Comparison of the Lipid Composition of Milk Fat Globules in Goat (Capra hircus) Milk during Different Lactations and Human Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Reagents and Chemicals
2.3. Protein Measurement
2.4. Particle Size and Total Lipid Content Analysis
2.5. Lipid Analysis Methods
2.5.1. Lipid Extraction
2.5.2. Lipid Analysis by UHPLC-Q-TOF-MS
2.5.3. Lipid Identification and Quantification
2.6. Statistical Analysis
3. Results and Discussion
3.1. Interface Protein Composition in Goat Milk from Different Lactation Periods and Human Milk
3.2. Size Distribution in Goat Milk from Different Lactation Periods and Human Milk
3.3. MFG Lipid Composition in Goat Milk from Different Lactation Periods and Human Milk
3.3.1. Reliability of Analytical Methods
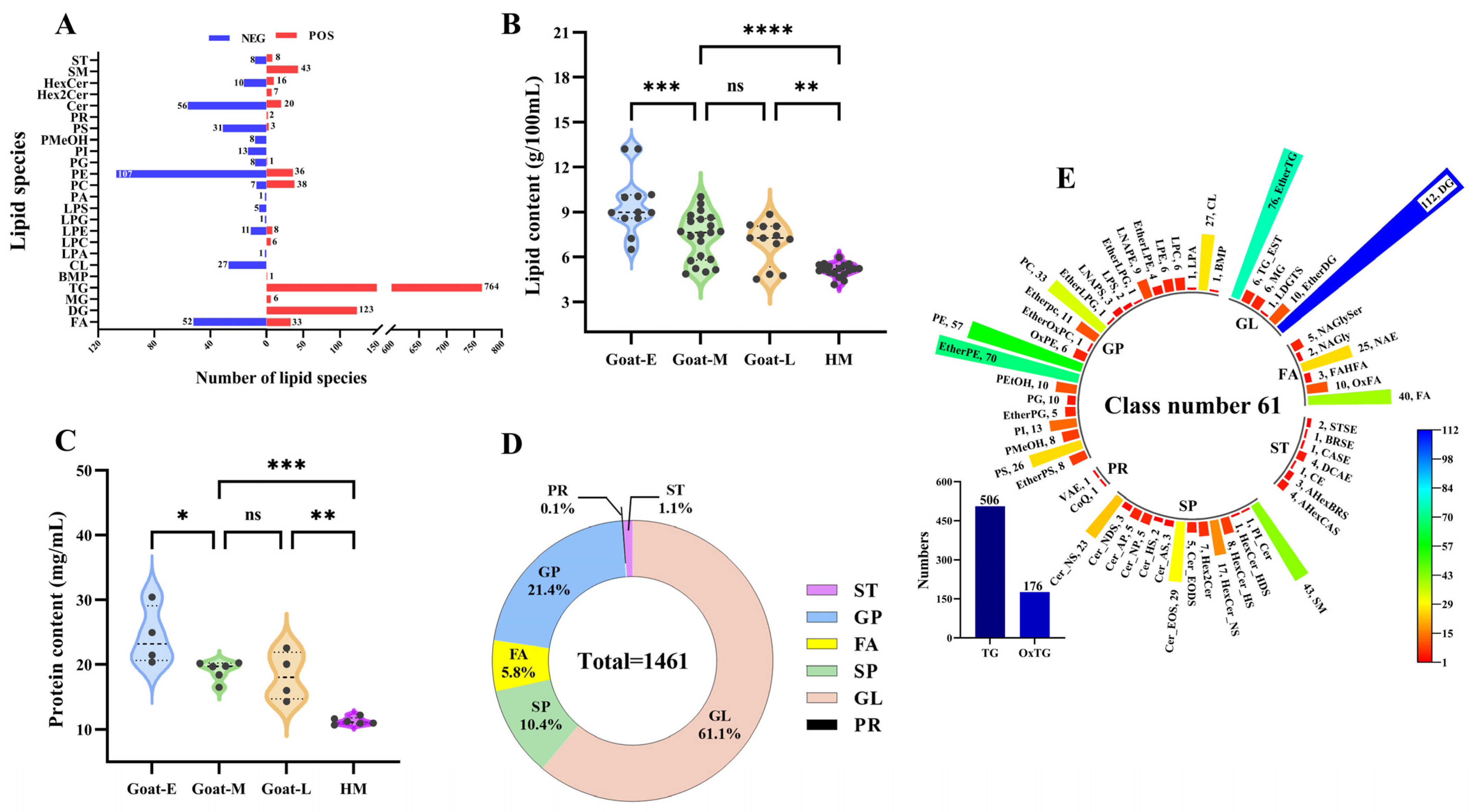
3.3.2. Total Lipid Levels and Species in Goat Milk and Human Milk
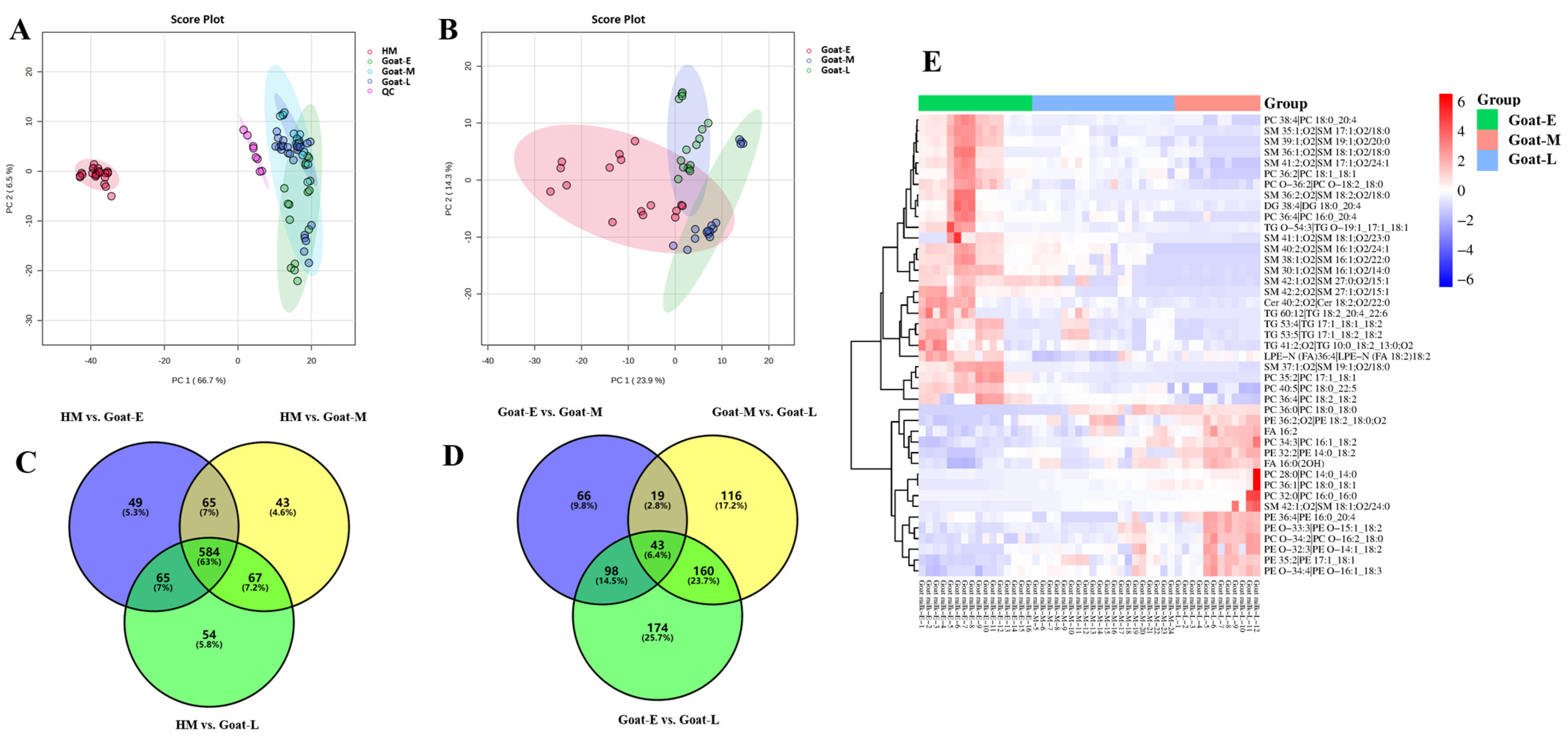
3.4. Difference in Lipids in Goat Milk and Human Milk
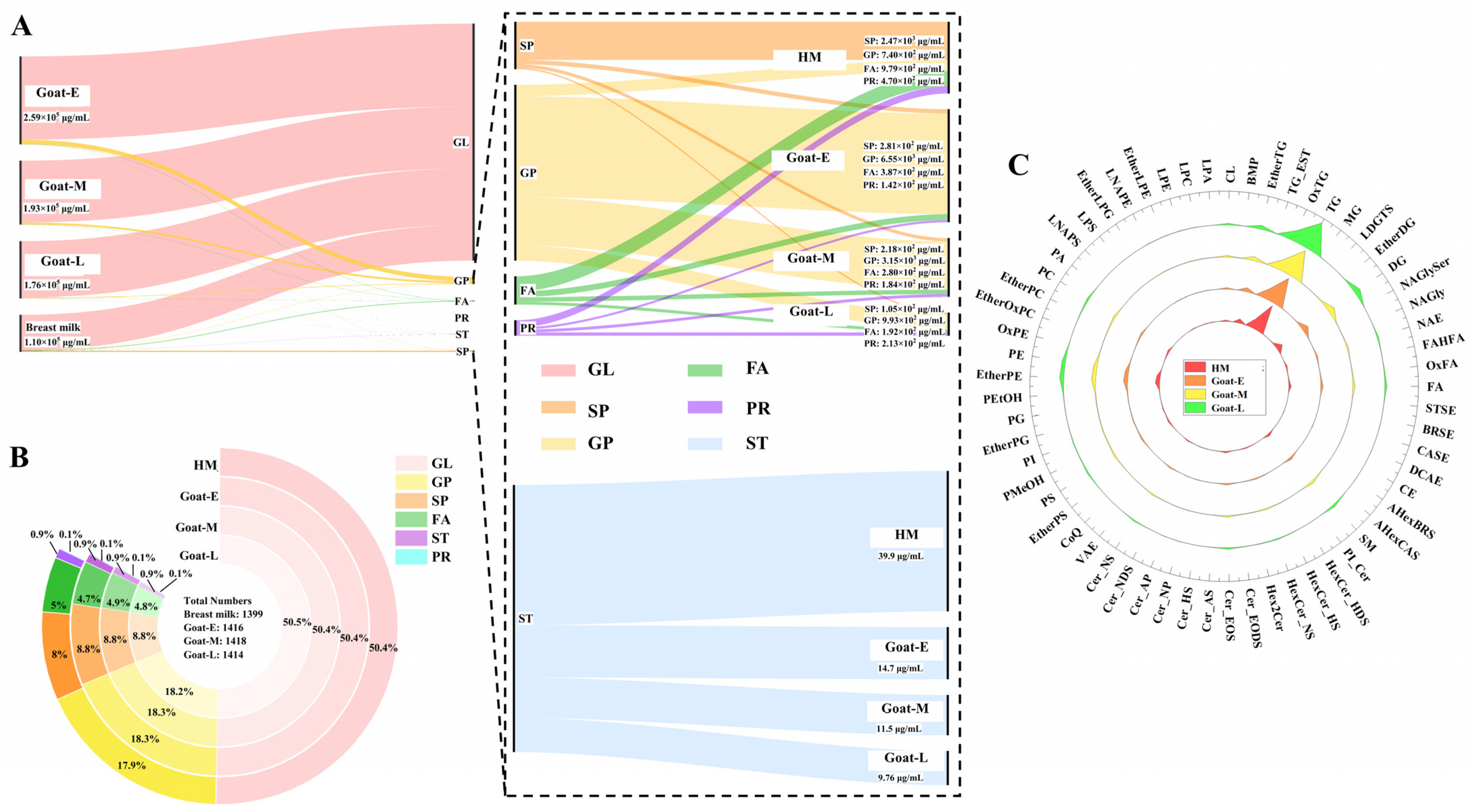
3.4.1. Difference in the Number and Content of Lipid Categories
3.4.2. Difference in the Number and Content of Lipid Subclasses
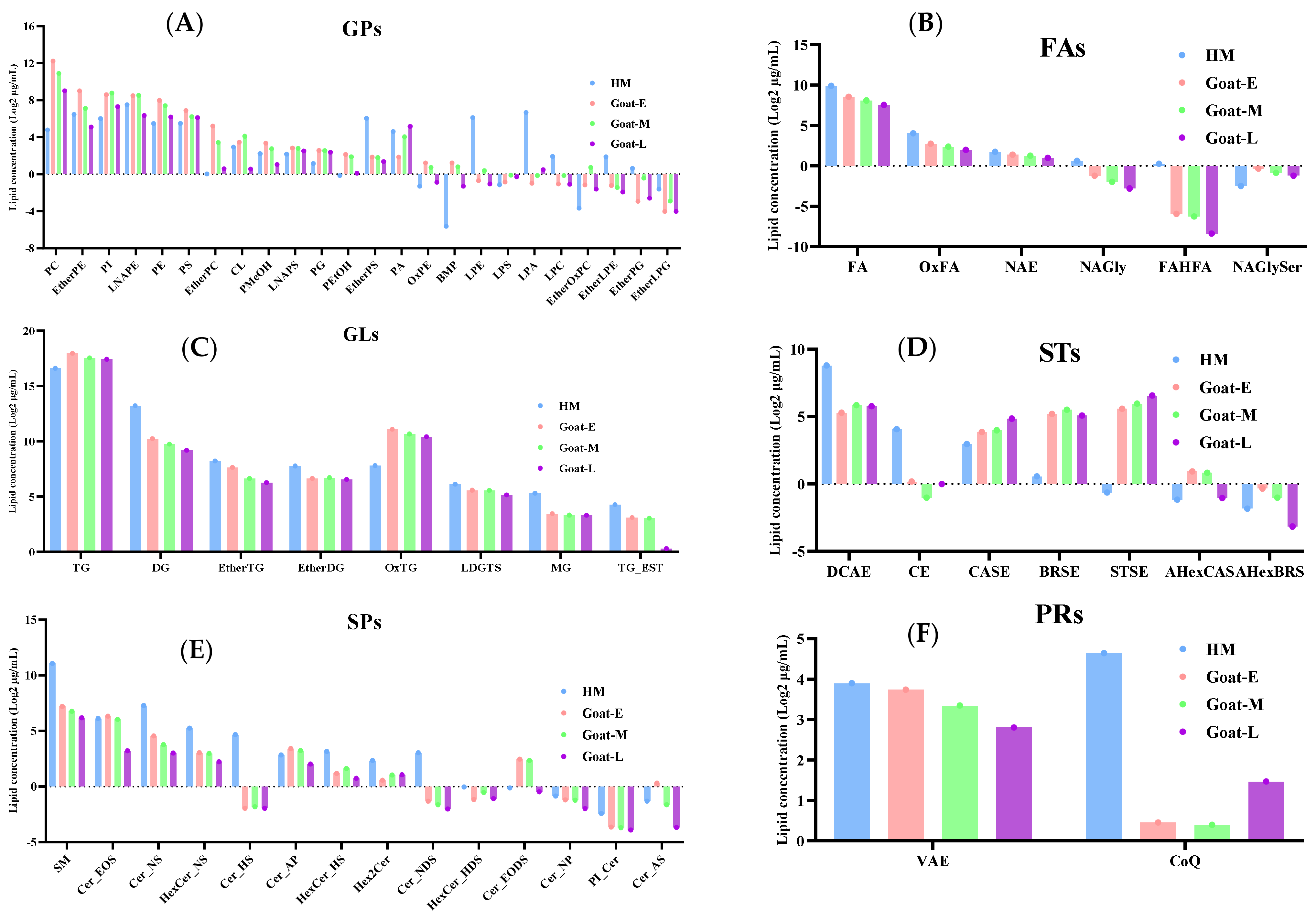
3.4.3. Differences in the Number and Content of Lipid Molecules
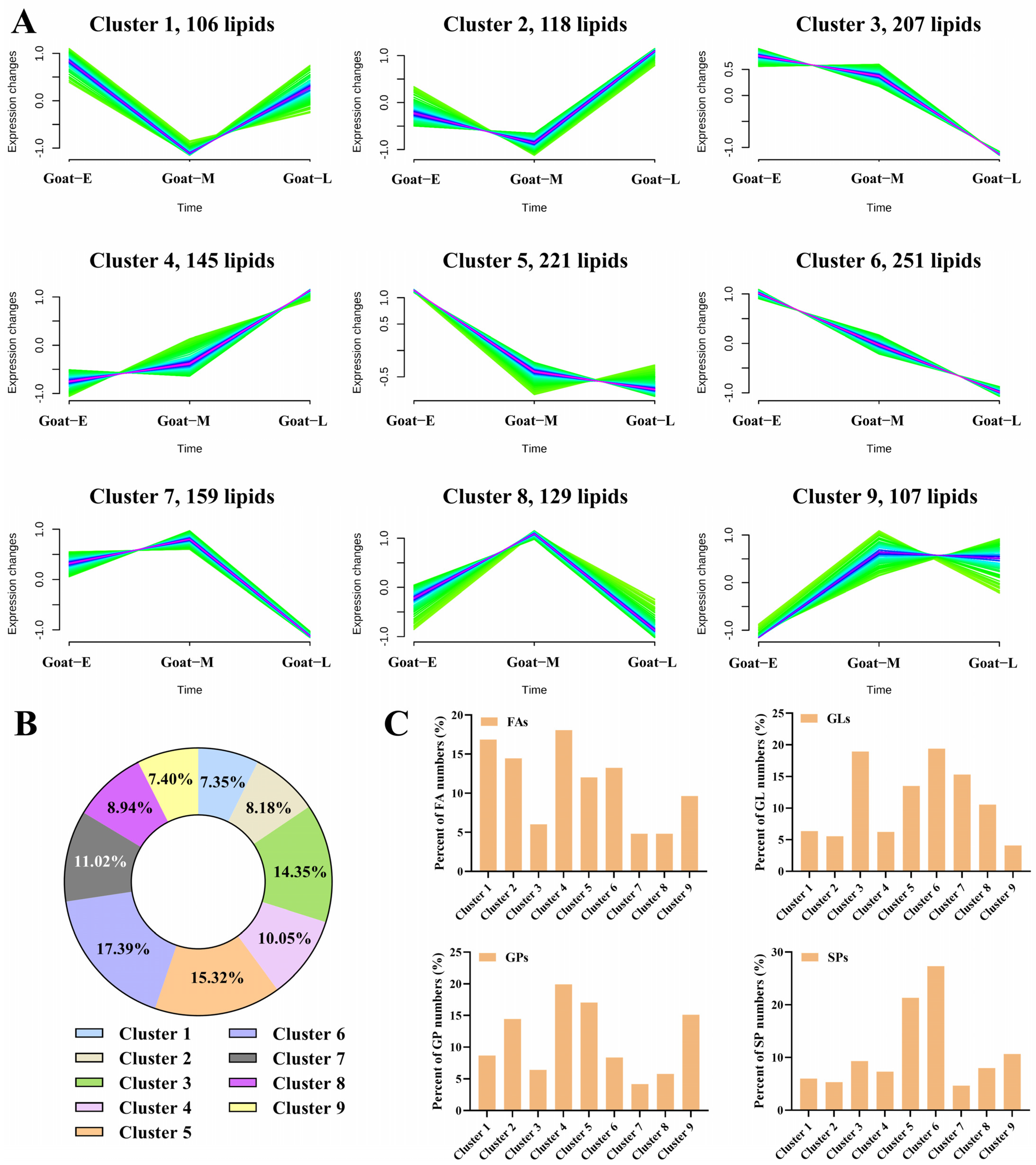
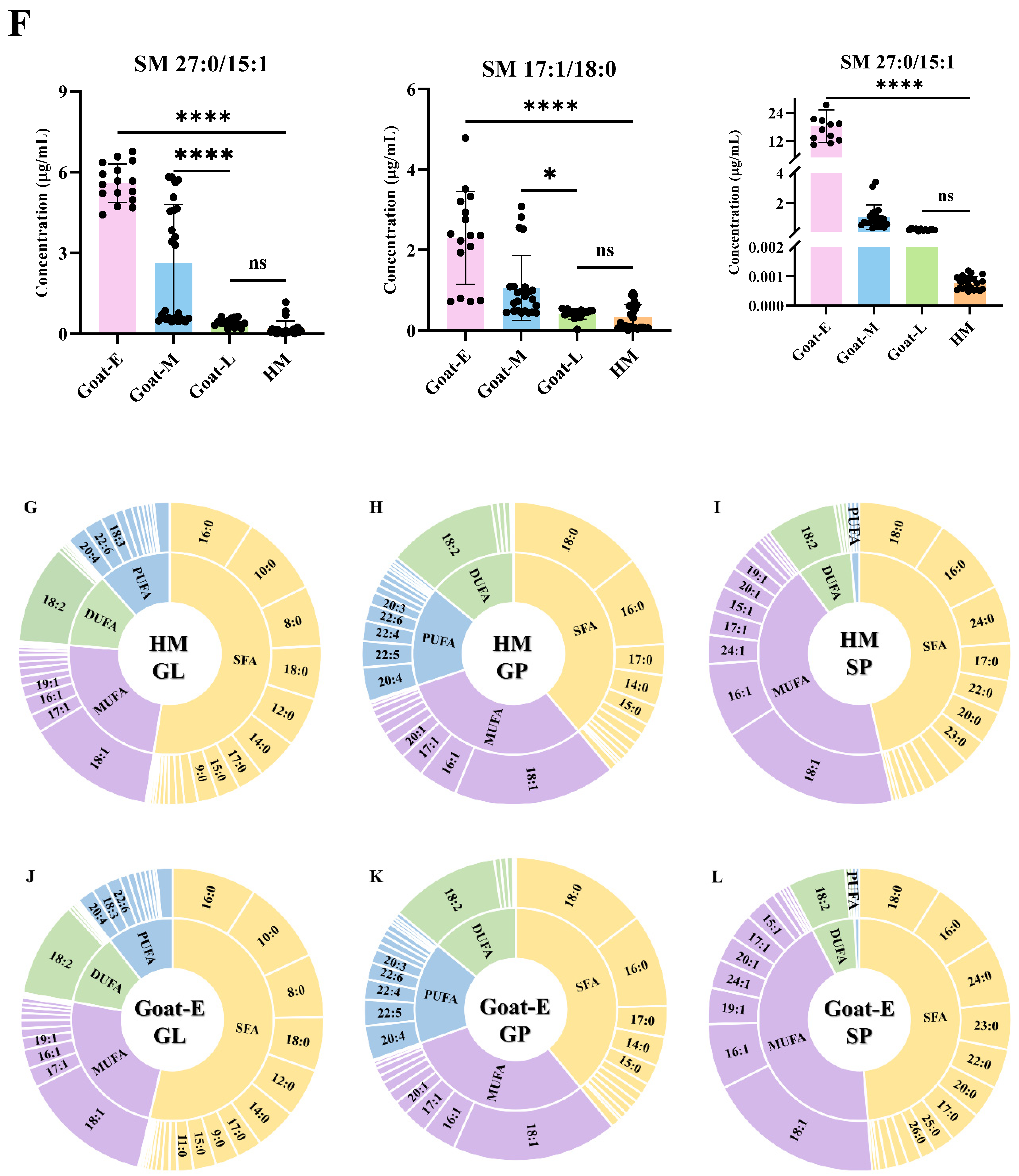
3.5. Differential Lipids in Goat Milk during Different Lactations and Human Milk
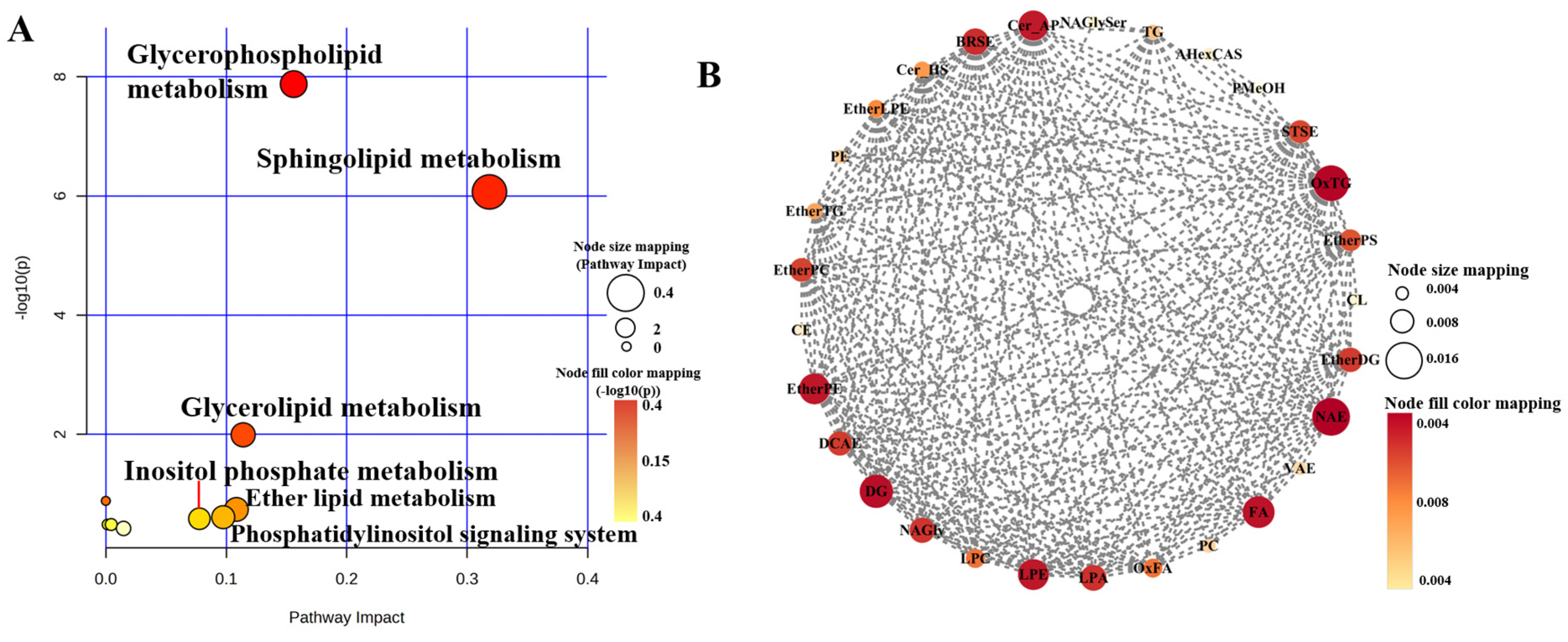
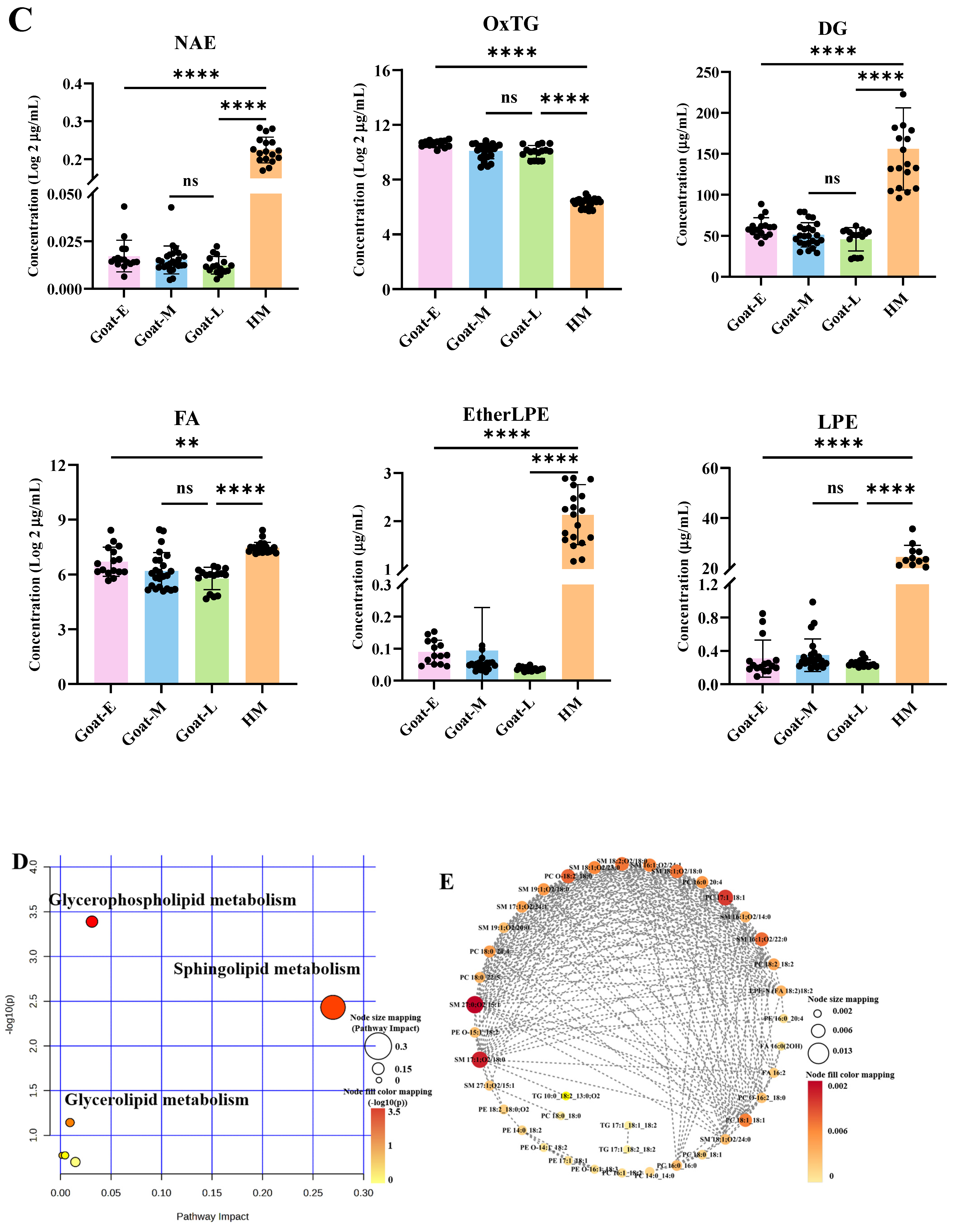
3.6. Analysis of Metabolic Pathways
3.7. Analysis of Fatty Acid Composition in GLs, GPs, and SPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, T.; Qi, C.; Dai, X.; Xia, Y.; Sun, C.; Sun, J.; Yu, R.; Zhou, Q.; Jin, Q.; Wei, W.; et al. Triacylglycerol Composition of Breast Milk during Different Lactation Stages. J. Agric. Food Chem. 2019, 67, 2272–2278. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, S.; Liu, L.; Pang, X.; Yang, Y.; Lu, J.; Lv, J. Differences in the Triacylglycerol and Fatty Acid Compositions of Human Colostrum and Mature Milk. J. Agric. Food Chem. 2018, 66, 4571–4579. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lei, T.; Li, G.; Liu, S.; Chu, X.; Hao, D.; Xiao, G.; Khan, A.A.; Haq, T.U.; Sameeh, M.Y.; et al. Rapid detection of micronutrient components in infant formula milk powder using near-infrared spectroscopy. Front. Nutr. 2023, 10, 1273374. [Google Scholar] [CrossRef]
