Anti-Inflammatory, Antioxidant, and Genoprotective Effects of Callus Cultures Obtained from the Pulp of Malus pumila cv Miller (Annurca Campana Apple)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Plant Material and Callus Cultures
2.3. Preparation of Extracts from Peel, Pulp, and Calli of Annurca Apple
2.4. Chemical Characterization of Extracts from Peel Pulp and Calli of Annurca Apple
2.5. DPPH Scavenging Activity
2.6. ABTS Scavenging Activity
2.7. ORAC Assay
2.8. Lipoxygenase Inhibition Assay
2.9. Nicking Assay
2.10. Cell Cultures
2.11. Griess Assay
2.12. Cell Viability Assay
2.13. Statistical Analyses
3. Results and Discussion
3.1. Callus Induction from Annurca Fruit Pulp and Chemical Characterization of Annurca Ethanolic Extracts
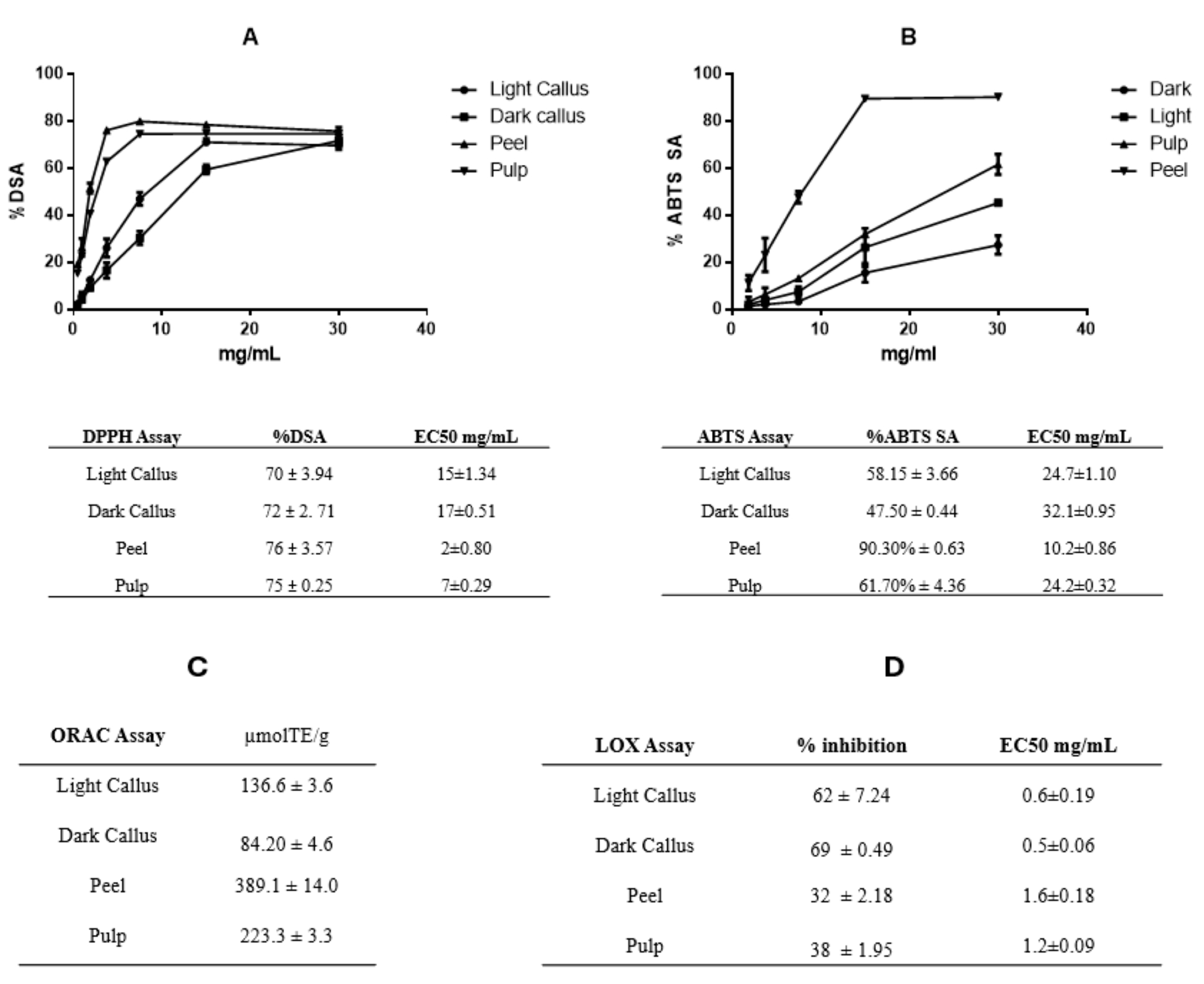
3.2. Antioxidant Capacity of Annurca Apple Extracts
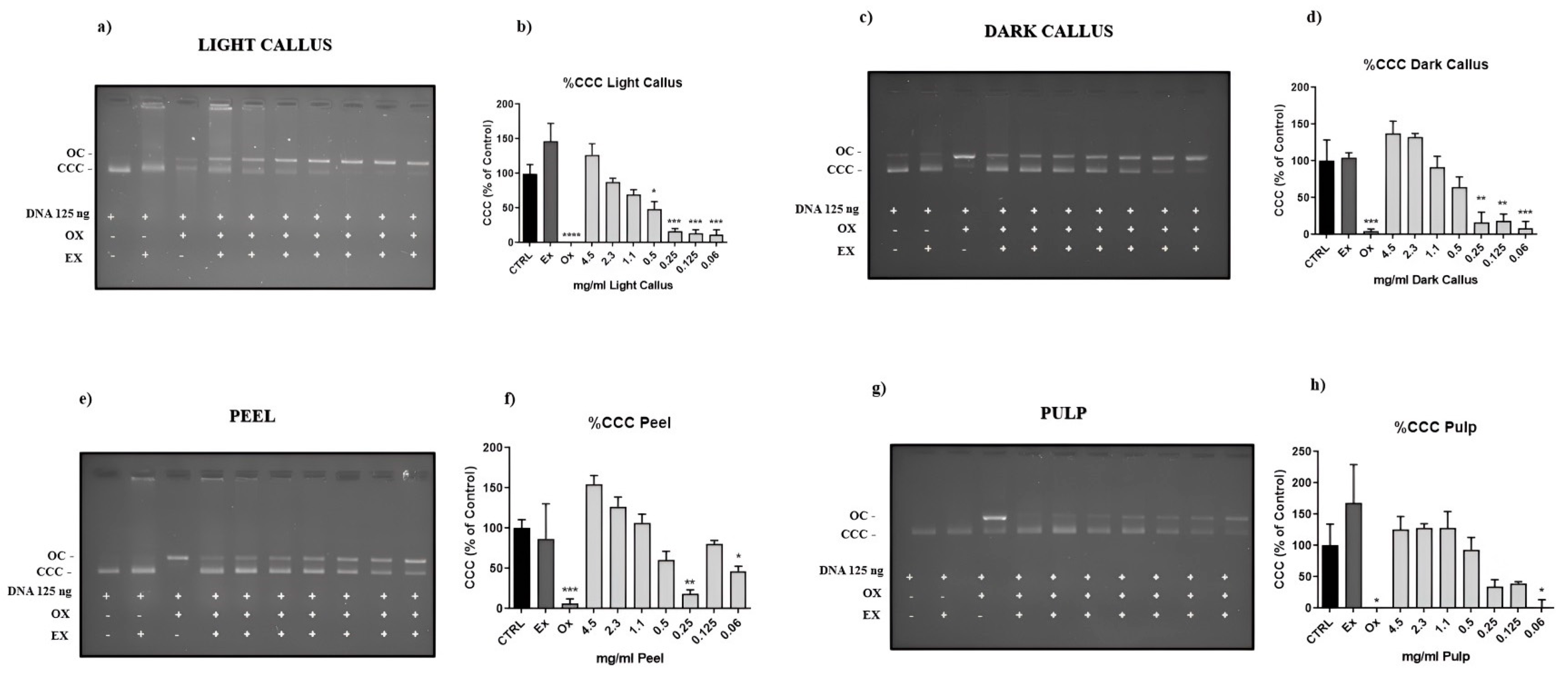
3.3. Genoprotective Activity of Annurca Apple Extracts
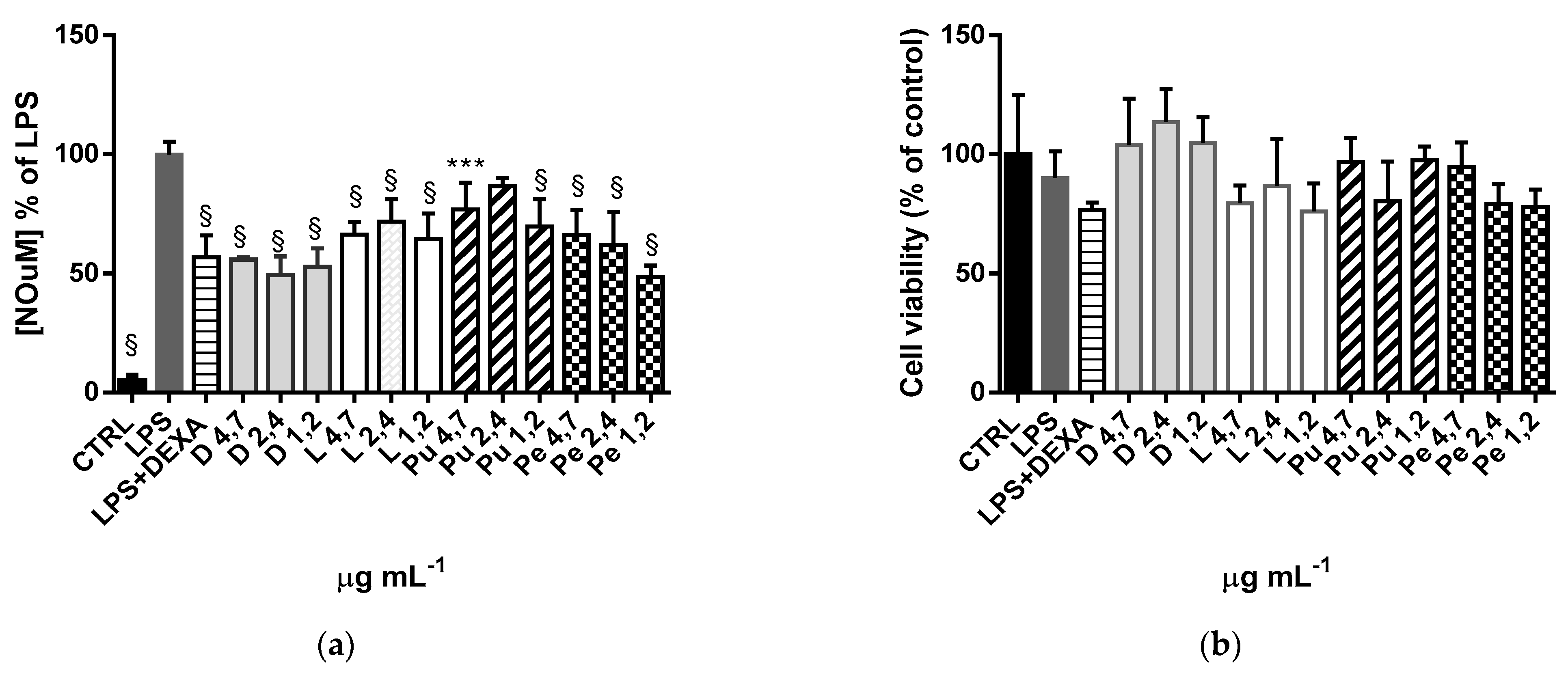
3.4. Anti-Inflammatory Activity of Annurca Apple Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hyson, D.A. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Pensec, F.; Bertsch, C. Fruit cuticular waxes as a source of biologically active triterpenoids. Phytochem. Rev. 2012, 11, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Lo Scalzo, R.; Testoni, A.; Genna, A. ‘Annurca’ apple fruit, a southern Italy apple cultivar: Textural properties and aroma composition. Food Chem. 2001, 49, 333–343. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Fiorentino, A.; Monaco, P.; Oriano, P.; Pacifico, S. Annurcoic acid: A new antioxidant ursane triterpene from fruits of cv. Annurca apple. Food Chem. 2006, 98, 285–290. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Scognamiglio, M.; Corrado, L.; Chiocchio, I.; Zampella, L.; Mastrobuoni, F.; Rega, P.; Scortichini, M.; Fiorentino, A.; Petriccione, M. Evaluation of different training systems on Annurca apple fruits revealed by agronomical, qualitative and NMR-based metabolomic approaches. Food Chem. 2017, 222, 18–27. [Google Scholar] [CrossRef]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef]
- Namdeo, A.G.; Ingawale, D.K. Ashwagandha: Advances in plant biotechnological approaches for propagation and production of bioactive compounds. J. Ethnopharmacol. 2021, 271, 113709. [Google Scholar] [CrossRef]
