Beyond the Bottle: Exploring Health-Promoting Compounds in Wine and Wine-Related Products—Extraction, Detection, Quantification, Aroma Properties, and Terroir Effects
Abstract
1. Introduction
2. Health-Promoting Phenolic Compounds
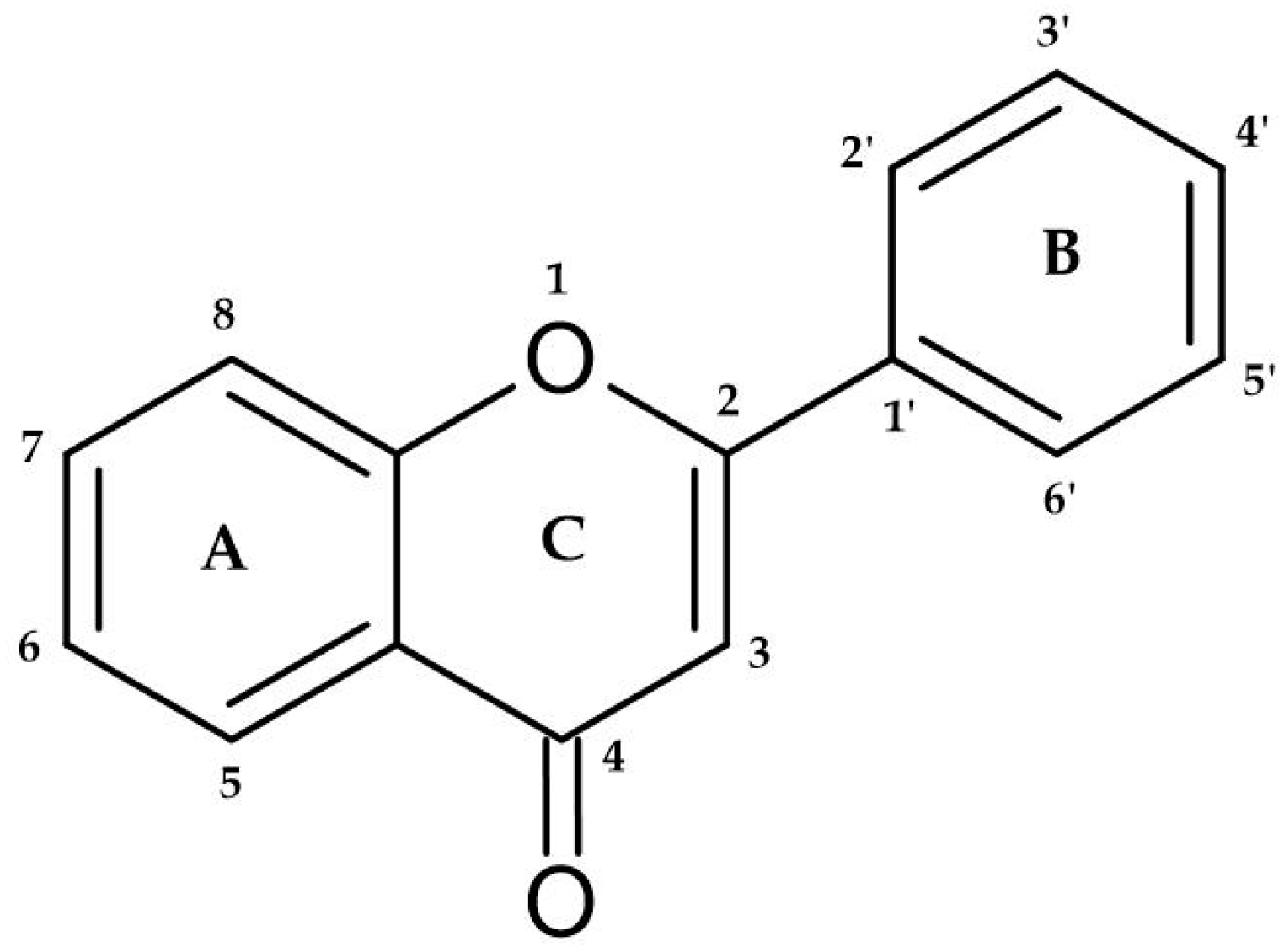
2.1. Flavonoids and Non-Flavonoids
2.2. Wine Compounds Produced by Fermentation
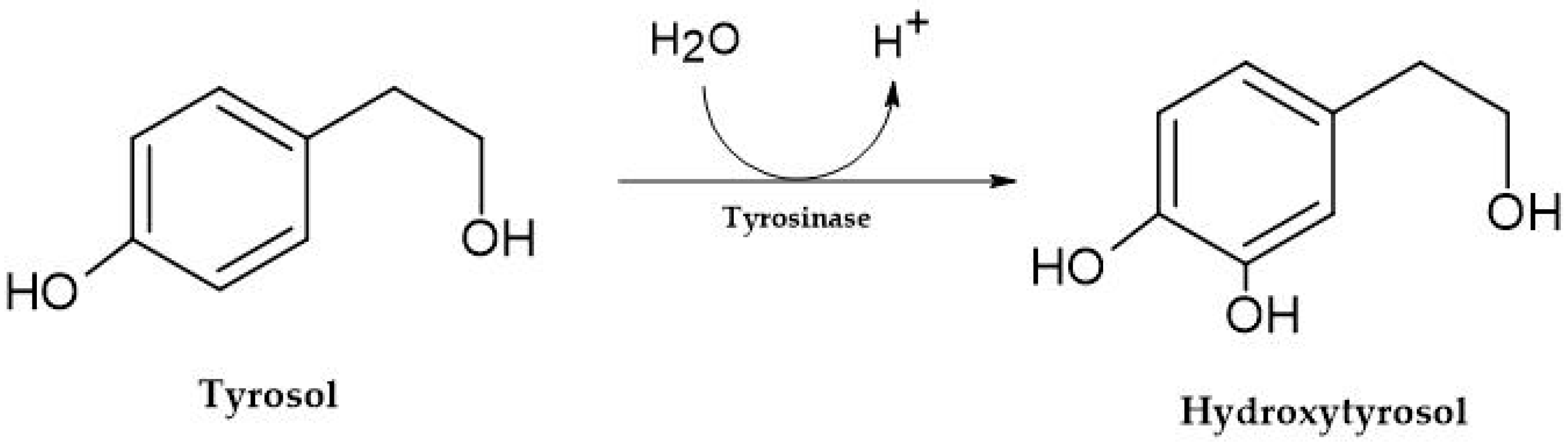
2.2.1. Tyrosol, Hydroxytyrosol, and Tryptophol
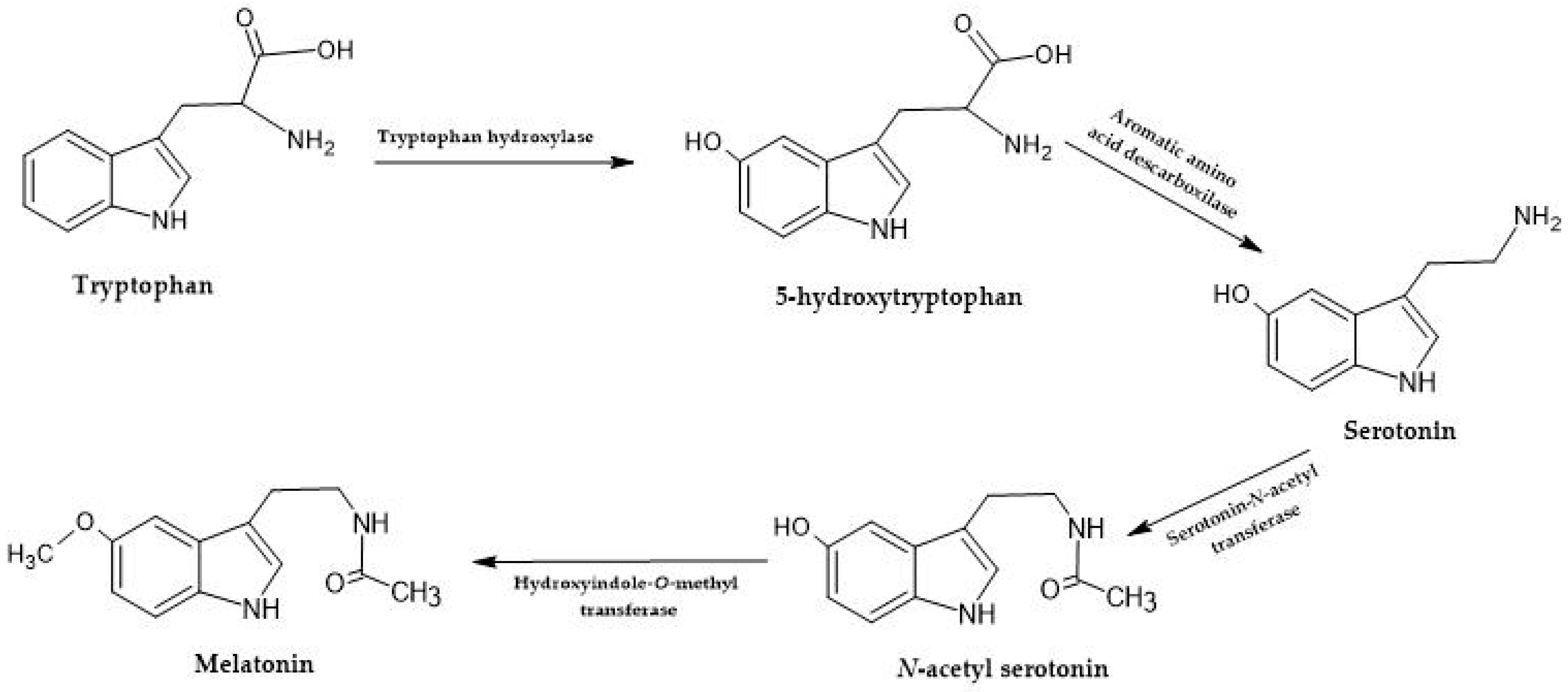
2.2.2. Melatonin and Serotonin
3. Health-Promoting Non-Phenolic Compounds
3.1. Glutathione
3.2. Vitamins
3.3. Minerals
4. Terroir and Its Impact on Health-Promoting Compounds Synthesis
5. Sensory Perception of Health-Promoting Compounds
5.1. Health-Promoting Compound’s Terroir-Dependent Sensory Attributes
5.2. Influence of pH, Saliva (Enzymes) Biochemistry, and Oral Microflora on the Sensory Perception of Health-Promoting Compounds
6. Extraction Techniques of Nutraceutical Compounds in Wine and Wine-Related Products
7. Separation and Identification of Health-Promoting Compounds
7.1. Chromatography Techniques
7.2. Spectroscopy Techniques
7.3. Enzymatic Methods and Antioxidant Assays
7.4. Electrochemical Techniques
| Method | Method Description | References |
|---|---|---|
| Amperometry | Measures the current produced during an electrochemical reaction between the analyte and the electrode. The analyte is oxidized or reduced at the working electrode, and then the current is measured by a potentiostat since the analyte concentration is directly proportional to the current. | [251,252,254] |
| Voltammetry | Voltammetry measures the redox potential of chemical species, with cyclic voltammetry commonly used in the food industry. It involves cycling the voltage between two values at a specific scan rate on a working electrode, allowing oxidation and reduction reactions. The resulting voltammogram shows peaks and valleys corresponding to the behavior of electroactive species in the sample. | [228,251,252] |
| Electrochemical impedance spectroscopy (EIS) | Electrochemical impedance spectroscopy (EIS) measures electrical impedance changes in response to an applied voltage across various frequencies. It determines impedance by analyzing the ratio of the applied voltage to the resulting current. | [16,255] |
8. Detection and Quantification of Health-Promoting Non-Phenolic Compounds
9. Final Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, S.; Chen, J.; Ruan, X.; Sun, Y.; Zhang, K.; Wang, X.; Li, X.; Gill, D.; Burgess, S.; Giovannucci, E.; et al. Smoking, alcohol consumption, and 24 gastrointestinal diseases: Mendelian randomization analysis. eLife 2023, 12, e84051. [Google Scholar] [CrossRef] [PubMed]
- Hrelia, S.; Di Renzo, L.; Bavaresco, L.; Bernardi, E.; Malaguti, M.; Giacosa, A. Moderate Wine Consumption and Health: A Narrative Review. Nutrients 2022, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Andarwulan, N.; Puspita, N.C.; Saraswati; Średnicka-Tober, D. Antioxidants Such as Flavonoids and Carotenoids in the Diet of Bogor, Indonesia Residents. Antioxidants 2021, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; González-Manzano, S.; González-Paramás, A.M. Wine, Polyphenols, and Mediterranean Diets. What Else Is There to Say? Molecules 2021, 26, 5537. [Google Scholar] [CrossRef] [PubMed]
- Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients 2022, 14, 2956. [Google Scholar] [CrossRef] [PubMed]
- Skoumas, J.; Pitsavos, C.; Panagiotakos, D.B.; Chrysohoou, C.; Zeimbekis, A.; Papaioannou, I.; Toutouza, M.; Toutouzas, P.; Stefanadis, C. Physical activity, high-density lipoprotein cholesterol and other lipids levels, in men and women from the ATTICA study. Lipids Health Dis. 2003, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Finelli, F.; Bonomo, M.G.; Giuzio, F.; Mang, S.M.; Capasso, A.; Salzano, G.; Sinicropi, M.; Saturnino, C.; Genovese, S. Nutraceutical Properties of Wine In Irpinia. Pharmacologyonline 2021, 3, 99–109. [Google Scholar]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean Diet: The Role of Long-Chain ω-3 Fatty Acids in Fish; Polyphenols in Fruits, Vegetables, Cereals, Coffee, Tea, Cacao and Wine; Probiotics and Vitamins in Prevention of Stroke, Age-Related Cognitive Decline, and Alzheimer Disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Visioli, F.; Panaite, S.-A.; Tomé-Carneiro, J. Wine’s Phenolic Compounds and Health: A Pythagorean View. Molecules 2020, 25, 4105. [Google Scholar] [CrossRef]
- Motilva, M.-J.; Macià, A.; Romero, M.-P.; Rubió, L.; Mercader, M.; González-Ferrero, C. Human Bioavailability and Metabolism of Phenolic Compounds from Red Wine Enriched with Free or Nano-Encapsulated Phenolic Extract. J. Funct. Foods 2016, 25, 80–93. [Google Scholar] [CrossRef]
- Muñoz-Bernal, Ó.A.; de la Rosa, L.A.; Rodrigo-García, J.; Martínez-Ruiz, N.R.; Sáyago-Ayerdi, S.; Rodriguez, L.; Fuentes, E.; Alvarez-Parrilla, E. Phytochemical Characterization and Antiplatelet Activity of Mexican Red Wines and Their By-products. S. Afr. J. Enol. Vitic. 2021, 42, 77. [Google Scholar] [CrossRef]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Wine as a Biological Fluid: History, Production, and Role in Disease Prevention. J. Clin. Lab. Anal. 1997, 11, 287–313. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Luján-Barroso, L.; Romieu, I.; Scalbert, A.; Slimani, N.; Hjartåker, A.; Engeset, D.; Skeie, G.; Overvad, K.; et al. Differences in Dietary In-takes, Food Sources and Determinants of Total Flavonoids between Mediterranean and Non-Mediterranean Countries Participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Br. J. Nutr. 2012, 109, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Lähteenmäki, L. Claiming Health in Food Products. Food Qual. Prefer. 2013, 27, 196–201. [Google Scholar] [CrossRef]
- Vilela, A.; Pinto, T. Grape Infusions: Between Nutraceutical and Green Chemistry. Sustain. Chem. 2021, 2, 441–466. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Santos, L. A Potential Valorization Strategy of Wine Industry by-Products and Their Application in Cosmetics—Case Study: Grape Pomace and Grapeseed. Molecules 2022, 27, 969. [Google Scholar] [CrossRef]
- El Kersh, D.M.; Hammad, G.; Donia, M.S.; Farag, M.A. A Comprehensive Review on Grape Juice Beverage in Context to Its Processing and Composition with Future Perspectives to Maximize Its Value. Food Bioprocess Technol. 2022, 16, 1–23. [Google Scholar] [CrossRef]
- Lambrechts, M.; van Velden, D.; Louw, L.; van Rensburg, P. Brandy and Cognac: Consumption, Sensory and Health Effects. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 456–461. [Google Scholar] [CrossRef]
- Antoniewicz, J.; Jakubczyk, K.; Kwiatkowski, P.; Maciejewska-Markiewicz, D.; Kochman, J.; Rębacz-Maron, E.; Janda-Milczarek, K. Analysis of Antioxidant Capacity and Antimicrobial Properties of Selected Polish Grape Vinegars Obtained by Spontaneous Fermentation. Molecules 2021, 26, 4727. [Google Scholar] [CrossRef]
- Kondo, T.; Kishi, M.; Fushimi, T.; Ugajin, S.; Kaga, T. Vinegar Intake Reduces Body Weight, Body Fat Mass, and Serum Triglyceride Levels in Obese Japanese Subjects. Biosci. Biotechnol. Biochem. 2009, 73, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Budak, N.H.; Aykin, E.; Seydim, A.C.; Greene, A.K.; Guzel-Seydim, Z.B. Functional Properties of Vinegar. J. Food Sci. 2014, 79, R757–R764. [Google Scholar] [CrossRef] [PubMed]
- Poklar Ulrih, N.; Opara, R.; Korošec, M.; Wondra, M.; Abram, V. Part II. Influence of Trans-Resveratrol Addition on the Sensory Properties of ‘Blaufränkisch’ Red Wine. Food Chem. Toxicol. 2020, 137, 111124. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bernal, A.; Vazquez-Flores, A.A.; de la Rosa, L.A.; Rodrigo-García, J.; Martínez-Ruiz, N.R.; Alvarez-Parrilla, E. Enriched Red Wine: Phenolic Profile, Sensory Evaluation and In Vitro Bioaccessibility of Phenolic Compounds. Foods 2023, 12, 1194. [Google Scholar] [CrossRef] [PubMed]
- Harvey, D. The Language of Analytical Chemistry. In Modern Analytical Chemistry, 1st ed.; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2000; pp. 35–44. [Google Scholar]
- International Organisation of Vine and Wine. Compendium of International Methods of Analysis of Wines and Musts, 2021st ed.; International Organization of Vine and Wine: Paris, France, 2021; Volume 1, Available online: https://www.oiv.int/public/medias/7907/oiv-vol1-compendium-of-international-methods-of-analysis.pdf (accessed on 4 September 2023).
