Abstract
Fuchsia hybrida (pena pena) and Alcea rosea L. (malvagoma) are predominant flowers in the “Horchata” infusion, a traditional beverage in southern Ecuador, to which some medicinal properties are attributed. However, there is very little published information about these two flower species. The current study aimed to obtain two dehydrated powders of these flowers and to determine their chemical composition, physicochemical and technological properties, polyphenols, and fatty acids profile. In both powdered flowers, carbohydrates predominated, with a significant content of dietary fiber and fructose. The fat content was low, mainly comprising polyunsaturated fats (62% pena pena and 52% malvagoma), with a significant presence of omega-3 (C18:3n-3,6,9) and omega-6 (C18:2n-6,9) fatty acids, showing a better n-6/n-3 balance in the malvagoma flowers. Pena pena flowers are highlighted by high anthocyanin and ellagic acid amounts, whereas malvagoma contains a high content of flavanones. In conclusion, the studied powder flowers, could be used in the formulation of new foods or as source of anthocyanins as food colorants.
1. Introduction
Horchata is a popular herbal infusion made from a mixture of 16 to 32 medicinal plants, commonly consumed in Southern Ecuador for its anti-inflammatory, analgesic, and diuretic properties [1,2]. Among the 20 culturally significant medicinal plant species used in this beverage, Alcea rosea and Fuchsia hybrida play a predominant role. Fuchsia hybrida Hort. T. ex Siebert & Voss, commonly known as “pena pena grande” or “pena pena roja”, is an introduced shrub that imparts aroma to the horchata drink and has various therapeutic uses, including anti-inflammatory, antiflu, cardiotonic, sedative, and for stomachaches [1]. On the other hand, Alcea rosea L. (also known as “malva goma”, “malva rosa”, or “malvón”) is an introduced herb that adds flavor to the drink and is known for its therapeutic properties such as expectorant, cooling, analgesic, anti-inflammatory, depurative, diuretic, and tonic effects [1,3].
Flowers constitute a vital component of plants, containing a wide array of bioactive compounds, including phytochemicals such as flavonoids (especially anthocyanins), phenolic acids, carotenoids (carotenes, xantophylls), chlorophylls, and numerous other constituents [4,5,6,7]. Extensive research has been dedicated to examining these components, driven by their profound impact on human health and their possession of antioxidant, anti-inflammatory, anticancer, anti-diabetic, and cardio-protective properties [8,9]. Edible flowers of various varieties have been studied worldwide to elucidate their nutritional composition, encompassing antioxidants, ascorbic acid, anthocyanins, phenolic compounds, carotenoids, chlorophylls, flavonoids, fatty acids, free sugars, vitamins, carotenoids, minerals, and organic acids [4,6,9,10,11,12]. Generally, the content of common components such as lipids, proteins, carbohydrates, and vitamins is like that found in vegetables [13].
Changing consumer habits and lifestyles have created a demand for functional and healthy foods, and natural additives [7,14], as consumers seek healthier and more attractive food options to improve their dietary aesthetics and diversify their sources of micronutrients [15]. In this context, edible flowers have emerged as a new trend in human nutrition. A recent review describes the use of edible flowers in the development of functional dairy products, either to fortify them or as a substitute for natural colorants in products such as yogurt, milk, curd, and probiotic drinks, incorporating flowers in the form of extracts, distilled water (maceration), powder, or syrup [16]. Gamage et al. [17] studied the blue pea flower for its significant content of delphinidin-3-(trans-p-coumaroyl)-glucoside, the predominant anthocyanin in this flower, and obtained a natural colorant that is more stable under thermal and refrigeration treatments than spirulina, which is the only approved natural food colorant in Europe. On the other hand, Hnin et al. [18] used rose flower powder as an ingredient to develop nutritional cookies at different levels. They demonstrated that the flower powder offered positive nutritional and sensory properties, including higher total phenolic and total anthocyanin contents, increased antioxidant activities, a higher color score, and an improved odor of cookies due to the associated rose flower fragrance.
In many countries worldwide, they are consumed for their potential health benefits or as part of traditional foods to enhance nutritional value, add fresh and exotic aromas, delicate flavors, visual appeal, and vibrant colors [19,20]. Furthermore, the utilization of these species can contribute to the valorization of local flora through the large-scale production of edible flowers, considering the increasing global demand for edible flowers and the growing number of producers and points of sale, including supermarkets, local markets, and online outlets [8,21]. The edible flowers segment holds value in the business market, as the industrial sector explores various possibilities for incorporating bioactive compounds from edible flowers into new food products. This can be achieved through the direct use of various floral parts (petals, stems, sepals) or by incorporating their extracts or essential oils [8,22]. The search for new food products also involves seeking new colors, textures, and flavors that can be achieved using edible flowers [23]. The edible flowers sector for human consumption is diverse, encompassing a wide range of value-added alternative food products, including drinks, beverages, dishes, preserves, jams, and more [24]. The food industry stands to benefit by meeting market demands for functional and healthy foods through the development of novel floral-based foods and formulations, thereby allowing for the valorization of previously unexplored or underexplored species [21]. Therefore, the current study aimed to obtain two flower powders from pena pena (Fuchsia hybrida) and malvagoma (Alcea rosea) and to determine their chemical composition, physicochemical and technological properties, and (poly)phenol and fatty acid profiles. The study results allowed the functional potential of both flowers to be revealed for use in the development of new foods.
2. Materials and Methods
2.1. Plant Material
The pena pena (Fuchsia hybrida) and malvagoma (Alcea rosea) flowers (Supplementary Figure S1) were purchased from a local market in Loja, Ecuador. The freshest flowers that were not wilted were selected and immediately dehydrated (40 °C, 36 h) in a tray dryer, Model DY-110H (LASSELE.CO, Danwon-gu, Republic of Korea). Subsequently, they were ground in an ultracentrifugal mill ZM 200 (Retsch GmbH, Hann, Germany) until obtaining a final particle size of <210 µm. The powdered flowers were vacuum sealed and packaged in hermetically sealed aluminum bags to protect them from light and moisture.
2.2. Chemical Composition
AOAC methods [25] were used to determine the moisture (No. 925.09), ash (No. 925.09), fat (No. 948.22), and protein (No. 935.11) content in the samples. Results were expressed as g/100 g of dehydrated powder. Dietary fiber (TDF) was determined using an enzymatic-gravimetric method based on the AOAC official method No. 985.29. Total carbohydrates were calculated by the difference.
2.3. Sugar Content
The sugar profile of powder flowers was carried out through HPLC following the method described by Lucas-González et al. [26]. In brief, the samples were homogenized with ultrapure water in an Ultra-Turrax at 12,000 rpm for 60 s with a solid–solvent relation of 1:40. Next, the samples were centrifugated at 5000× g for 10 min at 4 °C. Then, the supernatant was collected and filtered through a 0.45 μm Millipore filter (Millipore Corporation, Bedford, MA, USA). Detection and identification of samples were carried out through HPLC (Hewlett-Packard HP-1100 instrument (Hewlett-Packar, Woldbronn, Germany)) coupled with a UV–visible diode array detector G1315A (set at 210 nm) and a refractive index detector G-1362. A cation exchange column (Supelcogel C-610H, 300 × 7.8 mm; Supelco, Bellefonte, PA, USA) with a pre-column (Supelguard-H, 50 × 4.6 mm, Supelco) was used to separate compounds using a mobile-phase phosphoric acid (0.1% v/v). The HPLC conditions were: injected volume, 10 µL; flow rate, 0.5 mL min−1; temperature, 30 °C; and run time, 30 min. Standard glucose, fructose, and sucrose were used to identify compounds by comparing their retention time with sample peaks. A regression formula of standards was used to quantify sugar in samples. The sugar content was expressed as g 100 g−1 of sample.