- Rumiyati; Sismindari; Semiarti, E.; Milasari, A.F.; Sari, D.K.; Fitriana, N.; Galuh, S. Callus Induction from Various Organs of Dragon Fruit, Apple and Tomato on some Mediums. Pak. J. Biol. Sci. 2017, 20, 244–252. [Google Scholar] [CrossRef][Green Version]
- Baldelli, G.; De Santi, M.; Fraternale, D.; Brandi, G.; Fanelli, M.; Schiavano, G.F. Chemopreventive Potential of Apple Pulp Callus Against Colorectal Cancer Cell Proliferation and Tumorigenesis. J. Med. Food 2019, 22, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Verardo, G.; Gorassini, A.; Ricci, D.; Fraternale, D. High Triterpenic Acids Production in Callus Cultures from Fruit Pulp of Two Apple Varieties. Phytochem. Anal. 2017, 28, 5–15. [Google Scholar] [CrossRef]
- Verardo, G.; Gorassini, A.; Fraternale, D. New triterpenic acids produced in callus culture from the fruit pulp of Acca sellowiana (O. Berg) Burret. Food Res. Int. 2019, 119, 596–604. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, R.; Chiarantini, L.; Potenza, L.; Gorassini, A.; Verardo, G.; De Marco, R.; Benayada, L.; Stocchi, V.; Albertini, M.C.; Fraternale, D. High production of secondary metabolites and biological activities of Cydonia oblonga Mill. pulp fruit callus. J. Funct. Foods 2022, 94, 105133. [Google Scholar] [CrossRef]
- Saltarelli, R.; Palma, F.; Gioacchini, A.M.; Calcabrini, C.; Mancini, U.; De Bellis, R.; Stocchi, V.; Potenza, L. Phytochemical composition, antioxidant and antiproliferative activities and effects on nuclear DNA of ethanolic extract from an Italian mycelial isolate of Ganoderma lucidum. J. Ethnopharmacol. 2019, 231, 464–473. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Sicari, V.; Pellicanò, T.; Xiao, J.; Poiana, M.; Tundis, R. Comparative analysis of chemical composition, antioxidant and anti-proliferative activities of Italian Vitis vinifera by-products for a sustainable agro-industry. Food Chem. Toxicol. 2019, 127, 127–134. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, R.; Piacentini, M.P.; Meli, M.A.; Mattioli, M.; Menotta, M.; Mari, M.; Valentini, L.; Palomba, L.; Desideri, D.; Chiarantini, L. In vitro effects on calcium oxalate crystallization kinetics and crystal morphology of an aqueous extract from Ceterach officinarum: Analysis of a potential antilithiatic mechanism. PLoS ONE 2019, 14, e0218734. [Google Scholar] [CrossRef] [PubMed]
