Bioaccessibility of Carotenoids and Polyphenols in Organic Butternut Squash (Cucurbita moschata): Impact of Industrial Freezing Process
Abstract
1. Introduction
2. Materials and Methods
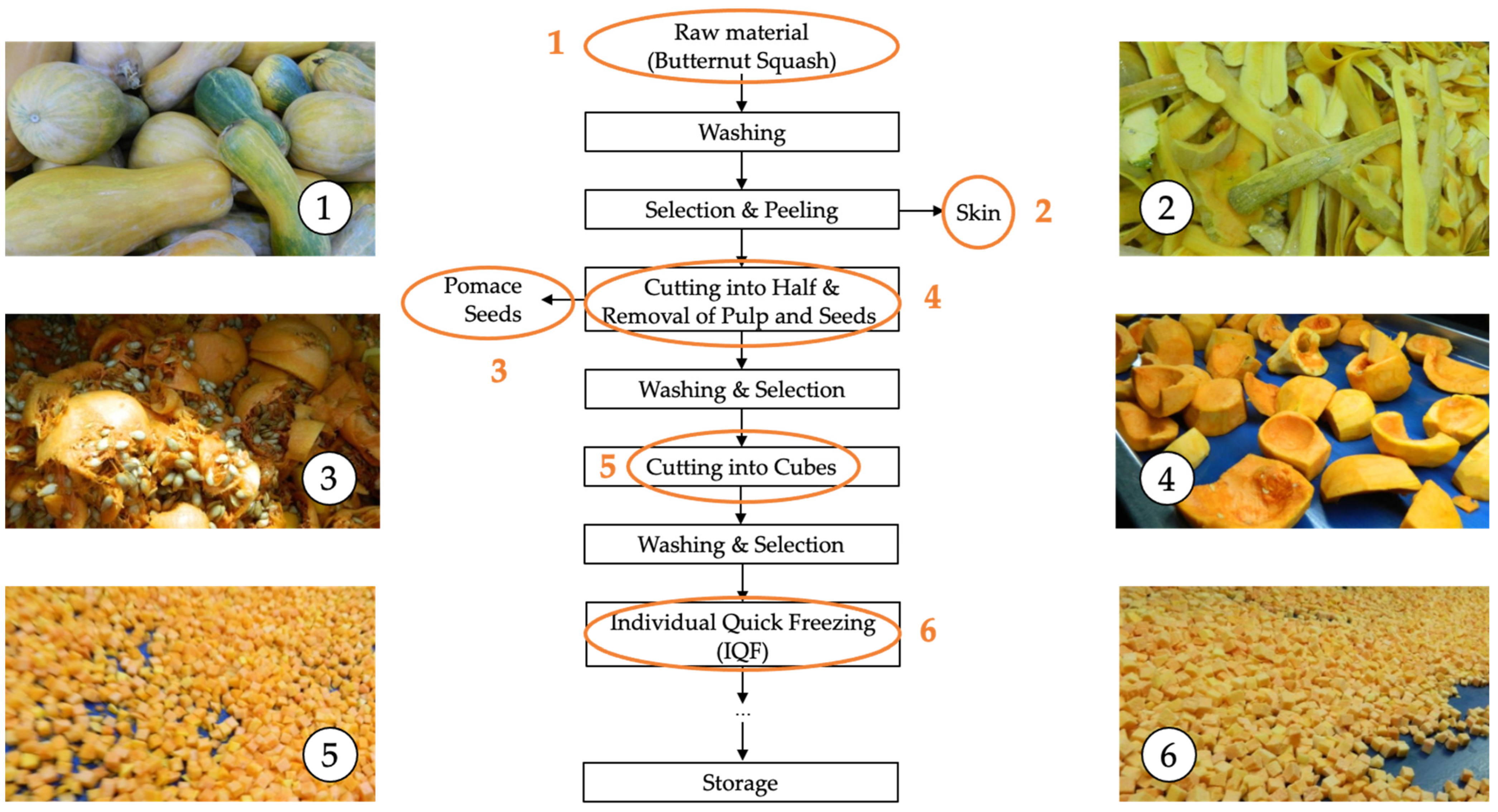
2.1. Materials
2.2. Chemicals
2.3. In Vitro Gastrointestinal Digestion
2.4. Carotenoids
2.4.1. Extraction
2.4.2. Identification and Quantification Using High Performance Liquid Chromatography-Photodiode Array Detector (HPLC-PDA)
2.5. Polyphenols
2.5.1. Extraction
2.5.2. Identification Using Ultra High Performance Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry (UPLC-ESI-MS/MS)
2.5.3. Quantification Using HPLC-PDA
2.6. Total Phenolic Content
2.7. Total Flavonoid Content
2.8. Total Antioxidant Capacity
2.8.1. ABTS Assay
2.8.2. Cupric Ion Reducing Antioxidant Capacity (CUPRAC) Assay
2.8.3. Ferric Ion Reducing Antioxidant Power (FRAP) Assay
2.8.4. DPPH Assay
2.9. Statistical Analysis
3. Results and Discussion
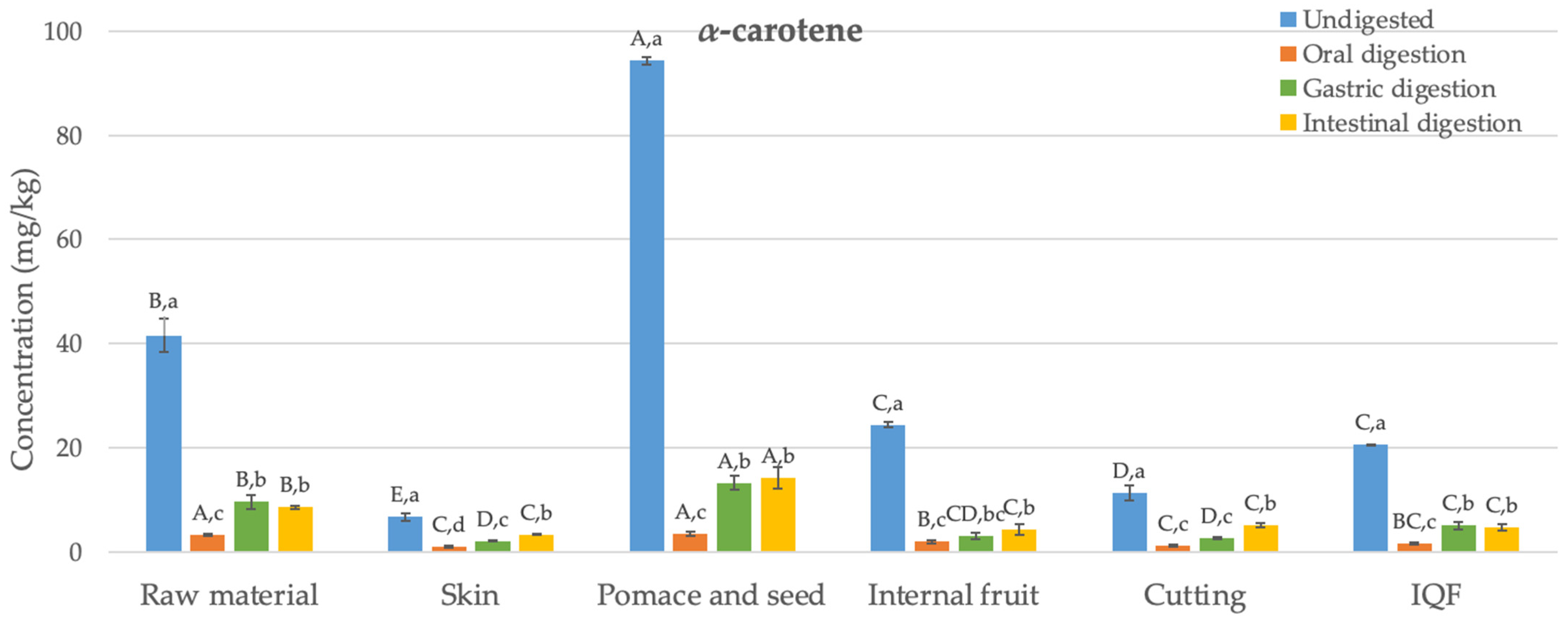
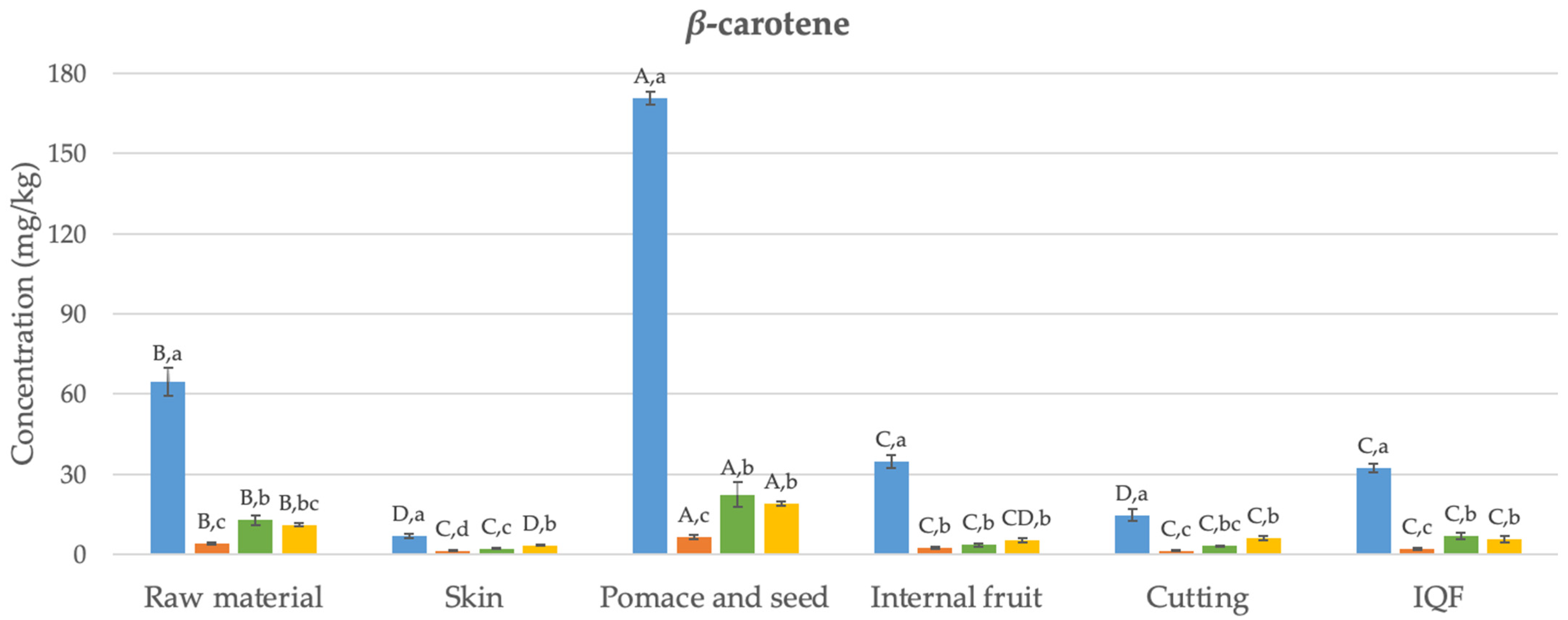
3.1. Carotenoids
3.2. Polyphenols
3.2.1. Flavonoids
3.2.2. Phenolic Acids