2.4. Fatty Acid Profile
Fat extraction was carried out as follows: 20 g of samples was mixed with hexane (1:4; w/v). The solution was submitted to one hour of ultrasonic bath plus 30 min of magnetic agitation. Then, the samples were centrifugated (10 min; 4 °C; 7200× g). The supernatant was evaporated under vacuum and the fat was resuspended in a 2–3 mL of hexane and filtered through a 0.45 μm Millipore filter (Millipore Corporation, Bedford, MA, USA). The hexane was evaporated again under vacuum and the remaining fat (around 100 mg) underwent a methylation procedure, previously described by Lucas-González et al. [27]. A Gas-Chromatographer HP-6890 (Woldbronn, Germany) equipped with a flame ionization detector (FID) and a Suprawax 280 capillary column (30 m × 0.25 μm film thickness × 0.25 mm i.d.; Tecknokroma, Barcelona, Spain) was used to identify fatty acids in the samples using the same conditions proposed by Botella-Martínez et al. [28]. Fatty acid methyl esters (FAMEs) standards (Supelco 37 component FAME Mix, Bellefonte, PA, USA) were used to identify compounds by comparing their retention time with the sample retention time. The results were expressed as mg/100 g of sample.
2.5. Free and Bound (Poly)phenol Profile
For the extraction of free (poly)phenols, the methodology described by Lucas-González et al. [27] was followed, while for obtained bound (poly)phenols, the procedure proposed by Mpofu et al. [29] with the modification carried out by Lucas-González et al. [27] was used. In brief, two grams of the samples were submitted to two extraction steps in sequence, first with an aqueous–methanol (20:80 v/v) and then with aqueous–acetone (30:70 v/v). In each extraction process, samples were sonicated (10 min in ultrasonic bath), centrifugated, and the collected supernatant evaporated under vacuum. The dry extract was resuspended in water and then purified with a C-18 Sep-Pak cartridge. The free (poly)phenols were collected in acidified methanol (MeOH: formic acid; 99:1; v:v). The pellet was subjected to an alkaline–acid extraction to extract bound (poly)phenols. In brief, the pellet was mixed with 50 mL of 4 M NaOH and left in darkness for 4 h. Afterward, the medium was acidified (pH 2.0) with 6 M HCl. The sample was centrifuged, and the supernatant was extracted with ethyl acetate. The organic solvent was dried under vacuum, and the sample was resuspended in methanol and filtered thought a 0.45 μm Millipore filter (Millipore Corporation, Bedford, MA, USA).
A Hewlett-Packard HPLC series 1200 instrument (Woldbronn, Germany) equipped with UV–visible diode array detector and coupled with a C18 Teknokroma column (Mediterranean sea18, 25 × 0.4 cm, 5 μm particle size; Teknokroma, Barcelona, Spain) was used to detect and elute compounds. The mobile phases were formic acid in water (1:90, v/v) as solvent A and acetonitrile as solvent B. The working conditions were 180 bar, gradient elution at 1 mL min−1 with the following gradient program: started with 95% A, 75% A at 20 min, 50% A at 40 min, 95% A at 45 min and 20 µL of injected volume. Four wavelengths were used, 280, 320, 360, and 520 nm, for the detection of (poly)phenols. The identification of compounds was performed by comparison of their retention time and absorbance spectrum with the standards (gallic acid, 4-hydroxycinnamic acid, ellagic acid, protocatechuic acid, sinapic acid, syringic acid, vanillic acid, vanillin, ferulic acid, caffeic acid, p-coumaric acid, chlorogenic acid, rosmarinic acid, catechin, epicatechin, gallocatechin gallate, gallocatechin-3-gallate, catechin-3-gallate, epicatechin-3-gallate, epigallocatechin-3-gallate, quercetin, kaempferol, myricetin, rutin, apigenin, luteolin, luteolin-7-O-glucoside, naringenin, hesperidin, neorecitrin, and neohesperidin, cyanidin-3-O-β-glucopyranoside, delphidin-3-O-β-glucopyranoside, malvidin, malvidin-3,5-O-β-glucopyranoside, malvidin-3-O-β-glucopyranoside, pelargonidin-3-O-β-glucopyranoside, peonidin-3-O-β-glucopyranoside, petunidin-3-O-β-glucopyranoside), which were eluted in the same conditions of samples. Quantification was carried out using a standard regression formula. The results were expressed as µg/g of sample.
2.6. Physicochemical Properties
A 10% (w/v) aqueous solution of flower powder samples was used to determine pH with a pH meter (pH/Ion Model, Eutech Instruments Pte Ltd., Singapore). Water activity was measured in Sprint TH-500 Novasina Thermoconstanter at 25 °C (Lachen, Switzerland). The color coordinates, L* (lightness), a* (±red-green), and b* (±yellow-blue), were measured in the CIELab color space with the help of CM-2600d colorimeter (Minolta Camera Co., Osaka, Japan). The colorimeter was used in SCI mode, D65 as an illuminant, and 10° as an observer. The aperture for illumination and measurement was 11 mm and 8 mm, respectively. The colorimetric values hue (h* = tan−1 b*/a*) and chroma (C* = (a*2 + b*2)1/2) were also calculated. Bulk density was expressed as weight of the sample in kg per unit volume of the powder flower (kg/m3).
2.7. Techno-Functional Properties
Oil holding capacity (OHC) was determined by mixing the sample with vegetable oil (1:10, w/v) for 30 min. After centrifugation at 4750 rpm using a CLAY ADAMS® Brand DYNAC® (Becton, Dickinson, MD, USA) centrifuge, the OHC was expressed as grams of oil held per gram of the sample [30]. Swelling capacity (SWC) was measured following the method described by Robertson et al. [31] with slight modifications. In brief, 0.1 g of the sample was weighed in a 10 mL graduated cylinder (graduated to 0.1 mL), and 10 mL of distilled water was added. The mixture was gently stirred and left at room temperature (20 °C) for 16 h. Then, the volume (mL) occupied by the sample was measured, and SWC was expressed as mL/g of sample.
2.8. Statistics Assay
The results are presented as mean ± standard deviation (SD). Each determination was carried out in triplicate. A one-way ANOVA was carried out using the statistical software SPSS 19.0 (SPSS Inc., Chicago, IL, USA) to compare results between samples. Tukey’s post hoc test was applied for comparisons of means. Significant differences were considered p < 0.05.