- Saltarelli, R.; Ceccaroli, P.; Iotti, M.; Zambonelli, A.; Buffalini, M.; Casadei, L.; Vallorani, L.; Stocchi, V. Biochemical Characterisation and Antioxidant Activity of Mycelium of Ganoderma lucidum from Central Italy. Food Chem. 2009, 116, 143–151. [Google Scholar] [CrossRef]
- Sadat Asadi, N.; Heidari, M.M.; Khatami, M. Protective effect of Berberis vulgaris on Fenton reaction-induced DNA cleavage. Avicenna J. Phytomed. 2019, 9, 213–220. [Google Scholar] [PubMed]
- Ferraro, M.G.; Piccolo, M.; Pezzella, A.; Guerra, F.; Maione, F.; Tenore, G.C.; Santamaria, R.; Irace, C.; Novellino, E. Promelanogenic Effects by an Annurca Apple-Based Natural Formulation in Human Primary Melanocytes. Clin. Cosmet. Investig. Dermatol. 2021, 14, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Nasso, R.; Pagliara, V.; D’Angelo, S.; Rullo, R.; Masullo, M.; Arcone, R. Annurca Apple Polyphenol Extract Affects Acetyl- Cholinesterase and Mono-Amine Oxidase In Vitro Enzyme Activity. Pharmaceuticals 2021, 14, 62. [Google Scholar] [CrossRef]
- Maisto, M.; Piccolo, V.; Novellino, E.; Schiano, E.; Iannuzzo, F.; Ciampaglia, R.; Summa, V.; Tenore, G.C. Optimization of Phlorizin Extraction from Annurca Apple Tree Leaves Using Response Surface Methodology. Antioxidants 2022, 11, 1933. [Google Scholar] [CrossRef]
- Orlandella, F.M.; Mirabelli, P.; De Stefano, A.E.; Iervolino, P.L.C.; Luciano, N.; D’Angelo, S.; Salvatore, G. Effects of Annurca Flesh Apple Polyphenols in Human Thyroid Cancer Cell Lines. Oxid. Med. Cell. Longev. 2022, 2022, 6268755. [Google Scholar] [CrossRef]
- Laezza, C.; Imbimbo, P.; D’Amelia, V.; Marzocchi, A.; Monti, D.M.; Di Loria, A.; Monti, S.M.; Novellino, E.; Tenore, G.C.; Rigano, M.M. Use of yeast extract to elicit a pulp-derived callus cultures from Annurca apple and potentiate its biological activity. J. Funct. Foods 2024, 112, 105988. [Google Scholar] [CrossRef]
- Ahmad, N.; Rab, A.; Ahmad, N. Light-induced biochemical variations in secondary metabolite production and antioxidant activity in callus cultures of Stevia rebaudiana (Bert). J. Photochem. Photobiol. B 2016, 154, 51–56. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, S.S.; Akbar, F.; Kanwal, F. Correlation of different spectral lights with biomass accumulation and production of antioxidant secondary metabolites in callus cultures of medicinally important Prunella vulgaris L. J. Photochem. Photobiol. B 2016, 159, 1–7. [Google Scholar] [CrossRef]
