Structural Characteristic, Strong Antioxidant, and Anti-Gastric Cancer Investigations on an Oleoresin from Ginger (Zingiber officinale var. roscoe)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Lines
2.3. CO2 Supercritical Fluid Extraction Condition for the Ginger Oleoresin
2.4. LC-QTOF-MS Analyses of the Ginger Oleoresin
2.5. GC-MS Analyses of the Volatile Compounds in the Ginger Oleoresin
2.6. Antioxidant Activities of the Ginger Oleoresin
2.7. Anticancer Activities of Ginger Oleoresin
2.8. Statistical Analyses
3. Results and Discussion
3.1. Identification of Chemical Compounds of Ginger Oleoresin Obtained by CO2 SFE
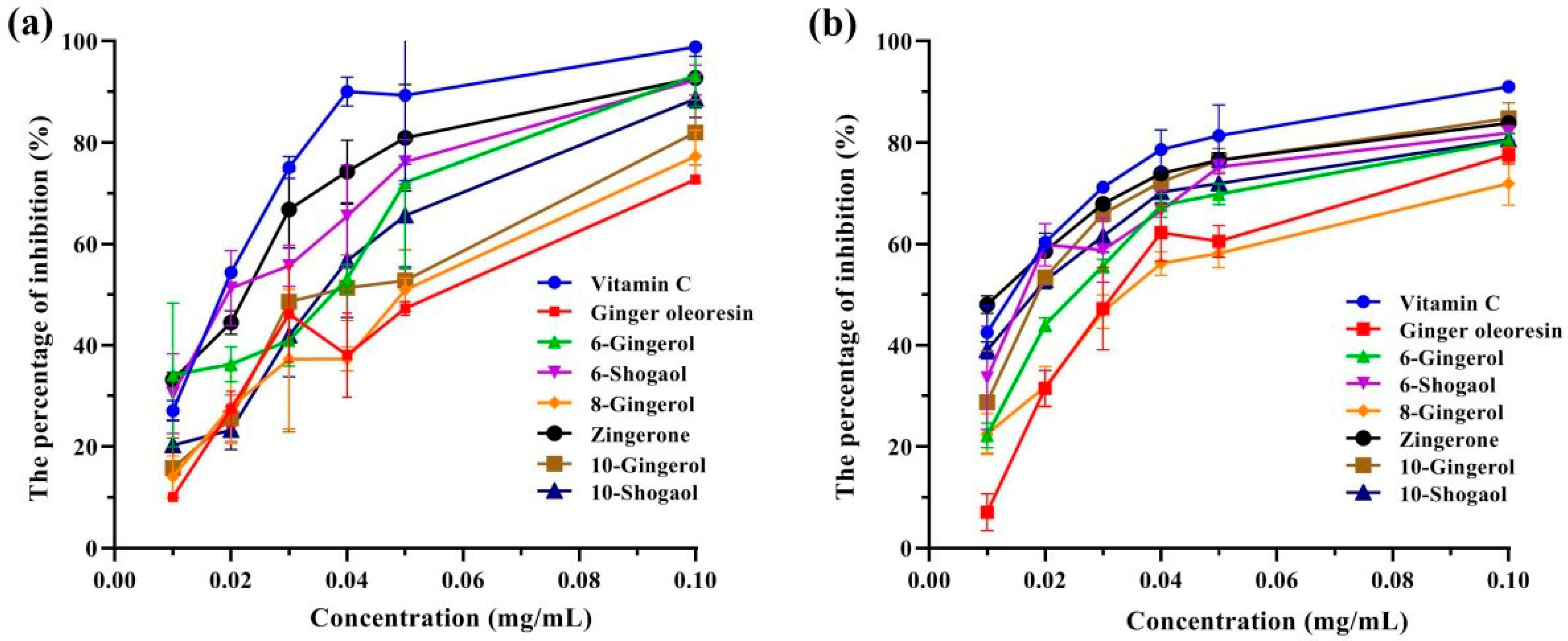
3.2. Antioxidant Activities of Ginger Oleoresin
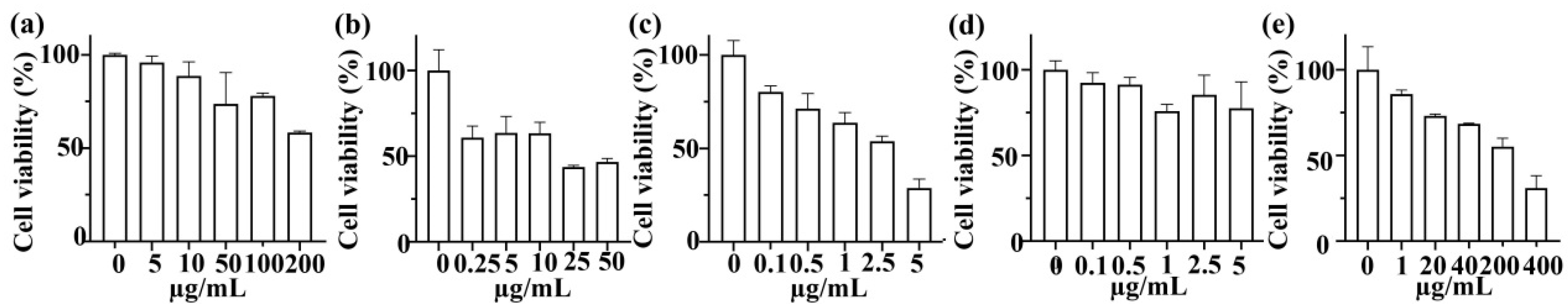
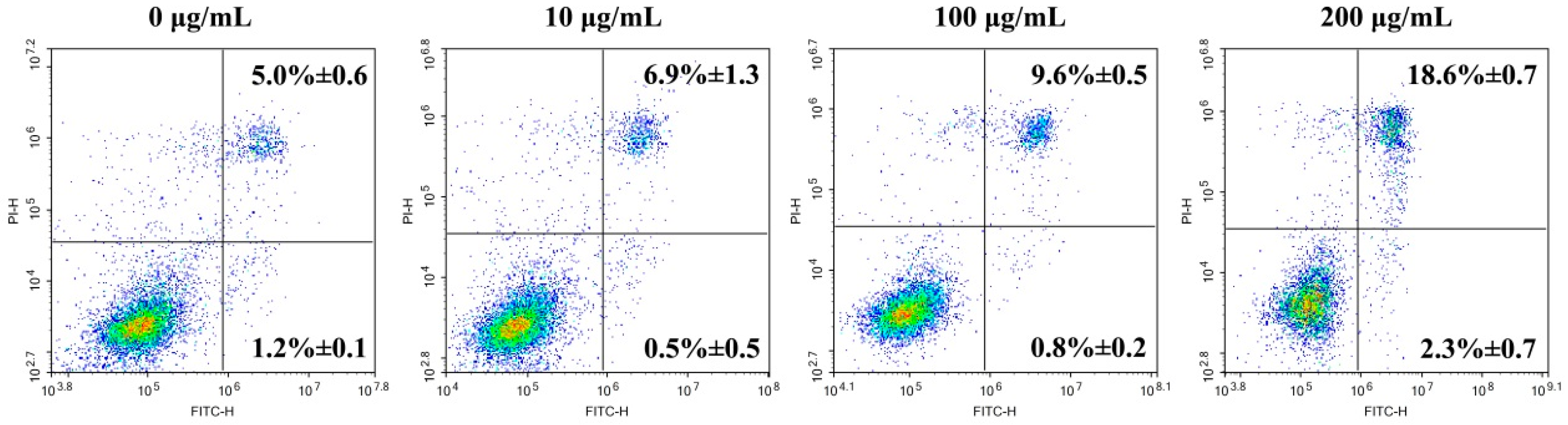
3.3. In Vitro Anticancer Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiyama, R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. J. Nutr. Biochem. 2020, 86, 108486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Zhao, R.; Wang, D.; Wang, L.; Zhang, Q.; Wei, S.J.; Lu, F.; Peng, W.; Wu, C.J. Ginger (Zingiber officinale Rosc.) and its bioactive components are potential resources for health beneficial agents. Phytother Res. 2021, 35, 711–742. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Masuku, N.P.; Paimo, O.K.; Lebelo, S.L. Ginger from Farmyard to Town: Nutritional and pharmacological applications. Front. Pharmacol. 2021, 12, 779352. [Google Scholar] [CrossRef] [PubMed]
- Syafitri, D.M.; Levita, J.; Mutakin, M.; Diantini, A. A Review: Is Ginger (Zingiber officinale var. Roscoe) Potential for future phytomedicine? Iran. J. Appl. Anim. Sci. 2018, 8, 30. [Google Scholar] [CrossRef]
- Dalsasso, R.R.; Valencia, G.A.; Monteiro, A.R. Impact of drying and extractions processes on the recovery of gingerols and shogaols, the main bioactive compounds of ginger. Food Res. Int. 2022, 154, 111043. [Google Scholar] [CrossRef]
- Lashgari, N.A.; Momeni Roudsari, N.; Khayatan, D.; Shayan, M.; Momtaz, S.; Roufogalis, B.D.; Abdolghaffari, A.H.; Sahebkar, A. Ginger and its constituents: Role in treatment of inflammatory bowel disease. Biofactors 2022, 48, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Kamaruddin, M.S.H.; Chong, G.H.; Daud, N.M.; Putra, N.R.; Salleh, L.M.; Suleiman, N. Bioactivities and green advanced extraction technologies of ginger oleoresin extracts: A review. Food Res. Int. 2023, 164, 112283. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.Z.; Zhang, J.G.; Hu, F.; Wei, Z.J. Antibacterial activity and mechanism of ginger essential oil against Escherichia coli and Staphylococcus aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef]
- Ballester, P.; Cerdá, B.; Arcusa, R.; García-Muñoz, A.M.; Marhuenda, J.; Zafrilla, P. Antioxidant activity in extracts from Zingiberaceae family: Cardamom, turmeric, and ginger. Molecules. 2023, 28, 4024. [Google Scholar] [CrossRef]
- Si, W.H.; Chen, Y.P.; Zhang, J.H.; Chen, Z.Y.; Chung, H.Y. Antioxidant activities of ginger extract and its constituents toward lipids. Food Chem. 2018, 239, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, D.S.; Albalawi, S.F.; Alghrid, S.T.; Alhwity, B.S.; Qushawy, M.; Mortagi, Y.; EI-Sherbiny, M.; Prabahar, K.; Elsherbiny, N. Ginger oil nanoemulsion formulation augments its antiproliferative effect in ehrlich solid tumor model. Foods 2023, 12, 4139. [Google Scholar] [CrossRef] [PubMed]
- de Lima, R.M.T.; Dos Reis, A.C.; de Menezes, A.P.M.; Santos, J.V.O.; de Oliveira Filho, J.W.G.; de Oliveira Ferreira, J.R.; de Alencar, M.V.O.B.; da Mata, A.M.O.F.; Khan, I.N.; Islam, A.; et al. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phytother. Res. 2018, 32, 1885–1907. [Google Scholar] [CrossRef] [PubMed]
- Suk, S.; Kwon, G.T.; Lee, E.; Jang, W.J.; Yang, H.; Kim, J.H.; Thimmegowda, N.R.; Chung, M.Y.; Kwon, J.Y.; Yang, S.; et al. Gingerenone A, a polyphenol present in ginger, suppresses obesity and adipose tissue inflammation in high-fat diet-fed mice. Mol. Nutr. Food Res. 2017, 61, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; Mani, J.S.; White, S.; Brown, P.; Naiker, M. Pungent and volatile constituents of dried Australian ginger. Curr. Res. Food Sci. 2021, 4, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Alberti, Á.; Riethmüller, E.; Béni, S. Characterization of diarylheptanoids: An emerging class of bioactive natural products. J. Pharm. Biomed. Anal. 2018, 147, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Kiran, C.R.; Chakka, A.K.; Padmakumari Amma, K.P.; Nirmala Menon, A.; Sree Kumar, M.M.; Venugopalan, V.V. Influence of cultivar and maturity at harvest on the essential oil composition, oleoresin and [6]-gingerol contents in fresh ginger from northeast India. J. Agric. Food Chem. 2013, 61, 4145–4454. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.; Haw, X.R.; Alkatib, H.H.; Sasidharan, S.; Marriott, P.J.; Wong, Y.F. Phytochemical constituents and antiproliferative activities of essential oils from four varieties of Malaysian Zingiber officinale Roscoe against human cervical cancer cell line. Plants 2022, 11, 1280. [Google Scholar] [CrossRef]
- Chen, M.C.; Zhu, Y.J.; Zhang, H.F.; Wang, J.P.; Liu, X.G.; Chen, Z.; Zheng, M.X.; Liu, B. Phenolic compounds and the biological effects of Puerh teas with long-term storage. Int. J. Food Prop. 2017, 20, 1715–1728. [Google Scholar] [CrossRef]
- van den Dool, H.; Kratz, P.D. A Generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Gunasena, M.T.; Rafi, A.; Mohd Zobir, S.A.; Hussein, M.Z.; Ali, A.; Kutawa, A.B.; Wahab, M.A.A.; Sulaiman, M.R.; Adzmi, F.; Ahmad, K. Phytochemicals profiling, antimicrobial activity and mechanism of action of essential oil extracted from ginger (Zingiber officinale Roscoe cv. Bentong) against Burkholderia glumae causative agent of bacterial panicle blight disease of rice. Plants 2022, 11, 1466. [Google Scholar] [CrossRef] [PubMed]
- Höferl, M.; Stoilova, I.; Wanner, J.; Schmidt, E.; Jirovetz, L.; Trifonova, D.; Stanchev, V.; Krastanov, A. Composition and comprehensive antioxidant activity of ginger (Zingiber officinale) essential oil from ecuador. Nat. Prod. Commun. 2015, 10, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Wohlmuth, H.; Smith, M.K.; Brooks, L.O.; Myers, S.P.; Leach, D.N. Essential oil composition of diploid and tetraploid clones of ginger (Zingiber officinale Roscoe) grown in Australia. J. Agric. Food Chem. 2006, 54, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, W.; Liang, W.; Breemen, R.B. Identification and quantification of gingerols and related compounds in ginger dietary supplements using high performance liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2009, 57, 10014–10021. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Q.; Timmermann, B.N.; Gang, D.R. Characterization and identification of diarylheptanoids in ginger (Zingiber officinale Rosc.) using high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 509–518. [Google Scholar] [CrossRef]
- Jolad, S.D.; Lantz, R.C.; Chen, G.J.; Bates, R.B.; Timmermann, B.N. Commercially processed dry ginger (Zingiber officinale): Composition and effects on LPS-stimulated PGE2 production. Phytochemistry 2005, 66, 1614–1635. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M. Electrochemistry of flavonoids: A comprehensive review. Int. J. Mol. Sci. 2023, 24, 15667. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Fu, Z.M.; Deng, G.; Guo, R.; Chen, D.F. Free radical scavenging potency of ellagic acid and its derivatives in multiple H+/e− processes. Phytochemistry 2020, 180, 112517. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef]
- Aeschbach, R.; Löliger, J.; Scott, B.C.; Murcia, A.; Butler, J.; Halliwell, B.; Aruoma, O.I. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem. Toxicol. 1994, 32, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Wang, X.Q.; Cao, G.; Sun, L.; Ho, R.J.Y.; Han, Y.Q.; Hong, Y.; Wu, D.L. Prediction of the potential mechanism of compound gingerol against liver cancer based on network pharmacology and experimental verification. J. Pharm. Pharmacol. 2022, 74, 869–886. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Park, S.; Song, K.S.; Bae, S.W.; Lee, J.S.; Jang, K.J.; Park, Y.M. Anticancer effects of 6-gingerol through downregulating iron transport and PD-L1 expression in non-small cell lung cancer cells. Cells 2023, 12, 2628. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.J.; Chen, X.; Luo, L.M.; Zhang, Q.; Gao, C.X.; Zhuang, X.B.; Yuan, S.J.; Qiao, T.K. [6]-Gingerol enhances the radiosensitivity of gastric cancer via G2/M phase arrest and apoptosis induction. Oncol. Rep. 2018, 39, 2252–2260. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.M.; Yao, X.H.; Hao, Y.H.; Pan, A.H.; Zhou, X.W. 8-Gingerol regulates colorectal cancer cell proliferation and migration through the EGFR/STAT/ERK pathway. Int. J. Oncol. 2020, 56, 390–397. [Google Scholar] [CrossRef]
- Wei, J.; Wang, R.; Lu, Y.; He, S.; Ding, Y. Flotillin-1 promotes progression and dampens chemosensitivity to cisplatin in gastric cancer via ERK and AKT signaling pathways. Eur. J. Pharmacol. 2022, 916, 174631. [Google Scholar] [CrossRef]

| Retention Time/min | Compound Identification | Molecular Formula | KI a | KI b | Relative Content (%) |
|---|---|---|---|---|---|
| 7.90 | Decanal | C10H20O | 1195 | 1208.8 | 0.56 ± 0.05 |
| 8.97 | α-citral | C10H16O | 1269 | 1275.7 | 0.14 ± 0.01 |
| 9.30 | 2-Undecanone | C11H22O | 1294 | 1296.3 | 0.12 ± 0.06 |
| 10.98 | α-Copaene | C15H22 | 1376 | 1391.7 | 0.58 ± 0.07 |
| 11.23 | β-Elemene | C15H22 | 1407 | 1405.3 | 0.57 ± 0.02 |
| 11.37 | Trans-α-Bergamotene | C15H22 | 1438 | 1413.2 | 0.36 ± 0.04 |
| 11.85 | β-ylangene | C15H22 | 1423 | 1438.6 | 0.18 ± 0.03 |
| 12.00 | γ-Elemene | C15H22 | 1418 | 1446.4 | 0.63 ± 0.08 |
| 12.28 | β-trans-bergamotene | C15H22 | 1490 | 1461.4 | 0.21 ± 0.14 |
| 12.35 | β-Farnesene | C15H22 | 1459 | 1465.2 | 0.52 ± 0.11 |
| 12.65 | Alloaromadendrene | C15H22 | 1457 | 1481.2 | 0.08 ±0.09 |
| 12.87 | α-curcumene | C15H22 | 1483 | 1492.8 | 8.11 ±0.64 |
| 13.12 | Zingiberene | C15H22 | 1493 | 1506.2 | 15.34 ± 1.08 |
| 13.27 | α-Farnesene | C15H22 | 1507 | 1514 | 7.09 ± 0.51 |
| 13.37 | β-Bisabolene | C15H22 | 1506 | 1519.3 | 6.92 ± 0.53 |
| 13.58 | (+)-epi-Bicyclosesquiphellandrene | C15H22 | 1521 | 1530.4 | 0.07 ± 0.14 |
| 13.69 | β-Sesquiphellandrene | C15H22 | 1525 | 1536 | 8.42 ± 0.53 |
| 13.80 | (E)-γ-Bisabolene | C15H22 | 1533 | 1542.2 | 0.24 ± 0.03 |
| 14.13 | Dodecanoic acid | C12H24O2 | 1570 | 1559.6 | 0.16 ± 0.01 |
| 14.75 | Cetene | C16H32 | 1587 | 1592.2 | 1.56 ± 0.13 |
| 14.88 | Hexadecane | C16H34 | 1600 | 1599.1 | 0.38 ± 0.03 |
| 16.07 | Zingerone | C11H14O3 | 1653 | 1663.1 | 8.79 ± 0.27 |
| 16.73 | Heptadecane | C17H36 | 1700 | 1698.1 | 0.23 ± 0.09 |
| 18.42 | 1-Octadecene | C18H36 | 1795 | 1791.9 | 0.57 ± 0.04 |
| 19.86 | Phthalic acid, isobutyl octyl ester | C20H30O4 | 1868 | 1874.6 | 1.04 ± 0.06 |
| 21.82 | (E)-1-(6,10-Dimethylundec-5-en-2-yl)-4-methylbenzene | C20H32 | 1991 | 1991.1 | 1.26 ± 0.13 |
| 23.95 | 6-Methyl-4,6-bis(4-methylpent-3-en-1-yl)cyclohexa-1,3-dienecarbaldehyde | C20H30O | 2113.4 | 2120.6 | 1.33 ± 0.17 |
| 24.70 | Cyclohexene, 4-(4-ethylcyclohexyl)-1-pentyl- | C19H34 | NA | 2161.4 | 0.14 ± 0.04 |
| 25.14 | 2-Methyl-3-(3-methyl-but-2-enyl)-2-(4-methyl-pent-3-enyl)-oxetane | C15H26O | NA | 2185.5 | 0.55 ± 0.06 |
| 25.26 | 1-Docosene | C22H44 | NA | 2192 | 0.32 ± 0.02 |
| 26.53 | 6-Isoshogaol | C17H24O3 | 2231.7 | 2259.7 | 7.47 ± 0.43 |
| 27.56 | 6-Shogaol | C17H24O3 | 2294 | 2319.2 | 18.62 ± 2.91 |
| 28.60 | Z-12-Pentacosene | C25H50 | NA | 2393.3 | 0.29 ± 0.18 |
| 30.16 | Pentacosane | C25H52 | 2500 | 2498 | 0.38 ± 0.14 |
| Compound | Retention Time (min) | MS | MS2 | Identification | Content (mg/g Ginger Oleoresin) |
|---|---|---|---|---|---|
| 1 | 29.20 | 373 | 179, 193, 165, 121 | 5-hydroxy-1,7, bis (4-hydroxy-3-methoxyphenyl)-3-heptanone | 23.67 ± 0.33 |
| 2 | 33.16 | 445 | 385, 325, 355 | 3,5-diacetoxy-7-(3, 4-dihydroxyphenyl)-1-(4-hydroxy-3-methoxyphenyl)heptane | 4.88 ± 0.18 |
| 3 | 33.59 | 293 | 193, 135, 178, 100, 275 | 6-gingerol | 82.93 ± 2.12 |
| 4 | 34.30 | 291 | 191, 176, 135, 162 | 1-dehydro-[6]-gingerol | 17.04 ± 0.19 |
| 5 | 37.01 | 321 | 289, 274, 193, 175, 160, 149, 134, 127 | 8-gingerol | 11.94 ± 0.37 |
| 6 | 37.75 | 289 | 274, 219, 191, 175, 160, 149, 134 | 1-dehydro-6-gingerdione | 44.93 ± 0.96 |
| 7 | 38.90 | 349 | 331, 289, 274, 193, 175, 155, 149, 134 | 10-gingerol | 52.63 ± 6.38 |
| 8 | 40.09 | 423 | 329, 149, 177, 287, 133 | dihydrocurcumin derivatives | 63.40 ± 2.90 |
| 9 | 42.06 | 345 | 277, 149, 195, 175, 163, 134 | 1-dehydro-[10]-gingerdione | 19.93 ± 1.59 |
| 10 | 42.59 | 277 | 259, 205, 195, 175, 149, 134 | 1-dehydro-[5]-gingerol | 10.12 ± 1.08 |
| 11 | 43.26 | 279 | 195, 175, 149 | 5-gingerol | 60.84 ± 1.77 |
| 12 | 43.80 | 255 | 237 | 6-(4-hydroxy-3-methoxyphenyl)hexane-1,2,4-triol | 17.16 ± 1.36 |
| 13 | 44.58 | 281 | 275, 149, 134 | 5-gingerdiol | 9.93 ± 2.16 |
| 14 | 46.46 | 283 | 175, 193 | 8-(4-hydroxy-3-methoxyphenyl)octane-3,4,6-triol | 4.16 ± 0.35 |
| No. | Compounds | Mass Weight | Half Maximal Inhibitory Concentration (IC50, µg/mL) | |
|---|---|---|---|---|
| ABTS | DPPH | |||
| 1 | Vitamin C | 176 | 17.0 ± 0.0 e | 13.0 ± 2.0 de |
| 2 | Ginger oleoresin | 48.0 ± 0.0 a | 35.7 ± 3.5 a | |
| 3 | 6-gingerol | 294 | 27.3 ± 5.1 cd | 25.0 ± 1.0 b |
| 4 | 6-shogaol | 276 | 20.7 ± 2.3 de | 17.7 ± 5.5 cd |
| 5 | 8-gingerol | 322 | 47.0 ± 6.1 a | 36.0 ± 4.4 a |
| 6 | Zingerone | 194 | 19.0 ± 1.0 de | 11.3 ± 0.6 e |
| 7 | 10-gingerol | 350 | 38.7 ± 10.1 b | 19.0 ± 2.6 c |
| 8 | 10-shogaol | 332 | 33.3 ± 1.5 bc | 16.7 ± 1.2 cde |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Lin, E.; Xiao, R.; Li, Z.; Liu, B.; Wang, J. Structural Characteristic, Strong Antioxidant, and Anti-Gastric Cancer Investigations on an Oleoresin from Ginger (Zingiber officinale var. roscoe). Foods 2024, 13, 1498. https://doi.org/10.3390/foods13101498
Chen M, Lin E, Xiao R, Li Z, Liu B, Wang J. Structural Characteristic, Strong Antioxidant, and Anti-Gastric Cancer Investigations on an Oleoresin from Ginger (Zingiber officinale var. roscoe). Foods. 2024; 13(10):1498. https://doi.org/10.3390/foods13101498
Chicago/Turabian StyleChen, Meichun, Enquan Lin, Rongfeng Xiao, Zuliang Li, Bo Liu, and Jieping Wang. 2024. "Structural Characteristic, Strong Antioxidant, and Anti-Gastric Cancer Investigations on an Oleoresin from Ginger (Zingiber officinale var. roscoe)" Foods 13, no. 10: 1498. https://doi.org/10.3390/foods13101498
APA StyleChen, M., Lin, E., Xiao, R., Li, Z., Liu, B., & Wang, J. (2024). Structural Characteristic, Strong Antioxidant, and Anti-Gastric Cancer Investigations on an Oleoresin from Ginger (Zingiber officinale var. roscoe). Foods, 13(10), 1498. https://doi.org/10.3390/foods13101498





