6-BA Delays the Senescence of Postharvest Cabbage Leaves by Inhibiting Respiratory Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
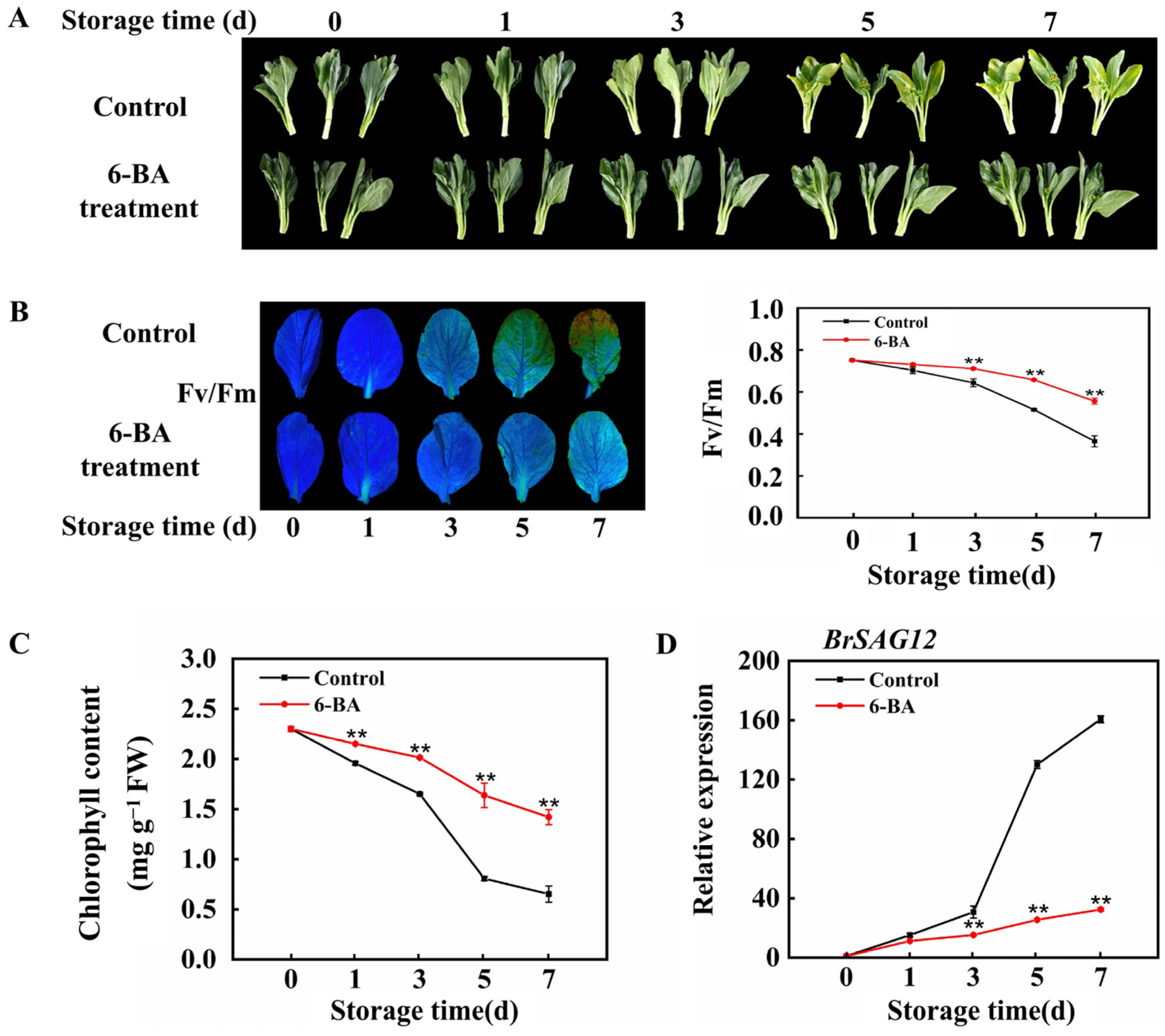
2.2. Total Chlorophyll Amount and Fv/Fm Relationships
2.3. Determination of the Respiration Rate
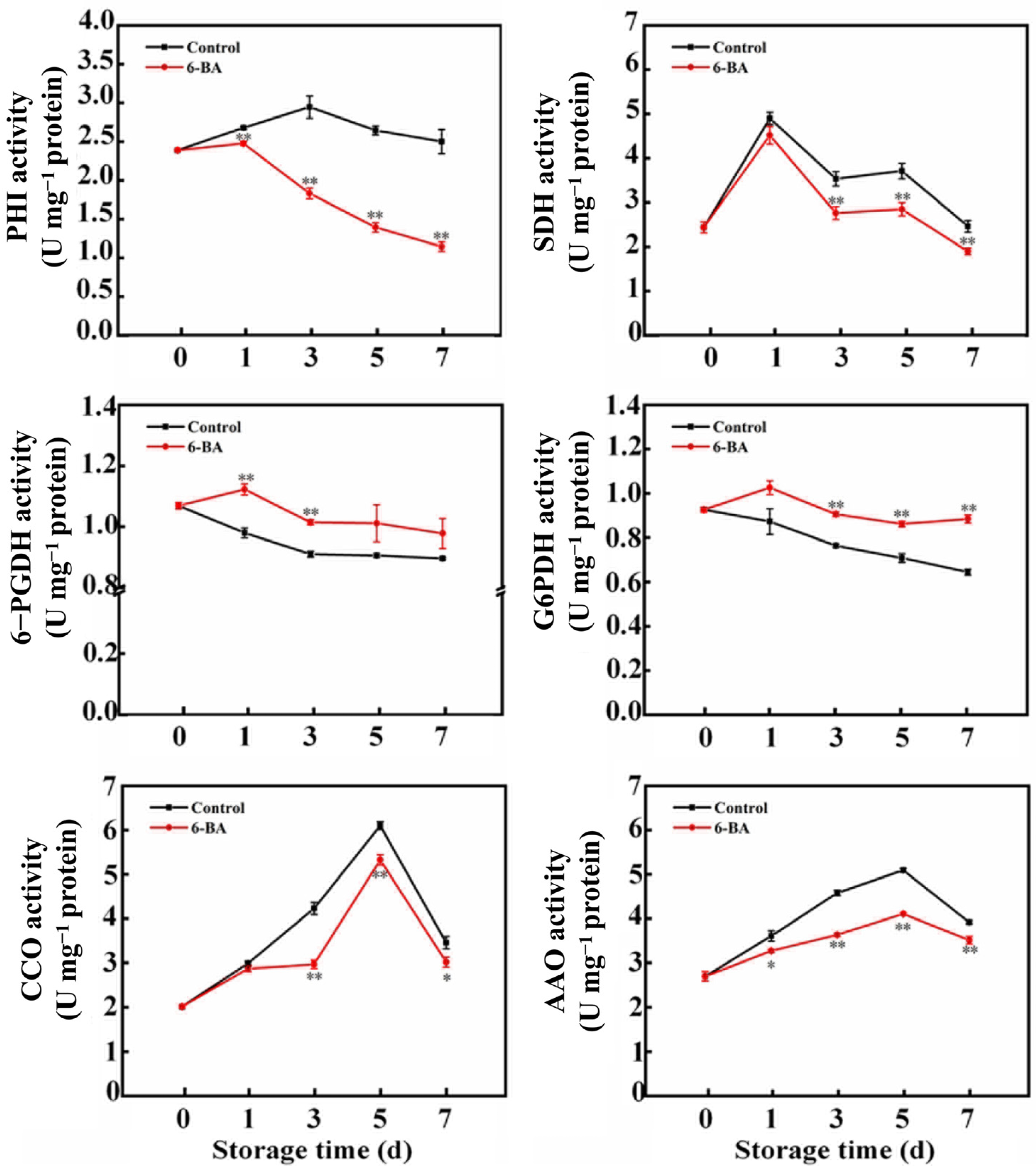
2.4. Assessment of Enzyme Activity Associated with Respiratory Metabolism
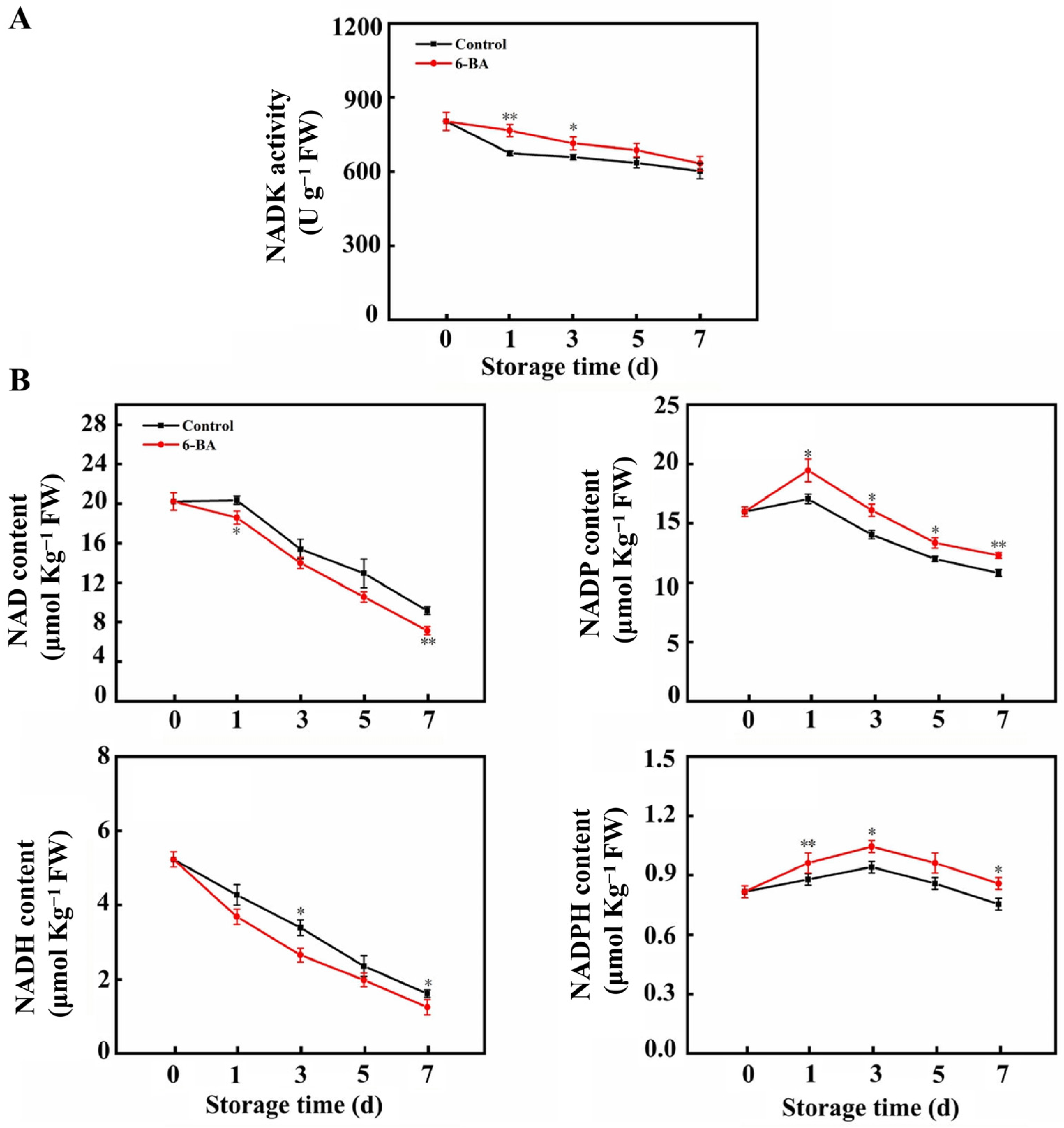
2.5. Measurement of NADK Enzyme Activity and the Levels of NAD(H) and NADP(H)
2.6. Determination of Total ATPase Activity and ATP Content
2.7. Real-Time Quantitative Polymerase Chain Reaction (qPCR)
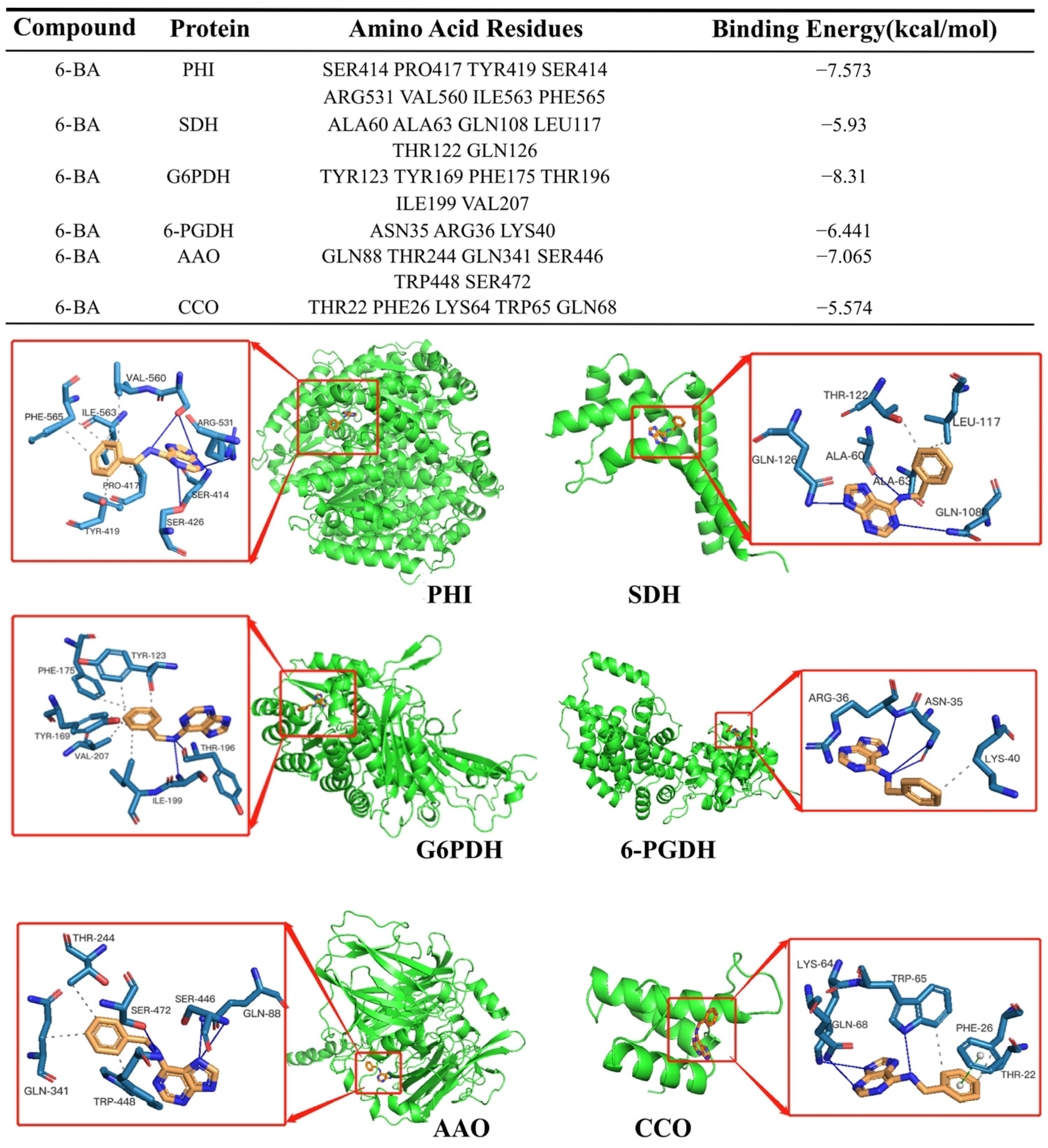
2.8. Molecular Docking
2.9. Statistical Analysis
3. Results
3.1. The Impact of External 6-BA Application on Postharvest Cabbage Leaf Senescence
3.2. The Impact of External 6-BA Application on Respiratory Metabolism
3.3. Impact of 6-BA on the Transcription Levels of Enzymes Involved in Respiratory Metabolism
3.4. The Impact of External 6-BA Application on NADK Activity and the Levels of NAD(H) and NADP(H)
3.5. The Impact of External Application of 6-BA on Total ATPase Activity, ATP Levels, and the Regulation of Genes Related to Energy Metabolism in Postharvest Cabbage