- Kapoor, S.; Raghuvanshi, R.; Bhardwaj, P.; Sood, H.; Saxena, S.; Chaurasia, O.P. Influence of light quality on growth, secondary metabolites production and antioxidant activity in callus culture of Rhodiola imbricata Edgew. J. Photochem. Photobiol. B 2018, 183, 258–265. [Google Scholar] [CrossRef]
- Younas, M.; Drouet, S.; Nadeem, M.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Differential accumulation of silymarin induced by exposure of Silybum marianum L. callus cultures to several specters of monochromatic lights. J. Photochem. Photobiol. B 2018, 184, 61–70. [Google Scholar] [CrossRef]
- Adil, M.; Haider Abbasi, B.; Ul Haq, I. Red light controlled callus morphogenetic patterns and secondary metabolites production in Withania somnifera L. Biotechnol. Rep. 2019, 24, e00380. [Google Scholar] [CrossRef]
- Kumar, S.S.; Arya, M.; Mahadevappa, P.; Giridhar, P. Influence of photoperiod on growth, bioactive compounds and antioxidant activity in callus cultures of Basella rubra L. J. Photochem. Photobiol. B 2020, 209, 111937. [Google Scholar] [CrossRef]
- Biswal, B.; Jena, B.; Giri, A.K.; Acharya, L. Monochromatic light elicited biomass accumulation, antioxidant activity, and secondary metabolite production in callus culture of Operculina turpethum (L.). Plant Cell Tissue Organ Cult. 2022, 149, 123–134. [Google Scholar] [CrossRef]
- Vergara Martínez, V.M.; Estrada-Soto, S.E.; Arellano-García, J.J.; Rivera-Leyva, J.C.; Castillo-España, P.; Flores, A.F.; Cardoso-Taketa, A.T.; Perea-Arango, I. Methyl Jasmonate and Salicylic Acid Enhanced the Production of Ursolic and Oleanolic Acid in Callus Cultures of Lepechinia caulescens. Pharmacogn. Mag. 2018, 13 (Suppl. S4), S886–S889. [Google Scholar]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Ivorra, M.D.; Paya, M.; Villar, A. Hypoglycemic and insulin release effects of tormentic acid: A new hypoglycemic natural product. Planta Med. 1998, 54, 282–285. [Google Scholar] [CrossRef]
- Wang, Y.L.; Sun, G.Y.; Zhang, Y.; He, J.J.; Zheng, S.; Lin, J.N. Tormentic acid inhibits H2O2-induced oxidative stress and inflammation in rat vascular smooth muscle cells via inhibition of the NF-κB signaling pathway. Mol. Med. Rep. 2016, 14, 3559–3564. [Google Scholar] [CrossRef]