- Onache, P.A.; Florea, A.; Geana, E.-I.; Ciucure, C.T.; Ionete, R.E.; Sumedrea, D.I.; Tița, O. Assessment of Bioactive Phenolic Compounds in Musts and the Corresponding Wines of White and Red Grape Varieties. Appl. Sci. 2023, 13, 5722. [Google Scholar] [CrossRef]
- Popîrdă, A.; Luchian, C.E.; Cotea, V.V.; Colibaba, L.C.; Scutarașu, E.C.; Toader, A.M. A Review of Representative Methods Used in Wine Authentication. Agriculture 2021, 11, 225. [Google Scholar] [CrossRef]
- Razungles, A. Extraction Technologies and Wine Quality. In Managing Wine Quality: Volume 2: Oenology and Wine Quality; Woodhead Publishing: Cambridge, UK, 2022; Volume 2, pp. 3–41. [Google Scholar] [CrossRef]
- Vidal-Casanella, O.; Núñez, O.; Granados, M.; Saurina, J.; Sentellas, S. Analytical Methods for Exploring Nutraceuticals Based on Phenolic Acids and Polyphenols. Appl. Sci. 2021, 11, 8276. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.-M. The Role of Metabolism (and the Microbiome) in Defining the Clinical Efficacy of Dietary Flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine—Stabili-zation and Treatments; John Wiley and Sons: Hoboken, NJ, USA, 2006; Available online: https://books.google.pt/books?id=a03C-aFy2jsC (accessed on 4 September 2023).
- Morata, A.; Escott, C.; Loira, I.; Del Fresno, J.M.; Gonzalez, C.; Suarez-Lepe, J.Á. Influence of Saccharomyces and non-Saccharomyces yeasts in the formation of pyranoanthocyanins and polymeric pigments during red winemaking. Molecules 2019, 24, 4490. [Google Scholar] [CrossRef]
- Ma, W.; Guo, A.; Zhang, Y.; Wang, H.; Liu, Y.; Li, H. A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 2014, 40, 6–19. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Contribution of non-Saccharomyces yeasts to wine freshness. A review. Biomolecules 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, M.; Perestrelo, R.; Barros, A.; Bilelo, M.; Morête, A.; Câmara, J.; Rocha, S. Allergic Asthma Exhaled Breath Metabolome: A Challenge for Comprehensive Two-Dimensional Gas Chromatography. J. Chromatogr. A 2012, 1254, 87–97. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and their variation in red wines II. Antho-cyanin-derived pigments and their color evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef] [PubMed]
- Ruta, L.L.; Farcasanu, I.C. Anthocyanins and Anthocyanin-Derived Products in Yeast-Fermented Beverages. Antioxidants 2019, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Ferreras-Charro, R.; García-Estévez, I.; Escribano-Bailón, M.-T. In search for flavonoid and colorimetric varietal markers of Vitis vinifera L. cv Rufete wines. Curr. Res. Food Sci. 2023, 6, 100467. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, P.; Ma, W.; Zeng, X.-A.; Fang, Z. Physicochemical properties, antioxidant activities and comprehensive phenolic profiles of tea-macerated Chardonnay wine and model wine. Food Chem. 2024, 436, 137748. [Google Scholar] [CrossRef]
- Artiushenko, O.; Zaitsev, V. Competing ligand exchange-solid phase extraction method of polyphenols from wine. Microchem. J. 2023, 191, 108917. [Google Scholar] [CrossRef]
- Benbouguerra, N.; Hornedo-Ortega, R.; Garcia, F.; El Khawand, T.; Saucier, C.; Richard, T. Stilbenes in grape berries and wine and their potential role as anti-obesity agents: A review. Trends Food Sci. Technol. 2021, 112, 362–381. [Google Scholar] [CrossRef]
- Nurmi, T.; Heinonen, S.; Mazur, W.; Deyama, T.; Nishibe, S.; Adlercreutz, H. Lignans in selected wines. Food Chem. 2003, 83, 303–309. [Google Scholar] [CrossRef]
- Jovanović-Cvetković, T.; Sredojević, M.; Natić, M.; Grbić, R.; Akšić, M.F.; Ercisli, S.; Cvetković, M. Exploration and Comparison of the Behavior of Some Indigenous and International Varieties (Vitis vinifera L.) Grown in Climatic Conditions of Herzegovina: The Influence of Variety and Vintage on Physico-Chemical Characteristics of Grapes. Plants 2023, 12, 695. [Google Scholar] [CrossRef] [PubMed]
- Radovanović, B.C.; Radovanović, A.N.; Souquet, J.-M. Phenolic Profile and Free Radical-Scavenging Activity of Cabernet Sauvi-gnon Wines of Different Geographical Origins from the Balkan Region. J. Sci. Food Agric. 2010, 90, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Vilela, A. Modulating Wine Pleasantness Throughout Wine-Yeast Co-Inoculation or Sequential Inoculation. Fermentation 2020, 6, 22. [Google Scholar] [CrossRef]
- Morata, A.; Vejarano, R.; Ridolfi, G.; Benito, S.; Palomero, F.; Uthurry, C.; Tesfaye, W.; González, C.; Suárez-Lepe, J. Reduction of 4-ethylphenol production in red wines using HCDC+ yeasts and cinnamyl esterases. Enzym. Microb. Technol. 2013, 52, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Espejo, F. Role of commercial enzymes in wine production: A critical review of recent research. J. Food Sci. Technol. 2020, 58, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Cosme, F.; Inês, A.; Vilela, A. Microbial and Commercial Enzymes Applied in the Beverage Production Process. Fermentation 2023, 9, 385. [Google Scholar] [CrossRef]
- Martínez, J.M.; Cebrián, G.; Álvarez, I.; Raso, J. Release of Mannoproteins during Saccharomyces cerevisiae Autolysis Induced by Pulsed Electric Field. Front. Microbiol. 2016, 7, 1435. [Google Scholar] [CrossRef]
- Vilela, A. The Importance of Yeasts on Fermentation Quality and Human Health-Promoting Compounds. Fermentation 2019, 5, 46. [Google Scholar] [CrossRef]
- Bordiga, M.; Lorenzo, C.; Pardo, F.; Salinas, M.R.; Travaglia, F.; Arlorio, M.; Coïsson, J.D.; Garde-Cerdán, T. Factors Influencing the Formation of Histaminol, Hydroxytyrosol, Tyrosol, and Tryptophol in Wine: Temperature, Alcoholic Degree, and Amino Ac-ids Concentration. Food Chem. 2016, 197, 1038–1045. [Google Scholar] [CrossRef]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Dong, Y.; Yu, M.; Wu, Y.; Xia, T.; Wang, L.; Song, K.; Zhang, C.; Lu, K.; Rahimnejad, S. Hydroxytyrosol Promotes the Mitochondrial Function through Activating Mitophagy. Antioxidants 2022, 11, 893. [Google Scholar] [CrossRef]
- Akbari, H.; Taghizadeh-Hesary, F. COVID-19 induced liver injury from a new perspective: Mitochondria. Mitochondrion 2023, 70, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Guo, J.; Ren, H.L.; Lu, S.; He, Z.; Chang, J.; Hu, X.; Shi, R.; Jin, Y.; Li, Y.; et al. Tyrosol Inhibits NF-ΚB Pathway in the Treatment of Enterotoxigenic Escherichia coli-Induced Diarrhea in Mice. Microb. Pathog. 2023, 176, 105944. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D.; Sayaf, K.; Colognesi, M.; Zanotto, I.; Russo, F.; De Martin, S. Tyrosol Attenuates Hepatic Steatosis and Fibrosis in a Mouse Model of NASH. Dig. Liver Dis. 2023, 55, S66. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Boronat, A.; Fitó, M.; De la Torre, R. Effects of White Wine and Tyrosol on Circulating Ceramides in Individuals at Cardiovascular Risk. Curr. Dev. Nutr. 2021, 5, 365. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Muñoz, F.; Urvieta, R.; Buscema, F.; Rasse, M.; Fontana, A.; Berli, F. Phenolic Characterization of Cabernet Sauvignon Wines from Different Geographical Indications of Mendoza, Argentina: Effects of Plant Material and Environment. Front. Sustain. Food Syst. 2021, 5, 700642. [Google Scholar] [CrossRef]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive Compounds in Wine: Resveratrol, Hydroxytyrosol and Melatonin: A Review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Gil, C.; Gómez-Cordovés, C. Tryptophol Content of Young Wines Made from Tempranillo, Garnacha, Viura and Airén Grapes. Food Chem. 1986, 22, 59–65. [Google Scholar] [CrossRef]
- Marhuenda, J.; Villaño, D.; Arcusa, R.; Zafrilla, P. Melatonin in Wine and Beer: Beneficial Effects. Molecules 2021, 26, 343. [Google Scholar] [CrossRef]
- Albu, C.; Letitia, E.R.; Gabriel-Lucian, R. Assessment of Melatonin and Its Precursors Content by an HPLC-MS/MS Method from Different Romanian Wines. ACS Omega 2020, 5, 27254–27260. [Google Scholar] [CrossRef] [PubMed]
- Sagonas, I.; Daoussis, D. Serotonin and Systemic Sclerosis. An Emerging Player in Pathogenesis. Jt. Bone Spine 2022, 89, 105309. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Naranjo, M.I.; Gil-Izquierdo, A.; Troncoso, A.M.; Cantos, E.; Garcia-Parrilla, M.C. Melatonin: A New Bioactive Com-pound in Wine. J. Food Compos. Anal. 2011, 24, 603–608. [Google Scholar] [CrossRef]
- Freedman, B.J. Sulphur Dioxide in Foods and Beverages: Its Use as a Preservative and Its Effect on Asthma. Respir. Med. 1980, 74, 128–134. [Google Scholar] [CrossRef]
- Lyu, X.; Del Prado, D.R.; Araujo, L.D.; Quek, S.-Y.; Kilmartin, P.A. Effect of glutathione addition at harvest on Sauvignon Blanc wines. Aust. J. Grape Wine Res. 2021, 27, 431–441. [Google Scholar] [CrossRef]