3. Results and Discussion
3.1. Chemical Composition
The chemical composition of both powder flower samples is presented in Table 1. Malvagoma flower powder exhibited the highest content in fat, proteins, ash, and fiber (p < 0.05). In contrast, the pena pena flower powder showed the largest amount of total carbohydrates and the monosaccharides fructose and glucose (p < 0.05).

Table 1.
Composition of flower powders (g 100 g−1).
Carbohydrates were the most abundant macronutrients in both dried petals. Considering that the contents of total soluble sugars significantly influence the taste of edible flowers, it was crucial to assess their content [32]. Rivas-García et al. [33] mentioned that the most prevalent monosaccharides in flowers are fructose, glucose, and sucrose. These findings are consistent with our results, where fructose predominates in both species, with the content being significantly higher in the pena pena powder.
The second most prevalent component was protein, followed by ash. The protein content of both studied flower powders, was similar to that reported for flowers such as Arbutus xalapensis (11.3 g/100 g dw) [34], Agave salmiana, Aloe vera, and Myrtillocactus geometrizans with 11.58, 11.85, and 12.53 g 100 g−1 dw, respectively [35]. There are also species with much higher protein values, such as Borage with 22.69 g 100 g−1 dw [12]. There are very few studies reported in the literature focusing on the amino acid profile of edible flowers and the presence or absence of essential amino acids in the edible flower [7]. Phenylalanine, leucine, and valine are the most abundant amino acids in some edible flowers. However, flowers like Cucurbita pepo, Erythrina americana, Erythrina caribaea, Yucca filifera, and Agave salmiana, lack the amino acid lysine. On the other hand, flowers such as Aloe vera, Euphorbia radians, and Arbutus xalapensis have tryptophan as the limiting amino acid [34].
The ash content of edible flowers is appreciable, and according to Fernandes et al. [36], it exhibits the highest variability in the total content, ranging from 2.6 to 15.9 g 100 g−1 dry weight for Madhuca indica and Cucurbita pepo, respectively, among 32 flowers studied. The ash content of the studied flower powders (Table 1) was at intermediate values of those mentioned. Considering that the ash content is related to mineral content, edible flowers have demonstrated significant mineral content, including potassium, calcium, magnesium, sodium, and phosphorus [36,37]. Therefore, further study of these minerals in edible flowers is important, as the mineral content in edible flowers can be higher than that found in common fruits and vegetables typically consumed daily [7].
Fat was the least abundant macronutrient. Although flowers generally have low fat content, other edible flowers with higher fat values have been reported, such as Aloe vera with 4.61 g 100 g−1 dw [38], Calendula with 5.33 g 100 g−1 dw [23], Borage, and Pansies with 4.93 and 5.17 g 100 g−1 dw, respectively [12].
The total dietary fiber content is notable in the two edible flowers, which is higher than that reported for Aloe vera (13.83 g 100 g−1 dw) [38] and Viola × wittrockiana Gams (17.24 g 100 g−1 dw) and lower than that of Camellia japónica L. (54.55 g 100 g−1 dw) and Centaurea cynaus L. (67.38 g 100 g−1 dw) [12]. Jakubczyk et al. [39] reported the fiber content of 12 edible flowers in their study, with Calendurla officinalis L. and Centaurea cyanus L. standing out at 62.33 and 53.06 g 100 g−1, respectively. In all the flowers, the insoluble fraction predominates (ranging from 8.69 to 57.54 g 100 g−1). Al-Snafi [3] mentions that Alcea rosea contained mucilages composed of glucuronic acid, galacturonic acid, rhamnose, and galactose, and that they are part of the high-molecular-weight fiber [40]. High fiber content has been associated with beneficial effects on human health, as it minimizes the risks of several conditions. The consumption of soluble dietary fiber (SDF) is a safe way to lower the risk of conditions such as high blood pressure, hypertension, cardiovascular disease, and diabetes [39,41,42].
3.2. Physicochemical Properties
The physicochemical properties of both studied flowers are presented in Table 2. The water content in fresh flowers is the main constituent, varying between 70% and 95%, resulting in higher water activity, which makes the edible flowers more perishable, lasting from 2 to 5 days after harvest [7,36,37], as they begin to discolor, wilt, and exhibit tissue darkening [36]. The drying process applied to the samples in the present study allowed for a reduction in water activity, which, together with pH, helps reduce the risk of microbial growth to extend their preservation [43].

Table 2.
Physicochemical and techno-functional properties of flower powders.
The pH values of both flowers are like those reported for Agave salmiana, Aloe vera, Erythrina americana, and Myrtillocactus geometrizans, which range between 4.35 and 5.58 [35]. Among the organic acids that determine the pH of flowers are malic, acetic, quinic, citric, and succinic acids, as demonstrated by Fernandes et al. [12] in their study. They found that malic acid was the major organic acid in Borago officinalis L., Camellia japonica L., and Viola × wittrockiana Gams, except in Centaurea cyanus L., where succinic acid was predominant. Similarly, Krzymińska et al. [44] found that malonic, succinic, acetic, and citric acids were the major organic acid components in five studied tulip petals.
The density of malvagoma flower powder was the highest, demonstrating that it is denser than pena pena flower powder. Ahmed et al. [45] reported values ranging from 510 to 640 kg/m3 and from 440 to 490 kg/m3 for Turkish and Indian lentil flours, indicating that with a larger particle size of 210 µm, which was used in our study, higher density values are obtained compared to smaller sizes (105 µm). This could be attributed to the fact that when smaller particle sizes are used, the content of protein and total starch decreases [46]. Knowing the density of a powdered ingredient, along with its moisture content, particle size, and shape, is important because they collectively influence the flowability. Flowability is a parameter that describes the ability to handle the powder for storage, transportation, dosing, and subsequent industrial processes involving its application [47].
Regarding to color attribute, according to Lucas-González et al. [48], the smaller the particle size of the material, the higher the values of lightness and hue, which they achieved with the particle size < 210 µm used in the present study. The L* value of the two flower powders was similar, but the a* value of pena pena flower powder was higher than that of malvagoma flower powder, which is related to the intense red color observed in this flower when fresh, a color that is retained in the dehydrated powder of this flower. The C* value of ‘pena pena,’ which displayed a vibrant color, was higher than that of malvagoma. Anthocyanins, chalcones, aurones, and some flavonols act as major flower pigments in many flowers [49]. Anthocyanins contribute significantly to the red, blue, and purple color of flowers [50].
3.3. Techno-Functional Properties
Techno-functional properties are important because they allow for the evaluation of how a particular ingredient behaves in the food matrix or in the body. These properties are influenced by chemical composition, especially the fiber content and particle size [48,51].
The oil holding capacity (OHC) is a property directly related to the cellulose content of dietary fiber [52]. Evaluating this property in an ingredient is important because it provides information about the mouthfeel and flavor retention of foods [42,45]. The values of OHC were similar in both studied flower powders, approaching values reported for other foods such as palm flower and leaves of smooth amaranth with 1.6 and 1.2 g/g, respectively [53]; apple pomace (1.33 g/g) and brewer’s spent grain (1.21 g/g) [54]; byproduct flours of two persimmon varieties (2.15 and 2.26 g/g for ‘Rojo Brillante’ flour and ‘Triumph’ flour, respectively) with a particle size < 210 µm. Our results suggest that the flower powders studied can be used as ingredients to stabilize foods with high fat contents.