- Babich, O.; Sukhikh, S.; Pungin, A.; Astahova, L.; Chupakhin, E.; Belova, D.; Prosekov, A.; Ivanova, S. Evaluation of the Conditions for the Cultivation of Callus Cultures of Hyssopus officinalis Regarding the Yield of Polyphenolic Compounds. Plants 2021, 10, 915. [Google Scholar] [CrossRef]
- Hariprasath, L.; Jegadeesh, R.; Arjun, P.; Raaman, N. In vitro propagation of Senecio candicans DC and comparative antioxidant properties of aqueous extracts of the in vivo plant and in vitro-derived callus. S. Afr. J. Bot. 2015, 98, 134–141. [Google Scholar] [CrossRef]
- Moon, T.C.; Lin, C.X.; Lee, J.S.; Kim, D.S.; Bae, K.; Son, K.H.; Kim, H.P.; Kang, S.S.; Son, J.K.; Chang, H.W. Antiinflammatory activity of astilbic acid from Astilbe chinensis. Biol. Pharm. Bull. 2005, 28, 24–26. [Google Scholar] [CrossRef]
- Vo, N.N.Q.; Nomura, Y.; Muranaka, T.; Fukushima, E.O. Structure-Activity Relationships of Pentacyclic Triterpenoids as Inhibitors of Cyclooxygenase and Lipoxygenase Enzymes. J. Nat. Prod. 2019, 82, 3311–3320. [Google Scholar] [CrossRef]
- Magiera, A.; Marchelak, A.; Michel, P.; Owczarek, A.; Olszewska, M.A. Lipophilic extracts from leaves, inflorescences and fruits of Prunus padus L. as potential sources of corosolic, ursolic and oleanolic acids with anti-inflammatory activity. Nat. Prod. Res. 2021, 35, 2263–2268. [Google Scholar] [CrossRef]
- Pace, S.; Zhang, K.; Jordan, P.M.; Bilancia, R.; Wang, W.; Börner, F.; Hofstetter, R.K.; Potenza, M.; Kretzer, C.; Gerstmeier, J.; et al. Anti-inflammatory celastrol promotes a switch from leukotriene biosynthesis to formation of specialized pro-resolving lipid mediators. Pharmacol. Res. 2021, 167, 105556. [Google Scholar] [CrossRef]
- Werz, O. Inhibition of 5-lipoxygenase product synthesis by natural compounds of plant origin. Planta Med. 2007, 73, 1331–1357. [Google Scholar] [CrossRef]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: Induction, repair, and significance. Mutat. Res. 2004, 567, 1–61. [Google Scholar] [CrossRef]
- Markesbery, W.R.; Lovell, M.A. DNA oxidation in Alzheimer’s disease. Antioxid. Redox Signal. 2006, 8, 2039–2045. [Google Scholar] [CrossRef]
- Lenart, P.; Krejci, L. DNA, the central molecule of aging. Mutat. Res. 2016, 786, 1–7. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef]