- OIV. Resolutions OIV-OENO 445-2015. In Proceedings of the 13th OIV General Assembly. Available online: https://www.oiv.int/public/medias/1686/oiv-oeno-445-2015-en.pdf (accessed on 25 May 2023).
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on the safety and efficacy of vitamin C (ascorbic acid and sodium calcium ascorbyl phosphate) as a feed additive for all animal species based on a dossier submitted by VITAC EEIG. EFSA J. 2013, 11, 3103. [Google Scholar] [CrossRef]
- European Chemicals Agency. Ascorbic Acid. 2023. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.000.061 (accessed on 21 November 2023).
- OIV. Maximum Acceptable Limits. 2023. Available online: https://www.oiv.int/standards/international-code-of-oenological-practices/annexes/maximum-acceptable-limits (accessed on 21 November 2023).
- Abdullah, M.; Jamil, R.T.; Attia, F.N. Vitamin C (Ascorbic Acid). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Dienes-Nagy, Á.; Vuichard, F.; Belcher, S.; Blackford, M.; Rösti, J.; Lorenzini, F. Simultaneous Quantification of Glutathione, Glutathione Disulfide and Glutathione-S-Sulfonate in Grape and Wine Using LC-MS/MS. Food Chem. 2022, 386, 132756. [Google Scholar] [CrossRef] [PubMed]
- Kritzinger, E.C.; Bauer, F.F.; du Toit, W.J. Role of Glutathione in Winemaking: A Review. J. Agric. Food Chem. 2013, 61, 269–277. [Google Scholar] [CrossRef]
- Lambert-Royo, M.I.; Ubeda, C.; Del Barrio-Galán, R.; Sieczkowski, N.; Canals, J.M.; Peña-Neira, A.; Cortiella, M.G.I. The Diversity of Effects of Yeast Derivatives during Sparkling Wine Aging. Food Chem. 2022, 390, 133174. [Google Scholar] [CrossRef]
- Webber, V.; Dutra, S.V.; Spinelli, F.R.; Carnieli, G.J.; Cardozo, A.; Vanderlinde, R. Effect of Glutathione during Bottle Storage of Sparkling Wine. Food Chem. 2017, 216, 254–259. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Julien, P.; Coelho, C.; Roullier-Gall, C.; Ballester, J.; Schmitt-Kopplin, P.; Gougeon, R.D. Impact of Glutathione on Wines Oxidative Stability: A Combined Sensory and Metabolomic Study. Front. Chem. 2018, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.C.; Deed, R.C. The Chemical Reaction of Glutathione and Trans-2-Hexenal in Grape Juice Media to Form Wine Aroma Precursors: The Impact of pH, Temperature, and Sulfur Dioxide. J. Agric. Food Chem. 2018, 66, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Binati, R.L.; Larini, I.; Salvetti, E.; Torriani, S. Glutathione Production by Non-Saccharomyces Yeasts and Its Impact on Winemaking: A Review. Food Res. Int. 2022, 156, 111333. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, D.; Roussis, I.G. Inhibition of the decline of linalool and α-terpineol in muscat wines by glutathione and N-acetylcysteine. Ital. J. Food Sci. 2001, 13, 413–419. [Google Scholar]
- Tsai, P.-C.; Araujo, L.D.; Tian, B. Varietal Aromas of Sauvignon Blanc: Impact of Oxidation and Antioxidants Used in Winemaking. Fermentation 2022, 8, 686. [Google Scholar] [CrossRef]
- Weschawalit, S.; Thongthip, S.; Phutrakool, P.; Asawanonda, P. Glutathione and Its Antiaging and Antimelanogenic Effects. Clin. Cosmet. Investig. Dermatol. 2017, 10, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Xie, W.; Kim, K.-H.; Rao Dronamraju, V.; Williams, J.; Vince, R.; More, S.S. Dipeptide of ψ-GSH Inhibits Oxidative Stress and Neuroinflammation in an Alzheimer’s Disease Mouse Model. Antioxidants 2022, 11, 1075. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wu, L.; Liu, Q.; Tan, F.; Wang, L.; Zhao, D.; Fang, X.; Liu, X.; Liu, J.; Han, H. Glutathione Improves Testicular Spermatogenesis through Inhibiting Oxidative Stress, Mitochondrial Damage, and Apoptosis Induced by Copper Deposition in Mice with Wilson Disease. Biomed. Pharm. 2023, 158, 114107. [Google Scholar] [CrossRef]
- Zasada, M.; Budzisz, E. Retinoids: Active Molecules Influencing Skin Structure Formation in Cosmetic and Dermatological Treatments. Adv. Dermatol. Allergol. 2019, 36, 392–397. [Google Scholar] [CrossRef]
- Fernández-Pachón, M.S.; Villaño, D.; García-Parrilla, M.C.; Troncoso, A.M. Antioxidant Activity of Wines and Relation with Their Polyphenolic Composition. Anal. Chim. Acta 2004, 513, 113–118. [Google Scholar] [CrossRef]
- Rodrigo, R.; Prat, H.; Passalacqua, W.; Araya, J.; Bächler, J.P. Decrease in Oxidative Stress through Supplementation of Vitamins C and E Is Associated with a Reduction in Blood Pressure in Patients with Essential Hypertension. Clin. Sci. 2008, 114, 625–634. [Google Scholar] [CrossRef]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total Antioxidant Capacity as a Tool to Assess Redox Status: Critical View and Experimental Data. Free. Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Evers, M.S.; Roullier-Gall, C.; Morge, C.; Sparrow, C.; Gobert, A.; Alexandre, H. Vitamins in Wine: Which, What for, and How Much? Compr. Rev. Food Sci. Food Saf. 2021, 20, 2991–3035. [Google Scholar] [CrossRef] [PubMed]
- Evers, M.S.; Hervé, A.; Morge, C.; Sparrow, C.; Gobert, A.; Roullier-Gall, C. Exploring the Unexplored: A Characterization of Vitamins and Vitamers in White Grape Musts by High-Performance Liquid Chromatography. Food Chem. 2023, 398, 133860. [Google Scholar] [CrossRef] [PubMed]
- Grindlay, G.; Mora, J.; Gras, L.; de Loos-Vollebregt, M.T.C. Atomic Spectrometry Methods for Wine Analysis: A Critical Evaluation and Discussion of Recent Applications. Anal. Chim. Acta 2011, 691, 18–32. [Google Scholar] [CrossRef]
- Huang, X.-M.; Huang, H.-B.; Wang, H.-C. Cell Walls of Loosening Skin in Post-Veraison Grape Berries Lose Structural Polysaccharides and Calcium While Accumulate Structural Proteins. Sci. Hortic. 2005, 104, 249–263. [Google Scholar] [CrossRef]
- McCormick, J.A.; Topf, J.; Tomacruz, I.D.; Grimm, P.R. A New Understanding of Potassium’s Influence Upon Human Health and Renal Physiology. Adv. Kidney Dis. Health 2023, 30, 137–147. [Google Scholar] [CrossRef]
- Kocyigit, E.; Akturk, M.; Koksal, E. Relationships between Serum and Dietary Magnesium, Calcium, and Metabolic Parameters in Women with Type 2 Diabetes Mellitus. Clin. Nutr. ESPEN 2023, 54, 304–310. [Google Scholar] [CrossRef]
- Soriano-Pérez, L.; Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Pedraza-Chaverri, J. Magnesium and Type 2 Diabetes Mellitus: Clini-cal and Molecular Mechanisms. Health Sci. Rev. 2022, 4, 100043. [Google Scholar] [CrossRef]
- Siddiqui, M.; Pasha, A.; Kochar, K.; Junarta, J.; Fischman, D.L. Effect of high potassium diet on cardiovascular mortality: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2022, 79, 1606. [Google Scholar] [CrossRef]
- Brescia, M.A.; Caldarola, V.; De Giglio, A.; Benedetti, D.; Fanizzi, F.P.; Sacco, A. Characterization of the geographical origin of Italian red wines based on traditional and nuclear magnetic resonance spectrometric determinations. Anal. Chim. Acta 2002, 458, 177–186. [Google Scholar] [CrossRef]
- Jos, A.; Moreno, I.; González, A.G.; López-Artíguez, M.; Cameán, A.M. Study of the mineral profile of Catalonian “brut” cava using atomic spectrometric methods. Eur. Food Res. Technol. 2004, 218, 448–451. [Google Scholar] [CrossRef]
- Paneque, P.; Morales, M.L.; Burgos, P.; Ponce, L.; Callejón, R.M. Elemental Characterisation of Andalusian Wine Vinegars with Protected Designation of Origin by ICP-OES and Chemometric Approach. Food Control 2017, 75, 203–210. [Google Scholar] [CrossRef]
- Versari, A.; Laurie, V.F.; Ricci, A.; Laghi, L.; Parpinello, G.P. Progress in Authentication, Typification and Traceability of Grapes and Wines by Chemometric Approaches. Food Res. Int. 2014, 60, 2–18. [Google Scholar] [CrossRef]
- Shimizu, H.; Akamatsu, F.; Kamada, A.; Koyama, K.; Iwashita, K.; Goto-Yamamoto, N. Variation in the Mineral Composition of Wine Produced Using Different Winemaking Techniques. J. Biosci. Bioeng. 2020, 130, 166–172. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, C.; Trégoat, O.; Choné, X.; Bois, B.; Pernet, D.; Gaudillère, J.-P. Vine Water Status Is a Key Factor in Grape Ripening and Vintage Quality for Red Bordeaux Wine. How Can It Be Assessed for Vineyard Management Purposes? OENO One 2009, 43, 121. [Google Scholar] [CrossRef]
- Seguin, G. Terroirs’ and Pedology of Wine Growing. Experientia 1986, 42, 861–873. [Google Scholar] [CrossRef]
- Bramley, R.G.V.; Hamilton, R.P. Terroir and Precision Viticulture: Are They Compatible? OENO One 2007, 41, 1–8. [Google Scholar] [CrossRef]
- White, R.E.; Balachandra, L.; Edis, R.; Chen, D. The Soil Component of Terroir. OENO One 2007, 41, 9. [Google Scholar] [CrossRef]
