Design, Optimization, and Modeling Study of Ultrasound-Assisted Extraction of Bioactive Compounds from Purple-Fleshed Sweet Potatoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Design of Experiments
2.4. Ultrasound-Assisted Extraction
2.5. Conventional Extraction
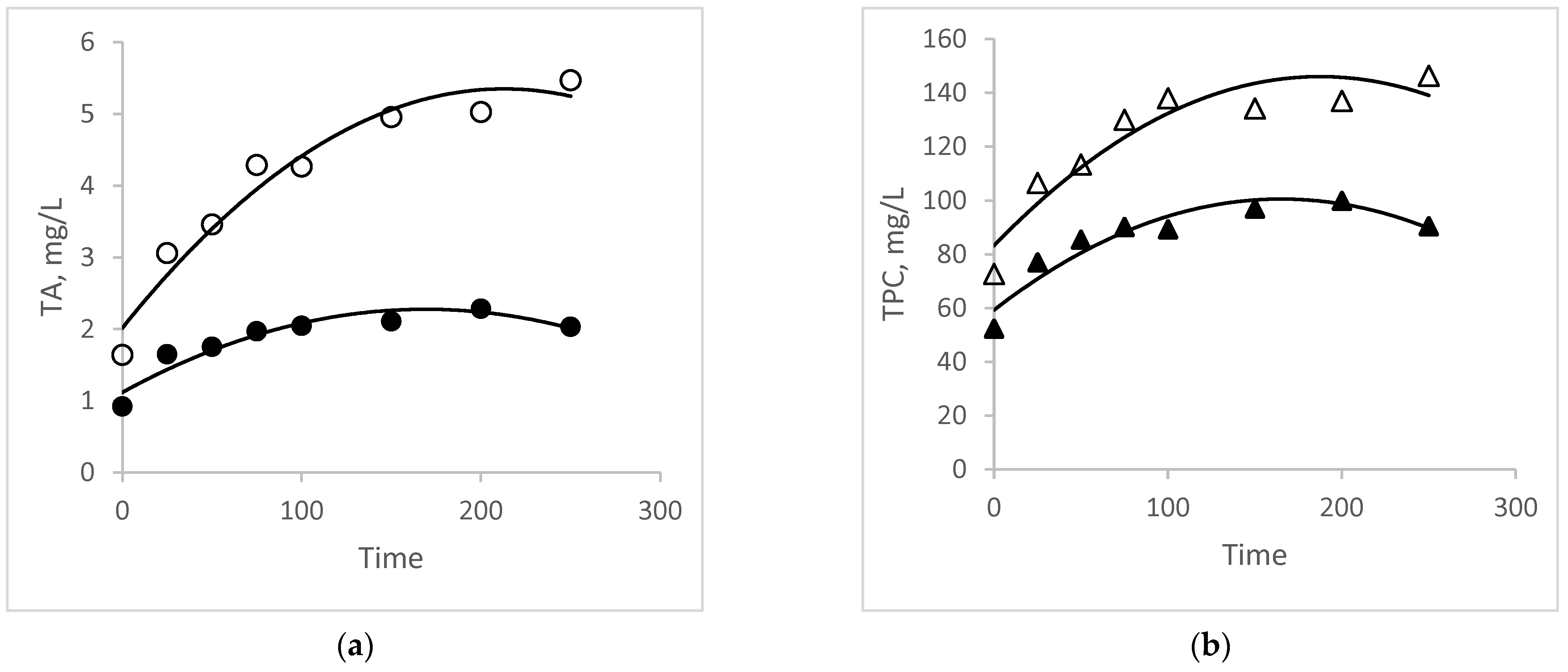
2.6. Kinetics and Modeling of Extraction
2.7. Total Anthocyanin (TA) Analysis
2.8. Total Phenolic Content (TPC) Analysis
2.9. Total Starch Content
2.10. Validation of Optimized UAE and Comparative Study of Extraction Efficiency
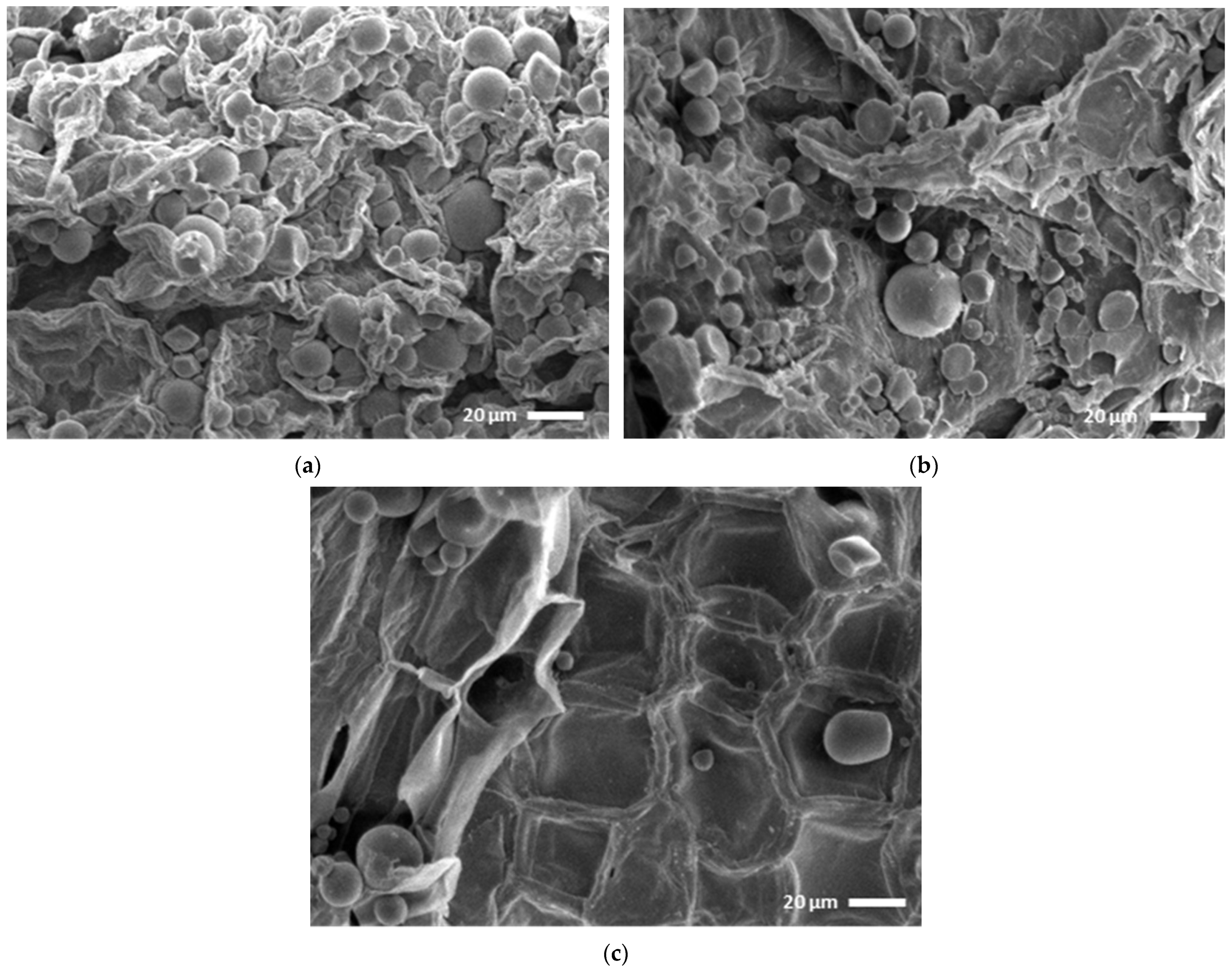
2.11. Scanning Electron Microscopy (SEM)
2.12. Statistical Analysis
3. Results and Discussion
3.1. Design and Optimization of Ultrasound-Assisted Extraction
3.2. Optimized Extraction Compared with Conventional Extraction
3.3. Comparative Study of Scanning Electron Microscopy
3.4. Comparative Study of Kinetics and Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yong, H.; Wang, X.; Sun, J.; Fang, Y.; Liu, J.; Jin, C. Comparison of the structural characterization and physicochemical properties of starches from seven purple sweet potato varieties cultivated in China. Int. J. Biol. Macromol. 2018, 120, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lin, C.; Chen, M.; Chiang, P. Stability and Quality of Anthocyanin in Purple Sweet Potato Extracts. Foods 2019, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition 2015, 31, 1301–1306. [Google Scholar] [CrossRef]
- He, B.; Zhang, L.L.; Yue, X.Y.; Liang, J.; Jiang, J.; Gao, X.L.; Yue, P.X. Optimization of Ultrasound-Assisted Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Technol. 2017, 66, 153–165. [Google Scholar] [CrossRef]
