, A.H.; Movagharnejad, K. Optimization and quality evaluation of infrared-dried kiwifruit slices. Food Sci. Nutr. 2020, 8, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Molla, E.T.; Teka, T.A.; Taye, A.H. Effects of solar tunnel drying zones and slice thickness on the drying characteristics of taro (Colocasia esculenta (L.) Schott) slice. Food Sci. Nutr. 2023, 11, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wu, M.; Wang, S.; Wang, W.; Zheng, Z. Design and Performance Verification of Infrared Combined Hot Air Drying Device. Trans. Chi. Soc. Agric. Eng. 2022, 53, 411–420. (In Chinese) [Google Scholar]
- Tan, J.; Yao, Y.; Wu, N.; Du, H.Y.; Xu, M.S.; Liao, M.F.; Zhao, Y.; Tu, Y.G. Color, physicochemical characteristics and antioxidant activities of preserved egg white pickled at different temperatures. Lwt 2022, 164, 113685. [Google Scholar] [CrossRef]
- Cao, X.H.; Zhang, M.; Mujumdar, A.S.; Zhong, Q.F. Evaluation of quality properties and water mobility in vacuum microwave-dried carrot slices using pulse-spouted bed with hot air. Dry. Technol. 2019, 37, 1087–1096. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, M.; Ren, G.; Zhou, S.; Zhao, L.; Xu, Y. Drying models and quality changes of rose subjected to infrared assisted spouted bed drying. Trans. Chi. Soc. Agric. Eng. 2020, 36, 238–245. (In Chinese) [Google Scholar]