- Tanaka, K.; Hamaguchi, Y.; Suzuki, S.; Enoki, S. Genomic Characterization of the Japanese Indigenous Wine Grape Vitis sp. cv. Koshu. Front. Plant Sci. 2020, 11, 532211. [Google Scholar] [CrossRef]
- Urvieta, R.; Jones, G.; Buscema, F.; Bottini, R.; Fontana, A. Terroir and vintage discrimination of Malbec wines based on phenolic composition across multiple sites in Mendoza, Argentina. Sci. Rep. 2021, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Prata-Sena, M.; Castro-Carvalho, B.M.; Nunes, S.; Amaral, B.; Silva, P. The terroir of Port wine: Two hundred and sixty years of history. Food Chem. 2018, 257, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Franco-Salas, A.; Peña-Fernández, A.; Valera-Martínez, D.L. Refrigeration Capacity and Effect of Ageing on the Operation of Cellulose Evaporative Cooling Pads, by Wind Tunnel Analysis. Int. J. Environ. Res. Public Health 2019, 16, 4690. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ma, Y.; Tian, X.; Li, J.-M.; Li, L.-X.; Tang, K.; Xu, Y. Chemosensory characteristics of regional Vidal icewines from China and Canada. Food Chem. 2018, 261, 66–74. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, I. Italy’s Native Wine Grape Terroirs, 1st ed.; University of California Press: Oakland, CA, USA, 2019. [Google Scholar]
- Priori, S.; Barbetti, R.; L’Abate, G.; Bucelli, P.; Storchi, P.; Costantini, E. Natural terroir units, Siena province, Tuscany. J. Maps 2014, 10, 466–477. [Google Scholar] [CrossRef]
- Priori, S.; Pellegrini, S.; Perria, R.; Puccioni, S.; Storchi, P.; Valboa, G.; Costantini, E.A. Scale effect of terroir under three contrasting vintages in the Chianti Classico area (Tuscany, Italy). Geoderma 2019, 334, 99–112. [Google Scholar] [CrossRef]
- Ghilardi, F.; Virano, A.; Prandi, M.; Borgogno-Mondino, E. Zonation of a Viticultural Territorial Context in Piemonte (NW Italy) to Support Terroir Identification: The Role of Pedological, Topographical and Climatic Factors. Land 2023, 12, 647. [Google Scholar] [CrossRef]
- Basso, M. Land-use changes triggered by the expansion of wine-growing areas: A study on the Municipalities in the Prosecco’s production zone (Italy). Land Use Policy 2019, 83, 390–402. [Google Scholar] [CrossRef]
- Tomasi, D.; Gaiotti, F.; Jones, G.V. The Power of the Terroir: The Case Study of Prosecco Wine; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Matus, J.T. Transcriptomic and Metabolomic Networks in the Grape Berry Illustrate That it Takes More Than Flavonoids to Fight Against Ultraviolet Radiation. Front. Plant. Sci. 2016, 7, 1337. [Google Scholar] [CrossRef]
- Schmidlin, L.; Poutaraud, A.; Claudel, P.; Mestre, P.; Prado, E.; Santos-Rosa, M.; Wiedemann-Merdinoglu, S.; Karst, F.; Merdinoglu, D.; Hugueney, P. A stress-inducible resveratrol o-methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiol. 2008, 148, 1630–1639. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Saucier, C.; Glories, Y. Grape and Wine Phenolics: History and Perspective. Am. J. Enol. Vitic. 2006, 57, 239–248. [Google Scholar] [CrossRef]
- Gerós, H.; Chaves, M.M.; Gil, H.M.; Delrot, S. Grapevine in a Changing Environment: A Molecular and Ecophysiological Perspective; John Wiley and Sons: Hoboken, NJ, USA, 2015; Available online: https://books.google.pt/books?id=ZCjvCQAAQBAJ (accessed on 4 September 2023).
- Pretty, J.N.; Brett, C.; Gee, D.; Hine, R.E.; Mason, C.F.; Morison, J.I.L.; Raven, H.; Rayment, M.D.; van der Bijl, G. An Assessment of the Total External Costs of UK Agriculture. Agric. Syst. 2000, 65, 113–136. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Roby, J.-P.; De Rességuier, L. Soil-Related Terroir Factors: A Review. OENO One 2018, 52, 173–188. [Google Scholar] [CrossRef]
- Sasaki, K.; Takase, H.; Matsuyama, S.; Kobayashi, H.; Matsuo, H.; Ikoma, G.; Takata, R. Effect of light exposure on linalool biosynthesis and accumulation in grape berries. Biosci. Biotechnol. Biochem. 2016, 80, 2376–2382. [Google Scholar] [CrossRef]
- Osrečak, M.; Karoglan, M.; Kozina, B. Influence of leaf removal and reflective mulch on phenolic composition and antioxidant activity of Merlot, Teran and Plavac mali wines (Vitis vinifera L.). Sci. Hortic. 2016, 209, 261–269. [Google Scholar] [CrossRef]
- Karaś, K.; Zioła-Frankowska, A.; Frankowski, M. Chemical speciation of aluminum in wine by LC–ICP–MS. Molecules 2020, 25, 1069. [Google Scholar] [CrossRef]
- Pinillos, V.; Chiamolera, F.M.; Ortiz, J.F.; Hueso, J.J.; Cuevas, J. Post-veraison regulated deficit irrigation in ‘Crimson Seedless’ table grape saves water and improves berry skin color. Agric. Water Manag. 2016, 165, 181–189. [Google Scholar] [CrossRef]
- Lang, C.P.; Merkt, N.; Klaiber, I.; Pfannstiel, J.; Zörb, C. Different forms of nitrogen application affect metabolite patterns in grapevine leaves and the sensory of wine. Plant Physiol. Biochem. 2019, 143, 308–319. [Google Scholar] [CrossRef]
- Wang, R.; Qi, Y.; Wu, J.; Shukla, M.K.; Sun, Q. Influence of the application of irrigated water-soluble calcium fertilizer on wine grape properties. PLoS ONE 2019, 14, e0222104. [Google Scholar] [CrossRef]
- Keller, M. The Science of Grapevines; Anatomy and Physiology; Elsevier Science; Academic press: Cambridge, MA, USA, 2010; Available online: https://books.google.pt/books?id=OzznbpjccZMC (accessed on 4 July 2023).
- Trégoat, O.; Gaudillère, J.-P.; Choné, X.; van Leeuwen, C. The Assessment of Vine Water and Nitrogen Uptake by Means of Physiological Indicators Influence on Vine Development and Berry Potential (Vitis vinifera L. Cv Merlot, 2000, Bordeaux). OENO One 2002, 36, 133. [Google Scholar] [CrossRef]
- Naselli, V.; Prestianni, R.; Badalamenti, N.; Matraxia, M.; Maggio, A.; Alfonzo, A.; Gaglio, R.; Vagnoli, P.; Settanni, L.; Bruno, M.; et al. Improving the Aromatic Profiles of Catarratto Wines: Impact of Metschnikowia pulcherrima and Glutathione-Rich Inactivated Yeasts. Antioxidants 2023, 12, 439. [Google Scholar] [CrossRef]
- Choné, X.; Lavigne-Cruège, V.; Tominaga, T.; Van Leeuwen, C.; Castagnède, C.; Saucier, C.; Dubourdieu, D. Effect of Vine Ni-trogen Status on Grape Aromatic Potential: Flavor Precursors (S-Cysteine Conjugates), Glutathione and Phenolic Content in Vitis vinifera L. Cv Sauvignon Blanc Grape Juice. OENO One 2006, 40, 1. [Google Scholar] [CrossRef]
- Helwi, P.; Guillaumie, S.; Thibon, C.; Keime, C.; Habran, A.; Hilbert, G.; Gomes, E.; Darriet, P.; Delrot, S.; van Leeuwen, C. Vine Nitrogen Status and Volatile Thiols and Their Precursors from Plot to Transcriptome Level. BMC Plant Biol. 2016, 16, 173. [Google Scholar] [CrossRef]
- Mundy, D.C. A Review of the Direct and Indirect Effects of Nitrogen on Botrytis Bunch Rot in Wine Grapes. New Zealand Plant Prot. 2008, 61, 306–310. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Dinis, L.-T.; Bernardo, S.; Conde, A.; Pimentel, D.; Ferreira, H.; Félix, L.; Gerós, H.; Correia, C.M.; Moutinho-Pereira, J. Kaolin Exogenous Application Boosts Antioxidant Capacity and Phenolic Content in Berries and Leaves of Grapevine under Summer Stress. J. Plant Physiol. 2016, 191, 45–53. [Google Scholar] [CrossRef]
- Cortell, J.M.; Kennedy, J.A. Effect of Shading on Accumulation of Flavonoid Compounds in (Vitis vinifera L.) Pinot Noir Fruit and Extraction in a Model System. J. Agric. Food Chem. 2006, 54, 8510–8520. [Google Scholar] [CrossRef]
- Dinis, L.-T.; Bernardo, S.; Matos, C.; Malheiro, A.; Flores, R.; Alves, S.; Costa, C.; Rocha, S.; Correia, C.; Luzio, A.; et al. Overview of Kaolin Outcomes from Vine to Wine: Cerceal White Variety Case Study. Agronomy 2020, 10, 1422. [Google Scholar] [CrossRef]
- Moran, W. Terroir—The human factor. Aust. NZ Wine Ind. J. 2001, 16, 32–51. [Google Scholar]
- Maltman, A. Minerality in Wine: A Geological Perspective. J. Wine Res. 2013, 24, 169–181. [Google Scholar] [CrossRef]
- Morris, J.R.; Sims, C.A.; Cawthon, D.L. Effects of Excessive Potassium Levels On PH, Acidity and Color of Fresh and Stored Grape Juice. Am. J. Enol. Vitic. 1983, 34, 35–39. [Google Scholar] [CrossRef]
- Soyer, J.P.; Molot, C. Fertilisation potassique et composition des moûts; Evolution durant la maturation du raisin. Prog. Agric. Vitic. 1993, 110, 174–177. [Google Scholar]
- van Leeuwen, C. Terroir: The Effect of the Physical Environment on Vine Growth, Grape Ripening and Wine Sensory Attributes. In Managing Wine Quality; Elsevier: Amsterdam, The Netherlands, 2010; pp. 273–315. [Google Scholar] [CrossRef]
- Goode, J. Wine Science: The Application of Science in Winemaking; Mitchell Beazley: London, UK, 2014; Available online: https://books.google.pt/books?id=RYoKngEACAAJ (accessed on 4 September 2023).