3.6. Molecular Docking Analysis of the Interaction between 6-BA and Respiratory Metabolic Enzymes
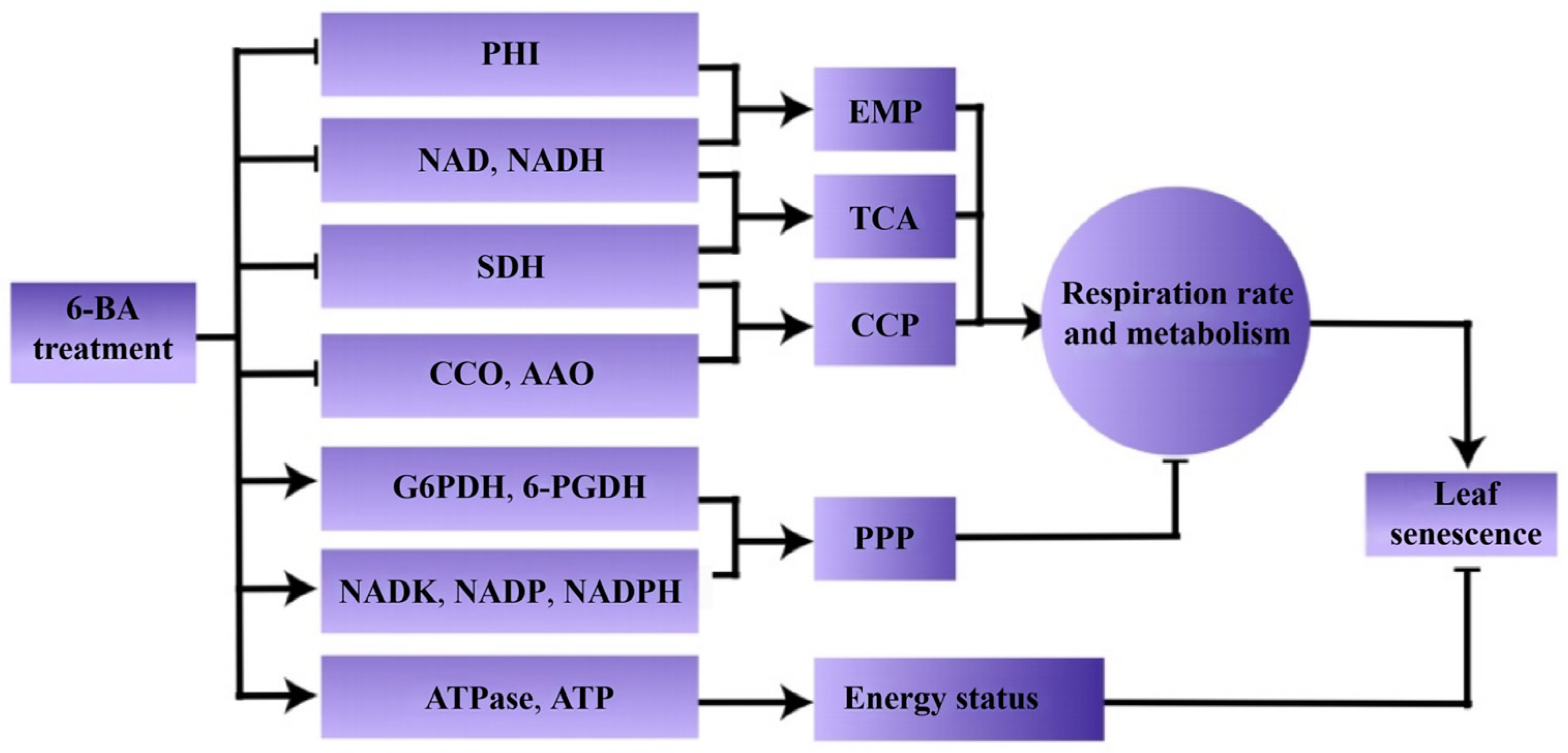
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brezeanu, C.; Brezeanu, P.M.; Stoleru, V.; Irimia, L.M.; Lipsa, F.D.; Teliban, G.C.; Ciobanu, M.M.; Murariu, F.; Puiu, I.; Branca, F.; et al. Nutritional value of new sweet pepper genotypes grown in organic system. Agriculture 2022, 12, 1863. [Google Scholar] [CrossRef]
- Wang, L.; Wen, M.; Chen, F.; Luo, Z.; Yin, J.; Chen, Y.; Huang, H. High oxygen atmospheric packaging (HOAP) reduces H2O2 production by regulating the accumulation of oxidative stress-related proteins in Chinese flowering cabbage. Postharvest Biol. Technol. 2020, 165, 111183. [Google Scholar] [CrossRef]
- Hoppu, U.; Puputti, S.; Sandell, M. Factors related to sensory properties and consumer acceptance of vegetables. Crit. Rev. Food Sci. Nutr. 2021, 61, 1751–1761. [Google Scholar] [CrossRef]
- Tan, X.L.; Fan, Z.Q.; Zeng, Z.X.; Shan, W.; Kuang, J.F.; Lu, W.J.; Su, X.G.; Tao, N.G.; Lakshmanan, P.; Chen, J.Y.; et al. Exogenous melatonin maintains leaf quality of postharvest Chinese flowering cabbage by modulating respiratory metabolism and energy status. Postharvest Biol. Technol. 2021, 177, 111524. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, P.; Liang, W.; Cheng, Q.; Mu, B.; Niu, F.; Yan, J.; Liu, C.; Xie, H.; Kav, N.N.V.; et al. A rapeseed WRKY transcription factor phosphorylated by CPK modulates cell death and leaf senescence by regulating the expression of ROS and SA-synthesis-related genes. J. Agric. Food Chem. 2020, 68, 7348–7359. [Google Scholar] [CrossRef]
- Sultana, N.; Islam, S.; Juhasz, A.; Ma, W. Wheat leaf senescence and its regulatory gene network. Crop J. 2021, 9, 703–717. [Google Scholar] [CrossRef]
- Li, L.; Lv, F.Y.; Guo, Y.Y.; Wang, Z.Q. Respiratory pathway metabolism and energy metabolism associated with senescence in postharvest Broccoli (Brassica oleracea L. var. italica) florets in response to O2/CO2 controlled atmospheres. Postharvest Biol. Technol. 2016, 111, 330–336. [Google Scholar] [CrossRef]
- Lin, Y.X.; Lin, Y.F.; Chen, Y.H.; Wang, H.; Shi, J.; Lin, H.T. Hydrogen peroxide induced changes in energy status and respiration metabolism of harvested longan fruit in relation to pericarp browning. J. Agric. Food Chem. 2016, 64, 4627–4632. [Google Scholar] [CrossRef]
- Lin, L.; Lin, Y.; Lin, H.; Lin, M.; Ritenour, M.A.; Chen, Y.; Wang, H.; Hung, Y.C.; Lin, Y. Comparison between ‘Fuyan’ and ‘Dongbi’ longans in aril breakdown and respiration metabolism. Postharvest Biol. Technol. 2019, 153, 176–182. [Google Scholar] [CrossRef]