- An, H.J.; Kim, I.T.; Park, H.J.; Kim, H.M.; Choi, J.H.; Lee, K.T. Tormentic acid, a triterpenoid saponin, isolated from Rosa rugosa, inhibited LPS-induced iNOS, COX-2, and TNF-α expression through inactivation of the nuclear factor-κb pathway in RAW 264.7 macrophages. Int. Immunopharmacol. 2011, 11, 504–510. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, J.N.; Han, S.N.; Kim, H.K. Ursolic acid isolated from guava leaves inhibits inflammatory mediators and reactive oxygen species in LPS-stimulated macrophages. Immunopharmacol. Immunotoxicol. 2015, 37, 228–235. [Google Scholar] [CrossRef]
- Nair, V.; Bang, W.Y.; Schreckinger, E.; Andarwulan, N.; Cisneros-Zevallos, L. Protective Role of Ternatin Anthocyanins and Quercetin Glycosides from Butterfly Pea (Clitoria ternatea Leguminosae) Blue Flower Petals against Lipopolysaccharide (LPS)-Induced Inflammation in Macrophage Cells. J. Agric. Food Chem. 2015, 63, 6355–6365. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, N.H.; Khan, I.; Yu, J.M.; Jung, H.G.; Kim, H.H.; Jang, J.Y.; Kim, H.J.; Kim, D.I.; Kwak, J.H.; et al. Anti-inflammatory Potential of Quercetin-3-O-β-D-(“2”-galloyl)-glucopyranoside and Quercetin Isolated from Diospyros kaki calyx via Suppression of MAP Signaling Molecules in LPS-induced RAW 264.7 Macrophages. J. Food Sci. 2016, 81, C2447–C2456. [Google Scholar] [CrossRef]
- Huang, H.T.; Liaw, C.C.; Chiou, C.T.; Kuo, Y.H.; Lee, K.T. Triterpene Acids from Mesona procumbens Exert Anti-inflammatory Effects on LPS-Stimulated Murine Macrophages by Regulating the MAPK Signaling Pathway. J. Agric. Food Chem. 2021, 69, 6271–6280. [Google Scholar] [CrossRef]
- Guo, F.; Tsao, R.; Li, C.; Wang, X.; Zhang, H.; Jiang, L.; Sun, Y.; Xiong, H. Polyphenol Content of Green Pea (Pisum sativum L.) Hull under In Vitro Digestion and Effects of Digestive Products on Anti-Inflammatory Activity and Intestinal Barrier in the Caco-2/Raw264.7 Coculture Model. J. Agric. Food Chem. 2022, 70, 3477–3488. [Google Scholar] [CrossRef]

| Compound | Light Callus | Dark Callus | Peel | Pulp |
|---|---|---|---|---|
| 3-O-Caffeoylquinic acid isomer | 0.05 ± 0.001 | n.d. | 0.18 ± 0.001 | 0.12 ± 0.001 |
| Procyanidin B-type dimer isomer | n.d. | n.d. | 7.38 ± 0.011 | 6.77 ± 0.006 |
| Procyanidin B-type trimer isomer | n.d. | n.d. | 3.87 ± 0.007 | 1.69 ± 0.008 |