Swelling capacity (SWC) is associated with the content of pectin, cellulose, and hemicellulose in the fiber [52]. Therefore, high levels of insoluble fiber, lower bulk density, smaller particle size, and a larger surface area may contribute to a higher SWC [55,56], as observed in the case of malvagoma flower powder, which had significantly higher SWC compared to pena pena flower powder. These values are notably higher than those reported by Requena et al. [53] for palm flower (12.8 mL/g) and smooth amaranth leaves (3.8 mL/g), as well as for commercial cereal fibers such as oat 600 (7.6 mL/g) and wheat (7.06 mL/g) [51]. In contrast, the SWC value for pena pena was like those this author reported for oat 401 (4.98 mL/g) and bamboo (5.69 mL/g).
Sources of soluble fiber are known for their superior hydration properties, including beta-glucan, pectin, gums, mucilages, and inulin. All these fiber components generally can hydrate well in the presence of water and form highly viscous solutions, even forming gels, as in the case of pectins [57,58]. In the case of malvagoma flower powder, which exhibits higher values in soluble water content, this could be related to its content of mucilages [3].
3.4. Fatty Acid Profile
The fatty acid profile of both pena pena and malvagoma flower powders can be seen in Table 3. Twenty-five fatty acids were identified and quantified, and malvagoma was the flower with the highest number of fatty acids detected. Both flower powder were rich in polyunsaturated fatty acids, 62% in the case of pena pena and 52% for malvagoma, results similar to those reported by Pires et al. [23] for rose, Calendula officinalis L., and Centaurea cyanus L. This differs from other flowers such as pansy (Viola × wittrockiana) petals [59], Anchusa azurea, Capparis spinosa, Cichorium intybus, Hedysarum coronarium, Malva sylvestris, Robinia pseudoacacia, Rosmarinus officinalis, and Sambucus nigra [19], where saturated fatty acids were predominant. Monounsaturated fatty acids were the least prevalent type of fatty acids present in both flower powders.

Table 3.
Fatty acid profile of flower powders (mg/100 g dw).
Regarding pena pena, linoleic acid (C18:2n6), followed by linolenic acid (C18:3n3) and palmitic acid (C16:0), were, in decreasing order, the most prevalent. Meanwhile, in malvagoma, the main fatty acids were linoleic acid, palmitic acid, and linoleic acid. Malvagoma oil also presented significant amounts of oleic acid and eicosadienoic acid. In other flowers, the content of palmitic acid was higher than that observed in the studied flowers, such as in the case of Viola × wittrockiana petals with a relative percentage of the mentioned acid of 36.41% [59]. In Helichrysum italicum flowers, the omega-6 PUFA linoleic acid was the most abundant fatty acid found (22.55% of total FA), and palmitic acid (C16:0) was the major SFA detected with a value of 1.19 μg/mg dw (16.45% of total FA) [60].
The Polyunsaturated Fatty Acid/Saturated Fatty Acid (PUFA/SFA) ratio is an index used to evaluate the impact of diet on cardiovascular health. Therefore, the higher this ratio, the more positive the effect [61]. The value of this index for pena pena was 2.18 and for malvagoma, it was 1.50, values higher than those reported by Fernandes, et al. [12] for blue borage (Borago officinalis L.), camellia (Camellia japonica L.), blue centaurea (Centaurea cyanus L.) and pansies (Viola × wittrockiana Gams.), which were below 0.45. Meanwhile, for other food matrices, values have been reported between 0.11 and 2.04 for meat, 0.50 to 1.62 for fish, 0.20 to 2.10 for shellfish, and between 0.02 and 0.175 for dietary products [61].
Another important index calculated is the ratio n-6/n-3 PUFA, which should be considerably improved regarding its potential role in cardiovascular disease. Numerous studies have highlighted the importance of the n-6/n-3 FA ratio, rather than the amount of each single fatty acid individually. Protective effects appear when the ratio is close to unity, while ensuring an adequate intake of essential fatty acids [62,63]. In the present study, the n-6/n-3 ratio for pena pena is 2.10, and for malvagoma, it is 0.71, demonstrating a better balance between these two groups of polyunsaturated fatty acids.
3.5. Bound and Free (Poly)phenol Profile of Powder Flowers
Supplementary Table S1 shows the 63 compounds detected in both studied flower powders along with their tentative identification. Among them, 20 were confirmed through a comparison with available standards. The other (poly)phenols were tentatively identified and quantified by comparing their absorbance spectrum with the standard and supporting this with the literature [64].
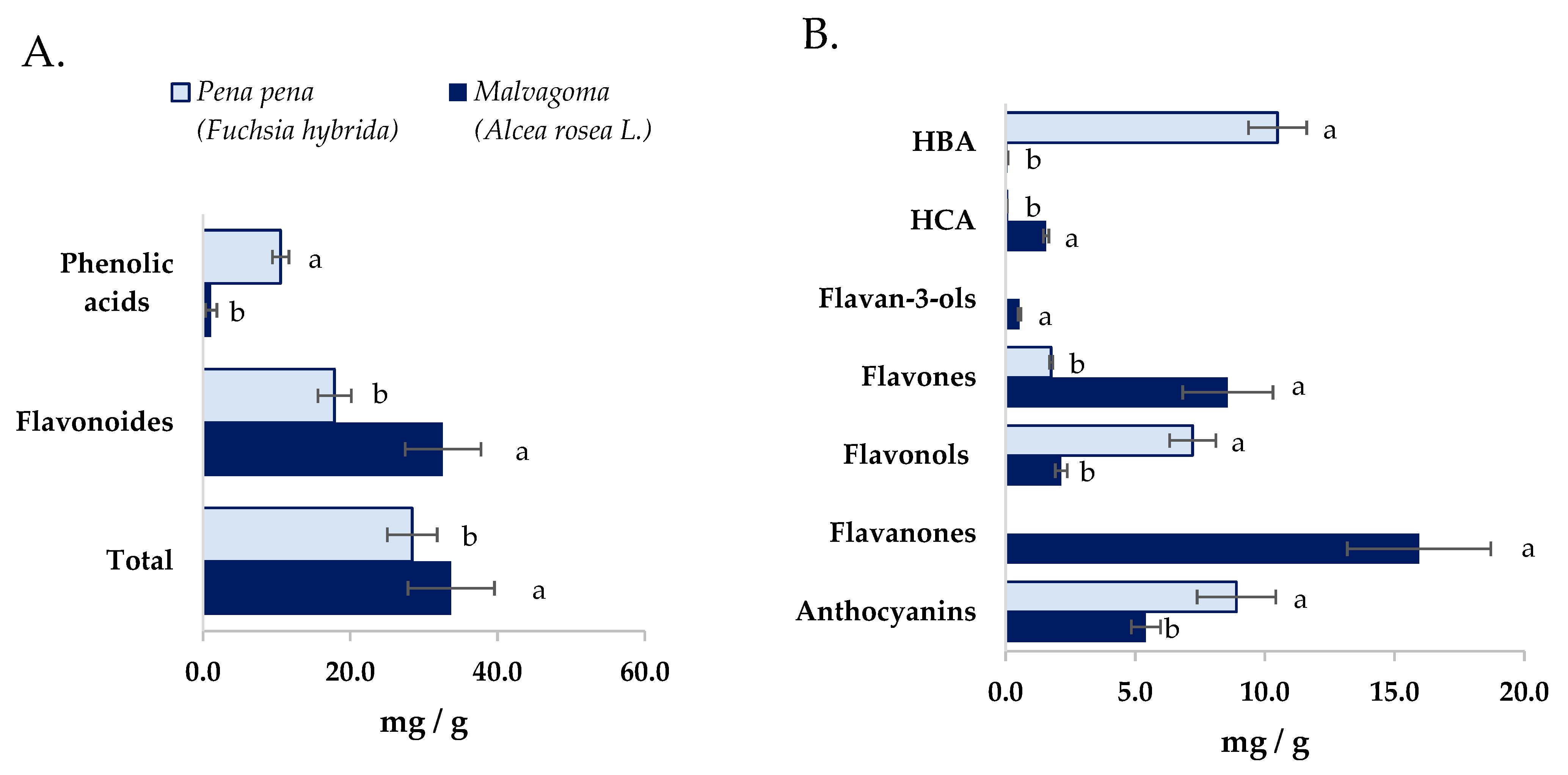
The total amount of polyphenols in both powder flowers samples was similar (p > 0.05) (Figure 1A), whereas the polyphenols profile was different between the studied flower powders. Malvagoma flower powder contained the highest diversity of polyphenols sub-families, seven against the four observed in pena pena flower powder (Figure 1B). Nevertheless, both flower powders presented more free polyphenols than bound polyphenols, and a higher number of phenolic acids in bound form than in free (Table 4 and Table 5). That fact could be expected since phenolic compounds are most frequently found in bound forms. Furthermore, in both samples, the hydroxycinnamic acids: ferulic caffeic, and p-coumaric, were detected in bound forms. These compounds are frequently found bound to cell walls and proteins in other vegetables like fruits and cereals [65,66].