- Robinson, J.; Harding, J.; Vouillamoz, J. Wine Grapes: A Complete Guide to 1368 Vine Varieties, Including Their Origins and Flavours; Penguin Books Ltd.: London, UK, 2013; Available online: https://books.google.pt/books?id=YGTnD2wGn94C (accessed on 5 June 2023).
- Robinson, J.; Harding, J. The Oxford Companion to Wine; Oxford University Press: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Friedel, M.; Frotscher, J.; Nitsch, M.; Hofmann, M.; Bogs, J.; Stoll, M.; Dietrich, H. Light Promotes Expression of Monoterpene and Flavonol Metabolic Genes and Enhances Flavour of Winegrape Berries (Vitis vinifera L. Cv. Riesling). Aust. J. Grape Wine Res. 2016, 22, 409–421. [Google Scholar] [CrossRef]
- Johnson, H.; Robinson, J. The World Atlas of Wine, 7th ed.; Octopus Books: Ottawa, ON, Canada, 2013; Available online: https://books.google.pt/books?id=0AXrwQEACAAJ (accessed on 5 June 2023).
- Marques, C.; Correia, E.; Dinis, L.-T.; Vilela, A. An overview of sensory characterization techniques: From classical descriptive analysis to the emergence of novel profiling methods. Foods 2022, 11, 255. [Google Scholar] [CrossRef]
- Gonzaga, L.; Capone, D.L.; Bastian, S.E.P.; Jeffery, D.W. Defining Wine Typicity: Sensory Characterisation and Consumer Perspectives. Aust. J. Grape Wine Res. 2021, 27, 246–256. [Google Scholar] [CrossRef]
- King, E.S.; Stoumen, M.; Buscema, F.; Hjelmeland, A.K.; Ebeler, S.E.; Heymann, H.; Boulton, R.B. Regional Sensory and Chemical Characteristics of Malbec Wines from Mendoza and California. Food Chem. 2014, 143, 256–267. [Google Scholar] [CrossRef]
- Geffroy, O.; Buissière, C.; Lempereur, V.; Chatelet, V. A Sensory, Chemical and Consumer Study of the Peppery Typicality of French Gamay Wines from Cool-Climate Vineyards. OENO One 2016, 50, 35. [Google Scholar] [CrossRef]
- Kustos, M.; Gambetta, J.M.; Jeffery, D.W.; Heymann, H.; Goodman, S.; Bastian, S.E.P. A Matter of Place: Sensory and Chemical Characterization of Fine Australian Chardonnay and Shiraz Wines of Provenance. Food Res. Int. 2020, 130, 108903. [Google Scholar] [CrossRef]
- Muñoz-González, C.; Feron, G.; Canon, F. Main Effects of Human Saliva on Flavour Perception and the Potential Contribution to Food Consumption. Proc. Nutr. Soc. 2018, 77, 423–431. [Google Scholar] [CrossRef]
- Duarte-Coimbra, S.; Forcina, G.; Pérez-Pardal, L.; Beja-Pereira, A. Characterization of Tongue Dorsum Microbiome in Wine Tas-ters. Food Res. Int. 2023, 163, 112259. [Google Scholar] [CrossRef]
- Martina, E.; Campanati, A.; Diotallevi, F.; Offidani, A. Saliva and Oral Diseases. J. Clin. Med. 2020, 9, 466. [Google Scholar] [CrossRef]
- Schwartz, M.; Neiers, F.; Feron, G.; Canon, F. The Relationship between Salivary Redox, Diet, and Food Flavor Perception. Fron-Tiers Nutr. 2021, 7, 612735. [Google Scholar] [CrossRef]
- Genovese, A.; Piombino, P.; Gambuti, A.; Moio, L. Simulation of Retronasal Aroma of White and Red Wine in a Model Mouth System. Investigating the Influence of Saliva on Volatile Compound Concentrations. Food Chem. 2009, 114, 100–107. [Google Scholar] [CrossRef]
- Mosca, A.C.; Chen, J. Food-Saliva Interactions: Mechanisms and Implications. Trends Food Sci. Technol. 2017, 66, 125–134. [Google Scholar] [CrossRef]
- Kubala, E.; Strzelecka, P.; Grzegocka, M.; Lietz-Kijak, D.; Gronwald, H.; Skomro, P.; Kijak, E. A Review of Selected Studies That Determine the Physical and Chemical Properties of Saliva in the Field of Dental Treatment. BioMed Res. Int. 2018, 2018, 6572381. [Google Scholar] [CrossRef]
- Schwartz, M.; Canon, F.; Feron, G.; Neiers, F.; Gamero, A. Impact of Oral Microbiota on Flavor Perception: From Food Processing to in-mouth Metabolization. Foods 2021, 10, 2006. [Google Scholar] [CrossRef]
- Gittings, S.; Turnbull, N.; Henry, B.; Roberts, C.J.; Gershkovich, P. Characterisation of Human Saliva as a Platform for Oral Dissolution Medium Development. Eur. J. Pharm. Biopharm. 2015, 91, 16–24. [Google Scholar] [CrossRef]
- Boehm, M.W.; Yakubov, G.E.; Stokes, J.R.; Baier, S.K. The Role of Saliva in Oral Processing: Reconsidering the Breakdown Path Paradigm. J. Text. Stud. 2020, 51, 67–77. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Espínola-Espínola, V.; López-Solís, R. Wine PH Prevails over Buffering Capacity of Human Saliva. J. Agric. Food Chem. 2016, 64, 8154–8159. [Google Scholar] [CrossRef]
- Liu, T.-T.; Liu, X.-T.; Chen, Q.-X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef]
- Santos, M.J.; Correia, E.; Vilela, A. Exploring the Impact of α-Amylase Enzyme Activity and pH on Flavor Perception of Alcohol-ic Drinks. Foods 2023, 12, 1018. [Google Scholar] [CrossRef]
- Morquecho-Campos, P.; Bikker, F.J.; Nazmi, K.; de Graaf, K.; Laine, M.L.; Boesveldt, S. Impact of Food Odors Signaling Specific Taste Qualities and Macronutrient Content on Saliva Secretion and Composition. Appetite 2019, 143, 104399. [Google Scholar] [CrossRef]
- Ali, N.; Nater, U.M. Salivary Alpha-Amylase as a Biomarker of Stress in Behavioral Medicine. Int. J. Behav. Med. 2020, 27, 337–342. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.D.; Mateo, S.V.; Tecles, F.; Hirtz, C.; Escribano, D.; Cerón, J.J. Changes Occurring on the Activity of Salivary Alpha-Amylase Proteoforms in Two Naturalistic Situations Using a Spectrophotometric Assay. Biology 2021, 10, 227. [Google Scholar] [CrossRef]
- Ployon, S.; Morzel, M.; Belloir, C.; Bonnotte, A.; Bourillot, E.; Briand, L.; Lesniewska, E.; Lherminier, J.; Aybeke, E.; Canon, F. Mechanisms of Astringency: Structural Alteration of the Oral Mucosal Pellicle by Dietary Tannins and Protective Effect of BPRPs. Food Chem. 2018, 253, 79–87. [Google Scholar] [CrossRef]
- Contreras-Aguilar, M.D.; Escribano, D.; Martínez-Subiela, S.; Martínez-Miró, S.; Rubio, M.; Tvarijonaviciute, A.; Tecles, F.; Cerón, J.J. Influence of the Way of Reporting Alpha-Amylase Values in Saliva in Different Naturalistic Situations: A Pilot Study. PLoS ONE 2017, 12, e0180100. [Google Scholar] [CrossRef]
- Canon, F.; Neiers, F.; Guichard, E. Saliva and Flavor Perception: Perspectives. J. Agric. Food Chem. 2018, 66, 7873–7879. [Google Scholar] [CrossRef]
- Jacob, J.J.; Suthindhiran, K. Immobilisation of Lipase Enzyme onto Bacterial Magnetosomes for Stain Removal. Biotechnol. Rep. 2020, 25, e00422. [Google Scholar] [CrossRef]
- Gordon, R.; Power, A.; Chapman, J.; Chandra, S.; Cozzolino, D. A Review on the Source of Lipids and Their Interactions during Beer Fermentation That Affect Beer Quality. Fermentation 2018, 4, 89. [Google Scholar] [CrossRef]
- Restrepo, S.; Espinoza, L.; Ceballos, A.; Urtubia, A. Production of Fatty Acids during Alcoholic Wine Fermentation under selected temperature and Aeration Conditions. Am. J. Enol. Vitic. 2019, 70, 169–176. [Google Scholar] [CrossRef]
- Schumaker, M.R.; Diako, C.; Castura, J.C.; Edwards, C.G.; Ross, C.F. Influence of Wine Composition on Consumer Perception and Acceptance of Brettanomyces Metabolites Using Temporal Check-All-That-Apply Methodology. Food Res. Int. 2019, 116, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Rabe, A.; Salazar, M.G.; Michalik, S.; Fuchs, S.; Welk, A.; Kocher, T.; Völker, U. Metaproteomics Analysis of Microbial Diversity of Human Saliva and Tongue Dorsum in Young Healthy Individuals. J. Oral Microbiol. 2019, 11, 1654786. [Google Scholar] [CrossRef] [PubMed]
- Rud, I.; Almli, V.L.; Berget, I.; Tzimorotas, D.; Varela, P. Taste Perception and Oral Microbiota: Recent Advances and Future Perspectives. Curr. Opin. Food Sci. 2023, 51, 101030. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Zorraquín-Peña, I.; González de Llano, D.; Bartolomé, B.; Moreno-Arribas, M.V. The Role of Wine and Food Polyphenols in Oral Health. Trends Food Sci. Technol. 2017, 69, 118–130. [Google Scholar] [CrossRef]
- Le Roy, C.I.; Wells, P.M.; Si, J.; Raes, J.; Bell, J.T.; Spector, T.D. Red Wine Consumption Associated with Increased Gut Microbiota α-Diversity in 3 Independent Cohorts. Gastroenterology 2020, 158, 270–272.e2. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients, and the consumer: A review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different Phenolic Compounds Activate Distinct Hu-man Bitter Taste Receptors. J. Agric. Food Chem. 2013, 61, 1525–1533. [Google Scholar] [CrossRef]
- Wooding, S.P.; Ramirez, V.A. Global Population Genetics and Diversity in the TAS2R Bitter Taste Receptor Family. Front. Genet. 2022, 13, 952299. [Google Scholar] [CrossRef]
- Wooding, S.P.; Ramirez, V.A.; Behrens, M. Bitter taste receptors: Genes, evolution and health. Evol. Med. Public Health 2021, 9, 431–447. [Google Scholar] [CrossRef]
- Temussi, P.A. The good taste of peptides. Pept. Sci. 2012, 18, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, S.; Kari, N.M.; Ismail, N.; Ismail, M.N.; Ahmad, F. Evaluation of taste active peptides and amino acids from anchovy proteins in fish sauce by in silico approach. Food Sci. Biotechnol. 2022, 31, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Bassoli, A.; Borgonovo, G.; Caremoli, F.; Mancuso, G. The Taste of D- and L-Amino Acids: In Vitro Binding Assays with Cloned Human Bitter (TAS2Rs) and Sweet (TAS1R2/TAS1R3) Receptors. Food Chem. 2014, 150, 27–33. [Google Scholar] [CrossRef] [PubMed]
