The Effect of Nanoscale Modification of Nisin by Different Milk-Derived Proteins on Its Physicochemical Properties and Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Different Milk Protein–Nisin Nanoparticles
2.3. Characterization of Different Milk Protein–Nisin Nanoparticles
2.4. Determination of Intermolecular Interaction Forces
2.5. Determination of Storage Stability
2.6. Determination of Emulsification Properties
2.7. Determination of Bacteriostatic Activity
2.7.1. Strain Recovery and Activation
2.7.2. Determination of the Zone of Inhibition
2.7.3. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.7.4. Determination of 24 h Short-Term Bacteriostatic Activity
2.7.5. Determination of 7 d Long-Term Bacteriostatic Activity
2.8. Effect of Milk Protein–Nisin Nanoparticles on Bacterial Cell Membranes
2.8.1. Determination of Leakage of Bacterial Contents
2.8.2. Observation of Bacterial Micromorphology
2.9. Statistical Analysis
3. Results and Discussion
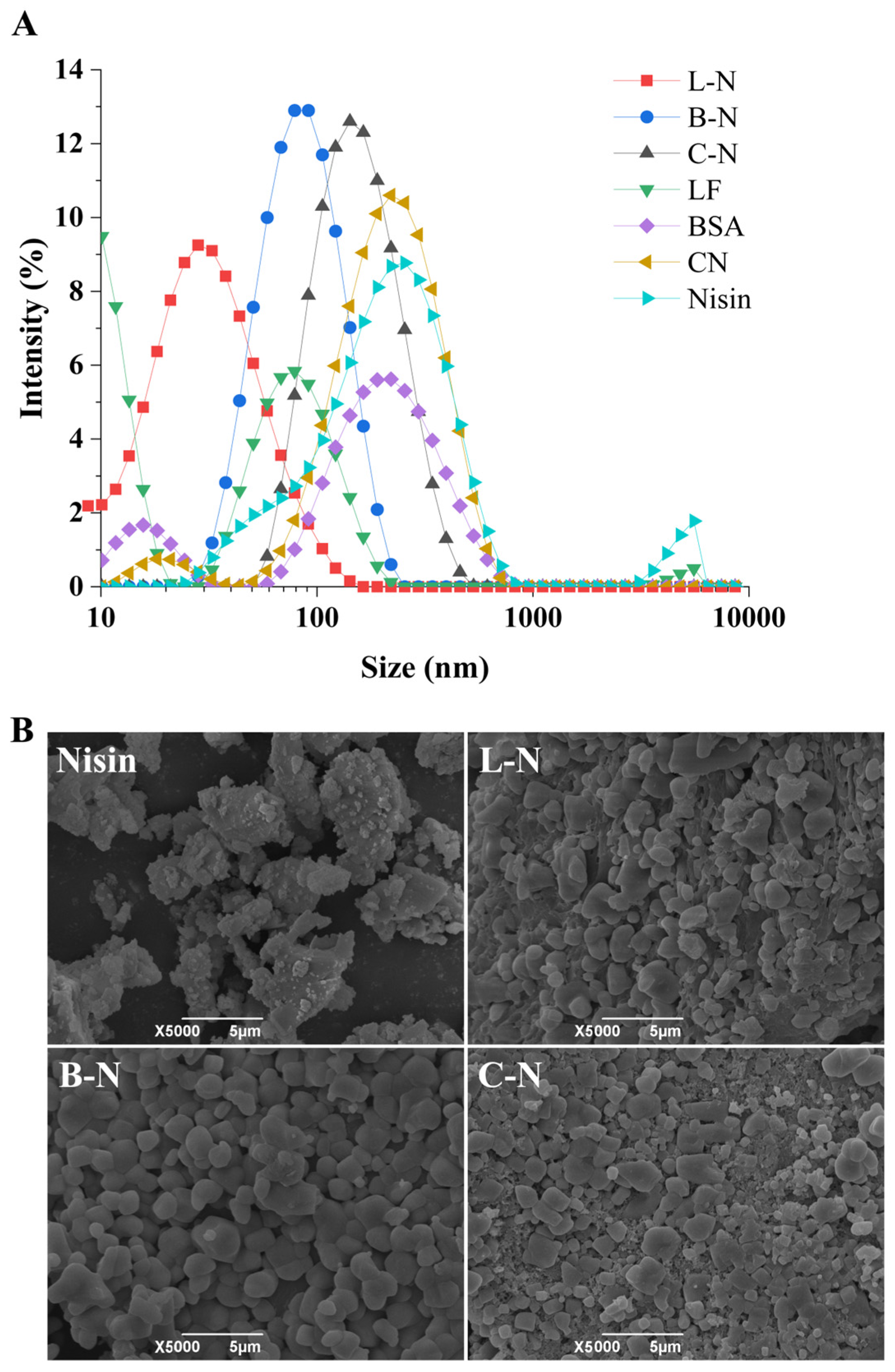
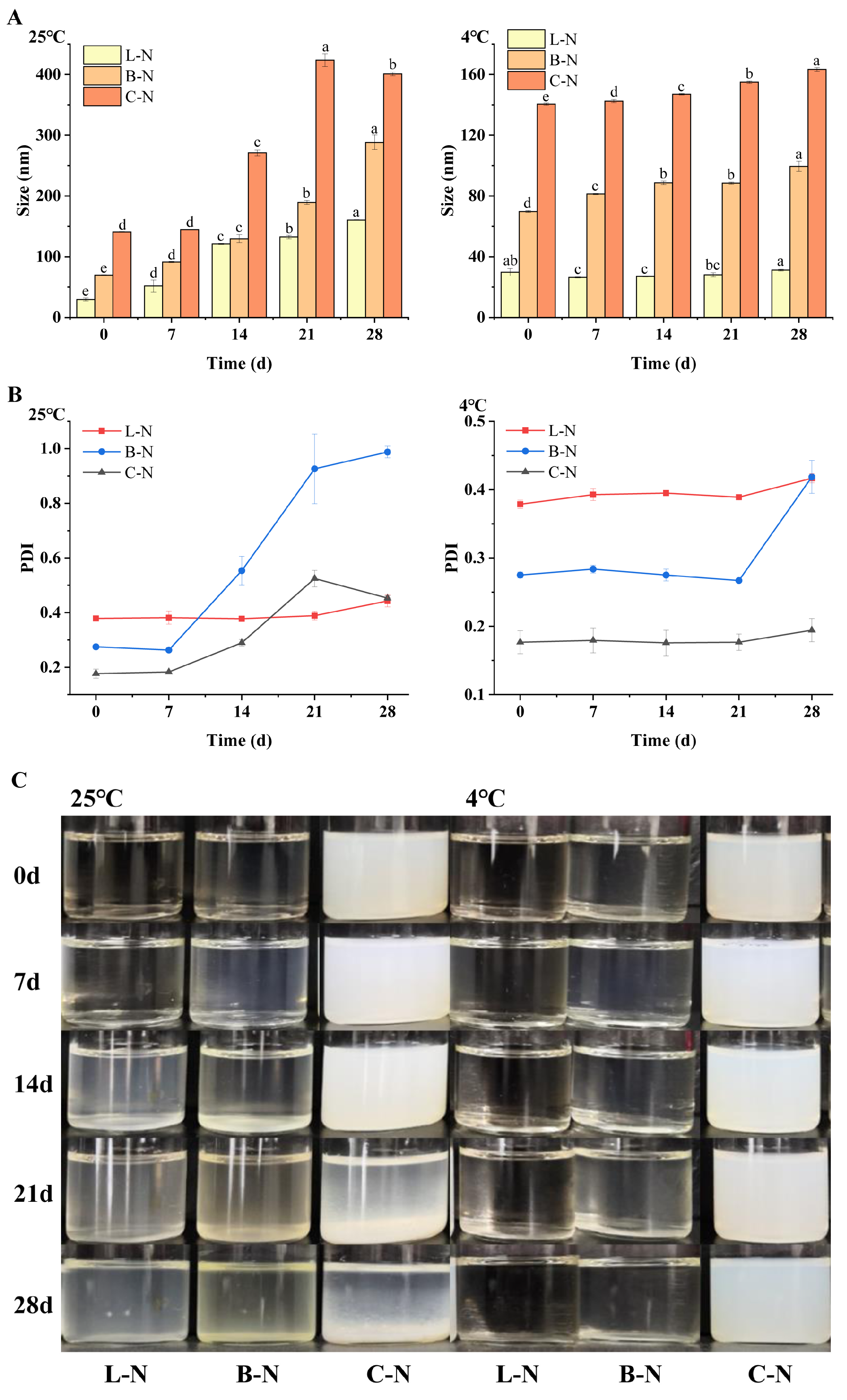
3.1. Particle Size and Apparent Morphology Observation
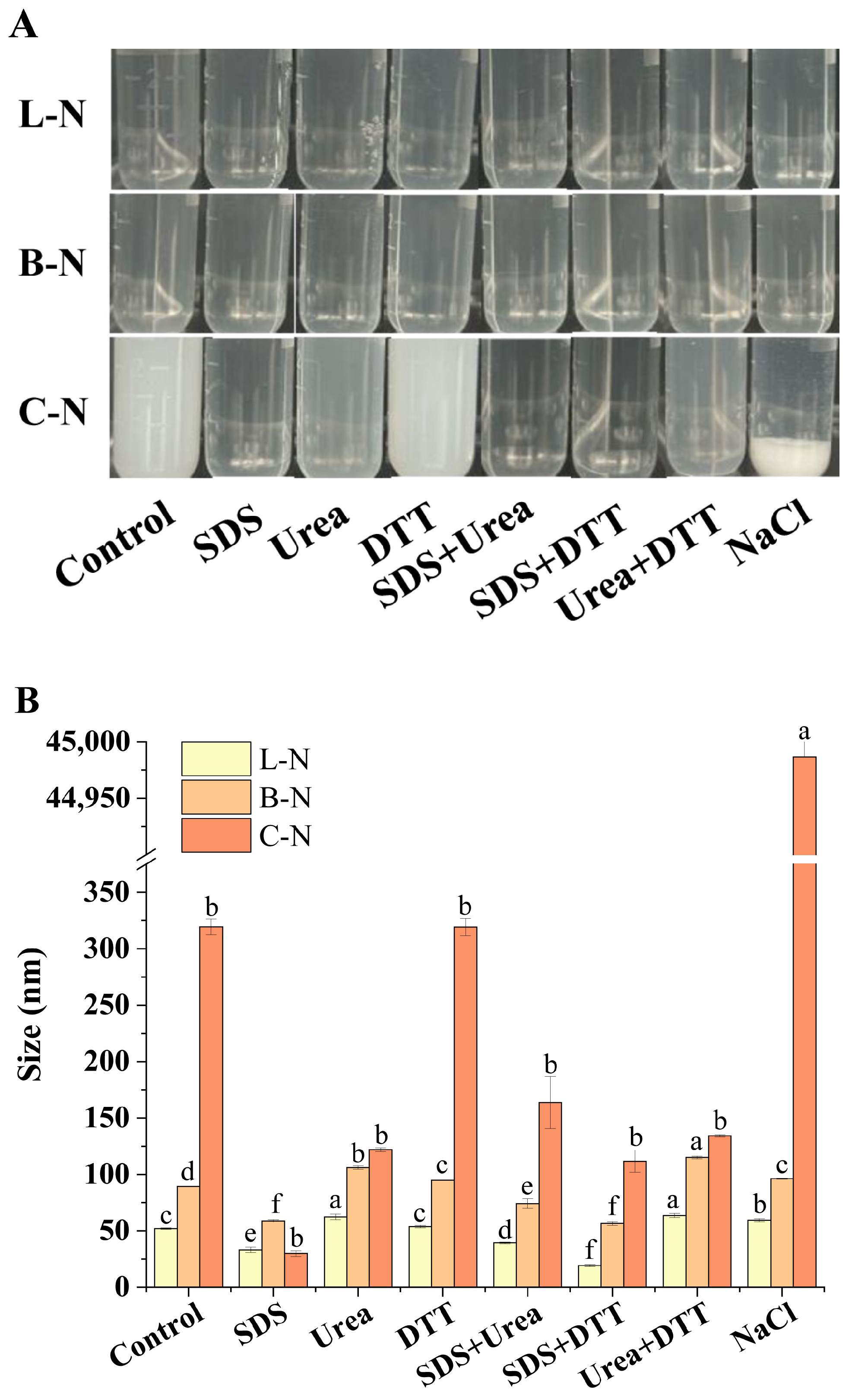
3.2. Analysis of Molecular Interactions among Nanoparticles
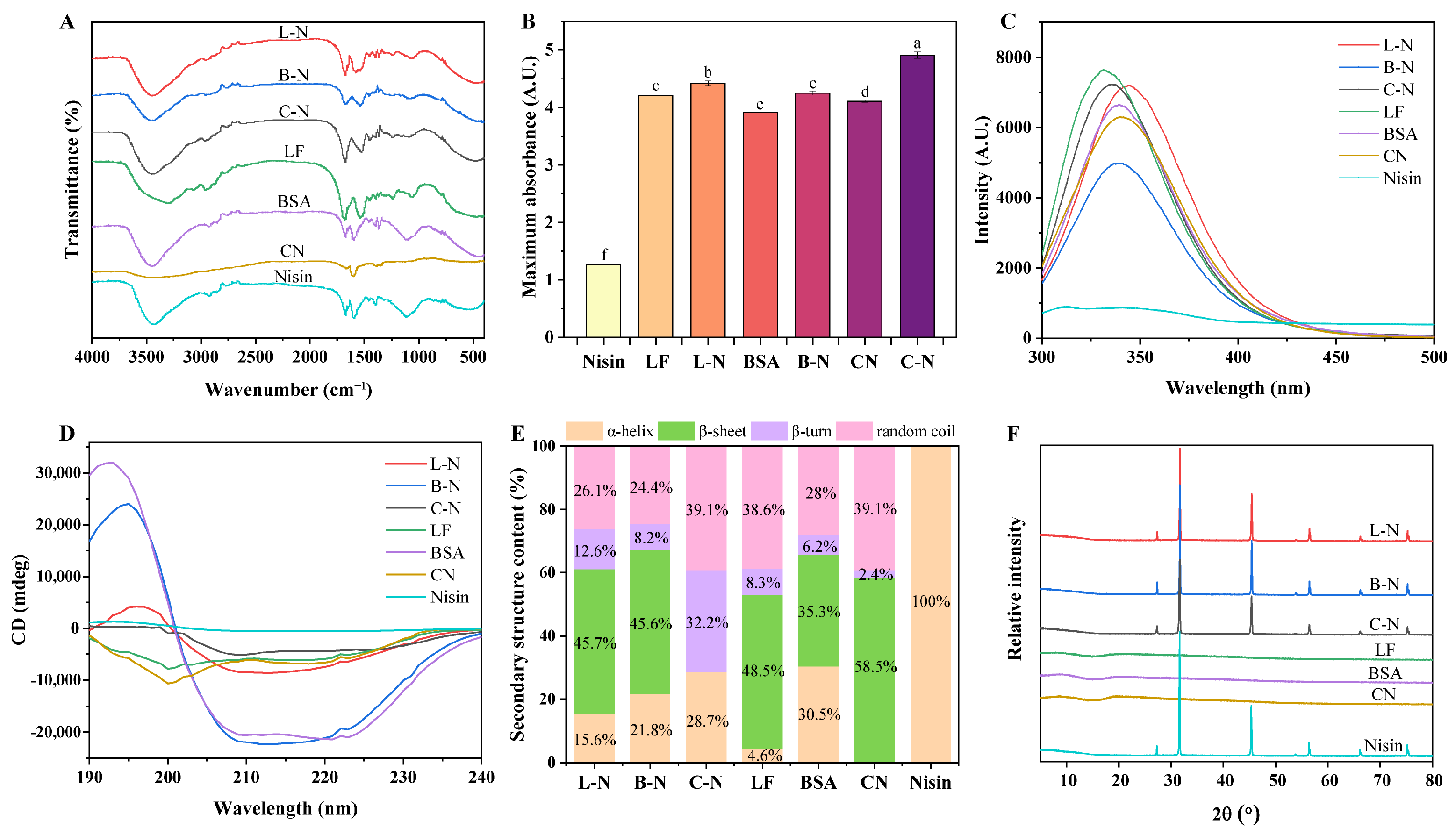
3.3. Spectral Analysis
3.3.1. Functional Group Analysis
3.3.2. UV Characteristic Analysis
3.3.3. Fluorescence Characteristic Analysis
3.3.4. Secondary Structural Analysis
3.3.5. Crystallinity Analysis
3.4. Stability Analysis
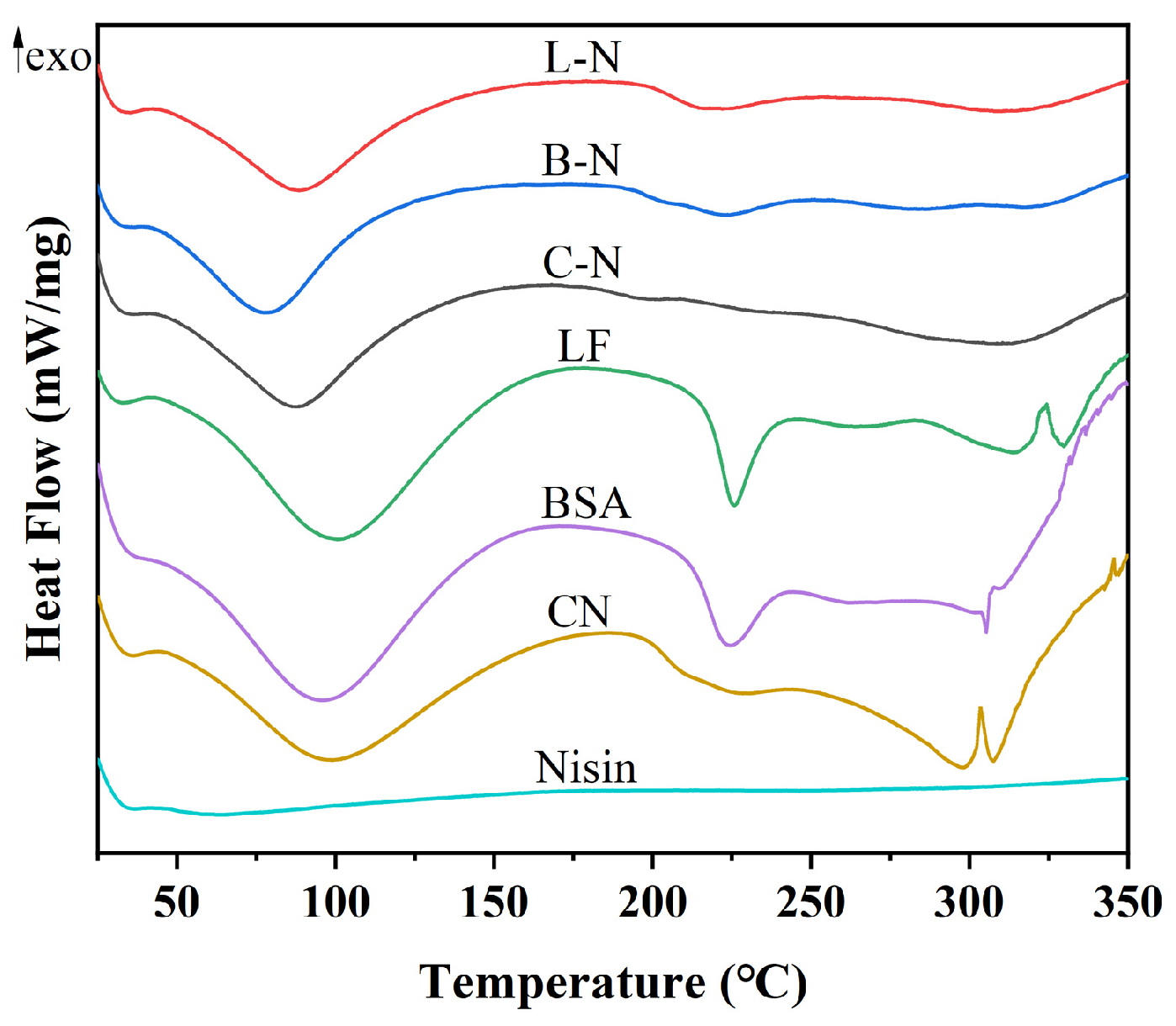
3.4.1. Thermal Stability
3.4.2. Storage Stability
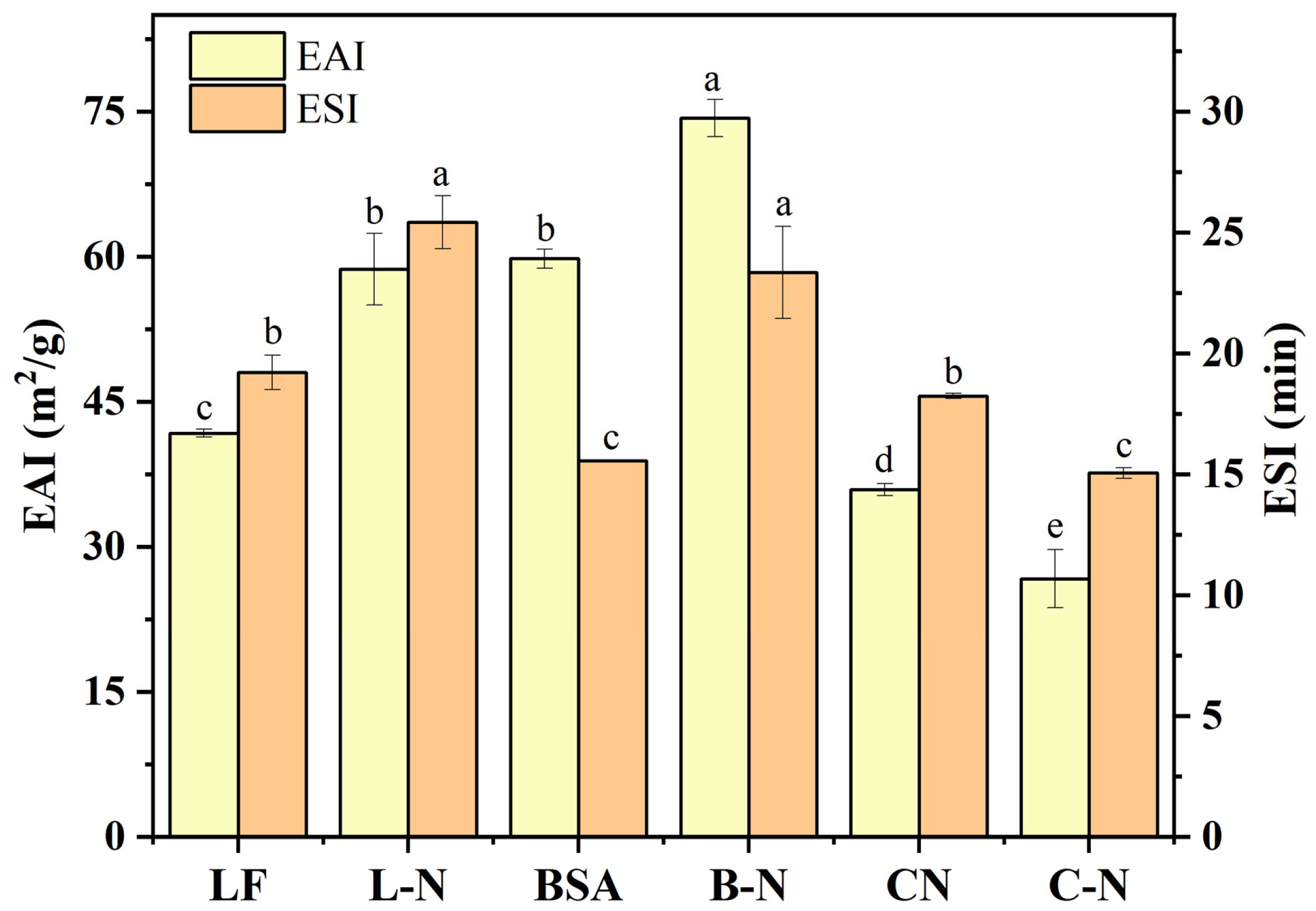
3.4.3. Emulsifying Properties
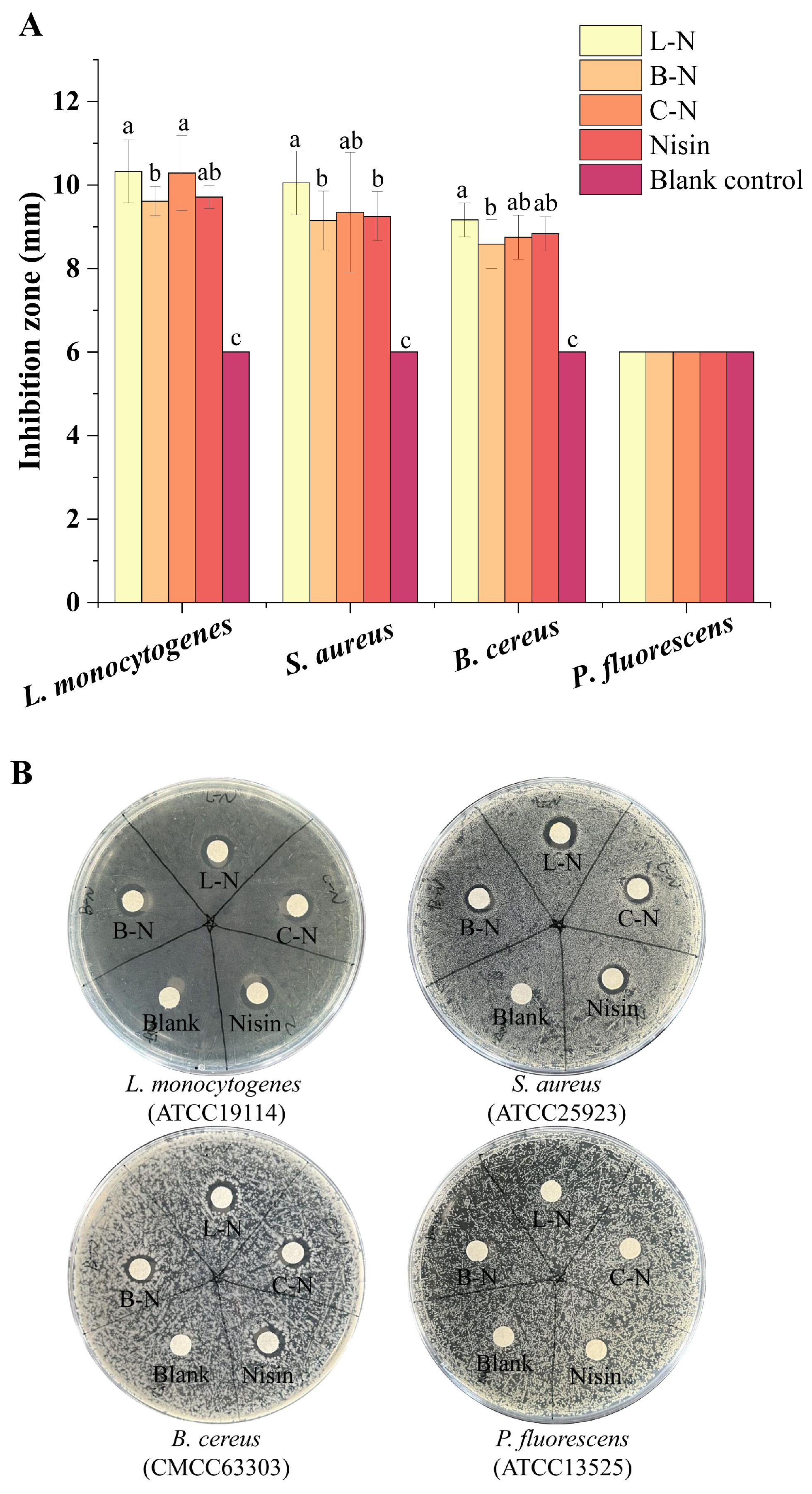
3.5. Bacteriostatic Activity of Nanoparticles
3.5.1. Bacteriostatic Circle
3.5.2. MIC and MBC
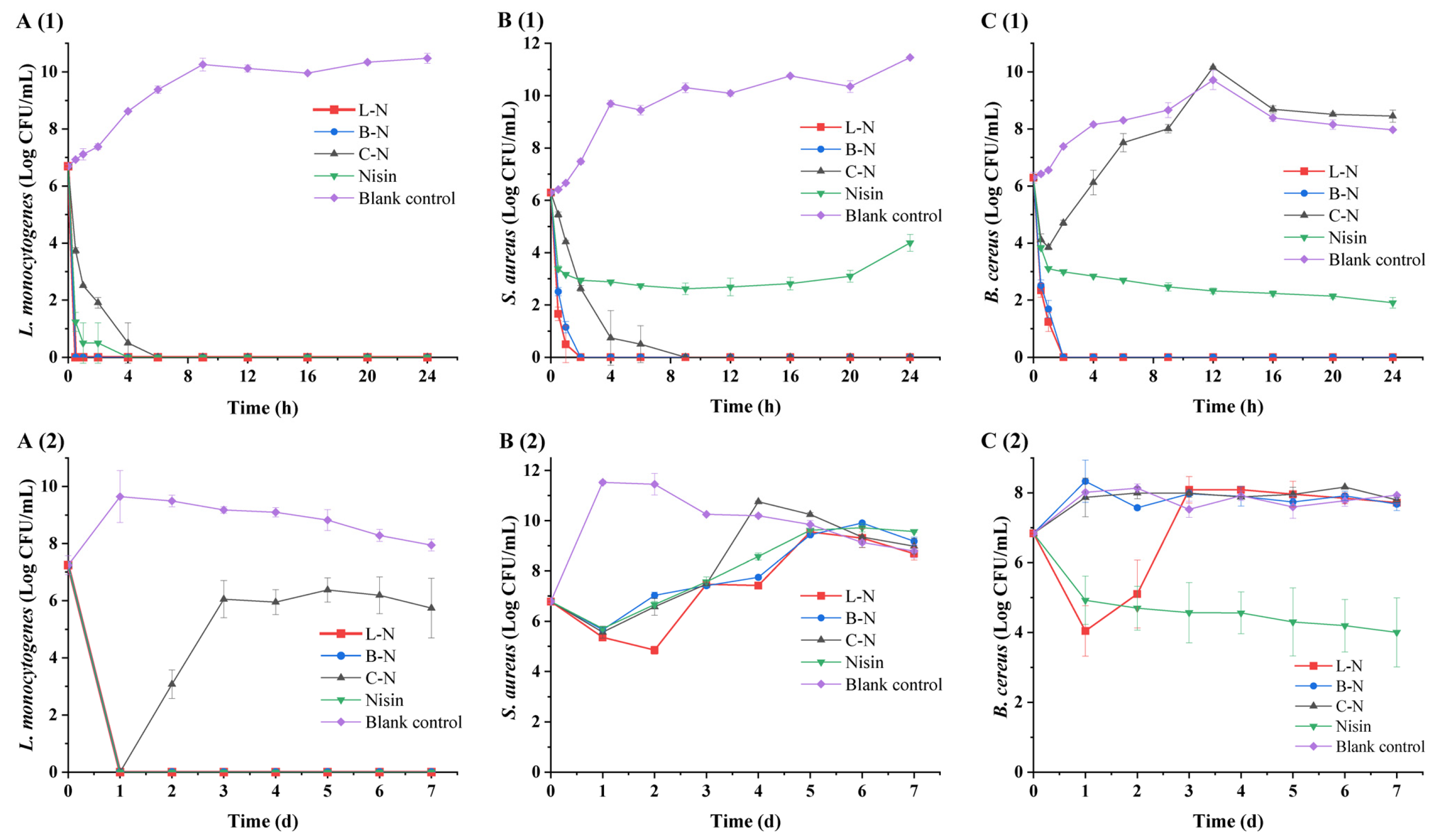
3.5.3. Twenty-Four-Hour Short-Term Bacteriostatic Activity and 7 d Long-Term Bacteriostatic Activity
3.6. Effect of Milk Protein–Nisin Nanoparticles on Bacterial Cell Membranes
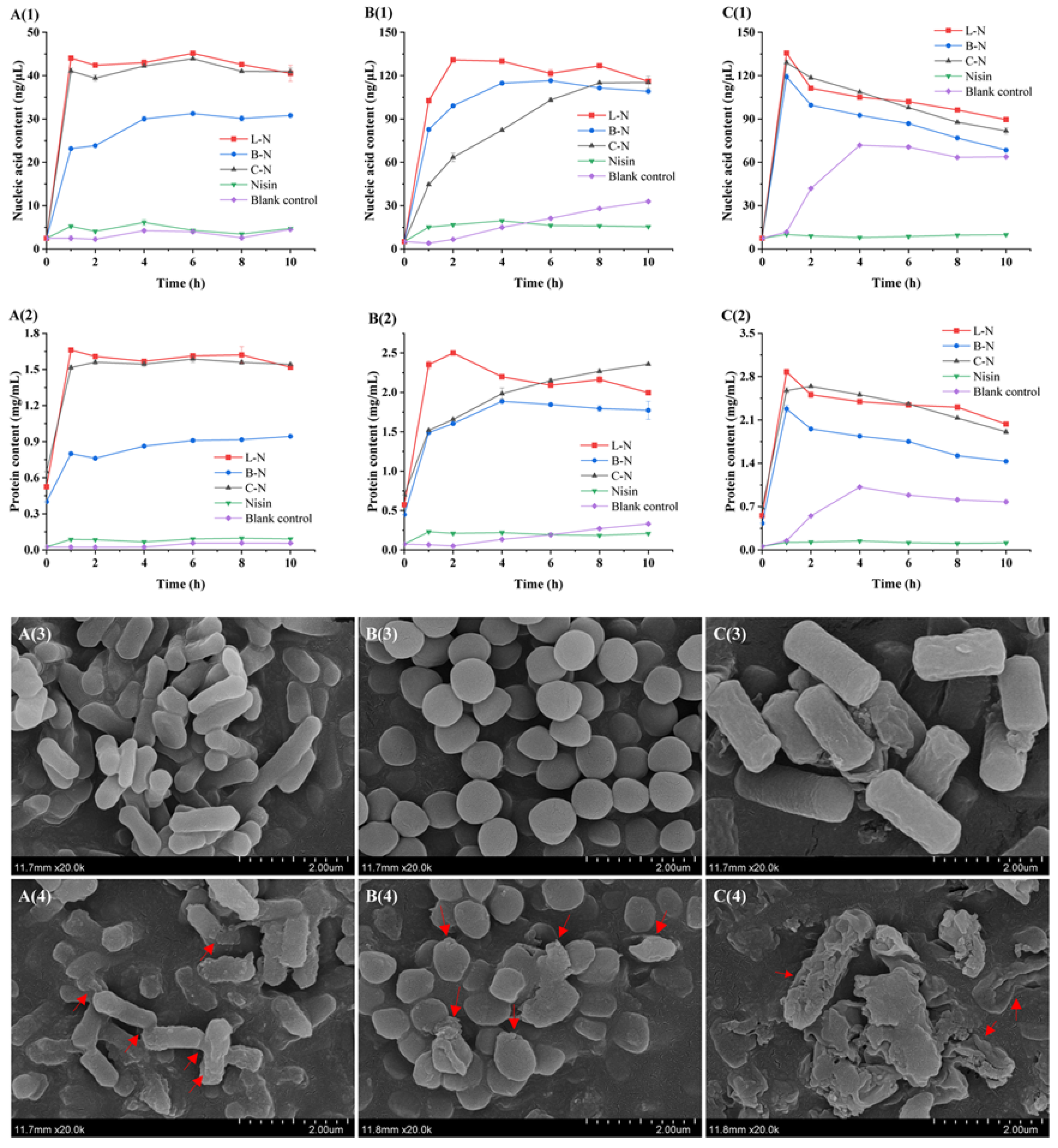
3.6.1. Analysis of Bacterial Content Leakage
3.6.2. Analysis of Bacterial Micromorphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malaczewska, J.; Kaczorek-Lukowska, E. Nisin-A lantibiotic with immunomodulatory properties: A review. Peptides 2021, 137, 170479. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yang, W.; Lei, B.; Zhao, F.; Guo, L.; Qian, J. Synergistic antimicrobial effect of nisin-octanoic acid nanoemulsions against E. coli and S. aureus. Arch. Microbiol. 2023, 205, 203. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.E.; Mitelut, A.C.; Rapa, M.; Popescu, P.A.; Draghici, M.C.; Geicu-Cristea, M.; Popa, M.E. Antimicrobial Active Packaging Containing Nisin for Preservation of Products of Animal Origin: An Overview. Foods 2022, 11, 3820. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; Fernandez, M.; Ross, R.P.; Hill, C. After a century of nisin research—Where are we now? FEMS Microbiol. Rev. 2023, 47, fuad023. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Singh, P.; Joshi, A.S.; Tabassum, N.; Jeong, G.-J.; Bamunuarachchi, N.I.; Mijakovic, I.; Kim, Y.-M. Multiple potential strategies for the application of nisin and derivatives. Crit. Rev. Microbiol. 2023, 49, 628–657. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.-S.; Hussein, D.E.; Algammal, A.M.; George, T.T.; Jeandet, P.; Al-Snafi, A.E.; Tiwari, A.; Pagnossa, J.P.; Lima, C.M.; Thorat, N.D.; et al. Application of natural antimicrobials in food preservation: Recent views. Food Control 2021, 130, 108324. [Google Scholar] [CrossRef]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Bedard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef] [PubMed]
- Hashad, R.A.; Singla, R.; Bhangu, S.K.; Jap, E.; Zhu, H.; Peleg, A.Y.; Blakeway, L.; Hagemeyer, C.E.; Cavalieri, F.; Ashokkumar, M.; et al. Chemoenzymatic surface decoration of Nisin-shelled nanoemulsions: Novel targeted drug-nanocarriers for cancer applications. Ultrason. Sonochem. 2022, 90, 106183. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Gomaa, A.; Subirade, M.; Kheadr, E.; St-Gelais, D.; Fliss, I. Novel design for alginate/resistant starch microcapsules controlling nisin release. Int. J. Biol. Macromol. 2020, 153, 1186–1192. [Google Scholar] [CrossRef]
- Wu, M.; Dong, Q.; Yan, H.; Song, Y.; Liu, Y.; Hirata, T.; Li, Z. Bacteriostatic potential of nisin and sesamol combination against Listeria monocytogenes in chilled raw tuna fillets. LWT-Food Sci. Technol. 2023, 183, 114924. [Google Scholar] [CrossRef]
- Peng, Z.; Xiong, T.; Huang, T.; Xu, X.; Fan, P.; Qiao, B.; Xie, M. Factors affecting production and effectiveness, performance improvement and mechanisms of action of bacteriocins as food preservative. Crit. Rev. Food Sci. Nutr. 2023, 63, 12294–12307. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Delshadi, R.; Jafari, S.M.; Williams, L. Nanoencapsulated nisin: An engineered natural antimicrobial system for the food industry. Trends Food Sci. Technol. 2019, 94, 20–31. [Google Scholar] [CrossRef]
- Eghbal, N.; Viton, C.; Gharsallaoui, A. Nano and microencapsulation of bacteriocins for food applications: A review. Food Biosci. 2022, 50 Pt B, 102173. [Google Scholar] [CrossRef]
- Wu, M.; Ma, Y.; Dou, X.; Aslam, M.Z.; Liu, Y.; Xia, X.; Yang, S.; Wang, X.; Qin, X.; Hirata, T.; et al. A review of potential antibacterial activities of nisin against Listeria monocytogenes: The combined use of nisin shows more advantages than single use. Food Res. Int. 2023, 164, 112363. [Google Scholar] [CrossRef] [PubMed]
- Niaz, T.; Shabbir, S.; Noor, T.; Imran, M. Antimicrobial and antibiofilm potential of bacteriocin loaded nano-vesicles functionalized with rhamnolipids against foodborne pathogens. LWT-Food Sci. Technol. 2019, 116, 108583. [Google Scholar] [CrossRef]
- Lein, H.; Lin, J.; Chen, Z.; Shi, Z.; Niu, D.; Zeng, X.; Zhou, L.; Han, Z. The behavior of whey protein isolate-curcumin complex at the oil-water interface. Food Hydrocoll. 2023, 145, 109046. [Google Scholar] [CrossRef]