3.3. Total Phenolics, Flavonoids and Antioxidant Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kulczyński, B.; Gramza-Michałowska, A.; Królczyk, J.B. Optimization of Extraction Conditions for the Antioxidant Potential of Different Pumpkin Varieties (Cucurbita maxima). Sustainability 2020, 12, 1305. [Google Scholar] [CrossRef]
- Mokhtar, M.; Bouamar, S.; Di Lorenzo, A.; Temporini, C.; Daglia, M.; Riazi, A. The Influence of Ripeness on the Phenolic Content, Antioxidant and Antimicrobial Activities of Pumpkins (Cucurbita moschata Duchesne). Molecules 2021, 26, 3623. [Google Scholar] [CrossRef]
- Huerta-Reyes, M.; Tavera-Hernández, R.; Alvarado-Sansininea, J.J.; Jiménez-Estrada, M. Selected Species of the Cucurbitaceae Family Used in Mexico for the Treatment of Diabetes Mellitus. Molecules 2022, 27, 3440. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Melim, C.; Neves, B.G.; Sharifi-Rad, J.; Calina, D.; Mamurova, A.; Cabral, C. Cucurbitacins as Potential Anticancer Agents: New Insights on Molecular Mechanisms. J. Transl. Med. 2022, 20, 630. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K.; et al. Cucurbita Plants: From Farm to Industry. Appl. Sci. 2019, 9, 3387. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization Corporate Statistical Database. Crops. Available online: http://www.fao.org/faostat/en/#data/qc/visualize (accessed on 1 November 2023).
- Antignani, A.; Francavilla, R.; Vania, A.; Leonardi, L.; Di Mauro, C.; Tezza, G.; Cristofori, F.; Dargenio, V.; Scotese, I.; Palma, F.; et al. Nutritional Assessment of Baby Food Available in Italy. Nutrients 2022, 14, 3722. [Google Scholar] [CrossRef]
- Grover, Y.; Negi, P.S. Recent Developments in Freezing of Fruits and Vegetables: Striving for Controlled Ice Nucleation and Crystallization with Enhanced Freezing Rates. J. Food Sci. 2023, 88, 4799–4826. [Google Scholar] [CrossRef] [PubMed]
- Ozdemirli, N.; Kamiloglu, S. Influence of Industrial Blanching, Cutting and Freezing Treatments on in Vitro Gastrointestinal Digestion Stability of Orange (Citrus sinensis L.) and Lemon (Citrus limon L.) Peel Polyphenols. J. Sci. Food Agric. 2023; early view. [Google Scholar] [CrossRef]
- Szabo, K.; Emőke Teleky, B.; Ranga, F.; Simon, E.; Lelia Pop, O.; Babalau-Fuss, V.; Kapsalis, N.; Cristian Vodnar, D. Bioaccessibility of Microencapsulated Carotenoids, Recovered from Tomato Processing Industrial by-Products, Using In Vitro Digestion Model. LWT 2021, 152, 112285. [Google Scholar] [CrossRef]
- Vulić, J.; Šeregelj, V.; Kalušević, A.; Lević, S.; Nedović, V.; Tumbas Šaponjac, V.; Čanadanović-Brunet, J.; Ćetković, G. Bioavailability and Bioactivity of Encapsulated Phenolics and Carotenoids Isolated from Red Pepper Waste. Molecules 2019, 24, 2837. [Google Scholar] [CrossRef]
- Gulsen, O.; Kaya, G.; Dalda-Sekerci, A. Status of Pumpkin Seed Production in Turkey. Curr. Trends Nat. Sci. 2017, 6, 54–59. [Google Scholar]
- Ünlükara, A.; Varol, İ.S.; Güneş, A. Effects of Various Fertilizers and Different Nitrogen Doses on Pumpkin Seed and Plant Water Consumption. Commun. Soil. Sci. Plant Anal. 2022, 53, 590–601. [Google Scholar] [CrossRef]
- UN. Sustainable Development Goals. 2020. Available online: https://www.un-page.org/page-and-sustainable-development-goals (accessed on 1 November 2023).
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Kamiloglu, S. Effect of Different Freezing Methods on the Bioaccessibility of Strawberry Polyphenols. Int. J. Food Sci. Technol. 2019, 54, 2652–2660. [Google Scholar] [CrossRef]
- Kamiloglu, S. Industrial Freezing Effects on the Content and Bioaccessibility of Spinach (Spinacia oleracea L.) Polyphenols. J. Sci. Food Agric. 2020, 100, 4190–4198. [Google Scholar] [CrossRef] [PubMed]
- Ozdemirli, N.; Kamiloglu, S. Changes in the Bioaccessibility of Citrus Polyphenols during Industrial Freezing Process. Int. J. Food Sci. Technol. 2023, 58, 5819–5828. [Google Scholar] [CrossRef]
- Vaz, A.A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Physicochemical Properties and Bioaccessibility of Phenolic Compounds of Dietary Fibre Concentrates from Vegetable By-Products. Foods 2022, 11, 2578. [Google Scholar] [CrossRef]
- Cangussu, L.B.; Melo, J.C.; Franca, A.S.; Oliveira, L.S. Chemical Characterization of Coffee Husks, a By-Product of Coffea Arabica Production. Foods 2021, 10, 3125. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Silva, T.B.R.; Lobo, L.A.; Domingues, R.M.C.P.; Perrone, D.; Monteiro, M. Bioaccessibility of Phenolic Compounds of Jaboticaba (Plinia jaboticaba) Peel and Seed after Simulated Gastrointestinal Digestion and Gut Microbiota Fermentation. J. Funct. Foods 2020, 67, 103851. [Google Scholar] [CrossRef]
- Soares Carneiro, T.; da Conceição Prudêncio Dutra, M.; Andrade Lima, D.; Júlia de Brito Araújo, A.; Lessa Constant, P.B.; dos Santos Lima, M. Phenolic Compounds in Peel, Seed and Cold Pressed Pink Pepper (Schinus terebinthifolia R.) Oil and Bioaccessibility of Peel Using a Digestion Model with Intestinal Barrier Simulation. Food Biosci. 2022, 49, 101930. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static In Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static In Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Barba, A.I.O.; Hurtado, M.C.; Mata, M.C.S.; Ruiz, V.F.; Tejada, M.L.S. de Application of a UV–Vis Detection-HPLC Method for a Rapid Determination of Lycopene and β-Carotene in Vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- Akpinar Bayizit, A.; Bekar, E.; Unal, T.T.; Celik, M.A.; Acoglu Celik, B.; Koc Alibasoglu, E.; Sahin Dilmenler, P.; Yolci Omeroglu, P.; Copur, O.U.; Kamiloglu, S. Investigating the Effect of Harvest Season on the Bioaccessibility of Bee Pollen Polyphenols by Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. Eur. Food Res. Technol. 2023, 249, 2529–2542. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant Capacity of Phenolic Phytochemicals from Various Cultivars of Plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.A. Factors Influencing the Antioxidant Activity Determined by the ABTS•+ Radical Cation Assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. Antioxidant and Free Radical Scavenging Activity of an Aqueous Extract of Coleus Aromaticus. Food Chem. 2006, 97, 109–114. [Google Scholar] [CrossRef]
- Zaccari, F.; Galietta, G. α-Carotene and β-Carotene Content in Raw and Cooked Pulp of Three Mature Stage Winter Squash “Type Butternut. ” Foods 2015, 4, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, J.L.; Turner, N.D.; King, S.R. Carotenoid Bioaccessibility from Nine Raw Carotenoid-storing Fruits and Vegetables Using an In Vitro Model. J. Sci. Food Agric. 2012, 92, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Azevedo-Meleiro, C.H.; Rodriguez-Amaya, D.B. Qualitative and Quantitative Differences in Carotenoid Composition among Cucurbita Moschata, Cucurbita Maxima, and Cucurbita Pepo. J. Agric. Food Chem. 2007, 55, 4027–4033. [Google Scholar] [CrossRef]
- Hussain, A.; Kausar, T.; Din, A.; Murtaza, M.A.; Jamil, M.A.; Noreen, S.; ur Rehman, H.; Shabbir, H.; Ramzan, M.A. Determination of Total Phenolic, Flavonoid, Carotenoid, and Mineral Contents in Peel, Flesh, and Seeds of Pumpkin (Cucurbita maxima). J. Food Process Preserv. 2021, 45, e15542. [Google Scholar] [CrossRef]
- Behsnilian, D.; Mayer-Miebach, E. Impact of Blanching, Freezing and Frozen Storage on the Carotenoid Profile of Carrot Slices (Daucus carota L. Cv. Nutri Red). Food Control 2017, 73, 761–767. [Google Scholar] [CrossRef]
- Wani, S.M.; Masoodi, F.A.; Haq, E.; Ahmad, M.; Ganai, S.A. Influence of Processing Methods and Storage on Phenolic Compounds and Carotenoids of Apricots. LWT 2020, 132, 109846. [Google Scholar] [CrossRef]
- Petry, F.C.; Mercadante, A.Z. Impact of In Vitro Digestion Phases on the Stability and Bioaccessibility of Carotenoids and Their Esters in Mandarin Pulps. Food Funct. 2017, 8, 3951–3963. [Google Scholar] [CrossRef]
- Low, D.Y.; D’Arcy, B.; Gidley, M.J. Mastication Effects on Carotenoid Bioaccessibility from Mango Fruit Tissue. Food Res. Int. 2015, 67, 238–246. [Google Scholar] [CrossRef]
- Kopec, R.E.; Gleize, B.; Borel, P.; Desmarchelier, C.; Caris-Veyrat, C. Are Lutein, Lycopene, and β-Carotene Lost through the Digestive Process? Food Funct. 2017, 8, 1494–1503. [Google Scholar] [CrossRef]
- Veda, S.; Platel, K.; Srinivasan, K. Varietal Differences in the Bioaccessibility of β-Carotene from Mango (Mangifera Indica) and Papaya (Carica papaya) Fruits. J. Agric. Food Chem. 2007, 55, 7931–7935. [Google Scholar] [CrossRef]
- Reboul, E.; Richelle, M.; Perrot, E.; Desmoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of Carotenoids and Vitamin E from Their Main Dietary Sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [Google Scholar] [CrossRef] [PubMed]
- Aschoff, J.K.; Kaufmann, S.; Kalkan, O.; Neidhart, S.; Carle, R.; Schweiggert, R.M. In Vitro Bioaccessibility of Carotenoids, Flavonoids, and Vitamin C from Differently Processed Oranges and Orange Juices [Citrus sinensis (L.) Osbeck]. J. Agric. Food Chem. 2015, 63, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Hornero-Méndez, D.; Mínguez-Mosquera, M.I. Bioaccessibility of Carotenes from Carrots: Effect of Cooking and Addition of Oil. Innov. Food Sci. Emerg. Technol. 2007, 8, 407–412. [Google Scholar] [CrossRef]
- Pugliese, A.; O’Callaghan, Y.; Tundis, R.; Galvin, K.; Menichini, F.; O’Brien, N.; Loizzo, M.R. In Vitro Investigation of the Bioaccessibility of Carotenoids from Raw, Frozen and Boiled Red Chili Peppers (Capsicum annuum). Eur. J. Nutr. 2014, 53, 501–510. [Google Scholar] [CrossRef]
- Pugliese, A.; Loizzo, M.R.; Tundis, R.; O’Callaghan, Y.; Galvin, K.; Menichini, F.; O’Brien, N. The Effect of Domestic Processing on the Content and Bioaccessibility of Carotenoids from Chili Peppers (Capsicum Species). Food Chem. 2013, 141, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Enneb, S.; Drine, S.; Bagues, M.; Triki, T.; Boussora, F.; Guasmi, F.; Nagaz, K.; Ferchichi, A. Phytochemical Profiles and Nutritional Composition of Squash (Cucurbita moschata D.) from Tunisia. S. Afr. J. Bot. 2020, 130, 165–171. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Király, L.; Al-Mansori, A.-N.A.; Younes, H.A.; Zeid, A.; Elsharkawy, M.M.; Behiry, S.I. Defense Responses and Metabolic Changes Involving Phenylpropanoid Pathway and PR Genes in Squash (Cucurbita pepo L.) Following Cucumber Mosaic Virus Infection. Plants 2022, 11, 1908. [Google Scholar] [CrossRef]
- Leichtweis, M.G.; Molina, A.K.; Pires, T.C.S.; Dias, M.I.; Calhelha, R.; Bachari, K.; Ziani, B.E.C.; Oliveira, M.B.P.P.; Pereira, C.; Barros, L. Biological Activity of Pumpkin Byproducts: Antimicrobial and Antioxidant Properties. Molecules 2022, 27, 8366. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, L.; Qi, Y.; Xie, C.; Zhao, W.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Effect of Processing on Polyphenols in Butternut Pumpkin (Cucurbita moschata). Food Biosci. 2022, 49, 101925. [Google Scholar] [CrossRef]
- Mansour, R.B.; Falleh, H.; Nefzi, N.; Dakhlaoui, S.; Selmi, S.; Hammami, M.; Barros, L.; Petropoulos, S.A.; Tarchoun, N.; Ksouri, R. Improved Recovery of Antioxidant Compounds from Refined Pumpkin Peel Extract: A Mixture Design Method Approach. Horticulturae 2023, 9, 1111. [Google Scholar] [CrossRef]
- Mansour, R.B.; Falleh, H.; Hammami, M.; Barros, L.; Petropoulos, S.A.; Tarchoun, N.; Ksouri, R. The Use of Response Surface Methodology to Optimize Assisted Extraction of Bioactive Compounds from Cucurbita Maxima Fruit By-Products. Processes 2023, 11, 1726. [Google Scholar] [CrossRef]
- Stryjecka, M.; Krochmal-Marczak, B.; Cebulak, T.; Kiełtyka-Dadasiewicz, A. Assessment of Phenolic Acid Content and Antioxidant Properties of the Pulp of Five Pumpkin Species Cultivated in Southeastern Poland. Int. J. Mol. Sci. 2023, 24, 8621. [Google Scholar] [CrossRef] [PubMed]
- Ng, Z.X.; See, A.N. Effect of in Vitro Digestion on the Total Polyphenol and Flavonoid, Antioxidant Activity and Carbohydrate Hydrolyzing Enzymes Inhibitory Potential of Selected Functional Plant-based Foods. J. Food Process Preserv. 2019, 43, e13903. [Google Scholar] [CrossRef]
- van der Sman, R.G.M. Impact of Processing Factors on Quality of Frozen Vegetables and Fruits. Food Eng. Rev. 2020, 12, 399–420. [Google Scholar] [CrossRef]
- Armesto, J.; Rocchetti, G.; Senizza, B.; Pateiro, M.; Barba, F.J.; Domínguez, R.; Lucini, L.; Lorenzo, J.M. Nutritional Characterization of Butternut Squash (Cucurbita moschata D.): Effect of Variety (Ariel vs. Pluto) and Farming Type (Conventional vs. Organic). Food Res. Int. 2020, 132, 109052. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Evaluation of Bioactivity of Butternut Squash (Cucurbita moschata D.) Seeds and Skin. Food Sci. Nutr. 2020, 8, 3252–3261. [Google Scholar] [CrossRef]
- Singh, J.; Singh, V.; Shukla, S.; Rai, A.K. Phenolic Content and Antioxidant Capacity of Selected Cucurbit Fruits Extracted with Different Solvents. J. Nutr. Food Sci. 2016, 6, 1000565. [Google Scholar] [CrossRef]
- Loncaric, A.; Dugalic, K.; Mihaljevic, I.; Jakobek, L.; Pilizota, V. Effects of Sugar Addition on Total Polyphenol Content and Antioxidant Activity of Frozen and Freeze-Dried Apple Purée. J. Agric. Food Chem. 2014, 62, 1674–1682. [Google Scholar] [CrossRef]
- Ketnawa, S.; Reginio, F.C., Jr.; Thuengtung, S.; Ogawa, Y. Changes in Bioactive Compounds and Antioxidant Activity of Plant-Based Foods by Gastrointestinal Digestion: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 4684–4705. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Lin, M.; Zheng, Y.; Chen, J. Effect of Roasting and in Vitro Digestion on Phenolic Profiles and Antioxidant Activity of Water-Soluble Extracts from Sesame. Food Chem. Toxicol. 2020, 139, 111239. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Soszka, A. Changes in Phenolic Compounds and Antioxidant Activity of Fruit Musts and Fruit Wines during Simulated Digestion. Molecules 2020, 25, 5574. [Google Scholar] [CrossRef] [PubMed]
- Caponio, G.; Noviello, M.; Calabrese, F.; Gambacorta, G.; Giannelli, G.; De Angelis, M. Effects of Grape Pomace Polyphenols and In Vitro Gastrointestinal Digestion on Antimicrobial Activity: Recovery of Bioactive Compounds. Antioxidants 2022, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Capanoglu, E.; Kamiloglu, S.; Cekic, S.D.; Baskan, K.S.; Avan, A.N.; Uzunboy, S.; Apak, R. Antioxidant Activity and Capacity Measurement. In Plant Antioxidants and Health; Ekiert, H.M., Ramawa, K.G., Arora, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 709–773. [Google Scholar]
| Retention Time | Ionization Mode | Mass (m/z) | MS2 Main Fragment (m/z) | Other Fragmental Ions (m/z) | Tentative Identification | Reference(s) |
|---|---|---|---|---|---|---|
| Flavonoids | ||||||
| 1.85 | ESI+ | 306.9 | 139.0 | 289.0 | Epigallocatechin | [52,53] |
| 2.44 | ESI- | 289.1 | 245.1 | 205.1 | Epicatechin | [49,50,51,52,53] |
| 3.37 | ESI- | 447.1 | 285.0 | 256.0 | Luteolin 7-O-glucoside (Cynaroside) | [48] |
| 3.38 | ESI- | 463.1 | 300.1 | 301.1 | Quercetin 3-O-galactoside (Hyperoside) | [48] |
| 3.43 | ESI- | 477.1 | 301.0 | 151.1 | Quercetin 3-O-glucuronide (Quercituron) | [51] |
| 3.81 | ESI- | 578.9 | 271.1 | 459.2 | Naringin | [48,49] |
| Phenolic acids | ||||||
| 1.74 | ESI- | 168.9 | 125.1 | 79.0 | Gallic acid | [49,51,52,53] |
| 2.01 | ESI- | 353.1 | 191.1 | 110.9 | Chlorogenic acid | [49,52,53,54] |
| 2.74 | ESI- | 197.0 | 123.1 | 121.1 | Syringic acid | [48,51,54] |
| 3.79 | ESI- | 192.9 | 134.1 | 178.1 | Ferulic acid | [48,51,52,53,54] |
| Sample | Undigested | Oral Digestion | Gastric Digestion | Intestinal Digestion | Bioaccessibility (%) |
|---|---|---|---|---|---|
| Epigallocatechin (mg/kg) | |||||
| Raw material | 58.7 ± 2.7 BC,a | 14.4 ± 3.0 C,c | 33.3 ± 6.9 B,bc | 45.2 ± 9.3 A,ab | 77 |
| Skin | 74.2 ± 6.0 B,a | 21.2 ± 0.3 B,c | 35.0 ± 1.6 B,b | 43.4 ± 3.5 A,b | 58 |
| Pomace and seed | 169.5 ± 10.2 A,a | 29.7 ± 0.1 A,c | 69.6 ± 12.6 A,b | 47.8 ± 6.9 A,bc | 28 |
| Internal fruit | 53.5 ± 1.6 CD | <LOQ | <LOQ | <LOQ | n/a |
| Cutting | 40.2 ± 3.5 D | <LOQ | <LOQ | <LOQ | n/a |
| IQF | 37.3 ± 1.1 D | <LOQ | <LOQ | <LOQ | n/a |
| Epicatechin (mg/kg) | |||||
| Raw material | 178.4 ± 5.8 BC,b | 49.5 ± 5.0 A,c | 58.2 ± 10.8 AB,c | 284.1 ± 51.9 B,a | 159 |
| Skin | 181.7 ± 6.0 B,a | 43.5 ± 3.8 AB,b | 73.5 ± 11.9 A,b | 173.2 ± 24.6 BC,a | 95 |
| Pomace and seed | 269.7 ± 19.7 A,b | 32.9 ± 5.8 BC,c | 71.1 ± 12.9 A,c | 454.0 ± 87.1 A,a | 168 |
| Internal fruit | 148.2 ± 3.2 C,a | 23.6 ± 6.0 CD,c | 27.6 ± 1.5 C,c | 105.8 ± 20.3 C,b | 71 |
| Cutting | 62.6 ± 1.4 D,a | 13.3 ± 2.7 D,b | 33.2 ± 5.5 BC,b | 80.3 ± 16.9 C,a | 128 |
| IQF | 74.8 ± 4.1 D,b | 14.6 ± 3.3 D,c | 24.8 ± 4.1 C,c | 117.5 ± 21.5 C,a | 157 |
| Luteolin 7-O-glucoside (mg/kg) | |||||
| Raw material | 5.1 ± 0.4 B | <LOQ | <LOQ | <LOQ | n/a |
| Skin | 6.3 ± 0.3 A | <LOQ | <LOQ | <LOQ | n/a |
| Pomace and seed | <LOQ | <LOQ | <LOQ | <LOQ | n/a |
| Internal fruit | 5.0 ± 0.1 B | <LOQ | <LOQ | <LOQ | n/a |
| Cutting | <LOQ | <LOQ | <LOQ | <LOQ | n/a |
| IQF | <LOQ | <LOQ | <LOQ | <LOQ | n/a |
| Quercetin 3-O-galactoside (mg/kg) | |||||
| Raw material | 18.0 ± 1.2 A | <LOQ | <LOQ | <LOQ | n/a |
| Skin | 16.4 ± 2.8 A | <LOQ | <LOQ | <LOQ | n/a |
| Pomace and seed | 13.3 ± 0.1 A | <LOQ | <LOQ | <LOQ | n/a |
| Internal fruit | 13.2 ± 0.1 A | <LOQ | <LOQ | <LOQ | n/a |
| Cutting | <LOQ | <LOQ | <LOQ | <LOQ | n/a |
| IQF | <LOQ | <LOQ | <LOQ | <LOQ | n/a |
| Quercetin 3-O-glucuronide (mg/kg) | |||||
| Raw material | 15.5 ± 0.8 A,b | 4.7 ± 1.2 A,c | 12.6 ± 2.1 A,b | 24.1 ± 1.8 A,a | 155 |
| Skin | 16.9 ± 0.1 A,ab | 3.1 ± 0.03 AB,c | 11.5 ± 2.8 A,b | 22.8 ± 3.0 A,a | 135 |
| Pomace and seed | 12.0 ± 0.1 B,b | 2.5 ± 0.01 B,d | 9.1 ± 0.1 A,c | 21.0 ± 0.6 A,a | 175 |
| Internal fruit | <LOQ | <LOQ | <LOQ | <LOQ | n/a |
| Cutting | <LOQ | <LOQ | <LOQ | <LOQ | n/a |
| IQF | <LOQ | <LOQ | <LOQ | <LOQ | n/a |
| Naringin (mg/kg) | |||||
| Raw material | 1.4 ± 0.04 B | <LOQ | <LOQ | <LOQ | n/a |
| Skin | <LOQ | <LOQ | <LOQ | <LOQ | n/a |
| Pomace and seed | 1.2 ± 0.04 C | <LOQ | <LOQ | <LOQ | n/a |
| Internal fruit | 1.6 ± 0.04 A | <LOQ | <LOQ | <LOQ | n/a |
| Cutting | 1.4 ± 0.02 B | <LOQ | <LOQ | <LOQ | n/a |
| IQF | 1.1 ± 0.01 C | <LOQ | <LOQ | <LOQ | n/a |
| Sample | Undigested | Oral Digestion | Gastric Digestion | Intestinal Digestion | Bioaccessibility (%) |
|---|---|---|---|---|---|
| Gallic acid (mg/kg) | |||||
| Raw material | 30.5 ± 1.1 B,a | 6.4 ± 2.0 A,b | 5.6 ± 1.3 A,b | <LOQ | n/a |
| Skin | 5.2 ± 0.3 C,a | 0.9 ± 0.2 B,c | 2.4 ± 0.3 B,b | <LOQ | n/a |
| Pomace and seed | 107.1 ± 4.2 A,a | 3.3 ± 0.2 B,c | 5.5 ± 1.1 A,bc | 12.7 ± 2.8 b | 12 |
| Internal fruit | 3.2 ± 0.1 C,a | 0.9 ± 0.2 B,b | <LOQ | <LOQ | n/a |
| Cutting | 3.9 ± 0.1 C,a | 0.5 ± 0.04 B,b | <LOQ | <LOQ | n/a |
| IQF | 1.7 ± 0.03 C,a | 0.6 ± 0.2 B,b | <LOQ | <LOQ | n/a |
| Chlorogenic acid (mg/kg) | |||||
| Raw material | 5.2 ± 0.3 B | <LOQ | <LOQ | <LOQ | n/a |
| Skin | 4.0 ± 0.02 C | <LOQ | <LOQ | <LOQ | n/a |
| Pomace and seed | 6.0 ± 0.2 A,b | 1.7 ± 0.2 c | 5.1 ± 0.5 b | 8.1 ± 0.7 a | 135 |
| Internal fruit | 4.3 ± 0.02 C,a | 1.0 ± 0.1 b | <LOQ | <LOQ | n/a |
| Cutting | 5.5 ± 0.2 AB,a | 1.7 ± 0.1 b | <LOQ | <LOQ | n/a |
| IQF | 4.3 ± 0.02 C,a | 1.1 ± 0.1 b | <LOQ | <LOQ | n/a |
| Syringic acid (mg/kg) | |||||
| Raw material | 43.7 ± 2.1 BC,a | 11.6 ± 1.1 d | 17.8 ± 2.1 A,c | 37.0 ± 1.6 B,b | 85 |
| Skin | 48.9 ± 3.8 B,a | 10.3 ± 0.8 b | 11.3 ± 0.8 B,b | 44.3 ± 8.1 B,a | 91 |
| Pomace and seed | 63.0 ± 4.4 A,b | 8.0 ± 1.3 c | 15.8 ± 2.0 A,c | 132.4 ± 20.1 A,a | 210 |
| Internal fruit | 36.2 ± 0.7 C,a | 5.9 ± 1.4 b | 8.6 ± 0.3 B,b | 29.4 ± 5.2 B,a | 81 |
| Cutting | 18.4 ± 1.8 D,ab | <LOQ | 8.9 ± 0.6 B,b | 27.5 ± 5.4 B,a | 149 |
| IQF | 20.0 ± 0.9 D,b | <LOQ | 11.4 ± 0.3 B,b | 32.0 ± 5.9 B,a | 160 |
| Ferulic acid (mg/kg) | |||||
| Raw material | 5.3 ± 0.4 A | <LOQ | <LOQ | <LOQ | n/a |
| Skin | 3.8 ± 0.1 B | <LOQ | <LOQ | <LOQ | n/a |
| Pomace and seed | 4.1 ± 0.03 B | <LOQ | <LOQ | <LOQ | n/a |
| Internal fruit | 3.8 ± 0.1 B | <LOQ | <LOQ | <LOQ | n/a |
| Cutting | 4.1 ± 0.1 B | <LOQ | <LOQ | <LOQ | n/a |
| IQF | 4.3 ± 0.1 B | <LOQ | <LOQ | <LOQ | n/a |
| Sample | Undigested | Oral Digestion | Gastric Digestion | Intestinal Digestion | Bioaccessibility (%) |
|---|---|---|---|---|---|
| Total phenolic content (mg GAE/100 g) | |||||
| Raw material | 57.9 ± 2.6 AB,b | 24.5 ± 1.9 B,c | 92.0 ± 2.4 B,a | 98.3 ± 9.7 AB,a | 170 |
| Skin | 49.6 ± 6.9 BC,b | 24.0 ± 0.2 B,c | 63.3 ± 3.5 CD,b | 85.2 ± 11.3 AB,a | 172 |
| Pomace and seed | 63.0 ± 6.0 A,b | 30.4 ± 1.9 A,c | 114.6 ± 6.4 A,a | 116.3 ± 22.0 A,a | 185 |
| Internal fruit | 44.8 ± 0.8 C,b | 19.4 ± 1.9 C,c | 68.6 ± 5.3 C,a | 74.1 ± 13.7 B,a | 165 |
| Cutting | 30.5 ± 1.3 D,bc | 14.9 ± 0.6 D,c | 47.8 ± 1.1 E,ab | 61.9 ± 17.1 B,a | 203 |
| IQF | 29.1 ± 5.1 D,b | 17.0 ± 0.7 CD,c | 53.9 ± 7.2 DE,a | 60.7 ± 2.0 B,a | 209 |
| Total flavonoid content (mg RE/100 g) | |||||
| Raw material | 7.6 ± 1.8 BC,bc | 3.8 ± 0.7 C,c | 9.5 ± 1.0 CD,b | 45.9 ± 2.8 B,a | 604 |
| Skin | 10.6 ± 0.8 AB,b | 6.7 ± 0.6 B,c | 22.0 ± 1.6 B,a | 13.3 ± 1.5 C,b | 125 |
| Pomace and seed | 13.1 ± 1.8 A,b | 10.5 ± 1.3 A,b | 30.1 ± 1.7 A,b | 122.4 ± 15.2 A,a | 934 |