- DuBoi, G.E. Molecular mechanism of sweetness sensation. Physiol. Behav. 2016, 164, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Von Molitor, E.; Riedel, K.; Krohn, M.; Hafner, M.; Rudolf, R.; Cesetti, T. Sweet Taste Is Complex: Signaling Cascades and Circuits Involved in Sweet Sensation. Front. Hum. Neurosci. 2021, 15, 667709. [Google Scholar] [CrossRef] [PubMed]
- Ömür-Özbek, P.; Dietrich, A.M.; Duncan, S.E.; Lee, Y.W. Role of Lipid Oxidation, Chelating Agents, and Antioxidants in Metallic Flavor Development in the Oral Cavity. J. Agric. Food Chem. 2012, 60, 2274–2280. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Tsao, R.; Li, Y.; Miao, M. Food Safety: Food Analysis Technologies/Techniques. In Encyclopedia of Agriculture and Food Systems; Academic Press: Cambridge, MA, USA, 2014; pp. 273–288. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zheng, Y.; Xia, M.L.; Wu, Y.N.; Liu, X.J.; Xie, S.K.; Wu, Y.F.; Wang, M. Knowledge Domain and Emerging Trends in Vinegar Research: A Bibliometric Review of the Literature from WOSCC. Foods 2020, 9, 166. [Google Scholar] [CrossRef]
- Chanioti, S.; Liadakis, G.; Tzia, C. Solid–Liquid Extraction. In Food Engineering Handbook; Varzakas, T., Tzia, C., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 253–286. [Google Scholar] [CrossRef]
- Rayess, Y.E.; Barakat, N.; Azzi-Achkouty, S. A Review of Polyphenols Extraction Technologies from Grapes and Byproducts. In Vitis Products: Composition, Health Benefits and Economic Valorization, 1st ed.; Botelho, R., Jordão, A., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2021; pp. 305–327. [Google Scholar]
- Marín, R.; Mejías, C.R.; García Moreno, M.V.; Rowe, F.G.; Barroso, C.G. Headspace solid-phase microextraction analysis of aroma compounds in vinegar. J. Chromatogr. A 2002, 967, 261–267. [Google Scholar] [CrossRef]
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Applications of Solid-Phase Microextraction in Food Analysis. J. Chromatogr. A 2000, 880, 35–62. [Google Scholar] [CrossRef]
- Chakka, A.K.; Babu, A.S. Bioactive Compounds of Winery Byproducts: Extraction Techniques and Their Potential Health Benefits. Appl. Food Res. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Vicaş, S.I.; Bandici, L.; Teuşdea, A.C.; Turcin, V.; Popa, D.; Bandici, G.E. The Bioactive Compounds, Antioxidant Capacity, and Color Intensity in Must and Wines Derived from Grapes Processed by Pulsed Electric Field. CyTA—J. Food 2017, 15, 553–562. [Google Scholar] [CrossRef]
- Coelho, M.C.; Ghalamara, S.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Innovation and Winemaking By-Product Valor-ization: An Ohmic Heating Approach. Processes 2023, 11, 495. [Google Scholar] [CrossRef]
- Camargo, A.C.; Regitano-d’Arce, M.A.B.; Biasoto, A.V.T.; Shahidi, F. Enzyme-Assisted Extraction of Phenolics from Winemaking byproducts: Antioxidant Potential and Inhibition of Alpha-Glucosidase and Lipase Activities. Food Chem. 2016, 212, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.S. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, A.; Smith, F.J. (Eds.) Chromatographic Methods; Springer: Dordrecht, The Netherlands, 1999. [Google Scholar] [CrossRef]
- Ettre, L.S. Chromatography: The Separation Technique of the 20th Century. Chromatographia 2000, 51, 7–17. [Google Scholar] [CrossRef]
- Holčapek, M.; Byrdwel, W.C. (Eds.) Handbook of Advanced Chromatography/Mass Spectrometry Techniques; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Seader, J.D.; Henley, E.J.; Rope, D.K. (Eds.) Separation Process Principles: Chemical and Biochemical Operations, 3rd ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Catani, M.; Felletti, S.; Franchina, A.F. Separation Techniques. In Metabolomics Perspectives: From Theory to Practical Application; Troisi, J., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 63–108. [Google Scholar] [CrossRef]
- Coskun, O. Separation Techniques: Chromatography. North Clin. Istanbul 2016, 3, 156–160. [Google Scholar] [CrossRef]
- Fekete, S.; Schappler, J.; Veuthey, J.L.; Guillaume, D. Current and Future Trends in UHPLC. Trends Anal. Chem. 2014, 63, 2–13. [Google Scholar] [CrossRef]
- François, I.; Sandra, K.; Sandra, P. Comprehensive Liquid Chromatography: Fundamental Aspects and Practical Considerations—A Review. Anal. Chim. Acta 2009, 641, 14–31. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Ferreira, I.C.F.R.; Carvalho, A.M.; Santos-Buelga, C. Use of HPLC–DAD–ESI/MS to Profile Phenolic Com-pounds in Edible Wild Greens from Portugal. Food Chem. 2011, 127, 169–173. [Google Scholar] [CrossRef]
- Petrova, O.E.; Karin, S. High-Performance Liquid Chromatography (HPLC)-Based Detection and Quantitation of Cellular c-Di-GMP. In c-di-GMP Signaling; Methods in Molecular Biology; Sauer, K., Ed.; Humana Press: New York, NY, USA, 2017; Volume 1657, pp. 33–43. [Google Scholar] [CrossRef]
- Guillaume, D.; Jean-Luc, V. Theory and Practice of UHPLC and UHPLC–MS. In Handbook of Advanced Chromatography/Mass Spectrometry Techniques; AOCS Press: Urbana, IL, USA, 2017; pp. 1–38. [Google Scholar] [CrossRef]
- Lukić, I.; Carlin, S.; Vrhovsek, U. Utility of Comprehensive GC×GC Gas Chromatography in Finding Varietal Markers among Volatile Compounds in Non-Aromatic Red Wines. Agronomy 2022, 12, 2512. [Google Scholar] [CrossRef]
- Dahman, Y. Generic Methodologies for Characterization. In Nanotechnology and Functional Materials for Engineers; Dahman, Y., Halim, C.M., Chan, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 19–45. [Google Scholar]
- Hofmann, A.; Clokie, S. Wilson and Walker’s Principles and Techniques of Biochemistry and Molecular Biology, 8th ed.; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Du Toit, W. The Role of UV-Visible Spectroscopy for Phenolic Compounds Quantification in Winemak-ing. In Frontiers and New Trends in the Science of Fermented Food and Beverages; Solís-Oviedo, R.L., de la Cruz Pech-Canul, Á., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Peak, D. Fourier Transform Infrared Spectroscopy. In Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; pp. 80–85. [Google Scholar] [CrossRef]
- Ríos-Reina, R.; Callejón, R.M.; Oliver-Pozo, C.; Amigo, J.M.; García-González, D.L. ATR-FTIR as a Potential Tool for Controlling High Quality Vinegar Categories. Food Control 2017, 78, 230–237. [Google Scholar] [CrossRef]
- Hassoun, A.; Måge, I.; Schmidt, W.F.; Temiz, H.T.; Li, L.; Kim, H.Y.; Nilsen, H.; Biancolillo, A.; Aït-Kaddour, A.; Sikorski, M.; et al. Fraud in Animal Origin Food Products: Ad-vances in Emerging Spectroscopic Detection Methods over the Past Five Years. Foods 2020, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.L.; Schmidt-Rohr, K. Quantitative Solid-State 13C NMR with Signal Enhancement by Multiple Cross Polarization. J. Magn. Reson. 2014, 239, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Singh, A. Nuclear Magnetic Resonance Spectroscopy. In Characterization of Polymers and Fibres; Elsevier: Amsterdam, The Netherlands, 2022; pp. 321–339. [Google Scholar] [CrossRef]
- Viskić, M.; Bandić, L.M.; Korenika, A.-M.J.; Jeromel, A. NMR in the Service of Wine Differentiation. Foods 2021, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Bumbrah, G.S.; Sharma, R.M. Raman Spectroscopy—Basic Principle, Instrumentation and Selected Applications for the Characterization of Drugs of Abuse. Egypt. J. Forensic. Sci. 2016, 6, 209–215. [Google Scholar] [CrossRef]
- Clarke, S.; Bosman, G.; du Toit, W.; Aleixandre-Tudo, J.L. White Wine Phenolics: Current Methods of Analysis. J. Sci. Food Agric. 2023, 103, 7–25. [Google Scholar] [CrossRef]
- Ember, K.J.; Hoeve, M.A.; McAughtrie, S.L.; Bergholt, M.S.; Dwyer, B.J.; Stevens, M.M.; Faulds, K.; Forbes, S.J.; Campbell, C.J. Raman spectroscopy and regenerative medicine: A review. NPJ Regen. Med. 2017, 2, 12. [Google Scholar] [CrossRef]
- Mellon, F.A. Mass Spectrometry|Principles and Instrumentation. In Encyclopedia of Food Sciences and Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 3739–3749. [Google Scholar] [CrossRef]
- Sleeman, R.; Carter, J.F. Mass Spectrometry|Overview. In Encyclopedia of Analytical Science, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 337–344. [Google Scholar] [CrossRef]
- Waddell, S.R. Mass Spectrometry. In Encyclopedia of Forensic Sciences, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 603–608. [Google Scholar] [CrossRef]
- Stauffer, E.; Dolan, J.A.; Newman, R. Gas Chromatography and Gas Chromatography—Mass Spectrometry. In Fire Debris Analysis; Academic Press: Cambridge, MA, USA, 2008; pp. 235–293. [Google Scholar] [CrossRef]
- Sugai, T. Mass and Charge Measurements on Heavy Ions. Mass Spectrom. 2017, 6, S0074. [Google Scholar] [CrossRef]
- Urban, P.L. Quantitative Mass Spectrometry: An Overview. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150382. [Google Scholar] [CrossRef]
- Xavier, M.; de Oliveira, T.; Portugal, I.B.M.; Padilha, C.V.S.; Padilha, F.F.; dos Santos Lima, M. New Trends in the Use of Enzymes for the Recovery of Polyphenols in Grape Byproducts. J. Food Biochem. 2021, 45, e13712. [Google Scholar] [CrossRef]
- Chailapakul, O.; Siangproh, W.; Jampasa, S.; Chaiyo, S.; Teengam, P.; Yakoh, A.; Pinyorospathum, C. Paper-Based Sensors for the Application of Biological Compound Detection. Comp. Anal Chem. 2020, 89, 31–62. [Google Scholar] [CrossRef]
- Alhajj, M.; Farhana, A. Enzyme-Linked Immunosorbent Assay. 2023. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25908411 (accessed on 4 September 2023).