- Ke, X.; Zhang, J.; Lai, S.; Chen, Q.; Zhang, Y.; Jiang, Y.; Mo, W.; Ren, Y. Quantitative analysis of cow whole milk and whey powder adulteration percentage in goat and sheep milk products by isotopic dilution-ultra-high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Pulina, G.; Milan, M.J.; Lavin, M.P.; Theodoridis, A.; Morin, E.; Capote, J.; Thomas, D.L.; Francesconi, A.H.D.; Caja, G. Invited review: Current production trends, farm structures, and economics of the dairy sheep and goat sectors. J. Dairy. Sci. 2018, 101, 6715–6729. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Zhao, Q.; Qin, Y.; Zhang, J. Lipidomics analysis for identifying the geographical origin and lactation stage of goat milk. Food Chem. 2020, 309, 125765. [Google Scholar] [CrossRef]
- Felice, V.D.; Owens, R.A.; Kennedy, D.; Hogan, S.A.; Lane, J.A. Comparative Structural and Compositional Analyses of Cow, Buffalo, Goat and Sheep Cream. Foods 2021, 10, 2643. [Google Scholar] [CrossRef] [PubMed]
- Kuchtík, J. Changes of physico-chemical characteristics, somatic cell count and curd quality during lactation and their relationships in Lacaune ewes. Mljekarstvo Dairy 2017, 67, 138–145. [Google Scholar] [CrossRef]
- Thum, C.; Roy, N.C.; Everett, D.W.; McNabb, W.C. Variation in milk fat globule size and composition: A source of bioactives for human health. Crit. Rev. Food Sci. Nutr. 2021, 63, 87–113. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, X.; Fu, F.; Aziz, T.; Li, G.; Zhao, J.; Shah, S.; Xiao, G.; Gong, J.; He, G. Assessing the effect of powder characteristics of infant milk on the compressibility of milk powder compression molding. Food Sci. Nutr. 2023, 11, 4625–4633. [Google Scholar] [CrossRef]
- Wang, Y.; Aziz, T.; Hu, G.; Liu, J.; Min, Z.; Zhennai, Y.; Alharbi, M.; Alshammari, A.; Alasmari, A.F. Production, purification, and characterization of a novel milk-clotting metalloproteinase produced by Bacillus velezensis YH-1 isolated from traditional Jiuqu. LWT 2023, 184, 115080. [Google Scholar] [CrossRef]