| Internal fruit | 7.1 ± 1.1 BC,b | 2.6 ± 0.4 C,c | 12.7 ± 0.8 C,a | 8.6 ± 1.2 C,b | 121 |
| Cutting | 6.9 ± 1.5 BC,a | 2.2 ± 0.7 C,b | 5.7 ± 1.0 D,a | 8.1 ± 1.2 C,a | 117 |
| IQF | 6.6 ± 1.1 C,a | 2.3 ± 0.8 C,b | 9.5 ± 2.6 CD,a | 8.1 ± 1.1 C,a | 123 |
| Total antioxidant capacity (mg TE/100 g) | |||||
| ABTS assay | |||||
| Raw material | 55.1 ± 0.2 B,a | 10.8 ± 0.2 B,c | 35.0 ± 1.7 B,b | 62.6 ± 12.8 B,a | 114 |
| Skin | 44.1 ± 1.9 C,a | 9.5 ± 0.1 CD,c | 31.0 ± 2.9 BC,b | 42.2 ± 2.9 BC,a | 96 |
| Pomace and seed | 77.2 ± 1.4 A,b | 24.9 ± 0.2 A,c | 76.4 ± 0.1 A,b | 140.4 ± 8.0 A,a | 182 |
| Internal fruit | 44.4 ± 1.1 C,a | 10.0 ± 0.3 C,c | 27.2 ± 3.5 BC,b | 38.1 ± 4.3 C,a | 86 |
| Cutting | 35.4 ± 1.4 D,a | 9.7 ± 0.4 C,c | 23.4 ± 1.3 C,b | 28.2 ± 7.2 C,ab | 80 |
| IQF | 37.3 ± 1.0 D,a | 8.9 ± 0.5 D,c | 25.9 ± 6.5 BC,ab | 25.3 ± 5.7 C,b | 68 |
| CUPRAC assay | |||||
| Raw material | 36.5 ± 3.2 B,b | 15.3 ± 0.8 B,c | 40.6 ± 5.3 B,b | 64.1 ± 8.4 B,a | 176 |
| Skin | 24.3 ± 2.5 CD,bc | 11.5 ± 2.4 B,c | 41.6 ± 6.1 B,ab | 53.9 ± 12.0 B,a | 222 |
| Pomace and seed | 56.2 ± 6.1 A,b | 27.0 ± 5.8 A,b | 63.5 ± 11.9 A,b | 166.4 ± 43.6 A,a | 296 |
| Internal fruit | 25.4 ± 3.4 C,bc | 16.3 ± 1.4 B,c | 27.2 ± 2.1 B,b | 46.6 ± 7.0 B,a | 183 |
| Cutting | 16.6 ± 0.1 CD,b | 9.9 ± 0.8 B,c | 27.6 ± 2.8 B,a | 32.6 ± 3.3 B,a | 196 |
| IQF | 15.2 ± 2.1 D,b | 9.9 ± 3.3 B,b | 26.7 ± 4.0 B,a | 23.9 ± 2.2 B,a | 157 |
| FRAP assay | |||||
| Raw material | 11.6 ± 0.4 B,b | 2.6 ± 0.1 B,d | 8.0 ± 0.3 B,c | 31.3 ± 0.2 B,a | 270 |
| Skin | 5.4 ± 0.03 C,b | 1.7 ± 0.03 C,c | 7.7 ± 0.6 B,a | 7.3 ± 1.0 C,a | 135 |
| Pomace and seed | 26.9 ± 1.5 A,b | 4.0 ± 0.2 A,c | 20.7 ± 1.5 A,b | 91.8 ± 10.4 A,a | 341 |
| Internal fruit | 4.1 ± 0.3 CD,b | 2.7 ± 0.1 B,c | 5.4 ± 0.3 C,a | 5.6 ± 0.2 C,a | 137 |
| Cutting | 3.1 ± 0.3 D,a | 1.2 ± 0.1 D,c | 3.7 ± 0.2 CD,a | 2.3 ± 0.5 C,b | 74 |
| IQF | 2.2 ± 0.2 D,a | 0.9 ± 0.1 D,c | 2.8 ± 0.1 D,a | 1.5 ± 0.4 C,b | 68 |
| DPPH assay | |||||
| Raw material | 7.9 ± 1.3 B,b | 2.5 ± 0.2 B,c | 6.2 ± 0.8 B,b | 11.9 ± 0.1 B,a | 151 |
| Skin | 1.4 ± 0.1 C,b | 1.8 ± 0.6 BC,b | 1.3 ± 0.2 C,b | 7.6 ± 1.6 BC,a | 543 |
| Pomace and seed | 17.2 ± 1.6 A,b | 6.8 ± 0.7 A,c | 17.9 ± 3.8 A,b | 29.5 ± 5.7 A,a | 172 |
| Internal fruit | 2.5 ± 0.4 C,c | 1.9 ± 0.1 BC,c | 4.2 ± 0.5 BC,b | 7.2 ± 0.8 BC,a | 288 |
| Cutting | 0.5 ± 0.1 C,b | 1.8 ± 0.5 BC,ab | 2.2 ± 0.03 BC,a | 3.1 ± 1.0 C,a | 620 |
| IQF | 2.3 ± 0.7 C,b | 1.1 ± 0.1 C,b | 2.8 ± 1.2 BC,b | 7.0 ± 0.6 BC,a | 304 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiloglu, S.; Koc Alibasoglu, E.; Acoglu Celik, B.; Celik, M.A.; Bekar, E.; Unal, T.T.; Kertis, B.; Akpinar Bayizit, A.; Yolci Omeroglu, P.; Copur, O.U. Bioaccessibility of Carotenoids and Polyphenols in Organic Butternut Squash (Cucurbita moschata): Impact of Industrial Freezing Process. Foods 2024, 13, 239. https://doi.org/10.3390/foods13020239
Kamiloglu S, Koc Alibasoglu E, Acoglu Celik B, Celik MA, Bekar E, Unal TT, Kertis B, Akpinar Bayizit A, Yolci Omeroglu P, Copur OU. Bioaccessibility of Carotenoids and Polyphenols in Organic Butternut Squash (Cucurbita moschata): Impact of Industrial Freezing Process. Foods. 2024; 13(2):239. https://doi.org/10.3390/foods13020239
Chicago/Turabian StyleKamiloglu, Senem, Elif Koc Alibasoglu, Busra Acoglu Celik, M. Alpgiray Celik, Erturk Bekar, Taha Turgut Unal, Buket Kertis, Arzu Akpinar Bayizit, Perihan Yolci Omeroglu, and O. Utku Copur. 2024. "Bioaccessibility of Carotenoids and Polyphenols in Organic Butternut Squash (Cucurbita moschata): Impact of Industrial Freezing Process" Foods 13, no. 2: 239. https://doi.org/10.3390/foods13020239
APA StyleKamiloglu, S., Koc Alibasoglu, E., Acoglu Celik, B., Celik, M. A., Bekar, E., Unal, T. T., Kertis, B., Akpinar Bayizit, A., Yolci Omeroglu, P., & Copur, O. U. (2024). Bioaccessibility of Carotenoids and Polyphenols in Organic Butternut Squash (Cucurbita moschata): Impact of Industrial Freezing Process. Foods, 13(2), 239. https://doi.org/10.3390/foods13020239