| Procyanidin B-type trimer isomer | n.d. | n.d. | 1.67 ± 0.018 | 0.90 ± 0.022 |
| Procyanidin B-type tetramer isomer | n.d. | n.d. | 0.83 ± 0.012 | 0.42 ± 0.001 |
| Procyanidin B-type trimer isomer | n.d. | n.d. | 4.27 ± 0.018 | 1.93 ± 0.021 |
| 4-O-Caffeoylquinic acid | 0.01 ± 0.001 | n.d. | 0.29 ± 0.005 | 0.09 ± 0.001 |
| (+) Catechin | n.d. | n.d. | 8.37 ± 0.035 | 7.32 ± 0.019 |
| Procyanidin B-type tetramer isomer | n.d. | n.d. | 3.93 ± 0.008 | 1.24 ± 0.009 |
| Procyanidin B2 | n.d. | n.d. | 23.26 ± 0.152 | 10.18 ± 0.021 |
| 5-O-Caffeoylquinic acid (chlorogenic acid) | 19.57 ± 0.034 | 0.10 ± 0.001 | 49.32 ± 0.110 | 61.97 ± 0.061 |
| Caffeoyl acid hexoside | 2.16 ± 0.005 | 3.46 ± 0.009 | 0.56 ± 0.003 | 0.39 ± 0.004 |
| 4-O-Caffeoylquinic acid isomer | 0.43 ± 0.001 | n.d. | 1.02 ± 0.001 | 0.84 ± 0.001 |
| Procyanidin B-type trimer isomer | n.d. | n.d. | 15.32 ± 0.098 | 6.93 ± 0.011 |
| 4-O-p-Coumaroylquinic acid isomer | 0.32 ± 0.003 | n.d. | 0.98 ± 0.011 | 0.46 ± 0.002 |
| Cyanidin hexoside | n.d. | n.d. | 12.27 ± 0.181 | n.d. |
| (-) Epicatechin | 0.17 ± 0.002 | 0.07 ± 0.001 | 44.62 ± 0.324 | 25.09 ± 0.093 |
| 5-O-Caffeoylquinic acid isomer | 0.51 ± 0.001 | n.d. | 1.53 ± 0.001 | 1.79 ± 0.005 |
| 5-O-p-Coumaroylquinic acid isomer | 0.29 ± 0.002 | 0.01 ± 0.001 | 1.08 ± 0.001 | 1.24 ± 0.001 |
| 4-O-p-Coumaroylquinic acid isomer | 0.44 ± 0.004 | n.d. | 2.84 ± 0.006 | 5.55 ± 0.013 |
| 5-O-p-Coumaroylquinic acid isomer | 0.04 ± 0.001 | n.d. | 0.45 ± 0.007 | 0.15 ± 0.002 |
| Procyanidin B-type dimer isomer | n.d. | n.d. | 2.41 ± 0.002 | 1.02 ± 0.003 |
| Quercetin 3-O-galactoside | 1.93 ± 0.020 | n.d. | 66.40 ± 0.151 | 0.14 ± 0.001 |
| Phloretin xyloglucoside isomer | n.d. | n.d. | 23.84 ± 0.034 | 15.26 ± 0.016 |
| Quercetin hexoside | 1.41 ± 0.001 | n.d. | 25.80 ± 0.037 | 0.79 ± 0.001 |
| Quercetin 3-O-xyloside | 0.25 ± 0.001 | n.d. | 14.60 ± 0.112 | 0.36 ± 0.004 |
| Phloretin xyloglucoside isomer | n.d. | n.d. | 1.12 ± 0.010 | 0.60 ± 0.005 |
| Quercetin 3-O-arabinoside | 0.03 ± 0.001 | n.d. | 6.60 ± 0.075 | 0.03 ± 0.001 |
| Phloridzin | 0.63 ± 0.010 | n.d. | 36.21 ± 0.061 | 1.71 ± 0.005 |
| Quercetin pentoside | 0.10 ± 0.001 | n.d. | 28.86 ± 0.064 | 0.28 ± 0.001 |
| Quercetin 3-O-rhamnoside | 0.04 ± 0.001 | n.d. | 13.23 ± 0.143 | 0.91 ± 0.009 |
| Total | 28.39 ± 0.003 | 3.64 ± 0.011 | 403.12 ± 0.493 | 156.16 ± 0.094 |