- Zhao, X.; Cai, M.; Yang, Z.-J.; Luo, T.-Q.; Sarwar, A.; Megrous, S.; Aziz, T.; Yang, Z.-N. Purification and characterization of a novel milk-clotting enzyme produced by Bacillus amyloliquefaciens GSBa-1. Eur. Food Res. Technol. 2019, 245, 2447–2457. [Google Scholar] [CrossRef]
- Zhao, H.; Ali, U.; Ren, Q.; Yao, M.; Lai, T.; Naz, S.; Aziz, T.; Sameeh, M.Y.; Zhang, M.; Yang, Z. Integrated metabolomic analysis of Lactiplantibacillus plantarum NMGL2 reveals its survival and response to combinational cold and acidic conditions during storage of fermented milk. Food Biosci. 2023, 54, 102833. [Google Scholar] [CrossRef]
- Fan, R.; Zhang, W.; Zhao, X.; Ji, Z.; Du, Q.; Han, R.; Wang, J.; Yang, Y. Changes in milk fat globule physical properties and milk fatty acid composition throughout the lactation cycle of Laoshan goat. J. Anim. Sci. 2023, 101, skad005. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Z.; Kang, F.; Wu, K.; Ni, H.; Han, Y.; Yang, Y.; Fu, T.; Yang, G.; Gao, T.; et al. Is milk fat globule size correlated with milk fat content in Ruminants? Food Chem. 2024, 439, 138101. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, C.; Chen, Z.; Zhang, X.; Han, X.; Chen, L.; Hu, J.; Zhou, P. Effect of cold storage on human milk fat globule membrane: Microstructure and proteomic analysis. Food Biosci. 2023, 55, 103096. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, L.; Zhu, Y.; Han, M.; Wang, Y.; Tang, J.; Zhou, P. Changes in Caprine Milk Fat Globule Membrane Proteins after Heat Treatment Using a Label-Free Proteomics Technique. Foods 2022, 11, 2705. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Liu, Q.; Zhao, J.; Qiao, W.; Liu, B.; Yang, B.; Chen, L. Comparison of phospholipid composition and microstructure of milk fat globules contained in human milk and infant formulae. Food Chem. 2023, 415, 135762. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Lu, J.; Zhu, H.; Wu, G.; Zhang, S.; Lv, J.; Wei, W.; Wang, X. Preparation and characterization of infant formula concentrates: Simulation with human milk lipid composition and fat globule size and distribution. J. Food Eng. 2023, 359, 111696. [Google Scholar] [CrossRef]