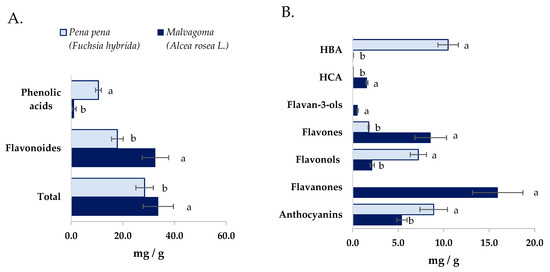
Figure 1.
(A). Phenolic acid, flavonoids, and total (sum) of (poly)phenols present in both studied flower powders. (B). Content of (poly)phenols divided by (poly)phenols sub-families present in both studied flower powders. Values with the same letter in the different figures are not statistically different (p > 0.05) for the same tested chemical compound. HBA: Hydroxybenzoic acids; HCA: Hydroxycinnamic acids.

Table 4.
(Poly)phenol profile of pena pena (Fuchsia hybrida) flower powder (µg/g).

Table 5.
(Poly)phenol profile of malvagoma (Alcea rosea L.) flower powder (µg/g).
Concerning the polyphenol profile of pena pena flower powders (Table 4), a total of thirty-five compounds were identified, encompassing ten flavonols, nine hydroxybenzoic acids, six anthocyanins, six flavones, and four hydroxycinnamic acids. Among the free compounds, pelargonidin-3-O-β-glucopyranoside, quercetin glycoside III, ellagic acid, and rutin emerged as the four most abundant, arranged in descending order. In the bound fraction, gallic acid was unequivocally prominent as the most abundant polyphenol. The most remarkable result was the high levels of anthocyanins and free ellagic acid and derivatives. In flowers of Sanguisorba officinalis, ellagic acid derivatives as ellagic acid hexosides and 3,3′,4′-O-trimethyl ellagic acid [67] were also reported.
Ellagic acid and ellagitannins are abundant in pomegranate, and their metabolic transformation by the human microbiota has been reported as beneficial to health. The content of ellagic acid in pena pena flower powders was close to ellagic acid vegetable sources like raspberry, cloudberry, and strawberry [68] and flowers like Tagetes erecta L.
In malvagoma flower powder, twenty-six polyphenols have been observed (Table 5). These include nine flavonols, nine hydroxycinnamic acids, eight anthocyanins, five hydroxybenzoic acids, three flavones, two flavanones, and one flavanol. Notably, in the free fraction, the predominant compounds were naringin glycoside I and II, with luteolin glycoside II following closely. Luteolin glycoside II were also the main compound quantified in bound form, followed by Gallo catechin gallate and caffeic acid. The number of phenolic acids was significantly lower than that shown in pena pena flower powder (p < 0.05) (Figure 1A) and observed more diversity of hydrocinnamic acids than hydroxybenzoic acids.
To the best of our knowledge, no previous studies have quantified the polyphenol composition of Fuchsia hybrida and Alcea rosea L. However, in horchata infusion, among the 23 compounds, containing malva esencia (Malva sp.), malva blanca (Althea officinalis L.), and pena pena (Fuchsia laxensis Kunth), thirteen quercetin glycoside were detected, like quercetin-O-pentoside, querecetin-O-galactoside, or methylquerce-tin-O-pentosyl-hexoside-O-hexoside [64]. Other authors have observed the abundance of quercetin and kaempferol glycosides in C. oleifera and C. polyodonta flowers [69].
In horchata infusion, two flavonones, hesperetin-O-caffeoyl-deoxyhexosyl-hexoside and naringenin hexoside, and two anthocyanins, Cyanidin-O-coumaroylhexosyl-O-hexoside and Cyanidin-O-coumaroylhexosyl-O-hexoside [68] were also detected.
As other edible flowers [70], pena pena and malvagoma can be considered a source of anthocyanins. Their values, especially those shown in pena pena flower powder, are higher than those shown in the main considered sources of anthocyanins, like berries, purple corn, cherries, plums, eggplant, wine, grapes, black carrots, red cabbage, and purple cauliflower, whose contents ranged between 1.0 and 14 mg/g [71]. Worldwide, the compound annual growth rate of anthocyanin food colorants is projected to be 4.8% from 2022 to 2032, owing to the widespread acceptance of natural pigments over synthetic ones [17,72,73]. In this sense, edible flowers have become a valuable resource for extracting pigments such as anthocyanins, betalains, carotenoids, and many other non-anthocyanin compounds that can be used in food formulation. The obtention of stable powder of flowers could contribute to the inclusion of them in functional foods or as a source of food colorants or in the intelligent package.
4. Conclusions
The petals of Alcea rosea and Fuchsia hybrida contain high levels of carbohydrates and proteins. Additionally, noteworthy is the content of dietary fiber, which grants better hydration properties to malvagoma due to the significantly higher levels of this nutrient.
The total unsaturated fatty acids exceeded the total saturated fatty acids in these flowers. Linoleic acid (C18:2; n 6,9) and linolenic acid (C18:3; n 3,6,9) were the major fatty acids found, followed by palmitic acid (C16:0). Both flowers are sources of polyphenols, especially anthocyanins. Pena pena flower powder can be considered a source of ellagic acids and derivatives, while malvagoma highlights the content of flavones and luteolin glycoside.