- Wang, L.; Song, J.; Liu, A.; Xiao, B.; Li, S.; Wen, Z.; Lu, Y.; Du, G. Research Progress of the Antiviral Bioactivities of Natural Flavonoids. Nat. Prod. Bioprospect. 2020, 10, 271–283. [Google Scholar] [CrossRef]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016, 197, 1292–1300. [Google Scholar] [CrossRef]
- Michalczyk, M.; Macura, R.; Matuszak, I. The effect of air-drying, freeze-drying and storage on the quality and antioxidant activity of some selected berries. J. Food Process. Preserv. 2009, 33, 11–21. [Google Scholar] [CrossRef]
- Ren, Z.F.; Yu, X.J.; Yagoub, A.A.; Fakayode, O.A.; Ma, H.L.; Sun, Y.H.; Zhou, C.S. Combinative effect of cutting orientation and drying techniques (hot air, vacuum, freeze and catalytic infrared drying) on the physicochemical properties of ginger (Zingiber officinale Roscoe). Lwt 2021, 144, 111238. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Murata, K.; Itoh, K.; Hanamoto, Y.; Masuda, M.; Moriyama, K.; Shintani, T.; Matsuda, H. Anti-hyperuricemia effects of extracts of immature Citrus unshiu fruit. J. Tradit. Med. 2011, 28, 10–15. [Google Scholar]
- Deepa, N.; Kaur, C.; George, B.; Singh, B.; Kapoor, H.C. Antioxidant constituents in some sweet pepper (Capsicum annuum L.) genotypes during maturity. Lwt 2007, 40, 121–129. [Google Scholar] [CrossRef]
- Lin, Z.; Shi, J.; Tan, J.; Lin, Z. Effect of Drying Temperature on Drying Efficiency, Energy Consumption and Flavor Quality of Xiangcha, a Kind of Green Tea. J. Food Sci. 2020, 41, 153–158. [Google Scholar]
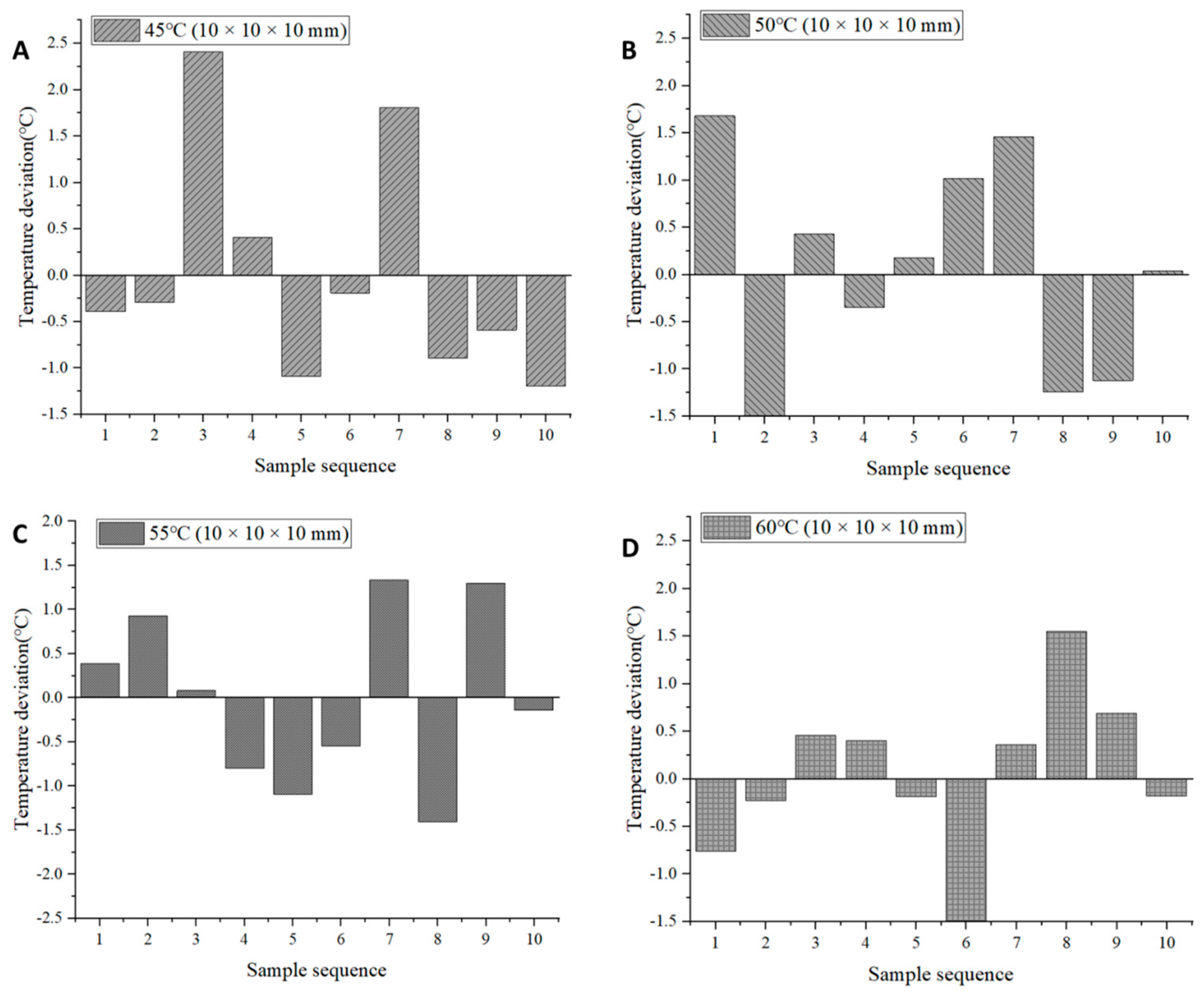
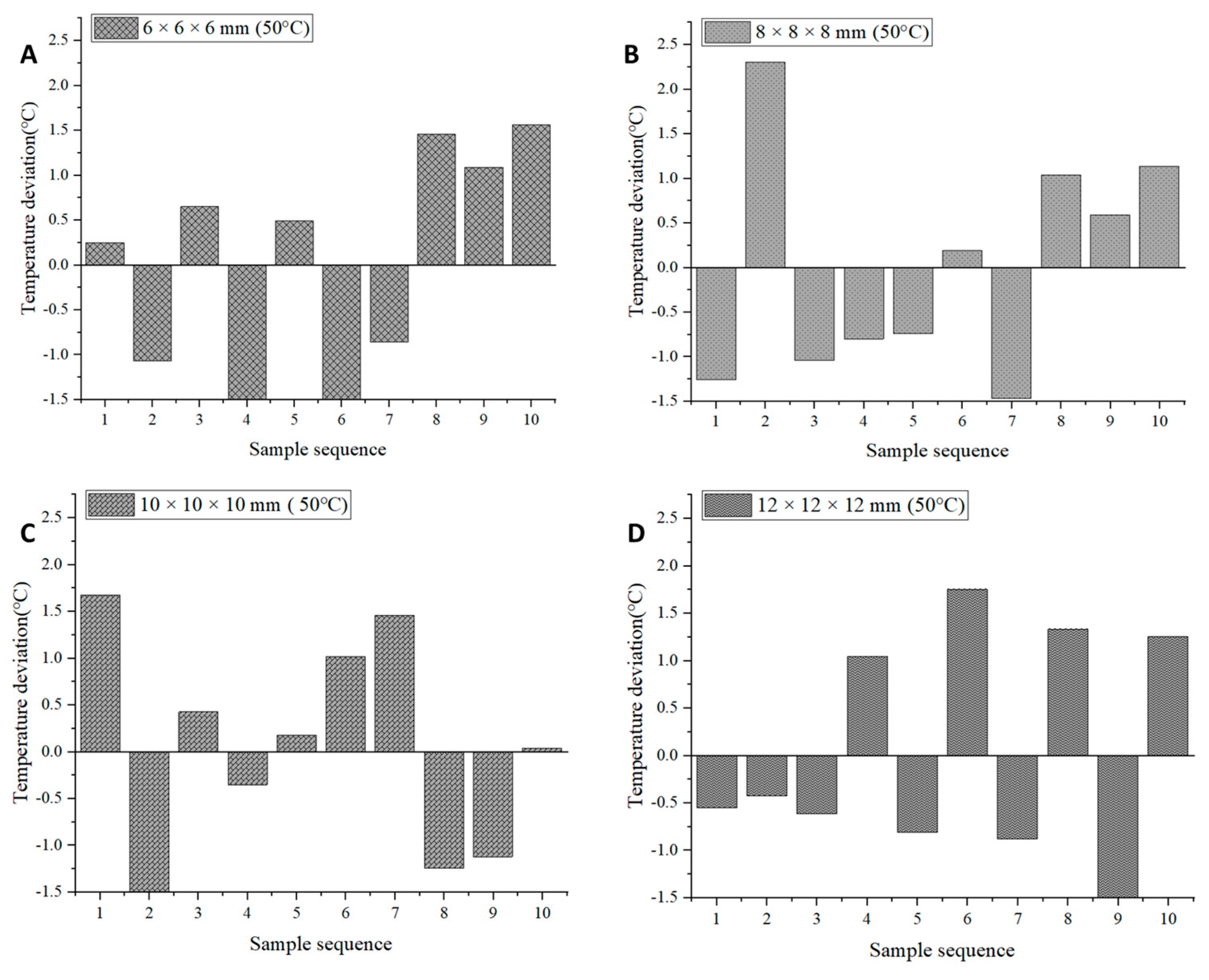