- Yu, J.; Wu, Y.; Yao, D.; Song, S.; Zhang, H.; Xu, X.; Cheong, L.-Z. The effects of maternal and perinatal factors on human milk lipids composition. J. Food Compos. Anal. 2023, 123, 105596. [Google Scholar] [CrossRef]
- Thum, C.; Wall, C.; Day, L.; Szeto, I.M.Y.; Li, F.; Yan, Y.; Barnett, M.P.G. Changes in Human Milk Fat Globule Composition Throughout Lactation: A Review. Front. Nutr. 2022, 12, 835856. [Google Scholar] [CrossRef] [PubMed]
- NY/T2835-2015; Technical Specification for Feeding and Management of Dairy Goat. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2015.
- Wang, S.; Liu, Z.; Song, Y.; Zhang, Y.; Zhao, L.; Zhang, L.; Lu, X.; Wang, H.; Zhang, X.; Zhang, J.; et al. Characterization and comparison of lipids from human and ewe colostrum based on lipidomics analysis. Food Chem. 2023, 400, 133998. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, L.; Tan, C.P.; Zheng, Z.; Liu, Y. Comparative Lipidomic Analysis Reveals the Lactational Changes in the Lipid Profiles of Chinese Human Milk. J. Agric. Food Chem. 2023, 71, 5403–5416. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, X.; Li, D.; Zhang, X.; Wei, W.; Jin, Q.; Wang, X. Development of in vitro digestion simulation of gastrointestinal tract to evaluate lipolysis and proteolysis: Comparison of infant model digestion of breast milk and adult model digestion of cow milk. Food Hydrocoll. 2023, 142, 108859. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, L.; Wu, Y.; Zhou, P. Changes in milk fat globule membrane proteome after pasteurization in human, bovine and caprine species. Food Chem. 2019, 279, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Michalski, M.C.; Briard, V.; Michel, F. Optical parameters of milk fat globules for laser light scattering measurements. Le Lait 2001, 81, 787–796. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Q.; Kang, S.; Cao, X.; Zheng, Y.; Wu, J.; Wu, R.; Shao, J.; Yang, M.; Yue, X. Characterization and comparison of lipids in bovine colostrum and mature milk based on UHPLC-QTOF-MS lipidomics. Food Res. Int. 2020, 136, 109490. [Google Scholar] [CrossRef] [PubMed]