- Li, L.; Kitazawa, H.; Wang, X.; Sun, H. Regulation of respiratory pathway and electron transport chain in relation to senescence of postharvest white mushroom (Agaricus bisporus) under high O2/CO2 controlled atmospheres. J. Agric. Food Chem. 2017, 65, 3351–3359. [Google Scholar] [CrossRef]
- Heidarvand, L.; Millar, A.H.; Taylor, N.L. Responses of the mitochondrial respiratory system to low temperature in plants. Crit. Rev. Plant Sci. 2017, 36, 217–240. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, B.; Peng, X.; Wang, J.; Li, Q.; Jin, J.; Ha, Y. Effects of chlorine dioxide treatment on respiration rate and ethylene synthesis of postharvest tomato fruit. Postharvest Biol. Technol. 2014, 93, 9–14. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, Y.; Lin, H.; Chen, Y.; Wang, H.; Shi, J. Application of propyl gallate alleviates pericarp browning in harvested longan fruit by modulating metabolisms of respiration and energy. Food Chem. 2018, 240, 863–869. [Google Scholar] [CrossRef]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef]
- Yang, W.; Shi, C.; Hu, Q.; Wu, Y.; Fang, D.; Pei, F.; Mariga, A.M. Nanocomposite packaging regulate respiration and energy metabolism in Flammulina velutipes. Postharvest Biol. Technol. 2019, 151, 119–126. [Google Scholar] [CrossRef]