The results obtained demonstrate the potential of these two flowers to be used in food formulation, potentially enhancing nutritional, sensory, and functional characteristics. Future research is necessary to delve deeper into the influence of transformation processes on functional components. Additionally, studies on digestibility are needed to understand the actual bioavailability of these components.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods13020237/s1.
Author Contributions
Conceptualization, R.L.-G. and M.C.-C.; methodology, R.L.-G., R.M.-E. and M.V.-M.; resources, J.Á.P.-Á.; writing—original draft preparation, R.L.-G. and M.C.-C.; writing—review and editing, R.L.-G., M.C.-C. and J.F.-L.; project administration, J.F.-L.; funding acquisition, J.Á.P.-Á. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are grateful to the Universidad Técnica Particular de Loja (UTPL) for supporting this research and open access publication. Raquel Lucas-González would like to thank the Spanish “Ministerio de Universidades” for his “Margarita Salas” postdoctoral fellowship (funded by the European Union—Next Generation EU).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rios, M.; Tinitana, F.; Jarrín-V, P.; Donoso, N.; Romero-Benavides, J.C. “Horchata” Drink in Southern Ecuador: Medicinal Plants and People’s Wellbeing. J. Ethnobiol. Ethnomed. 2017, 13, 18. [Google Scholar] [CrossRef]
- Guevara, M.; Proaño, A.; Tejera, E.; Ballesteros, I.; Sánchez, M.E.; Granda-Albuja, M.G.; Freire, B.; Chisaguano, A.M.; Debut, A.; Vizuete, K.; et al. Protective Effect of the Medicinal Herb Infusion “Horchata” against Oxidative Damage in Cigarette Smokers: An Ex Vivo Study. Food Chem. Toxicol. 2020, 143, 111538. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. The Pharmaceutical Importance of Althaea Officinalis and Althaea Rosea: A Review Medicinal Plants with Cardiovascular Effects View Project Medicinal Plants with Respiratory Effects View Project the Pharmaceutical Importance of Althaea Officinalis and Althaea Rosea: A Review. Int. J. PharmTech Res. 2013, 5, 1378–1385. [Google Scholar]
- Dujmović, M.; Radman, S.; Opačić, N.; Fabek Uher, S.; Mikuličin, V.; Voća, S.; Šic Žlabur, J. Edible Flower Species as a Promising Source of Specialized Metabolites. Plants 2022, 11, 2529. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, B.; Huang, W.; Amrouche, A.T.; Maurizio, B.; Simal-Gandara, J.; Tundis, R.; Xiao, J.; Zou, L.; Lu, B. Edible Flowers as Functional Raw Materials: A Review on Anti-Aging Properties. Trends Food Sci. Technol. 2020, 106, 30–47. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Xiao, Y.; Fu, N.L. Antioxidant Capacities and Total Phenolic Contents of 30 Flowers. Ind. Crops Prod. 2018, 111, 430–445. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Badwaik, L.S.; Annapure, U.; Casanova, F.; Alaskar, K. A Review on the Journey of Edible Flowers from Farm to Consumer’s Plate. Appl. Food Res. 2023, 3, 100312. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. An Overview on the Market of Edible Flowers. Food Rev. Int. 2020, 36, 258–275. [Google Scholar] [CrossRef]
- Janarny, G.; Ranaweera, K.K.D.S.; Gunathilake, K.D.P.P. Antioxidant Activities of Hydro-Methanolic Extracts of Sri Lankan Edible Flowers. Biocatal. Agric. Biotechnol. 2021, 35, 102081. [Google Scholar] [CrossRef]
- Fan, J.; Zhu, W.; Kang, H.; Ma, H.; Tao, G. Flavonoid Constituents and Antioxidant Capacity in Flowers of Different Zhongyuan Tree Penoy Cultivars. J. Funct. Foods 2012, 4, 147–157. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Jurikova, T.; Neugebauerova, J.; Vabkova, J. Edible Flowers—A New Promising Source of Mineral Elements in Human Nutrition. Molecules 2012, 17, 6672–6683. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Ramalhosa, E.; Pereira, J.A.; Saraiva, J.A.; Casal, S. Borage, Camellia, Centaurea and Pansies: Nutritional, Fatty Acids, Free Sugars, Vitamin E, Carotenoids and Organic Acids Characterization. Food Res. Int. 2020, 132, 109070. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, J.; Rop, O. Fresh Edible Flowers of Ornamental Plants—A New Source of Nutraceutical Foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Chen, N.H.; Wei, S. Factors Influencing Consumers’ Attitudes towards the Consumption of Edible Flowers. Food Qual. Prefer. 2017, 56, 93–100. [Google Scholar] [CrossRef]
- Pires, E.O., Jr.; Di Gioia, F.; Rouphael, Y.; Ferreira, I.C.F.R.; Caleja, C.; Barros, L.; Petropoulos, S.A. The Compositional Aspects of Edible Flowers as an Emerging Horticultural Product. Molecules 2021, 26, 6940. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.; Poonia, A. Bioactive Compounds, Nanoparticles Synthesis, Health Benefits and Potential Utilization of Edible Flowers for the Development of Functional Dairy Products: A Review. J. Food Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Gamage, G.C.V.; Goh, J.K.; Choo, W.S. Natural Blue Colourant Preparations from Blue Pea Flower and Spirulina: A Comparison Stability Study. Food Chem. Adv. 2023, 3, 100457. [Google Scholar] [CrossRef]
- Hnin, K.K.; Zhang, M.; Wang, B. Development of Nutritional Properties in Cookies with the Incorporation of Different Levels of Rose Flower Powder by Microwave-Vacuum Drying. Dry. Technol. 2021, 39, 1136–1148. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Tenuta, M.C.; Menichini, F.; Xiao, J.; Tundis, R. Edible Flowers: A Rich Source of Phytochemicals with Antioxidant and Hypoglycemic Properties. J. Agric. Food Chem. 2016, 64, 2467–2474. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible Flowers as Sources of Phenolic Compounds with Bioactive Potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef]
- de O. Pires, E., Jr.; Di Gioia, F.; Rouphael, Y.; García-Caparrós, P.; Tzortzakis, N.; Ferreira, I.C.F.R.; Barros, L.; Petropoulos, S.A.; Caleja, C. Edible Flowers as an Emerging Horticultural Product: A Review on Sensorial Properties, Mineral and Aroma Profile. Trends Food Sci. Technol. 2023, 137, 31–54. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, B.; Xu, B. An Update on the Health Benefits Promoted by Edible Flowers and Involved Mechanisms. Food Chem. 2021, 340, 127940. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Ferreira, I.C.F.R. Nutritional and Chemical Characterization of Edible Petals and Corresponding Infusions: Valorization as New Food Ingredients. Food Chem. 2017, 220, 337–343. [Google Scholar] [CrossRef]