| Compound | Light Callus | Dark Callus | Peel | Pulp |
|---|---|---|---|---|
| β-Sitosterol | 148.52 ± 0.85 | 174.69 ± 1.47 | 44.61 ± 0.46 | 32.04 ± 0.25 |
| Uvaol | 13.45 ± 0.04 | 13.13 ± 0.13 | n.d. | n.d. |
| Oleanolic acid | 49.80 ± 0.45 | 34.92 ± 0.18 | 163.91 ± 0.83 | 3.95 ± 0.02 |
| Ursolic acid | 210.40 ± 1.29 | 161.48 ± 0.43 | 864.08 ± 0.90 | 21.40 ± 0.19 |
| Maslinic acid | 276.61 ± 0.88 | 269.74 ± 2.08 | 17.78 ± 0.13 | n.d. |
| Corosolic acid | 321.53 ± 0.84 | 201.67 ± 0.88 | 42.71 ± 0.22 | n.d. |
| Pomolic acid | 14.88 ± 0.01 | 16.57 ± 0.06 | 50.46 ± 0.27 | n.d. |
| Annurcoic acid | 251.89 ± 2.29 | 381.55 ± 2.54 | 128.36 ± 0.56 | 1.31 ± 0.01 |
| Tormentic acid | 1152.75 ± 10.35 | 1032.97 ± 7.05 | 16.25 ± 0.23 | 0.42 ± 0.01 |
| Σ triterpenic acid | 2277.85 ± 7.16 | 2098.91 ± 7.30 | 1283.55 ± 0.87 | 27.08 ± 0.22 |
| Total | 2439.82 ± 8.05 | 2286.73 ± 8.64 | 1328.15 ± 1.32 | 59.12 ± 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubitosa, F.; Fraternale, D.; Benayada, L.; De Bellis, R.; Gorassini, A.; Saltarelli, R.; Donati Zeppa, S.; Potenza, L. Anti-Inflammatory, Antioxidant, and Genoprotective Effects of Callus Cultures Obtained from the Pulp of Malus pumila cv Miller (Annurca Campana Apple). Foods 2024, 13, 2036. https://doi.org/10.3390/foods13132036
Gubitosa F, Fraternale D, Benayada L, De Bellis R, Gorassini A, Saltarelli R, Donati Zeppa S, Potenza L. Anti-Inflammatory, Antioxidant, and Genoprotective Effects of Callus Cultures Obtained from the Pulp of Malus pumila cv Miller (Annurca Campana Apple). Foods. 2024; 13(13):2036. https://doi.org/10.3390/foods13132036
Chicago/Turabian StyleGubitosa, Federica, Daniele Fraternale, Leila Benayada, Roberta De Bellis, Andrea Gorassini, Roberta Saltarelli, Sabrina Donati Zeppa, and Lucia Potenza. 2024. "Anti-Inflammatory, Antioxidant, and Genoprotective Effects of Callus Cultures Obtained from the Pulp of Malus pumila cv Miller (Annurca Campana Apple)" Foods 13, no. 13: 2036. https://doi.org/10.3390/foods13132036
APA StyleGubitosa, F., Fraternale, D., Benayada, L., De Bellis, R., Gorassini, A., Saltarelli, R., Donati Zeppa, S., & Potenza, L. (2024). Anti-Inflammatory, Antioxidant, and Genoprotective Effects of Callus Cultures Obtained from the Pulp of Malus pumila cv Miller (Annurca Campana Apple). Foods, 13(13), 2036. https://doi.org/10.3390/foods13132036