- Zhu, L.; Hu, H.; Luo, S.; Wu, Z.; Li, P. Melatonin delaying senescence of postharvest broccoli by regulating respiratory metabolism and antioxidant activity. Trans. Chin. Soc. Agric. Eng. 2018, 34, 300–308. [Google Scholar]
- Shu, C.; Zhang, W.; Zhao, H.; Cao, J.; Jiang, W. Chlorogenic acid treatment alleviates the adverse physiological responses of vibration injury in apple fruit through the regulation of energy metabolism. Postharvest Biol. Technol. 2020, 159, 110997. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Jannatizadeh, A.; Luo, Z.; Paliyath, G. Ensuring sufficient intracellular ATP supplying and friendly extracellular ATP signaling attenuates stresses, delays senescence and maintains quality in horticultural crops during postharvest life. Trends Food Sci. Technol. 2018, 76, 67–81. [Google Scholar] [CrossRef]
- Klingenberg, M. The ADP and ATP transport in mitochondria and its carrier. Biochim. Biophys. Acta 2008, 1778, 1978–2021. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, H.; Lin, M.; Lin, Y.; Chen, Y.; Wang, H.; Lin, Y.; Shi, J. Lasiodiplodia theobromae (Pat.) Griff. & Maubl. reduced energy status and ATPase activity and its relation to disease development and pericarp browning of harvested longan fruit. Food Chem. 2019, 275, 239–245. [Google Scholar] [CrossRef]