- Purohit, S.R.; Rana, S.S.; Idrishi, R.; Sharma, V.; Ghosh, P. A Review on Nutritional, Bioactive, Toxicological Properties and Preservation of Edible Flowers. Future Foods 2021, 4, 100078. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Rockville, MA, USA, 2007; Volume 1, ISBN 0935584781. [Google Scholar]
- Lucas-González, R.; Fernández-López, J.; Pérez-Álvarez, J.Á.; Viuda-Martos, M. Effect of Particle Size on Phytochemical Composition and Antioxidant Properties of Two Persimmon Flours from Diospyros Kaki Thunb. Vars. ‘Rojo Brillante’ and ‘Triumph’ Co-Products. J. Sci. Food Agric. 2018, 98, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Lucas-González, R.; Pérez-álvarez, J.Á.; Viuda-Martos, M.; Fernández-López, J. Pork Liver Pâté Enriched with Persimmon Coproducts: Effect of in Vitro Gastrointestinal Digestion on Its Fatty Acid and Polyphenol Profile Stability. Nutrients 2021, 13, 1332. [Google Scholar] [CrossRef]
- Botella-Martínez, C.; Lucas-Gonzalez, R.; Ballester-Costa, C.; Pérez-Álvarez, J.Á.; Fernández-López, J.; Delgado-Ospina, J.; Chaves-López, C.; Viuda-Martos, M. Ghanaian Cocoa (Theobroma cacao L.) Bean Shells Coproducts: Effect of Particle Size on Chemical Composition, Bioactive Compound Content and Antioxidant Activity. Agronomy 2021, 11, 401. [Google Scholar] [CrossRef]
- Mpofu, A.; Sapirstein, H.D.; Beta, T. Genotype and Environmental Variation in Phenolic Content, Phenolic Acid Composition, and Antioxidant Activity of Hard Spring Wheat. J. Agric. Food Chem. 2006, 54, 1265–1270. [Google Scholar] [CrossRef]
- Chau, C.F.; Huang, Y.L. Comparison of the Chemical Composition and Physicochemical Properties of Different Fibers Prepared from the Peel of Citrus sinensis L. Cv. Liucheng. J. Agric. Food Chem. 2003, 51, 2615–2618. [Google Scholar] [CrossRef]
- Robertson, J.A.; de Monredon, F.D.; Dysseler, P.; Guillon, F.; Amado, R.; Thibault, J.-F. Hydration Properties of Dietary Fibre and Resistant Starch: A European Collaborative Study. LWT—Food Sci. Technol. 2000, 33, 72–79. [Google Scholar] [CrossRef]
- Zhang, X.K.; Cao, G.H.; Bi, Y.; Liu, X.H.; Yin, H.M.; Zuo, J.F.; Xu, W.; Li, H.D.; He, S.; Zhou, X.H. Comprehensive Analysis of 34 Edible Flowers by the Determination of Nutritional Composition and Antioxidant Capacity Planted in Yunnan Province China. Molecules 2023, 28, 5260. [Google Scholar] [CrossRef] [PubMed]
- Rivas-García, L.; Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Forbes-Hernández, T.Y.; Varela-López, A.; Llopis, J.; Sánchez-González, C.; Quiles, J.L. Edible Flowers as a Health Promoter: An Evidence-Based Review. Trends Food Sci. Technol. 2021, 117, 46–59. [Google Scholar] [CrossRef]
- Sotelo, A.; López-García, S.; Basurto-Peña, F. Content of Nutrient and Antinutrient in Edible Flowers of Wild Plants in Mexico. Plant Foods Hum. Human. Nutr. 2007, 62, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-Espinoza, J.M.; Gutiérrez-Tlahque, J.; Santiago-Saenz, Y.O.; Aguirre-Mancilla, C.L.; Reyes-Fuentes, M.; López-Palestina, C.U. Nutritional Composition, Bioactive Compounds and Antioxidant Activity of Wild Edible Flowers Consumed in Semiarid Regions of Mexico. Plant Foods Hum. Human. Nutr. 2020, 75, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible Flowers: A Review of the Nutritional, Antioxidant, Antimicrobial Properties and Effects on Human Health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Rezende, F.A.G.G.; Moura, M.A.F.; Dominguete, L.C.B.; Sande, D. Edible Flowers: Bioactive Profile and Its Potential to Be Used in Food Development. Food Res. Int. 2020, 129, 108868. [Google Scholar] [CrossRef]
- López-Cervantes, J.; Sánchez-Machado, D.I.; Cruz-Flores, P.; Mariscal-Domínguez, M.F.; Servín de la Mora-López, G.; Campas-Baypoli, O.N. Antioxidant Capacity, Proximate Composition, and Lipid Constituents of Aloe Vera Flowers. J. Appl. Res. Med. Aromat. Plants 2018, 10, 93–98. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Koprowska, K.; Gottschling, A.; Janda-milczarek, K. Edible Flowers as a Source of Dietary Fibre (Total, Insoluble and Soluble) as a Potential Athlete’s Dietary Supplement. Nutrients 2022, 14, 2470. [Google Scholar] [CrossRef]
- Stribling, P.; Ibrahim, F. Dietary Fibre Definition Revisited—The Case of Low Molecular Weight Carbohydrates. Clin. Nutr. ESPEN 2023, 55, 340–356. [Google Scholar] [CrossRef]
- Rachkeeree, A.; Kantadoung, K.; Suksathan, R.; Puangpradab, R.; Page, P.A.; Sommano, S.R. Nutritional Compositions and Phytochemical Properties of the Edible Flowers from Selected Zingiberaceae Found in Thailand. Front. Nutr. 2018, 5, 3. [Google Scholar] [CrossRef]
- Siddiqui, H.; Sultan, Z.; Yousuf, O.; Malik, M.; Younis, K. A Review of the Health Benefits, Functional Properties, and Ultrasound-Assisted Dietary Fiber Extraction. Bioact. Carbohydr. Diet. Fibre 2023, 30, 100356. [Google Scholar] [CrossRef]
- Lima, V.; Pinto, C.A.; Saraiva, J.A. The Dependence of Microbial Inactivation by Emergent Nonthermal Processing Technologies on PH and Water Activity. Innov. Food Sci. Emerg. Technol. 2023, 89, 103460. [Google Scholar] [CrossRef]
- Krzymińska, A.; Gąsecka, M.; Magdziak, Z. Content of Phenolic Compounds and Organic Acids in the Flowers of Selected Tulipa Gesneriana Cultivars. Molecules 2020, 25, 5627. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Taher, A.; Mulla, M.Z.; Al-Hazza, A.; Luciano, G. Effect of Sieve Particle Size on Functional, Thermal, Rheological and Pasting Properties of Indian and Turkish Lentil Flour. J. Food Eng. 2016, 186, 34–41. [Google Scholar] [CrossRef]
- Ahmed, J.; Al-Jassar, S.; Thomas, L. A Comparison in Rheological, Thermal, and Structural Properties between Indian Basmati and Egyptian Giza Rice Flour Dispersions as Influenced by Particle Size. Food Hydrocoll. 2015, 48, 72–83. [Google Scholar] [CrossRef]