- Han, S.; Yang, J.; Choi, K.; Kim, J.; Adhikari, K.; Lee, J. Chemical Analysis of Commercial White Wines and Its Relationship with Consumer Acceptability. Foods 2022, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Stucki, A.O.; Barton-Maclaren, T.S.; Bhuller, Y.; Henriquez, J.E.; Henry, T.R.; Hirn, C.; Miller-Holt, J.; Nagy, E.G.; Perron, M.M.; Ratzlaff, D.E.; et al. Use of New Approach Methodologies (NAMs) to Meet Regulatory Requirements for the Assessment of Industrial Chemicals and Pesticides for Effects on Human Health. Front. Toxicol. 2022, 4, 964553. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.B. Application of UV–VIS Spectrophotometry for Chemical Analysis. In Chemical Analysis and Material Characterization by Spectrophotometry; Elsevier: New York, NY, USA, 2020; pp. 79–145. [Google Scholar] [CrossRef]
- Olszowy, M.; Dawidowicz, A.L. Is It Possible to Use the DPPH and ABTS Methods for Reliable Estimation of Antioxidant Pow-er of Colored Compounds? Chem. Pap. 2018, 72, 393–400. [Google Scholar] [CrossRef]
- Gusti, A.M.T.; Qusti, S.Y.; Alshammari, E.M.; Toraih, E.A.; Fawzy, M.S. Antioxidants-Related Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), Glutathione-S-Transferase (GST), and Nitric Oxide Synthase (NOS) Gene Variants Analysis in an Obese Population: A Preliminary Case-Control Study. Antioxidants 2021, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Siddique, T.; Deng, H.X.; Ajroud-Driss, S. Motor Neuron Disease. In Emery and Rimoin’s Principles and Practice of Medical Genetics, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–22. [Google Scholar] [CrossRef]
- Tekos, F.; Makri, S.; Skaperda, Z.V.; Patouna, A.; Terizi, K.; Kyriazis, I.D.; Kotseridis, Y.; Mikropoulou, E.V.; Papaefstathiou, G.; Halabalaki, M.; et al. Assessment of Antioxidant and Anti-mutagenic Properties of Red and White Wine Extracts In Vitro. Metabolites 2021, 11, 436. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Ortiz, R.; Antilén, M.; Speisky, H.; Aliaga, M.E.; López-Alarcón, C.; Baugh, S. Application of a Microplate-Based ORAC-Pyrogallol Red Assay for the Estimation of Antioxidant Capacity: First Action 2012.03. J. AOAC Int. 2012, 95, 1558–1561. [Google Scholar] [CrossRef]
- Danilewicz, J.C. Folin-Ciocalteu, FRAP, and DPPH Assays for Measuring Polyphenol Concentration in White Wine. Am. J. Enol. Vitic. 2015, 66, 463–471. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Tang, K.; Rao, Z.; Chen, J. Effects of Different Aging Methods on the Phenolic Compounds and Antioxidant Activity of Red Wine. Fermentation 2022, 8, 592. [Google Scholar] [CrossRef]
- Dávalos, A.; Bartolomé, B.; Gómez-Cordovés, C. Antioxidant Properties of Commercial Grape Juices and Vinegars. Food Chem. 2005, 93, 325–330. [Google Scholar] [CrossRef]
- Haque, M.A.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Electrochemical Methods to Evaluate the Antioxidant Activity and Capacity of Foods: A Review. Electroanalysis 2021, 33, 1419–1435. [Google Scholar] [CrossRef]
- Hernanz-Vila, D.; Jara-Palacios, M.J.; Escudero-Gilete, M.L.; Heredia, F.J. Applications of Voltammetric Analysis to Wine Products. In Applications of the Voltammetry; InTechOpen: London, UK, 2017; Volume 109. [Google Scholar] [CrossRef]
- Datta, S.; Kanjilal, B.; Sarkar, P. Electrochemical Sensor for Detection of Polyphenols in Tea and Wine with Differential Pulse Voltammetry and Electrochemical Impedance Spectroscopy Utilizing Tyrosinase and Gold Nanoparticles Decorated Biomembrane. J. Electrochem. Soc. 2017, 164, B118–B126. [Google Scholar] [CrossRef]
- Gomes, S.A.S.S.; Nogueira, J.M.F.; Rebelo, M.J.F. An Amperometric Biosensor for Polyphenolic Compounds in Red Wine. Biosens. Bioelectron. 2004, 20, 1211–1216. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.A.; Tu-Maung, N.; Cheng, K.; Wang, B.; Baeumner, A.J.; Kraft, C.E. Thiamine Assays—Advances, Challenges, and Ca-veats. ChemistryOpen 2017, 6, 178–191. [Google Scholar] [CrossRef]
- Ward, R.E.; Legako, J.F. Traditional Methods for Mineral Analysis. In Food Analysis; Nielsen, S.S., Ed.; Food Science Text Series; Springer: Berlin/Heidelberg, Germany, 2017; pp. 371–386. [Google Scholar] [CrossRef]
- Buldini, P.L.; Silvano, C.; Sharma, J.L. Determination of Transition Metals in Wine by IC, DPASV-DPCSV, and ZGFAAS Coupled with UV Photolysis. J. Agric. Food Chem. 1999, 47, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcon, M.; Velasco, C.; Jodral, A.; Terrés, C.; Olalla, M.; Lopez, H.; Lopez, M.C. Copper, Zinc, Calcium and Magnesium Content of Alcoholic Beverages and byproducts from Spain: Nutritional Supply. Food Addit. Contam. 2007, 24, 685–694. [Google Scholar] [CrossRef]
- Olaoye, O.A.; Ubbor, S.C.; Uduma, E.A. Determination of Vitamins, Minerals, and Microbial Loads of Fortified Nonalcoholic Beverage (Kunun Zaki) Produced from Millet. Food Sci. Nutr. 2006, 4, 96–102. [Google Scholar] [CrossRef]
- Maciel, J.V.; Souza, M.M.; Silva, L.O.; Dias, D. Direct Determination of Zn, Cd, Pb and Cu in Wine by Differential Pulse Anodic Stripping Voltammetry. Beverages 2019, 5, 6. [Google Scholar] [CrossRef]
- Baroni, M.V.; Naranjo, R.D.P.; García-Ferreyra, C.; Otaiza, S.; Wunderlin, D.A. How Good Antioxidant Is the Red Wine? Comparison of Some in Vitro and in Vivo Methods to Assess the Antioxidant Capacity of Argentinean Red Wines. LWT—Food Sci. Technol. 2012, 47, 1–7. [Google Scholar] [CrossRef]
- Pizzorno, J. Glutathione! Integr. Med. A Clin. J. 2014, 13, 8–12. [Google Scholar]
- Drava, G.; Minganti, V. Mineral Composition of Organic and Conventional White Wines from Italy. Heliyon 2019, 5, e02464. [Google Scholar] [CrossRef] [PubMed]
- Gajek, M.; Pawlaczyk, A.; Szynkowska-Jozwik, M.I. Multi-Elemental Analysis of Wine Samples in Relation to Their Type, Origin, and Grape Variety. Molecules 2021, 26, 214. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Stozhko, N.Y.; Belysheva, G.M.; Inzhevatova, O.V.; Kolyadina, L.I.; Cremisini, C.; Galletti, M. Determination of Heavy Metals in Wines by Anodic Stripping Voltammetry with Thick-Film Modified Electrode. Anal. Chim. Acta 2004, 514, 227–234. [Google Scholar] [CrossRef]
- Schaffer, S.; Rimbach, G.; Pieper, D.; Hommen, N.; Fischer, A.; Birringer, M.; Seidel, U. Minerals and Trace Elements in 990 Beverages and Their Contribution to Dietary Reference Values for German Con-sumers. Nutrients 2022, 14, 4899. [Google Scholar] [CrossRef]
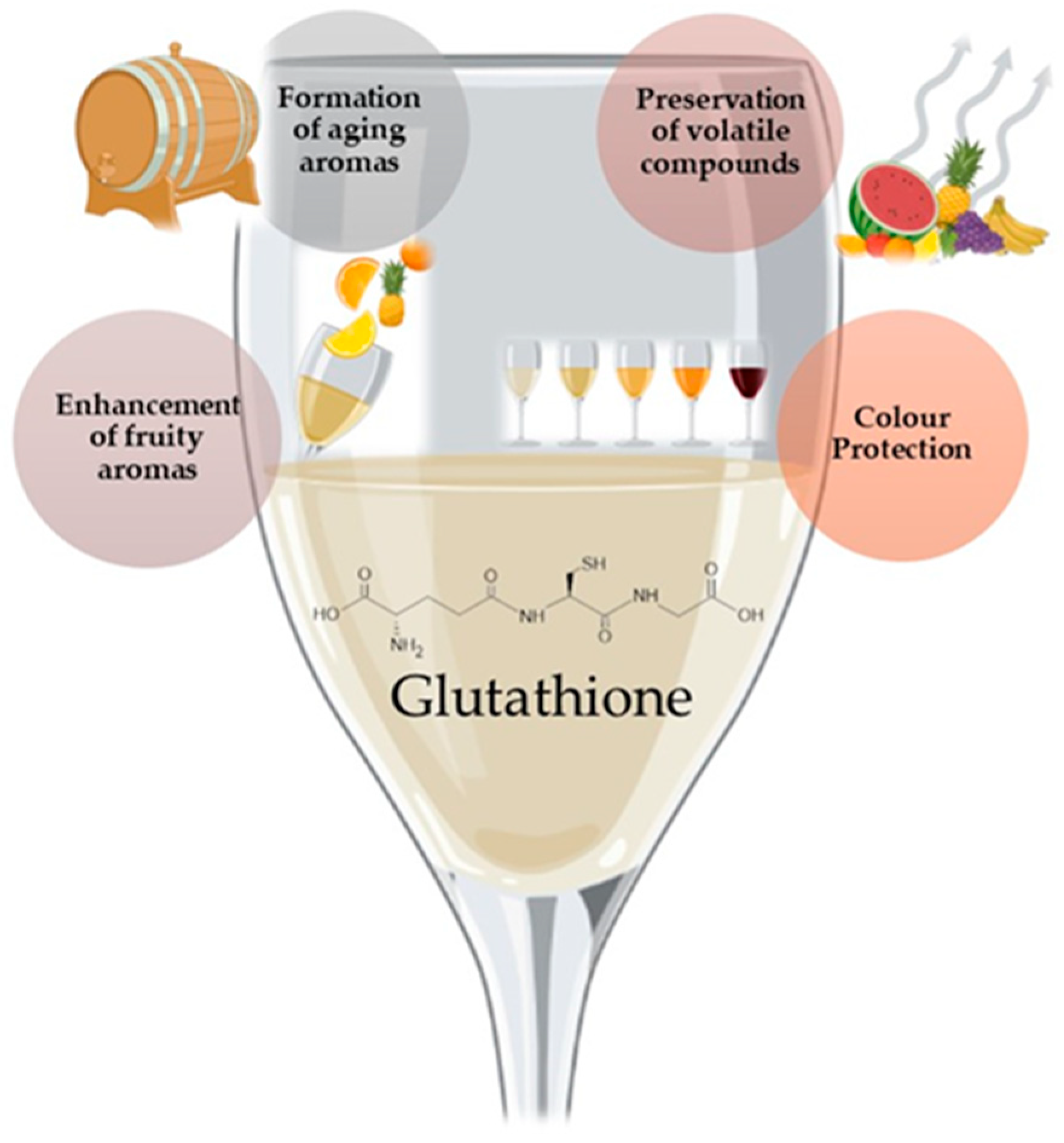
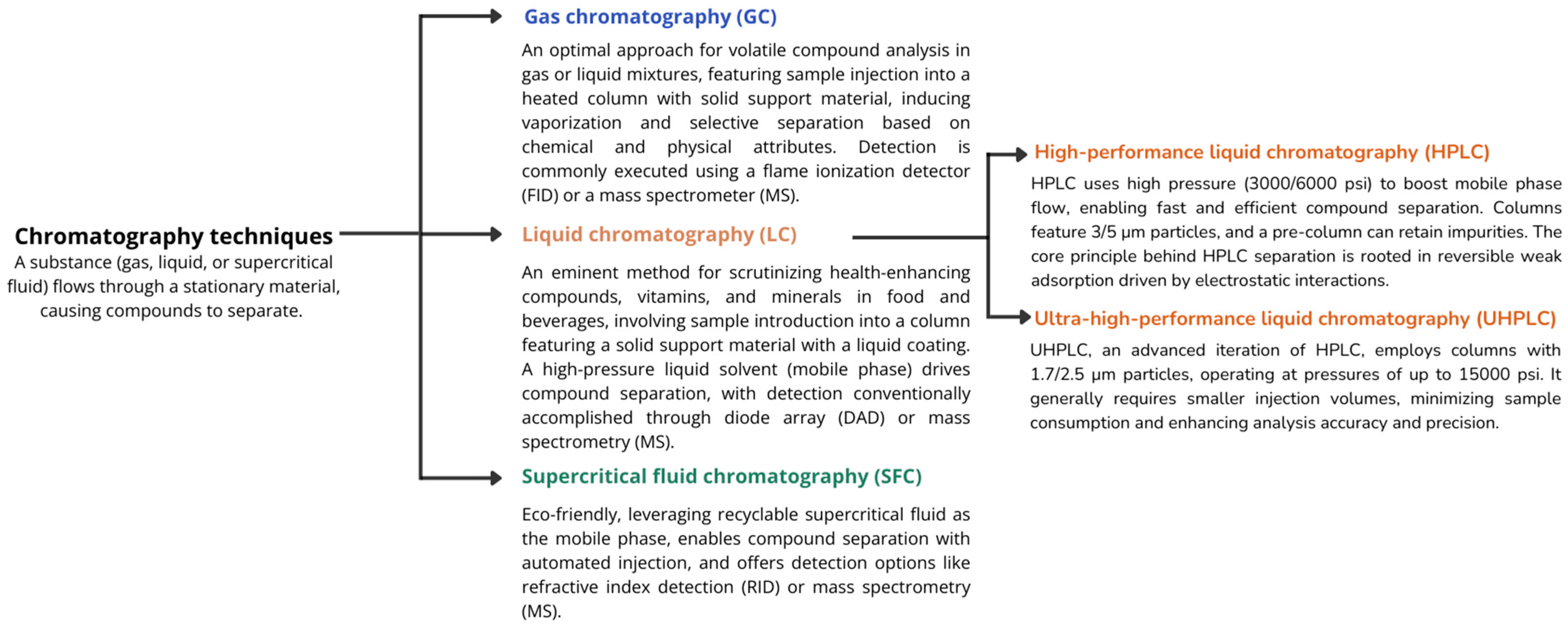
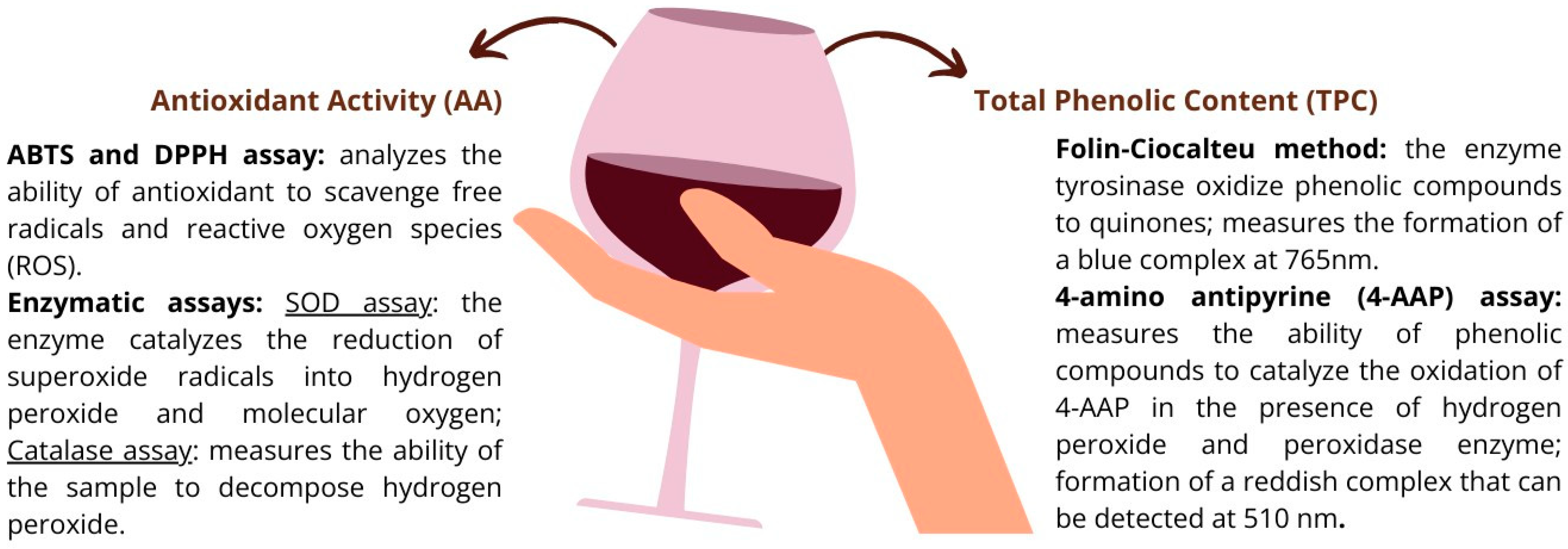
| Group | Designation | Matrix |
|---|---|---|
| Flavonoids | Anthocyanins | Yeast-fermented beverages [39] Wine and grape [40] |
| Flavonols | Rufete and Tempranillo wine [41] Flavored wine [42] Wine and grape [40] | |
| Flavanols | Rufete and Tempranillo wine [41] Wine and grape [40] | |
| Flavones | Flavored wine [42] Wine and grape [40] | |
| Flavanones | Wine [43] Wine and grape [40] | |
| Flavanes | Wine and grape [40] | |
| Flavononols | Wine and grape [40] | |
| Chalcones/ Dihydrochalcones | Wine and grape [40] | |
| Non-flavonoids | Phenolic acids | Wine and grape [40] |
| Tannins | Wine and grape [40] | |
| Stilbenes | Wine and grape [40,44] | |
| Coumarins | Wine and grape [40] | |
| Phenyl ethanol derivatives | Wine and grape [40] | |
| Lignans | Wine and grape [40] Wine [45] | |
| Neolignans | Wine and grape [40] |
| Group | Designation | Matrix | (mg/L) [92] | (mg/L) [93] |
|---|---|---|---|---|
| Vit. C | Ascorbic acid | Grape musts | 30–572 | |
| Vit. B1 | Thiamine | Grape musts | 0.08–1.20 | 0.89–3.51 |
| Vit. B2 | Riboflavin | Grape musts White Wine Red Wine | 0.003–1.45 0.008–1.33 0.00047–0.0019 | -- |
| Vit. B3 | Niacin | Grape musts Wines | 0.79–8.80 0.11–0.42 | 0.02–3.24 |
| Vit. B5 | Pantothenic acid | Grape musts Grapes | 0.00016–10−50 6.8–8.5 | 0.12–2.70 |
| Vit. B6 | Pyridoxine | Grape musts Red grapes White grapes | 0.14–2.9 1.25 0.88 | 0.93–6.94 |
| Vit. B7 | Biotin | Red grape juices White grape juices Grape musts | 0.00285 0.00147 0.0001–0.060 | -- |
| Vit. B8 | Inositol | White wines Red wines | 220–730 290–334 | -- |
| Vit. B9 | Folic acid | Grape musts Wines | 0.003–0.05 0.0004–0.0045 | -- |
| Techniques | Solvent | Method Description | References |