- SG, B.G.; Minami, Y.; Gowda, D.; Chiba, H.; Hui, S.P. Detection and characterization of lipids in eleven species of fish by non-targeted liquid chromatography/mass spectrometry. Food Chem. 2022, 393, 133402. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Liu, L.; da Zhang, H.; Zhang, Y.; Hao Chang, Y.; Zhu, Q.P. Comparative lipidomics analysis of human, bovine and caprine milk by UHPLC-Q-TOF-MS. Food Chem 2020, 310, 125865. [Google Scholar] [CrossRef]
- Fontecha, J.; Brink, L.; Wu, S.; Pouliot, Y.; Visioli, F.; Jiménez-Flores, R. Sources, Production, and Clinical Treatments of Milk Fat Globule Membrane for Infant Nutrition and Well-Being. Nutrients 2020, 12, 1607. [Google Scholar] [CrossRef] [PubMed]
- Liu Tingting, Z.G.; Liu, L. Recent progress in milk fat globule membrane. J. Dairy. Sci. Technol. 2019, 42, 45–50. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, L.; Tian, S.; Li, X.; Hussain, M.; Li, C.; Zhang, L.; Zhang, Q.; Leng, Y.; Jiang, S.; et al. Comparative analysis of interfacial composition and structure of fat globules in human milk and infant formulas. Food Hydrocoll. 2022, 124, 107290. [Google Scholar] [CrossRef]
- Martin, H.M.; Hancock, J.T.; Salisbury, V.; Harrison, R. Role of Xanthine Oxidoreductase as an Antimicrobial Agent. Infect. Immun. 2004, 72, 4933–4939. [Google Scholar] [CrossRef]
- Zou, Z.; Duley, J.A.; Cowley, D.M.; Bansal, N. Enzymes Indigenous to Milk: Xanthine Oxidoreductase. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Oxford, UK, 2022; pp. 701–705. [Google Scholar]
- Liang, C.; Yu, Z.; Zhu, G.; Li, Y.; Sun, X.; Jiang, H.; Du, Q.; Fan, R.; Wang, J.; Yang, Y.; et al. Changes in milk fat globule membrane proteins along lactation stage of Laoshan dairy goat. J. Integr. Agric. 2024, 23, 1737–1748. [Google Scholar] [CrossRef]
- Huang, Y.; Wei, T.; Chen, F.; Tan, C.; Gong, Z.; Wang, F.; Deng, Z.; Li, J. Effects of various thermal treatments on interfacial composition and physical properties of bovine milk fat globules. Food Res. Int. 2023, 167, 112580. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y. Analysis of Whey and Milk Fat Globule Membrane Proteomes and Digestion Properties in Goat/Cow Milk by Proteomics Technologies. Ph.D. Thesis, Jilin University, Changchun, China, 2020. [Google Scholar]