- Düsenberg, B.; Schmidt, J.; Sensoy, I.; Bück, A. Flowability of Plant Based Food Powders: Almond, Chestnut, Chickpea, Coconut, Hazelnut and Rice. J. Food Eng. 2023, 357, 111606. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Pérez-Álvarez, J.Á.; Fernández-López, J. Evaluation of Particle Size Influence on Proximate Composition, Physicochemical, Techno-Functional and Physio-Functional Properties of Flours Obtained from Persimmon (Diospyros Kaki Trumb.) Coproducts. Plant Foods Hum. Human. Nutr. 2017, 72, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T. Contribution to Flower Colors of Flavonoids Including Anthocyanins: A Review. Nat. Prod. Commun. 2015, 10, 529–544. [Google Scholar] [CrossRef]
- Han, M.L.; Zhao, Y.H.; Meng, J.X.; Yin, J.; Li, H.H. Analysis of Physicochemical and Antioxidant Properties of Malus Spp. Petals Reveals Factors Involved in Flower Color Change and Market Value. Sci. Hortic. 2023, 310, 111688. [Google Scholar] [CrossRef]
- Rosell, C.M.; Santos, E.; Collar, C. Physico-Chemical Properties of Commercial Fibres from Different Sources: A Comparative Approach. Food Res. Int. 2009, 42, 176–184. [Google Scholar] [CrossRef]
- He, C.-A.; Qi, J.-R.; Liao, J.-S.; Song, Y.-T.; Wu, C.-L. lin Excellent Hydration Properties and Oil Holding Capacity of Citrus Fiber: Effects of Component Variation and Microstructure. Food Hydrocoll. 2023, 144, 108988. [Google Scholar] [CrossRef]
- Requena, M.C.; González, C.N.A.; Barragán, L.A.P.; Correia, T.; Esquivel, J.C.C.; Herrera, R.R. Functional and Physico-Chemical Properties of Six Desert-Sources of Dietary Fiber. Food Biosci. 2016, 16, 26–31. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; O’Shea, N.; Gallagher, E. Rheological Properties of Wheat Dough Supplemented with Functional By-Products of Food Processing: Brewer’s Spent Grain and Apple Pomace. J. Food Eng. 2013, 116, 362–368. [Google Scholar] [CrossRef]
- Wuttipalakorn, P.; Srichumpuang, W.; Chiewchan, N. Effects of Pretreatment and Drying on Composition and Bitterness of High-Dietary-Fiber Powder from Lime Residues. Dry. Technol. 2009, 27, 133–142. [Google Scholar] [CrossRef]
- Ma, M.M.; Mu, T.H. Effects of Extraction Methods and Particle Size Distribution on the Structural, Physicochemical, and Functional Properties of Dietary Fiber from Deoiled Cumin. Food Chem. 2016, 194, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Escudero, E.; González, P. La Fibra Dietética Correspondencia. Nutr. Hosp. 2006, 21, 61–72. [Google Scholar]
- Mudgil, D.; Barak, S. Classification, Technological Properties, and Sustainable Sources. In Dietary Fiber: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 27–58. ISBN 9780128164952. [Google Scholar]
- da Silva, L.A.; Fischer, S.Z.; Zambiazi, R.C. Proximal Composition, Bioactive Compounds Content and Color Preference of Viola x Wittrockiana Flowers. Int. J. Gastron. Food Sci. 2020, 22, 100236. [Google Scholar] [CrossRef]
- Primitivo, M.J.; Neves, M.; Pires, C.L.; Cruz, P.F.; Brito, C.; Rodrigues, A.C.; de Carvalho, C.C.C.R.; Mortimer, M.M.; Moreno, M.J.; Brito, R.M.M.; et al. Edible Flowers of Helichrysum Italicum: Composition, Nutritive Value, and Bioactivities. Food Res. Int. 2022, 157, 111399. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Hammad, S.; Pu, S.; Jones, P.J. Current Evidence Supporting the Link Between Dietary Fatty Acids and Cardiovascular Disease. Lipids 2016, 51, 507–517. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Guevara, M.; Tejera, E.; Iturralde, G.A.; Jaramillo-Vivanco, T.; Granda-Albuja, M.G.; Granja-Albuja, S.; Santos-Buelga, C.; González-Paramás, A.M.; Álvarez-Suarez, J.M. Anti-Inflammatory Effect of the Medicinal Herbal Mixture Infusion, Horchata, from Southern Ecuador against LPS-Induced Cytotoxic Damage in RAW 264.7 Macrophages. Food Chem. Toxicol. 2019, 131, 110594. [Google Scholar] [CrossRef] [PubMed]
- Lucas-González, R.; Pérez-Álvarez, J.Á.; Moscaritolo, S.; Fernández-López, J.; Sacchetti, G.; Viuda-Martos, M. Evaluation of Polyphenol Bioaccessibility and Kinetic of Starch Digestion of Spaghetti with Persimmon (Dyospyros Kaki) Flours Coproducts during In Vitro Gastrointestinal Digestion. Food Chem. 2021, 338, 128142. [Google Scholar] [CrossRef] [PubMed]
- Lucas-González, R.; Díez-Riquelme, V.; Viuda-Martos, M.; Pérez-Álvarez, J.Á.; Sánchez-Zapata, E.; Fernández-López, J. Effect of the Food Matrix on the (Poly)Phenol Stability of Different Plant-Based Meat Products and Their Main Ingredients after In Vitro Gastrointestinal Digestion. Food Funct. 2023, 14, 10796–10813. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Oszmiański, J.; Rapak, A.; Ochmian, I. Profile and Content of Phenolic Compounds in Leaves, Flowers, Roots, and Stalks of Sanguisorba officinalis L. Determined with the LC-DAD-ESI-QTOF-MS/MS Analysis and Their In Vitro Antioxidant, Antidiabetic, Antiproliferative Potency. Pharmaceuticals 2020, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxid. Med. Cell Longev. 2022, 2022, 3848084. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Xia, C.; Feng, S.; Chen, T.; Zhou, L.; Liu, L.; Kong, Q.; Yang, H.; Ding, C. Assessment of Free and Bound Phenolics in the Flowers and Floral Organs of Two Camellia Species Flower and Their Antioxidant Activities. Food Biosci. 2022, 49, 101905. [Google Scholar] [CrossRef]
- Teixeira, M.; Tao, W.; Fernandes, A.; Faria, A.; Ferreira, I.M.P.L.V.O.; He, J.; de Freitas, V.; Mateus, N.; Oliveira, H. Anthocyanin-Rich Edible Flowers, Current Understanding of a Potential New Trend in Dietary Patterns. Trends Food Sci. Technol. 2023, 138, 708–725. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Nabi, B.G.; Mukhtar, K.; Ahmed, W.; Manzoor, M.F.; Ranjha, M.M.A.N.; Kieliszek, M.; Bhat, Z.F.; Aadil, R.M. Natural Pigments: Anthocyanins, Carotenoids, Chlorophylls, and Betalains as Colorants in Food Products. Food Biosci. 2023, 52, 102403. [Google Scholar] [CrossRef]
- Martins, N.; Roriz, C.L.; Morales, P.; Barros, L.; Ferreira, I.C.F.R. Coloring Attributes of Betalains: A Key Emphasis on Stability and Future Applications. Food Funct. 2017, 8, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).