- Kianfar, E. Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnol. 2021, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.M.; Bhandari, B.R.; Bansal, N. Functionality of bovine milk proteins and other factors in foaming properties of milk: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 4800–4820. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, S.P.; Kostic, A.Z.; Milincic, D.D.D.; Stanojevic, A.B.B.; Pesic, M.B. Composition of proteins in fresh whey as waste in tofu processing. J. Environ. Sci. Health Part B-Pestic. Food Contam. Agric. Wastes 2023, 58, 10–20. [Google Scholar] [CrossRef]
- Cao, X.; Ren, Y.; Lu, Q.; Wang, K.; Wu, Y.; Wang, Y.; Zhang, Y.; Cui, X.-S.; Yang, Z.; Chen, Z. Lactoferrin: A glycoprotein that plays an active role in human health. Front. Nutr. 2023, 9, 1018336. [Google Scholar] [CrossRef]
- Ohradanova-Repic, A.; Prazenicova, R.; Gebetsberger, L.; Moskalets, T.; Skrabana, R.; Cehlar, O.; Tajti, G.; Stockinger, H.; Leksa, V. Time to Kill and Time to Heal: The Multifaceted Role of Lactoferrin and Lactoferricin in Host Defense. Pharmaceutics 2023, 15, 1056. [Google Scholar] [CrossRef]
- Xu, J.; Huang, Y.; Wei, Y.; Weng, X.; Wei, X. Study on the Interaction Mechanism of Theaflavin with Whey Protein: Multi-Spectroscopy Analysis and Molecular Docking. Foods 2023, 12, 1637. [Google Scholar] [CrossRef] [PubMed]
- Hasanvand, E.; Razavi, S.M.A. Fabrication and characterisation of milk proteins-chitosan complex coacervates. Int. Dairy J. 2023, 145, 105716. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H.; El-Shibiny, S. Formation and potential uses of milk proteins as nano delivery vehicles for nutraceuticals: A review. Int. J. Dairy Technol. 2012, 65, 13–21. [Google Scholar] [CrossRef]
- Tomadoni, B.; Capello, C.; Valencia, G.A.; Gutiérrez, T.J. Self-assembled proteins for food applications: A review. Trends Food Sci. Technol. 2020, 101, 1–16. [Google Scholar] [CrossRef]
- Zeng, R.; Lv, C.; Wang, C.; Zhao, G. Bionanomaterials based on protein self-assembly: Design and applications in biotechnology. Biotechnol. Adv. 2021, 52, 107835. [Google Scholar] [CrossRef]
- Anema, S.G. Spontaneous interaction of lactoferrin with casein micelles or individual caseins. J. R. Soc. N. Zealand 2018, 48, 89–110. [Google Scholar] [CrossRef]
- Yang, W.; Liang, X.; Xu, L.; Deng, C.; Jin, W.; Wang, X.; Kong, Y.; Duan, M.; Nei, Y.; Zeng, J.; et al. Structures, fabrication mechanisms, and emulsifying properties of self-assembled and spray-dried ternary complexes based on lactoferrin, oat β-glucan and curcumin: A comparison study. Food Res. Int. 2020, 131, 109048. [Google Scholar] [CrossRef]
- Comez, L.; Gentili, P.L.; Paolantoni, M.; Paciaroni, A.; Sassi, P. Heat-induced self-assembling of BSA at the isoelectric point. Int. J. Biol. Macromol. 2021, 177, 40–47. [Google Scholar] [CrossRef]