- Chen, J.; Du, J.; Li, M.L.; Li, C. Degradation kinetics and pathways of red raspberry anthocyanins in model and juice systems and their correlation with color and antioxidant changes during storage. LWT 2020, 128, 109448. [Google Scholar] [CrossRef]
- Sharma, R.J.; Gupta, R.C.; Singh, S.; Bansal, A.K.; Singh, I.P. Stability of anthocyanins- and anthocyanidins-enriched extracts, and formulations of fruit pulp of Eugenia jambolana (‘jamun’). Food Chem. 2016, 190, 808–817. [Google Scholar] [CrossRef]
- Strieder, M.M.; Silva, E.K.; Meireles, M.A.A. Specific Energy: A New Approach to Ultrasound-assisted Extraction of Natural Colorants. Food Public Health 2019, 9, 45–52. [Google Scholar] [CrossRef]
- Lv, X.; Mu, J.; Wang, W.; Liu, Y.; Lu, X.; Sun, J.; Wang, J.; Ma, Q. Effects and mechanism of natural phenolic acids/fatty acids on copigmentation of purple sweet potato anthocyanins. Curr. Res. Food Sci. 2022, 5, 1243–1250. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Choi, J.H.; Choi, J.M.; Chung, Y.C.; Jeong, H.G. Protective mechanisms of anthocyanins from purple sweet potato against tert-butyl hydroperoxide-induced hepatotoxicity. Food Chem. Toxicol. 2011, 49, 2081–2089. [Google Scholar] [CrossRef]
- Han, Y.; Guo, Y.; Cui, S.W.; Li, H.; Shan, Y.; Wang, H. Purple Sweet Potato Extract extends lifespan by activating autophagy pathway in male Drosophila melanogaster. Exp. Gerontol. 2021, 144, 111190. [Google Scholar] [CrossRef]
- Okolie, C.L.; Akanbi, T.O.; Mason, B.; Udenigwe, C.C.; Aryee, A.N.A. