Effect of pH and Shear on Heat-Induced Changes in Milk Protein Concentrate Suspensions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Treatment
2.3. Particle Size and Zeta Potential Measurements
2.4. Fourier Transform Infrared (FTIR) Analysis
2.5. Reverse Phase-High Performance Liquid Chromatography Analysis
2.6. Statistical Analysis
3. Results
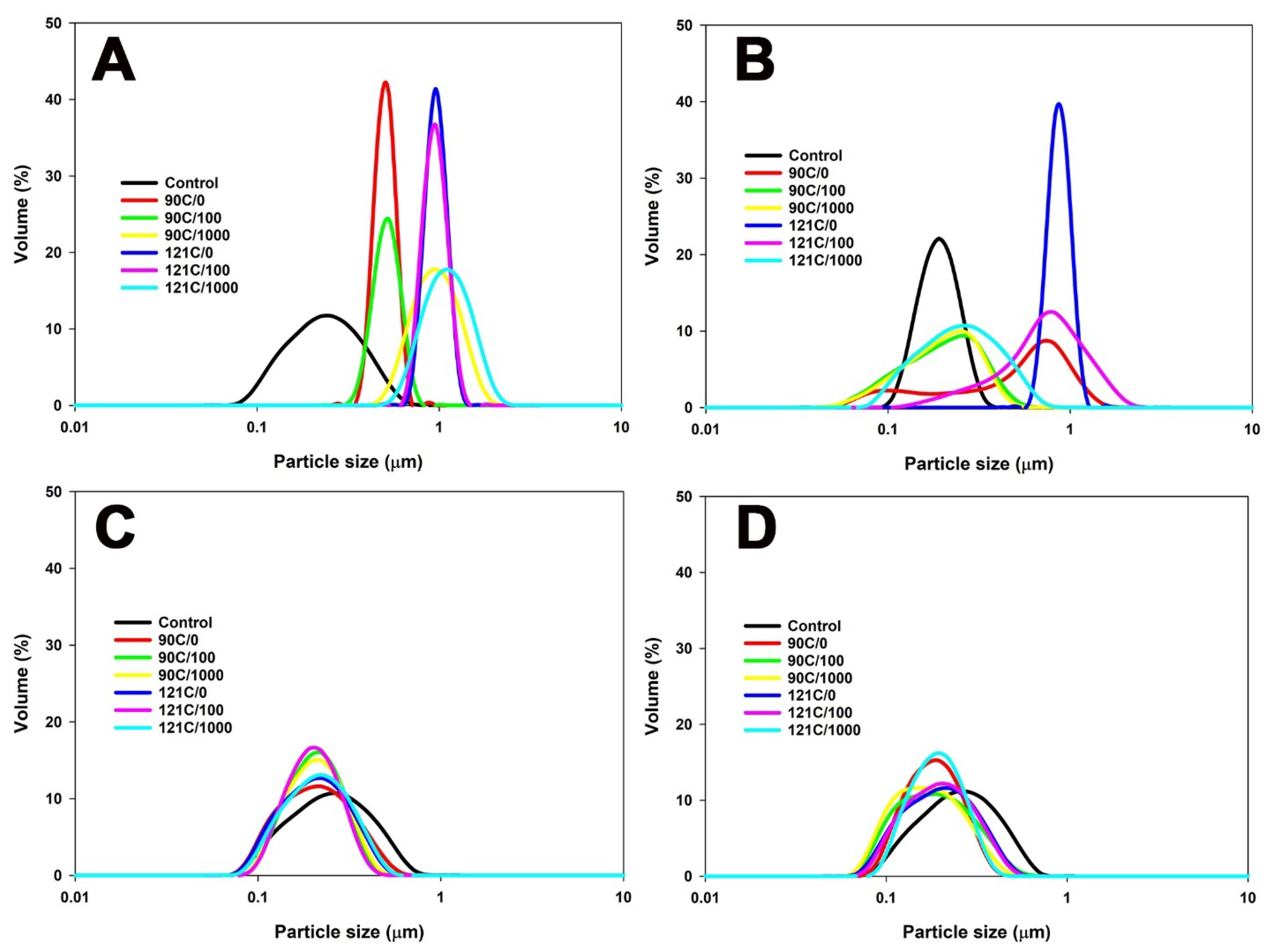
3.1. Particle Size Distribution and Zeta Potential of MPC Suspensions
3.2. Partitioning of Proteins in MPC Suspensions
3.2.1. Changes in Protein Content within the Non-Sedimentable Fraction
3.2.2. Changes in the Content of Aggregated Protein
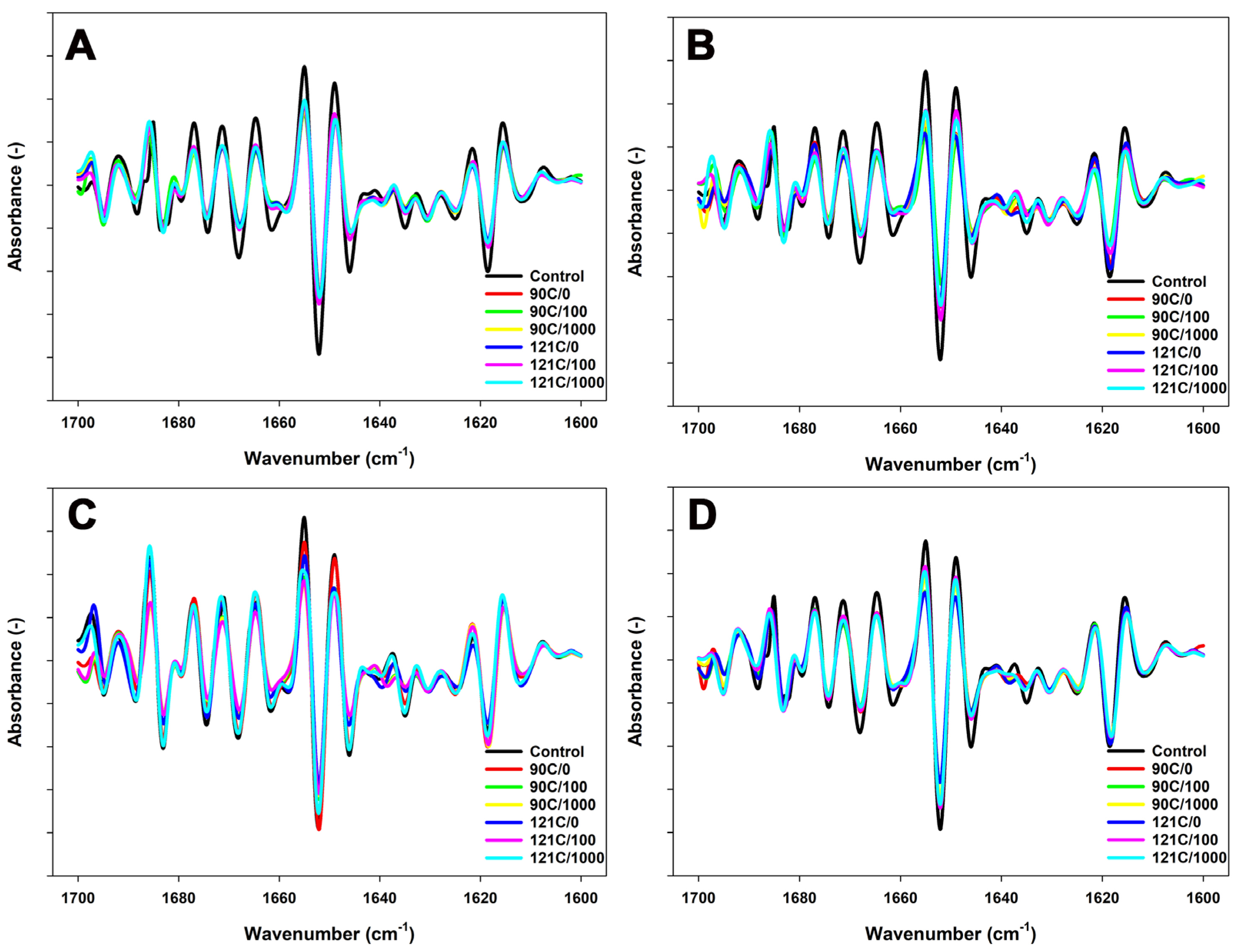
3.3. Conformational Properties of Proteins in MPC Suspensions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bastian, E.; Collinge, S.; Ernstrom, C. Ultrafiltration: Partitioning of milk constituents into permeate and retentate. J. Dairy Sci. 1991, 74, 2423–2434. [Google Scholar] [CrossRef]
- Green, M.L.; Scott, K.J.; Anderson, M.; Griffin, M.C.; Griffin, F.A. Chemical characterization of milk concentrated by ultrafiltration. J. Dairy Res. 1984, 51, 267–278. [Google Scholar] [CrossRef]
- Singh, J.; Prakash, S.; Bhandari, B.; Bansal, N. Ultra high temperature (UHT) stability of casein-whey protein mixtures at high protein content: Heat induced protein interactions. Food Res. Int. 2019, 116, 103–113. [Google Scholar] [CrossRef]
- Agarwal, S.; Beausire, R.L.; Patel, S.; Patel, H. Innovative uses of milk protein concentrates in product development. J. Food Sci. 2015, 80, A23–A29. [Google Scholar] [CrossRef] [PubMed]
- Mediwaththe, A.; Chandrapala, J.; Vasiljevic, T. Shear-induced behaviour of native milk proteins heated at temperatures above 80 °C. Int. Dairy J. 2018, 77, 29–37. [Google Scholar] [CrossRef]
- Singh, H. Heat stability of milk. Int. J. Dairy Technol. 2004, 57, 111–119. [Google Scholar] [CrossRef]
- Ozcan, T.; Horne, D.S.; Lucey, J.A. Yogurt made from milk heated at different pH values. J. Dairy Sci. 2015, 98, 6749–6758. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.V.; Megemont, M.; Gazi, I.; Kelly, A.L.; Huppertz, T.; O’Mahony, J.A. Heat stability of reconstituted milk protein concentrate powders. Int. Dairy J. 2014, 37, 104–110. [Google Scholar] [CrossRef]
- Crowley, S.V.; Boudin, M.; Chen, B.; Gazi, I.; Huppertz, T.; Kelly, A.L.; O’Mahony, J.A. Stability of milk protein concentrate suspensions to in-container sterilisation heating conditions. Int. Dairy J. 2015, 50, 45–49. [Google Scholar] [CrossRef]
- Olivares, M.L.; Berli, C.L.A.; Zorrilla, S.E. Rheological modelling of dispersions of casein micelles considered as microgel particles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 337–342. [Google Scholar] [CrossRef]
- Ranadheera, C.; Liyanaarachchi, W.; Dissanayake, M.; Chandrapala, J.; Huppertz, T.; Vasiljevic, T. Impact of shear and pH on properties of casein micelles in milk protein concentrate. LWT 2019, 108, 370–376. [Google Scholar] [CrossRef]
- Liyanaarachchi, W.; Vasiljevic, T. Caseins and their interactions that modify heat aggregation of whey proteins in commercial dairy mixtures. Int. Dairy J. 2018, 83, 43–51. [Google Scholar] [CrossRef]
- Liu, D.Z.; Weeks, M.G.; Dunstan, D.E.; Martin, G.J. Temperature-dependent dynamics of bovine casein micelles in the range 10–40 °C. Food Chem. 2013, 141, 4081–4086. [Google Scholar] [CrossRef] [PubMed]
- Mediwaththe, A.; Huppertz, T.; Chandrapala, J.; Vasiljevic, T. Heat-Induced Changes in κ-Carrageenan-Containing Chocolate-Flavoured Milk Protein Concentrate Suspensions under Controlled Shearing. Foods 2023, 12, 4404. [Google Scholar] [CrossRef] [PubMed]
- Mediwaththe, A.; Bogahawaththa, D.; Grewal, M.K.; Chandrapala, J.; Vasiljevic, T. Structural changes of native milk proteins subjected to controlled shearing and heating. Food Res. Int. 2018, 114, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Mediwaththe, A.; Huppertz, T.; Chandrapala, J.; Vasiljevic, T. Effect of protein content on heat stability of reconstituted milk protein concentrate under controlled shearing. Foods 2024, 13, 263. [Google Scholar] [CrossRef]
- Crowley, S.V. Physicochemical Characterisation of Protein Ingredients Prepared from Milk by Ultrafiltration or Microfiltration for Application in Formulated Nutritional Products. Ph.D. Thesis, University College Cork, Cork, Ireland, 2016. [Google Scholar]
- Anema, S.G. The turbidity of heated milk in relation to particle size and protein distributions between the casein micelles and the serum phase. Int. Dairy J. 2023, 147, 105771. [Google Scholar] [CrossRef]
- Anema, S.G.; Lowe, E.K.; Lee, S.K. Effect of pH at heating on the acid-induced aggregation of casein micelles in reconstituted skim milk. LWT Food Sci. Technol. 2004, 37, 779–787. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.-M.; Xiong, S.-B.; Xie, B.-J.; Qin, L.-H. Role of secondary structures in the gelation of porcine myosin at different pH values. Meat Sci. 2008, 80, 632–639. [Google Scholar] [CrossRef]
- Singh, H.; Latham, J.M. Heat stability of milk: Aggregation and dissociation of protein at ultra-high temperatures. Int. Dairy J. 1993, 3, 225–237. [Google Scholar] [CrossRef]
- Ahmad, S.; Piot, M.; Rousseau, F.; Grongnet, J.F.; Gaucheron, F. Physico-chemical changes in casein micelles of buffalo and cow milks as a function of alkalinisation. Dairy Sci. Technol. 2009, 89, 387–403. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.; Zhang, H.; Guo, H.; Wang, P.; Zhu, Y.; Ren, F. pH treatment as an effective tool to select the functional and structural properties of yak milk caseins. J. Dairy Sci. 2013, 96, 5494–5500. [Google Scholar] [CrossRef] [PubMed]
- Vaia, B.; Smiddy, M.A.; Kelly, A.L.; Huppertz, T. Solvent-mediated disruption of bovine casein micelles at alkaline pH. J. Agric. Food Chem. 2006, 54, 8288–8293. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, T.; Vaia, B.; Smiddy, M.A. Reformation of casein particles from alkaline-disrupted casein micelles. J. Dairy Res. 2008, 75, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Sinaga, H.; Bansal, N.; Bhandari, B. Effects of milk pH alteration on casein micelle size and gelation properties of milk. Int. J. Food Prop. 2017, 20, 179–197. [Google Scholar] [CrossRef]
- Aydogdu, T. Investigations of the Complex Relationships between Minerals, pH, and Heat Stability in Milk Protein Systems. Ph.D. Thesis, University College Cork, Cork, Ireland, 2023. [Google Scholar]
- Arp, P.; Mason, S. Chains of spheres in shear and electric fields. Colloid Polym. Sci. 1977, 255, 1165–1173. [Google Scholar] [CrossRef]
- Kim, J.; Kramer, T.A. Improved orthokinetic coagulation model for fractal colloids: Aggregation and breakup. Chem. Eng. Sci. 2006, 61, 45–53. [Google Scholar] [CrossRef]
- Steventon, A.; Donald, A.; Gladden, L. Thermal aggregation of whey protein concentrates under fluid shear conditions. In Biochemistry of Milk Products; Elsevier: Amsterdam, The Netherlands, 2005; pp. 133–142. [Google Scholar]
| pH | Temperature (°C) | Particle Size (nm) | ||
|---|---|---|---|---|
| 0 s−1 | 100 s−1 | 1000 s−1 | ||
| 6.1 | 20 | 235 E | ||
| 90 | 714 Cc | 850 Bb | 1974 Ba | |
| 121 | 1632 Ac | 1842 Ab | 2132 Aa | |
| 6.4 | 20 | 224 F | ||
| 90 | 384 Da | 330 Dc | 338 Db | |
| 121 | 1146 Ba | 796 Cb | 498 Cc | |
| 6.8 | 20 | 207 H | ||
| 90 | 182 Ja | 180 Ea | 178 Ea | |
| 121 | 179 Ja | 178 Ea | 177 Ea | |
| 7.5 | 20 | 214 G | ||
| 90 | 196 Ia | 174 Eb | 166 Fc | |
| 121 | 184 Ja | 180 Ea | 170 Fb | |
| SEMpooled * | 0.675 | |||
| pH | Temperature (°C) | Zeta Potential (mV) | ||
|---|---|---|---|---|
| 0 s−1 | 100 s−1 | 1000 s−1 | ||
| 6.1 | 20 | −18.4 AB | ||
| 90 | −19.0 ABa | −19.0 BCa | −20.2 ABa | |
| 121 | −19.8 ABa | −18.4 Aba | −16.5 Ab | |
| 6.4 | 20 | −18.6 AB | ||
| 90 | −17.8 Aa | −17.5 Aa | −18.9 ABb | |
| 121 | −18.3 Aa | −19.5 BCb | −20.5 ABc | |
| 6.8 | 20 | −21.2 C | ||
| 90 | −21.2 Ca | −21.2 Da | −21.1 Ba | |
| 121 | −20.8 Ca | −19.9 CDa | −21.1 Ba | |
| 7.5 | 20 | −25.3 D | ||
| 90 | −26.0 Da | −26.4 Ea | −26.8 Ca | |
| 121 | −25.1 Da | −25.9 Ea | −25.5 Ca | |
| SEMpooled * | 0.419 | |||
| pH | Temperature (°C) | Shear Rate (s−1) | % Protein Content in Non-Sedimentable Fraction | |||||
|---|---|---|---|---|---|---|---|---|
| αs1-CN | αs2-CN | β-CN | κ-CN | β-LG | α-LA | |||
| 6.1 | 20 | 0 | 2.4 g | 3.6 h | 5.0 j | 12.5 k | 70.5 c | 76.0 fgh |
| 90 | 0 | 0.0 | 0.0 | 0.0 | 6.0 m | 41.7 i | 52.4 kl | |
| 90 | 100 | 0.0 | 0.0 | 0.0 | 4.5 m | 30.0 j | 20 n | |
| 90 | 1000 | 0.0 | 0.0 | 0.0 | 3.0 m | 12.0 l | 11.1 o | |
| 121 | 0 | 0.0 | 0.0 | 0.0 | 4.3 m | 23.3 k | 39.4 m | |
| 121 | 100 | 0.0 | 0.0 | 0.0 | 4.7 m | 22.2 k | 16.7 n | |
| 121 | 1000 | 0.0 | 0.0 | 0.0 | 3.0 m | 20 k | 8.0 o | |
| 6.4 | 20 | 0 | 4.3 g | 9.3 g | 8.3 ij | 12.5 k | 85.0 b | 81.3 de |
| 90 | 0 | 0.0 | 1.7 h | 2.7 j | 12.1 k | 52.8 gh | 65.1 j | |
| 90 | 100 | 0.0 | 13.6 g | 8.6 ij | 40.5 hi | 57.3 fg | 71.8 hi | |
| 90 | 1000 | 0.0 | 10.7 g | 11.4 i | 62.5 cd | 68.6 c | 81.9 de | |
| 121 | 0 | 0.0 | 0.0 | 0.0 | 7.1 lm | 41.5 i | 57.5 k | |
| 121 | 100 | 0.0 | 0.0 | 0.0 | 25.0 j | 53.3 gh | 65.5 j | |
| 121 | 1000 | 0.0 | 0.0 | 0.0 | 36.4 i | 65.6 cde | 73.1 ghi | |
| 6.8 | 20 | 0 | 15.0 f | 20.0 f | 20.0 h | 28.0 j | 97.0 a | 98.0 a |
| 90 | 0 | 16.5 ef | 24.8 def | 28.7 fg | 36.2 i | 51.1 h | 68.0 ij | |
| 90 | 100 | 21.1 cde | 22.5 ef | 33.7 def | 46.7 fg | 66.3 cd | 76.1 fgh | |
| 90 | 1000 | 22.6 bcd | 25.0 d | 41.8 b | 63.0 cd | 70.0 c | 77.0 efg | |
| 121 | 0 | 20.5 cdef | 29.4 cd | 35.5 cde | 42.3 gh | 40.5 i | 49.6 l | |
| 121 | 100 | 23.5 bcd | 34.0 bc | 53.3 a | 56.5 e | 60.4 efg | 71.6 hi | |
| 121 | 1000 | 23.8 bcd | 34.4 bc | 57.0 a | 57.3 e | 62.9 def | 77.5 efg | |
| 7.5 | 20 | 0 | 21.3 cde | 21.7 ef | 26.4 g | 49.5 f | 81.9 b | 90.5 b |
| 90 | 0 | 19.8 def | 25.0 def | 31.0 efg | 58.2 de | 53.3 gh | 81.8 de | |
| 90 | 100 | 25.0 bc | 25.9 de | 40.0 bc | 65.6 c | 68.9 c | 85.4 bcd | |
| 90 | 1000 | 33.3 a | 38.7 b | 42.7 b | 73.7 b | 67.1 cd | 89.8 bc | |
| 121 | 0 | 26.8 b | 27.8 d | 38.8 bcd | 63.4 c | 42.9 i | 68.9 ij | |
| 121 | 100 | 22.2 bcd | 42.1 a | 54.7 a | 81.6 a | 51.7 h | 78.6 ef | |
| 121 | 1000 | 36.2 a | 43.9 a | 56.7 a | 85.9 a | 54.9 gh | 82.9 cde | |
| SEMpooled * | 0.143 | 0.034 | 0.210 | 0.085 | 0.120 | 0.097 | ||
| pH | Temperature (°C) | Shear Rate (s−1) | % Protein Content in Sedimentable Fraction | |||||
|---|---|---|---|---|---|---|---|---|
| αs1-CN | αs2-CN | β-CN | κ-CN | β-LG | α-LA | |||
| 6.1 | 20 | 0 | 85.7 b | 81.0 c | 80.0 d | 52.5 d | 14.1 ij | 15.7 gh |
| 90 | 0 | 99.8 a | 89.7 b | 85.0 c | 68.8 c | 43.3 d | 37.5 e | |
| 90 | 100 | 100.0 a | 98.5 a | 96.0 a | 73.2 c | 55.0 c | 55.0 c | |
| 90 | 1000 | 100.0 a | 99.2 a | 98.0 a | 80.0 b | 68.7 b | 61.1 b | |
| 121 | 0 | 98.5 a | 99.7 a | 90.0 b | 78.3 b | 52.2 c | 46.2 d | |
| 121 | 100 | 100.0 a | 100.0 a | 98.0 a | 80.0 b | 64.4 b | 58.3 b | |
| 121 | 1000 | 100.0 a | 100.0 a | 100.0 a | 85.0 a | 74.0 a | 67.0 a | |
| 6.4 | 20 | 0 | 61.3 e | 41.9 f | 58.3 g | 36.3 e | 2.0 k | 4.4 jkl |
| 90 | 0 | 75.7 d | 75.9 d | 64.8 f | 50.0 d | 22.3 fg | 11.8 hi | |
| 90 | 100 | 80.8 c | 72.7 d | 68.5 f | 24.3 gh | 17.4 ghi | 9.7 ij | |
| 90 | 1000 | 79.1 cd | 71.4 d | 70.5 ef | 12.5 i | 10.4 j | 5.5 jkl | |
| 121 | 0 | 98.7 a | 81.0 c | 70.0 ef | 73.8 c | 31.4 e | 27.5 f | |
| 121 | 100 | 100.0 a | 73.5 d | 73.6 e | 33.3 ef | 22.9 fg | 18.5 g | |
| 121 | 1000 | 100.0 a | 62.5 e | 75.0 e | 30 f | 11.1 j | 11.6 hi | |
| 6.8 | 20 | 0 | 5.0 h | 7.0 l | 8.0 k | 10.0 ij | 2.0 k | 1.0 l |
| 90 | 0 | 19.3 g | 23.9 gh | 18.4 i | 25.3 g | 21.1 fgh | 15.1 gh | |
| 90 | 100 | 7.8 h | 19.1 hi | 14.1 ij | 18.5 h | 10.5 j | 7.6 ijk | |
| 90 | 1000 | 5.4 h | 15.2 ij | 8.8 k | 10.0 ij | 10.0 j | 12.0 hi | |
| 121 | 0 | 25.1 f | 28.2 g | 25.6 h | 35.6 e | 24.8 f | 19.6 g | |
| 121 | 100 | 5.1 h | 5.3 l | 2.2 l | 23.5 gh | 8.8 j | 7.4 ijk | |
| 121 | 1000 | 3.2 i | 5.8 l | 1.1 l | 19.8 h | 9.3 j | 9.0 ij | |
| 7.5 | 20 | 0 | 6.1 h | 9.6 k | 8.3 k | 10.8 ij | 4.2 k | 3.6 k |
| 90 | 0 | 7.7 h | 14.3 ijk | 18.4 i | 13.8 i | 18.0 ghi | 3.4 k | |
| 90 | 100 | 4.5 h | 12.9 jk | 16.7 i | 2.1 k | 9.5 j | 2.4 k | |
| 90 | 1000 | 4.6 h | 9.9 kl | 10.6 jk | 4.0 k | 10.0 hi | 2.5 k | |
| 121 | 0 | 8.5 h | 16.5 ij | 25.5 h | 13.5 i | 16.5 hi | 1.3 l | |
| 121 | 100 | 7.8 h | 7.4 l | 17.4 i | 6.2 jk | 13.3 ij | 1.4 l | |
| 121 | 1000 | 5.3 h | 7.1 l | 11.1 jk | 2.1 k | 10.9 j | 0.9 l | |
| SEMpooled * | 0.093 | 0.125 | 0.115 | 0.145 | 0.085 | 0.098 | ||
| pH | Temp (°C) | Intramolecular β-Sheets (1615–1637) | Random Coils (1638–1645) | α-Helix (1646–1664) | β-Turns (1665–1681) | Aggregated β-Sheets (1682–1700) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 s−1 | 100 s−1 | 1000 s−1 | 0 s−1 | 100 s−1 | 1000 s−1 | 0 s−1 | 100 s−1 | 1000 s−1 | 0 s−1 | 100 s−1 | 1000 s−1 | 0 s−1 | 100 s−1 | 1000 s−1 | ||
| 6.1 | 20 | 31.85 EF | 4.31 F | 18.31 E | 35.14 C | 10.39 B | ||||||||||
| 90 | 28.70 Ga | 21.97 Eb | 18.72 Db | 5.11 Fc | 10.35 Fb | 12.81 Ga | 15.49 Fa | 10.51 Eb | 6.53 Dc | 37.19 Bc | 38.83 Bb | 41.81 Ba | 13.51 Ac | 18.34 Ab | 20.13 Ba | |
| 121 | 23.01 Ha | 19.88 Fb | 10.94 Eb | 5.25 Fc | 6.88 Gb | 11.53 Ga | 10.51 Ga | 6.82 Fb | 5.99 Db | 47.65 Aa | 48.55 Aa | 49.01 Aa | 13.58 Ac | 17.87 Ab | 22.53 Aa | |
| 6.4 | 20 | 36.71 C | 4.17 F | 21.15 CD | 29.14 E | 8.83 C | ||||||||||
| 90 | 33.11 DEa | 29.42 Cb | 24.43 Cc | 5.15 Fc | 11.11 Fb | 18.35 Fa | 17.30 Ea | 12.81 Db | 10.30 Cc | 33.93 Da | 32.53 Da | 30.81 Cb | 10.51 Bc | 14.13 Bb | 16.11 Ca | |
| 121 | 30.56 Fa | 27.44 Db | 24.32 Cc | 7.28 Ec | 14.28 Eb | 22.38 Ea | 11.53 Ga | 10.83 Eab | 9.95 Cc | 37.80 Ba | 35.21 Cb | 30.82 Dc | 12.83 Aa | 12.24 Ca | 12.53 Da | |
| 6.8 | 20 | 45.01 A | 5.22 F | 27.01 A | 19.21 H | 3.55 G | ||||||||||
| 90 | 40.77 Ba | 27.40 Db | 24.19 Cc | 8.31 Ec | 20.15 Db | 27.18 Da | 21.59 Ca | 20.31 Aa | 14.83 ABb | 23.50 Gc | 27.32 Eb | 30.52 Ca | 5.83 DEa | 4.82 Fab | 3.28 Fb | |
| 121 | 36.07 Ca | 14.96 Gb | 11.53 Ec | 19.65 Cc | 22.51 Cb | 30.04 Ca | 10.31 Gb | 18.51 Ba | 11.35 Cb | 27.0 Fc | 38.51 Bb | 42.81 Ba | 6.97 Da | 5.51 Fab | 4.27 Fb | |
| 7.5 | 20 | 40.04 B | 12.54 D | 25.51 B | 17.38 I | 4.53 EF | ||||||||||
| 90 | 37.53 Ca | 33.28 Ab | 30.85 Ac | 22.28 Bc | 28.23 Bb | 38.01 Aa | 22.57 Ca | 20.11 Ab | 15.32 Ac | 12.51 Ja | 10.53 Fb | 5.51 Dc | 5.11 Ec | 7.85 Db | 10.31 Ea | |
| 121 | 34.45 Da | 31.52 Bb | 28.85 Bc | 28.51 Ac | 32.31 Ab | 36.21 Ba | 19.91 Da | 16.83 Cb | 13.58 Bc | 10.30 Ka | 8.81 Gb | 6.15 Dc | 6.83 Dc | 10.53 Db | 15.21 Ca | |
| SEMpooled * | 0.667 | 0.782 | 0.531 | 0.634 | 0.367 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mediwaththe, A.; Huppertz, T.; Chandrapala, J.; Vasiljevic, T. Effect of pH and Shear on Heat-Induced Changes in Milk Protein Concentrate Suspensions. Foods 2024, 13, 1517. https://doi.org/10.3390/foods13101517
Mediwaththe A, Huppertz T, Chandrapala J, Vasiljevic T. Effect of pH and Shear on Heat-Induced Changes in Milk Protein Concentrate Suspensions. Foods. 2024; 13(10):1517. https://doi.org/10.3390/foods13101517
Chicago/Turabian StyleMediwaththe, Anushka, Thom Huppertz, Jayani Chandrapala, and Todor Vasiljevic. 2024. "Effect of pH and Shear on Heat-Induced Changes in Milk Protein Concentrate Suspensions" Foods 13, no. 10: 1517. https://doi.org/10.3390/foods13101517
APA StyleMediwaththe, A., Huppertz, T., Chandrapala, J., & Vasiljevic, T. (2024). Effect of pH and Shear on Heat-Induced Changes in Milk Protein Concentrate Suspensions. Foods, 13(10), 1517. https://doi.org/10.3390/foods13101517