- Ouyang, Y.; Chen, L.; Qian, L.; Lin, X.; Fan, X.; Teng, H.; Cao, H. Fabrication of caseins nanoparticles to improve the stability of cyanidin 3,-O-glucoside. Food Chem. 2020, 317, 126418. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, F.; Zhao, M.; Lin, L.; Ning, Z.; Sun, B. Soy peptide nanoparticles by ultrasound-induced self-assembly of large peptide aggregates and their role on emulsion stability. Food Hydrocoll. 2018, 74, 62–71. [Google Scholar] [CrossRef]
- Lin, L.; Luo, C.; Li, C.; Chen, X.; Cui, H. A Novel Biocompatible Ternary Nanoparticle with High Antibacterial Activity: Synthesis, Characterization, and Its Application in Beef Preservation. Foods 2022, 11, 438. [Google Scholar] [CrossRef]
- Sui, X.; Sun, H.; Qi, B.; Zhang, M.; Li, Y.; Jiang, L. Functional and conformational changes to soy proteins accompanying anthocyanins: Focus on covalent and non-covalent interactions. Food Chem. 2018, 245, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Dadmohammadi, Y.; Davachi, S.M.; Torabi, H.; Li, P.; Pomon, B.; Meletharayil, G.; Kapoor, R.; Abbaspourrad, A. Improvement of lactoferrin thermal stability by complex coacervation using soy soluble polysaccharides. Food Hydrocoll. 2022, 131, 107736. [Google Scholar] [CrossRef]
- Pandit, R.; Rai, M.; Santos, C.A. Enhanced antimicrobial activity of the food-protecting nisin peptide by bioconjugation with silver nanoparticles. Environ. Chem. Lett. 2017, 15, 443–452. [Google Scholar] [CrossRef]
- Bi, R.; Yue, L.; Niazi, S.; Khan, I.M.; Sun, D.; Wang, B.; Wang, Z.; Jiang, Q.; Xia, W. Facile synthesis and antibacterial activity of geraniol conjugated chitosan oligosaccharide derivatives. Carbohydr. Polym. 2021, 251, 117099. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, S.; Han, J.; Wu, J.; You, L.; Shi, X.; Wang, S. Synergistic antibacterial activity and mechanism of action of nisin/carvacrol combination against Staphylococcus aureus and their application in the infecting pasteurized milk. Food Chem. 2022, 380, 132009. [Google Scholar] [CrossRef]
- Huang, C.-M.; Chen, C.-H.; Pornpattananangkul, D.; Zhang, L.; Chan, M.; Hsieh, M.-F.; Zhang, L. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials 2011, 32, 214–221. [Google Scholar] [CrossRef]
- Wang, X.; Qian, J.; Yin, J.; Gong, F.; Guo, H. Preparation and antibacterial properties of high-methoxy pectin oligosaccharide -nisin nanoparticles. Eur. Polym. J. 2023, 200, 112472. [Google Scholar] [CrossRef]
- Cai, Z.; Chen, L.; Yu, X.; Yagoub, A.E.A.; Okonkwo, C.E.; Zhou, C. Effect of molecular weight of chitosan on the formation and properties of zein-nisin-chitosan nanocomplexes. Carbohydr. Polym. 2022, 292, 119664. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ibarra-Sanchez, L.A.; Miller, M.J.; Lee, Y. Fabrication of zein-modified starch nanoparticle complexes via microfluidic chip and encapsulation of nisin. Curr. Res. Food Sci. 2022, 5, 1110–1117. [Google Scholar] [CrossRef]
- Li, R.; Zeng, Z.; Fu, G.; Wan, Y.; Liu, C.; McClements, D.J. Formation and characterization of tannic acid/beta-glucan complexes: Influence of pH, ionic strength, and temperature. Food Res. Int. 2019, 120, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Krivorotova, T.; Cirkovas, A.; Maciulyte, S.; Staneviciene, R.; Budriene, S.; Serviene, E.; Sereikaite, J. Nisin-loaded pectin nanoparticles for food preservation. Food Hydrocoll. 2016, 54, 49–56. [Google Scholar] [CrossRef]
- Algharib, S.A.; Dawood, A.; Zhou, K.; Chen, D.; Li, C.; Meng, K.; Maa, M.K.; Ahmed, S.; Huang, L.; Xie, S. Designing, structural determination and biological effects of rifaximin loaded chitosan-carboxymethyl chitosan nanogel. Carbohydr. Polym. 2020, 248, 116782. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Xiao, Y.; Xia, L.; Li, L.; Jiang, S.; Jiang, S.; Wang, H. Colorimetric films incorporated with nisin and anthocyanins of pomegranate/Clitoria ternatea for shrimp freshness monitoring and retaining. Food Packag. Shelf Life 2022, 33, 100898. [Google Scholar] [CrossRef]
- Abid, Y.; Gharsallaoui, A.; Dumas, E.; Ghnimi, S.; Attia, H.; Azabou, S. Spray-drying microencapsulation of nisin by complexation with exopolysaccharides produced by probiotic Bacillus tequilensis-GM and Leuconostoc citreum-BMS. Colloids Surf. B-Biointerfaces 2019, 181, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, M.; Liu, H.; Liu, X. Properties of whey protein isolation/konjac glucomannan composite gels: Effects of deacetylation degrees. Int. J. Biol. Macromol. 2023, 238, 124138. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, L.; Zhang, H.; Liu, G.; Qin, X. Preparation, Characterization and Antioxidant Activity of Glycosylated Whey Protein Isolate/Proanthocyanidin Compounds. Foods 2023, 12, 2153. [Google Scholar] [CrossRef]
- Xiao, Y.; Qi, P.X.; Wickham, E.D. Interactions, induced by heating, of whey protein isolate (WPI) with sugar beet pectin (SBP) in solution: Comparisons with a dry-state Maillard reaction. Food Hydrocoll. 2018, 83, 61–71. [Google Scholar] [CrossRef]
- Amara, C.B.; Kim, L.; Oulahal, N.; Degraeve, P.; Gharsallaoui, A. Using complexation for the microencapsulation of nisin in biopolymer matrices by spray-drying. Food Chem. 2017, 236, 32–40. [Google Scholar] [CrossRef]
- Yu, X.; Cai, X.; Luo, L.; Wang, J.; Ma, M.; Wang, M.; Zeng, L. Influence of tea polyphenol and bovine serum albumin on tea cream formation by multiple spectroscopy methods and molecular docking. Food Chem. 2020, 333, 127432. [Google Scholar] [CrossRef]
- Liu, H.; Han, G.; Zhang, H.; Liu, Q.; Kong, B. Improving the physical and oxidative stability of emulsions based on the interfacial electrostatic effects between porcine bone protein hydrolysates and porcine bone protein hydrolysate-rutin conjugates. Food Hydrocoll. 2019, 94, 418–427. [Google Scholar] [CrossRef]
- Karbasi, M.; Askari, G. Modification of whey protein microgel particles with mono- oligo- and polysaccharides through the Maillard reaction: Effects on structural and techno-functional properties. Food Struct. 2021, 28, 100184. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Pei, Y.; Xiong, W.; Xu, W.; Li, B.; Li, J. Effect of substitution degree on carboxymethylcellulose interaction with lysozyme. Food Hydrocoll. 2017, 62, 222–229. [Google Scholar] [CrossRef]
- Wu, X.; Liu, A.; Wang, W.; Ye, R. Improved mechanical properties and thermal-stability of collagen fiber based film by crosslinking with casein, keratin or SPI: Effect of crosslinking process and concentrations of proteins. Int. J. Biol. Macromol. 2018, 109, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Pan, M.-H.; Chiou, Y.-S.; Li, Z.; Wei, S.; Yin, X.; Ding, B. Insights from alpha-Lactoalbumin and beta-Lactoglobulin into mechanisms of nanoliposome-whey protein interactions. Food Hydrocoll. 2022, 125, 107436. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Y.; Wang, Q.; Zhao, X.; Yang, H.; Gong, F.; Guo, H. Preparation and antimicrobial activity of pectin-chitosan embedding nisin microcapsules. Eur. Polym. J. 2021, 157, 110676. [Google Scholar] [CrossRef]
- Hu, Y.; Xing, K.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Xu, X.; Wang, J.; et al. Cyclodextrin carboxylate improves the stability and activity of nisin in a wider range of application conditions. Npj Sci. Food 2023, 7, 20. [Google Scholar] [CrossRef]
- Bonaccorso, A.; Carbone, C.; Tomasello, B.; Italiani, P.; Musumeci, T.; Puglisi, G.; Pignatello, R. Optimization of dextran sulfate/poly-L-lysine based nanogels polyelectrolyte complex for intranasal ovalbumin delivery. J. Drug Deliv. Sci. Technol. 2021, 65, 102678. [Google Scholar] [CrossRef]
- Cui, H.Y.; Wu, J.; Li, C.Z.; Lin, L. Anti-listeria effects of chitosan-coated nisin-silica liposome on Cheddar cheese. J. Dairy Sci. 2016, 99, 8598–8606. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jia, L.; Xie, Y.; Ma, W.; Yan, Z.; Liu, F.; Deng, J.; Zhu, A.; Siwei, X.; Su, W.; et al. Lyophilization process optimization and molecular dynamics simulation of mRNA-LNPs for SARS-CoV-2 vaccine. Npj Vaccines 2023, 8, 153. [Google Scholar] [CrossRef]
- Fathordoobady, F.; Sannikova, N.; Guo, Y.; Singh, A.; Kitts, D.D.; Pratap-Singh, A. Comparing microfluidics and ultrasonication as formulation methods for developing hempseed oil nanoemulsions for oral delivery applications. Sci. Rep. 2021, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Gruškienė, R.; Galinskaitė, A.; Kavleiskaja, T.; Stanevičienė, R.; Servienė, E.; Sereikaitė, J. Fucoidan as a carrier of antimicrobial peptide: Preparation and characterization of nisin-loaded particles. LWT 2024, 191, 115598. [Google Scholar] [CrossRef]
- Cheng, J.; Dudu, O.E.; Zhang, J.; Wang, Y.; Meng, L.; Wei, W.; Li, X.; Yan, T. Impact of binding interaction modes between whey protein concentrate and quercetin on protein structural and functional characteristics. Food Hydrocoll. 2023, 142, 108787. [Google Scholar] [CrossRef]
- Cheng, B.; Lin, J.; Zou, J.; Zhuang, Y.; Zhang, G.; Huang, B.; Fei, P. Preparation of curcumin-loaded pectin-nisin copolymer emulsion and evaluation of its stability. Int. J. Biol. Macromol. 2024, 254, 127812. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo Furtado, G.; Mantovani, R.A.; Consoli, L.; Hubinger, M.D.; da Cunha, R.L. Structural and emulsifying properties of sodium caseinate and lactoferrin influenced by ultrasound process. Food Hydrocoll. 2017, 63, 178–188. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Liu, Z.; Zhi, L.; Jiao, B.; Hu, H.; Ma, X.; Agyei, D.; Shi, A. Plant protein-based emulsifiers: Mechanisms, techniques for emulsification enhancement and applications. Food Hydrocoll. 2023, 144, 109008. [Google Scholar] [CrossRef]
- Östbring, K.; Matos, M.; Marefati, A.; Ahlström, C.; Gutiérrez, G. The effect of ph and storage temperature on the stability of emulsions stabilized by rapeseed proteins. Foods 2021, 10, 1657. [Google Scholar] [CrossRef]
- Li, Q.; Montalban-Lopez, M.; Kuipers, O.P. Increasing the Antimicrobial Activity of Nisin-Based Lantibiotics against Gram-Negative Pathogens. Appl. Environ. Microbiol. 2018, 84, e00052-18. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.D.; Ribeiro, A.R.; Silva, C.; Antunes, J.C.; Felgueiras, H.P. Combinatory effect of nisin antimicrobial peptide with bioactive molecules: A review. J. Drug Deliv. Sci. Technol. 2023, 91, 105246. [Google Scholar] [CrossRef]
- Liu, W.; Huang, K.; Tan, Z.; Wang, C.; Wen, T.; Huang, L.; Hang, F.; Xie, C.; Wang, S.; Li, K. Application of nisin-embedded pectin microcapsules for ‘Guiqi’mango fruit postharvest preservation. Food Packag. Shelf Life 2024, 42, 101261. [Google Scholar] [CrossRef]
- Carey, A.B.; Ashenden, A.; Koper, I. Model architectures for bacterial membranes. Biophys. Rev. 2022, 14, 111–143. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, J.; Yang, L.; Shi, F.; Yang, L.; Ye, M. Antibacterial activity and a membrane damage mechanism of Lachnum YM30 melanin against Vibrio parahaemolyticus and Staphylococcus aureus. Food Control 2017, 73, 1445–1451. [Google Scholar] [CrossRef]
- Khan, I.; Tango, C.N.; Miskeen, S.; Oh, D.H. Evaluation of nisin-loaded chitosan-monomethyl fumaric acid nanoparticles as a direct food additive. Carbohydr. Polym. 2018, 184, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Liang, Q.; Chen, H.; Zhou, Q.; Chi, Z.; Rashid, A.; Ma, H.; Ren, X. Insights into ultrasonic treatment on the properties of pullulan/oat protein/nisin composite film: Mechanical, structural and physicochemical properties. Food Chem. 2023, 402, 134237. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pan, D.; Yi, J.; Hao, L.; Kang, Q.; Liu, X.; Lu, L.; Lu, J. Protective effect of β-cyclodextrin on stability of nisin and corresponding interactions involved. Carbohydr. Polym. 2019, 223, 115115. [Google Scholar] [CrossRef]
- Quichaba, M.B.; Moreira, T.F.M.; De Oliveira, A.; De Carvalho, A.S.; De Menezes, J.L.; Gonçalves, O.H.; de Abreu Filho, B.A.; Leimann, F.V. Biopreservatives against foodborne bacteria: Combined effect of nisin and nanoncapsulated curcumin and co-encapsulation of nisin and curcumin. J. Food Sci. Technol.-Mysore 2023, 60, 581–589. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, H.; Tao, H.; Yu, B.; Cui, B.; Wang, Y. Ultrasound improves the physicochemical and foam properties of whey protein microgel. Front. Nutr. 2023, 10, 1140737. [Google Scholar] [CrossRef]
- Wu, C.; Zhi, Z.; Duan, M.; Sun, J.; Jiang, H.; Pang, J. Insights into the formation of carboxymethyl chitosan-nisin nanogels for sustainable antibacterial activity. Food Chem. 2023, 402, 134260. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shen, C.; Rao, J.; Li, J.; Yang, X.; Zhang, H.; Li, J.; Fawole, O.A.; Wu, D.; Chen, K. Biodegradable gelatin/pullulan aerogel modified by a green strategy: Characterization and antimicrobial activity. Food Packag. Shelf Life 2022, 34, 100957. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, D.; Yu, D.; Regenstein, J.M.; Jiang, Q.; Dong, J.; Chen, W.; Xia, W. Modulating physicochemical, antimicrobial and release properties of chitosan/zein bilayer films with curcumin/nisin-loaded pectin nanoparticles. Food Hydrocoll. 2022, 133, 107955. [Google Scholar] [CrossRef]
| Samples | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| L-N | 15.6 ± 1.1 d | 45.7 ± 4.5 b | 12.6 ± 2.3 b | 26.1 ± 1.0 bc |
| B-N | 21.8 ± 0.1 c | 45.6 ± 1.0 b | 8.2 ± 0.1 c | 24.4 ± 1.0 c |
| C-N | 28.7 ± 2.7 b | 0 ± 0 d | 32.2 ± 0.1 a | 39.1 ± 2.8 a |
| LF | 4.6 ± 2.1 e | 48.5 ± 5.9 b | 8.3 ± 3.1 c | 38.6 ± 0.8 a |
| BSA | 30.5 ± 1.4 b | 35.3 ± 1.0 c | 6.2 ± 0.8 c | 28.0 ± 0.6 b |
| CN | 0 ± 0 f | 58.5 ± 0.3 a | 2.4 ± 0 d | 39.1 ± 0.3 a |
| Nisin | 100 ± 0 a | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d |
| Samples | MIC (mg/mL) | MBC (mg/mL) | ||||
|---|---|---|---|---|---|---|
| L. monocytogenes | S. aureus | B. cereus | L. monocytogenes | S. aureus | B. cereus | |
| L-N | 0.625 | 0.625 | 1.25 | 1.25 | 1.25 | 1.25 |
| B-N | 0.625 | 0.625 | 1.25 | 1.25 | 1.25 | 1.25 |
| C-N | 1.25 | 2.5 | 2.5 | 2.5 | 2.5 | / |
| Nisin | 0.625 | 0.625 | 1.25 | 0.625 | 1.25 | 1.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, R.; Huang, X.; Bao, Y.; Wang, X.; Yi, H.; Lu, Y. The Effect of Nanoscale Modification of Nisin by Different Milk-Derived Proteins on Its Physicochemical Properties and Antibacterial Activity. Foods 2024, 13, 1606. https://doi.org/10.3390/foods13111606
Wang J, Liu R, Huang X, Bao Y, Wang X, Yi H, Lu Y. The Effect of Nanoscale Modification of Nisin by Different Milk-Derived Proteins on Its Physicochemical Properties and Antibacterial Activity. Foods. 2024; 13(11):1606. https://doi.org/10.3390/foods13111606
Chicago/Turabian StyleWang, Jing, Rui Liu, Xiaoyang Huang, Yuexin Bao, Xiaohong Wang, Huaxi Yi, and Youyou Lu. 2024. "The Effect of Nanoscale Modification of Nisin by Different Milk-Derived Proteins on Its Physicochemical Properties and Antibacterial Activity" Foods 13, no. 11: 1606. https://doi.org/10.3390/foods13111606
APA StyleWang, J., Liu, R., Huang, X., Bao, Y., Wang, X., Yi, H., & Lu, Y. (2024). The Effect of Nanoscale Modification of Nisin by Different Milk-Derived Proteins on Its Physicochemical Properties and Antibacterial Activity. Foods, 13(11), 1606. https://doi.org/10.3390/foods13111606