- Jia, L.E.; Liu, S.; Duan, X.M.; Zhang, C.; Wu, Z.H.; Liu, M.C.; Guo, S.G.; Zuo, J.H.; Wang, L.B. 6-benzylaminopurine treatment maintains the quality of Chinese chive (Allium tuberosum Rottler ex Spreng.) by enhancing antioxidant enzyme activity. J. Integr. Agric. 2017, 16, 1968–1977. [Google Scholar] [CrossRef]
- Wang, C.M.; Yang, Y.Y.; Chen, N.H.; Zeng, Z.X.; Ji, S.J.; Shan, W.; Kuang, J.F.; Lu, W.J.; Su, X.G.; Chen, J.Y.; et al. Physiological and transcription analyses reveal regulatory pathways of 6-benzylaminopurine delaying leaf senescence and maintaining quality in postharvest Chinese flowering cabbage. Food Res. Int. 2022, 157, 111455. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Y.; Li, J. Effects of 6-benzylaminopurine–calcium chloride–salicylic acid on yellowing and reactive oxygen metabolism of Broccoli. Trans. Tianjin Univ. 2018, 24, 318–325. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Zhu, M.; Li, F. Exogenous 6-benzyladenine improves waterlogging tolerance in maize seedlings by mitigating oxidative stress and upregulating the ascorbate-glutathione cycle. Front. Plant Sci. 2021, 12, 680376. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Tan, X.L.; Shan, W.; Kuang, J.F.; Lu, W.J.; Lin, H.T.; Su, X.G.; Lakshmanan, P.; Zhao, M.L.; Chen, J.Y. Involvement of BrNAC041 in ABA-GA antagonism in the leaf senescence of Chinese flowering cabbage. Postharvest Biol. Technol. 2020, 168, 111254. [Google Scholar] [CrossRef]
- Baker, C.M.; Atzori, A. AlphaFold: Deep learning, drug discovery and the protein structure revolution: Medicinal chemistry and chemical biology highlights. Chimia 2022, 76, 364–366. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Studer, G.; Rempfer, C.; Waterhouse, A.M.; Gumienny, R.; Haas, J.; Schwede, T. QMEANDisCo-distance constraints applied on model quality estimation. Bioinformatics 2020, 36, 1765–1771. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock vina 1.2.0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Yun, Z.; Hu, M.; Liu, J.; Jiang, Y.; Zhang, Z. Melatonin enhances cold tolerance by regulating energy and proline metabolism in litchi fruit. Foods 2020, 9, 454. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, H.; Lin, Y.; Lin, Y.; Hung, Y.C.; Chen, Y.; Wang, H.; Shi, J. Energy status regulates disease development and respiratory metabolism of Lasiodiplodia theobromae (Pat.) Griff. & Maubl.