- Tari, N.R.; Fan, M.Z.; Archbold, T.; Kristo, E.; Guri, A.; Arranz, E.; Corredig, M. Effect of milk protein composition of a model infant formula on the physicochemical properties of in vivo gastric digestates. J. Dairy. Sci. 2018, 101, 2851–2861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, Z.; Liu, C.; Liu, Y. Lipid Profiling and Microstructure Characteristics of Goat Milk Fat from Different Stages of Lactation. J. Agric. Food Chem. 2020, 68, 7204–7213. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Padhi, E.; Hasegawa, Y.; Larke, J.; Parenti, M.; Wang, A.; Hernell, O.; Lönnerdal, B.; Slupsky, C. Compositional Dynamics of the Milk Fat Globule and Its Role in Infant Development. Front. Pediatr. 2018, 6, 313. [Google Scholar] [CrossRef]
- Song, S.; Liu, T.T.; Liang, X.; Liu, Z.Y.; Yishake, D.; Lu, X.T.; Yang, M.T.; Man, Q.Q.; Zhang, J.; Zhu, H.L. Profiling of phospholipid molecular species in human breast milk of Chinese mothers and comprehensive analysis of phospholipidomic characteristics at different lactation stages. Food Chem. 2021, 348, 129091. [Google Scholar] [CrossRef]
- Rowlett, V.W.; Mallampalli, V.; Karlstaedt, A.; Dowhan, W.; Taegtmeyer, H.; Margolin, W.; Vitrac, H. Impact of Membrane Phospholipid Alterations in Escherichia coli on Cellular Function and Bacterial Stress Adaptation. J. Bacteriol. 2017, 199, e00849-16. [Google Scholar] [CrossRef] [PubMed]
- Thiam, A.R.; Farese Jr, R.V.; Walther, T.C. The biophysics and cell biology of lipid droplets. Nat. Rev. Mol. Cell Biol. 2013, 14, 775–786. [Google Scholar] [CrossRef] [PubMed]
- McManaman, J.L. Formation of milk lipids: A molecular perspective. Clin. Lipidol. 2009, 4, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Okahashi, N.; Tsugawa, H.; Ogata, Y.; Ikeda, K.; Suda, W.; Arai, H.; Hattori, M.; Arita, M. Elucidation of Gut Microbiota-Associated Lipids Using LC-MS/MS and 16S rRNA Sequence Analyses. iScience 2020, 23, 101841. [Google Scholar] [CrossRef] [PubMed]
- Sakane, F.; Mizuno, S.; Takahashi, D.; Sakai, H. Where do substrates of diacylglycerol kinases come from? Diacylglycerol kinases utilize diacylglycerol species supplied from phosphatidylinositol turnover-independent pathways. Adv. Biol. Regul. 2018, 67, 101–108. [Google Scholar] [CrossRef]