| Samples | Average Temperature (°C) | CV (%) | Average Temperature (°C)−Set Temperature (°C) |
|---|---|---|---|
| 45 °C (10 × 10 × 10 mm) | 45.49 | 2.67 | 0.49 |
| 50 °C (10 × 10 × 10 mm) | 50.38 | 2.47 | 0.38 |
| 55 °C (10 × 10 × 10 mm) | 54.71 | 1.81 | −0.29 |
| 60 °C (10 × 10 × 10 mm) | 59.88 | 1.65 | −0.12 |
| 6 × 6 × 6 mm (50 °C) | 49.55 | 2.62 | −0.46 |
| 8 × 8 × 8 mm (50 °C) | 50.44 | 2.48 | 0.44 |
| 12 × 12 × 12 mm (50 °C) | 50.72 | 2.52 | 0.72 |
| Samples | L* Value | a* Value | b* Value | ∆E |
|---|---|---|---|---|
| Fresh samples | 84.96 ± 1.57 c | 0.91 ± 1.17 ab | 6.52 ± 0.09 e | |
| 45 °C (10 × 10 × 10 mm) | 86.89 ± 0.03 b | 1.89 ± 0.04 a | 10.99 ± 0.05 b | 3.82 ± 0.03 cd |
| 50 °C (10 × 10 × 10 mm) | 87.59 ± 0.03 b | 1.58 ± 0.05 ab | 8.2 ± 0.69 d | 2.83 ± 0.05 e |
| 55 °C (10 × 10 × 10 mm) | 87.42 ± 0.05 b | 1.48 ± 0.04 ab | 10.43 ± 0.04 b | 2.99 ± 0.03 de |
| 60 °C (10 × 10 × 10 mm) | 86.98 ± 0.03 b | 1.38 ± 0.03 ab | 12.02 ± 0.04 a | 4.76 ± 0.04 bc |
| 6 × 6 × 6 mm (50 °C) | 90.89 ± 0.44 a | 1.01 ± 0.07 b | 8.74 ± 0.05 cd | 6.08 ± 0.44 a |
| 8 × 8 × 8 mm (50 °C) | 89.89 ± 1.03 a | 1.16 ± 0.23 ab | 8.72 ± 0.59 cd | 5 ± 1.01 b |
| 12 × 12 × 12 mm (50 °C) | 89.97 ± 0.55 a | 0.91 ± 0.05 b | 9.23 ± 0.17 c | 5.28 ± 0.58 ab |
| Samples | TPC (mg GAE/g DM) | TFC (mg RE/g DM) | DPPH Scavenging Capacity (mg Vc/g DM) | ABTS Scavenging Capacity (mg Vc/g DM) |
|---|---|---|---|---|
| 45 °C (10 × 10 × 10 mm) | 0.39 ± 0.04 ab | 1.87 ± 0.23 b | 1.54 ± 0.01 ab | 6.7 ± 0.02 a |