-infected longan fruit. Food Chem. 2017, 231, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, Y.; Lin, H.; Ritenour, M.A.; Shi, J.; Zhang, S.; Chen, Y.; Wang, H. Hydrogen peroxide-induced pericarp browning of harvested longan fruit in association with energy metabolism. Food Chem. 2017, 225, 31–36. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Yang, Y.; Yu, J.; Liu, Z.; Wei, W.; Chen, J.; Zhu, J.; Huang, R. 6-BA Delays the Senescence of Postharvest Cabbage Leaves by Inhibiting Respiratory Metabolism. Foods 2024, 13, 1607. https://doi.org/10.3390/foods13111607
Wang C, Yang Y, Yu J, Liu Z, Wei W, Chen J, Zhu J, Huang R. 6-BA Delays the Senescence of Postharvest Cabbage Leaves by Inhibiting Respiratory Metabolism. Foods. 2024; 13(11):1607. https://doi.org/10.3390/foods13111607
Chicago/Turabian StyleWang, Cimei, Yingying Yang, Jieting Yu, Zongli Liu, Wei Wei, Jianye Chen, Jianhua Zhu, and Riming Huang. 2024. "6-BA Delays the Senescence of Postharvest Cabbage Leaves by Inhibiting Respiratory Metabolism" Foods 13, no. 11: 1607. https://doi.org/10.3390/foods13111607
APA StyleWang, C., Yang, Y., Yu, J., Liu, Z., Wei, W., Chen, J., Zhu, J., & Huang, R. (2024). 6-BA Delays the Senescence of Postharvest Cabbage Leaves by Inhibiting Respiratory Metabolism. Foods, 13(11), 1607. https://doi.org/10.3390/foods13111607