- Bednarska-Makaruk, M.; Ługowska, A. Chapter 22—Rare monogenic disorders of cholesterol metabolism. In Cholesterol; Bukiya, A.N., Dopico, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 553–607. [Google Scholar]
- Yang, M.-T.; Lan, Q.-Y.; Liang, X.; Mao, Y.-Y.; Cai, X.-K.; Tian, F.; Liu, Z.-Y.; Li, X.; Zhao, Y.-R.; Zhu, H.-L. Lactational Changes of Phospholipids Content and Composition in Chinese Breast Milk. Nutrients 2022, 14, 1539. [Google Scholar] [CrossRef]
- Escoté, X.; Fajas, L. Metabolic adaptation to cancer growth: From the cell to the organism. Cancer Lett. 2015, 356, 171–175. [Google Scholar] [CrossRef]
- Wang, S.; Song, Y.; He, R.; Du, G.; Zhang, L.; Zhang, B.; Zhang, J.; Zhao, L.; Zhang, J.; Ge, W. A new insight into the polar lipid composition in mature breast milk and ewe milk with comparative lipidomics analysis. Food Res. Int. 2023, 170, 112977. [Google Scholar] [CrossRef]
- Ma, H.; He, Z.; Chen, J.; Zhang, X.; Song, P. Identifying of biomarkers associated with gastric cancer based on 11 topological analysis methods of CytoHubba. Sci. Rep. 2021, 11, 1331. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, W.-d.; Li, T.; Zhou, Z.; Luo, M.; Chen, X.; Cai, Y.; Zhu, Z.-J. Four-Dimensional Untargeted Profiling of N-Acylethanolamine Lipids in the Mouse Brain Using Ion Mobility–Mass Spectrometry. Anal. Chem. 2022, 94, 12472–12480. [Google Scholar] [CrossRef]
- Criscuolo, A.; Nepachalovich, P.; Garcia-del Rio, D.F.; Lange, M.; Ni, Z.; Baroni, M.; Cruciani, G.; Goracci, L.; Blüher, M.; Fedorova, M. Analytical and computational workflow for in-depth analysis of oxidized complex lipids in blood plasma. Nature Communications 2022, 13, 6547. [Google Scholar] [CrossRef] [PubMed]
- Custers, E.M.E.; Kiliaan, J. Dietary lipids from body to brain. Prog. Lipid Res. 2022, 85, 101144. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Vasconi, M.; Moretti, V.M.; Bellagamba, F. Fatty Acid Profile in Goat Milk from High- and Low-Input Conventional and Organic Systems. Animals 2019, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Potočki, S. Potential health benefits of sphingolipids in milk and dairy products. Mljekarstvo 2016, 66, 251–261. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Chen, R.; Qiao, Y.; Zhang, Q.; Li, Q.; Wang, X.; Pan, Y.; Li, S.; Wang, Z. Analysis for lipid nutrient differences in the milk of 13 species from a quantitative non-targeted lipidomics perspective. Food Chem. X 2023, 20, 101024. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kang, S.; Jung, B.-M.; Yi, H.; Jung, J.A.; Chang, N. Breast milk fatty acid composition and fatty acid intake of lactating mothers in South Korea. Br. J. Nutr. 2017, 117, 556–561. [Google Scholar] [CrossRef]
- Mazzocchi, A.; D’Oria, V.; Cosmi, V.; Bettocchi, S.; Milani, G.; Silano, M.; Agostoni, C. The Role of Lipids in Human Milk and Infant Formulae. Nutrients 2018, 10, 567. [Google Scholar] [CrossRef]

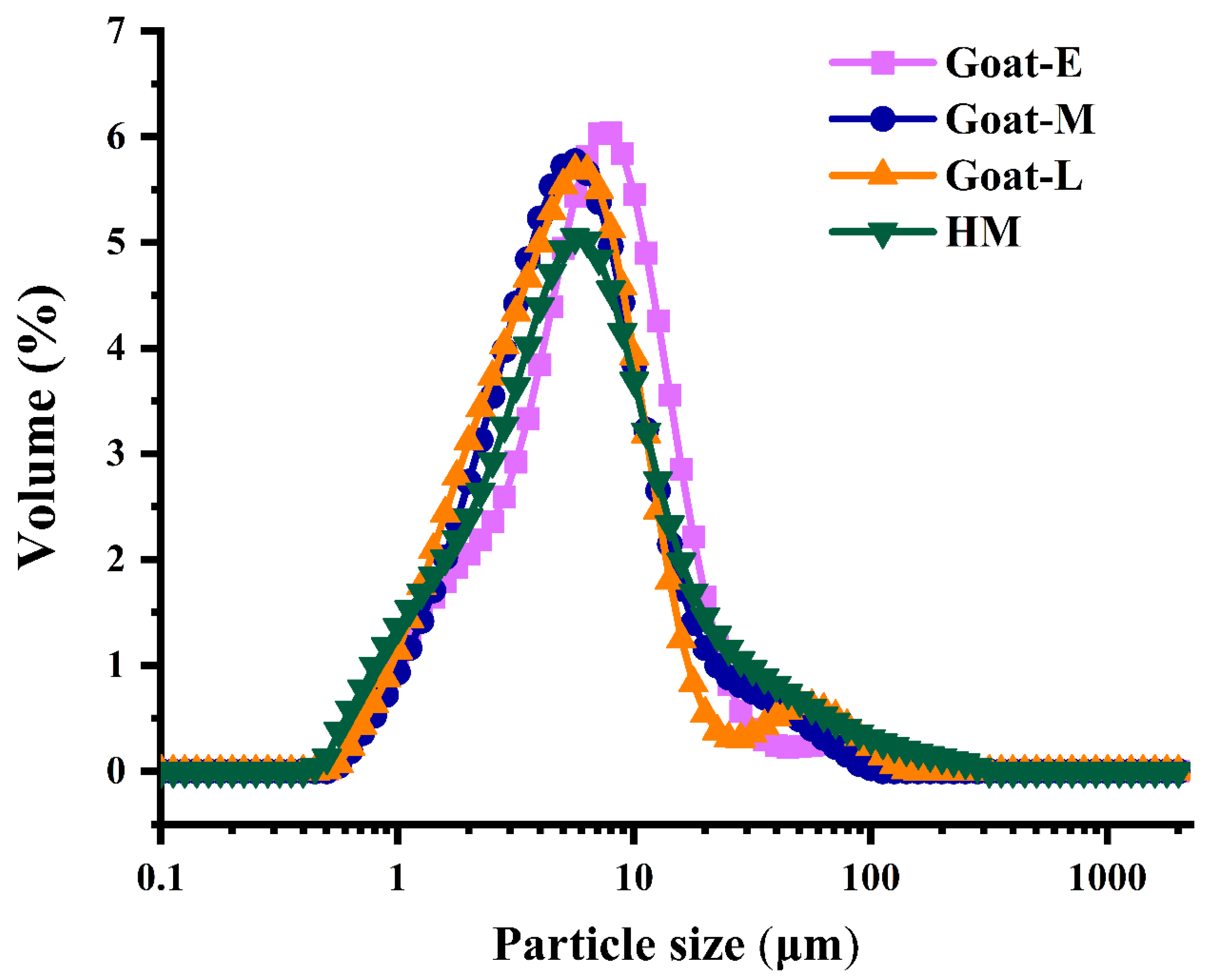
| Sample | Goat-E | Goat-M | Goat-L | HM |
|---|---|---|---|---|
| Particle size D4,3 (μm) | 6.03 ± 0.01 a | 5.17 ± 0.33 a | 5.31 ± 0.25 a | 4.70 ± 0.15 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, G.; Wang, T.; Li, X.; Gu, J.; Jia, Q.; Wang, Z.; Li, H.; Qian, Y.; Qiu, J. Comparison of the Lipid Composition of Milk Fat Globules in Goat (Capra hircus) Milk during Different Lactations and Human Milk. Foods 2024, 13, 1618. https://doi.org/10.3390/foods13111618
Liao G, Wang T, Li X, Gu J, Jia Q, Wang Z, Li H, Qian Y, Qiu J. Comparison of the Lipid Composition of Milk Fat Globules in Goat (Capra hircus) Milk during Different Lactations and Human Milk. Foods. 2024; 13(11):1618. https://doi.org/10.3390/foods13111618
Chicago/Turabian StyleLiao, Guangqin, Tiancai Wang, Xiabing Li, Jingyi Gu, Qi Jia, Zishuang Wang, Houru Li, Yongzhong Qian, and Jing Qiu. 2024. "Comparison of the Lipid Composition of Milk Fat Globules in Goat (Capra hircus) Milk during Different Lactations and Human Milk" Foods 13, no. 11: 1618. https://doi.org/10.3390/foods13111618
APA StyleLiao, G., Wang, T., Li, X., Gu, J., Jia, Q., Wang, Z., Li, H., Qian, Y., & Qiu, J. (2024). Comparison of the Lipid Composition of Milk Fat Globules in Goat (Capra hircus) Milk during Different Lactations and Human Milk. Foods, 13(11), 1618. https://doi.org/10.3390/foods13111618