| 50 °C (10 × 10 × 10 mm) | 0.43 ± 0.01 a | 2.5 ± 0.01 a | 1.55 ± 0.01 a | 7 ± 0.18 a |
| 55 °C (10 × 10 × 10 mm) | 0.25 ± 0.01 ef | 2.02 ± 0.01 b | 1.47 ± 0.02 c | 5.54 ± 0.01 b |
| 60 °C (10 × 10 × 10 mm) | 0.22 ± 0.01 f | 1.29 ± 0.03 d | 1.43 ± 0.03 d | 4.61 ± 0.95 c |
| 6 × 6 × 6 mm (50 °C) | 0.32 ± 0.47 cd | 1.19 ± 0.02 d | 1.49 ± 0.01 c | 3.4 ± 0.10 d |
| 8 × 8 × 8 mm (50 °C) | 0.38 ± 0.02 bc | 1.51 ± 0.05 c | 1.52 ± 0.01 b | 4.64 ± 0.02 c |
| 12 × 12 × 12 mm (50 °C) | 0.29 ± 0.01 de | 1.5 ± 0.12 c | 1.54 ± 0.01 ab | 4.82 ± 0.28 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Yu, F. Efficient Production of High-Quality Infrared-Assisted Spouted Bed-Dried Areca taro Based on the Drying Temperature and Cutting Size Control. Foods 2024, 13, 260. https://doi.org/10.3390/foods13020260
Zhao Y, Yu F. Efficient Production of High-Quality Infrared-Assisted Spouted Bed-Dried Areca taro Based on the Drying Temperature and Cutting Size Control. Foods. 2024; 13(2):260. https://doi.org/10.3390/foods13020260
Chicago/Turabian StyleZhao, Yitong, and Fanqianhui Yu. 2024. "Efficient Production of High-Quality Infrared-Assisted Spouted Bed-Dried Areca taro Based on the Drying Temperature and Cutting Size Control" Foods 13, no. 2: 260. https://doi.org/10.3390/foods13020260
APA StyleZhao, Y., & Yu, F. (2024). Efficient Production of High-Quality Infrared-Assisted Spouted Bed-Dried Areca taro Based on the Drying Temperature and Cutting Size Control. Foods, 13(2), 260. https://doi.org/10.3390/foods13020260