|---|---|---|---|
| SPME | Solvent-free | A method based on the principle of adsorption/absorption and desorption through a silica fiber. | [199,200] |
| UAE | Organic solvent | Uses high-frequency ultrasonic waves to extract compounds due to high pressure and temperature. | [16,198,201] |
| MAE | Organic solvent | The sample is heated in a microwave oven, and the boiled solvent extracts the desired compounds. | |
| SFE | Supercritical fluids (SF) * | The sample, placed in a vessel, is submitted to an SF under high pressure and temperature that dissolves the target compound in the fluid. When the conditions decrease, the extract is collected. | [16,198,201] |
| PLE or ASE | Water or organic solvent | The compounds are extracted by applying controlled pressure and temperature to the solvent. | [16,198,201] |
| PEF | Solvent-free | A green, non-thermal method that uses electrodes and a solid electrical field to create stress in the cell membrane, leading to the formation of pores (irreversible or not). | [201,202] |
| OH | Solvent-free | An environmentally friendly alternative that combines electrical and thermal treatment to damage the cell membrane and increase the extraction of phenolic compounds. | [201,203] |
| EAE | Solvent-free | Enzymes allow the leaking of phenolic compounds or the recovery of these compounds from cell vacuoles, especially the insoluble-bound phenolics. | [16,204] |
| Technique | Method Description | References |
|---|---|---|
| UV-Vis spectrophotometry | Measures light absorption or transmission by a sample at specific wavelengths in the ultraviolet and visible regions (UV-Vis) of the electromagnetic spectrum (200–800 nm), usually following the Beer–Lambert law, which relates absorbance to concentration. | [219,220] |
| Fourier-transform infrared spectroscopy (FTIR) | Identifies chemical structure and functional groups in a compound by measuring their interaction with infrared light. FTIR instruments include transmission, reflection, and ATR (Attenuated Total Reflectance) types, each suited for different sample types. Transmission passes light through a thin sample, reflection reflects it off the sample’s surface, and ATR presses the sample against a diamond crystal. | [221,222] |
| Nuclear magnetic resonance (NMR) | Studying the properties of atomic nuclei in molecules, this analytical technique involves placing the sample in a strong magnetic field and applying a radiofrequency pulse. This process excites the nuclei, causing them to emit energy and produce a detectable signal in the spectroscope. The method is versatile, used for determining the structure of organic compounds, identifying substances through unique spectra, quantifying compound concentrations, and studying molecular dynamics in various scientific fields. Fourier-transform nuclear magnetic resonance (FT-NMR) is a common approach within this technique. It allows for more efficient data acquisition, improved spectral resolution, and enhanced sensitivity, making it a valuable tool for detailed molecular analysis. | [223,224,225,226] |
| Raman spectroscopy | Weigh the vibrational mode of molecules when a sample is illuminated with monochromatic light; a small portion of the scattered light shifts. This technique can be used to analyze samples in situ, without sample preparation or separation, and is highly selective because it can distinguish between different chemical species. | [227,228,229] |
| Mass spectrometry | Provides structural information about the separated compounds in combination with chromatographic techniques (hyphenated method). The compounds are ionized and passed through the equipment, which separates ions based on their mass-to-charge ratio (m/z), determining the target compounds’ molecular weight and chemical structure. | [230,231,232,233,234,235] |
| Technique | Type | Compounds | Description | References |
|---|---|---|---|---|
| Acid titrations | Chemical | Water-soluble vitamins | Acid-base (redox) titrations can be used to determine the content or concentration of certain vitamins. However, the specific method and procedure can vary depending on the analyzed vitamin. | [92] |
| Atomic absorption spectrophotometer (AAS) | Spectroscopic | Minerals (principally sodium, calcium, magnesium, and iron) | Analyze elements by measuring the absorption of light by atomized samples. It quantifies element concentration based on the amount of light absorbed by the atoms in the ground state. The sample is atomized using a flame or graphite furnace, and a specific wavelength of light is passed through it. The absorbed light is detected, and the absorbance corresponds to the element concentration in the sample. | [92,257,258,259,260] |
| Differential pulse anodic stripping voltammetry (DPASV) | Electrochemical | Minerals | A sensitive and selective method that analyzes trace metals and electroactive species in a sample. It involves the analyte accumulation on an electrode, followed by applying a voltage pulse to cause oxidation and stripping the analyte from the electrode surface. The resulting current peak is measured and used to determine the analyte concentration in the sample. | [258,259,261] |
| Enzyme glutathione peroxidase assay | Enzymatic | Glutathione | The enzyme catalyzes the reduction of hydrogen peroxide and other hydroperoxides in the presence of glutathione. | [262,263]. |
| Inductively coupled plasma–optical emission spectroscopy (ICP-OES) | Spectroscopic | Minerals | This analytical method uses an inductively coupled plasma (high-temperature ionization source) to atomize and ionize the sample, then detect emitted light at specific wavelengths. It is handy for elemental analysis in a wide range of samples. | [257,261,264,265] |
| Inductively coupled plasma–mass spectrometer (ICP-MS) | Spectroscopic | Minerals | Employs an inductively coupled plasma as a high-temperature ionization source to atomize and ionize the sample. The ionized species are then introduced into a mass spectrometer, separated based on their m/z, and detected. This technique allows for the precise measurement of elemental and isotopic composition in a wide range of samples, offering high sensitivity and the ability to analyze multiple elements simultaneously. | [257,258,265,266,267] |
| Microbiological assay (MA) | Biological | Vitamins | These assays rely on the growth response of specific microorganisms (usually lactic acid bacteria), which require the vitamin to be analyzed for their growth and metabolism. The principle behind these assays is that the amount of change observed is directly proportional to the concentration of the vitamin in the sample. | [92] |
| Thiochrome assay | Chemical | Vitamin B1 | Thiamine is converted to thiochrome in the presence of a thiol reagent and an oxidizing agent. The thiochrome formed exhibits fluorescence, which can be measured and correlated with the thiamine concentration in the sample. | [92,258] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, C.; Dinis, L.-T.; Santos, M.J.; Mota, J.; Vilela, A. Beyond the Bottle: Exploring Health-Promoting Compounds in Wine and Wine-Related Products—Extraction, Detection, Quantification, Aroma Properties, and Terroir Effects. Foods 2023, 12, 4277. https://doi.org/10.3390/foods12234277
Marques C, Dinis L-T, Santos MJ, Mota J, Vilela A. Beyond the Bottle: Exploring Health-Promoting Compounds in Wine and Wine-Related Products—Extraction, Detection, Quantification, Aroma Properties, and Terroir Effects. Foods. 2023; 12(23):4277. https://doi.org/10.3390/foods12234277
Chicago/Turabian StyleMarques, Catarina, Lia-Tânia Dinis, Maria João Santos, João Mota, and Alice Vilela. 2023. "Beyond the Bottle: Exploring Health-Promoting Compounds in Wine and Wine-Related Products—Extraction, Detection, Quantification, Aroma Properties, and Terroir Effects" Foods 12, no. 23: 4277. https://doi.org/10.3390/foods12234277
APA StyleMarques, C., Dinis, L.-T., Santos, M. J., Mota, J., & Vilela, A. (2023). Beyond the Bottle: Exploring Health-Promoting Compounds in Wine and Wine-Related Products—Extraction, Detection, Quantification, Aroma Properties, and Terroir Effects. Foods, 12(23), 4277. https://doi.org/10.3390/foods12234277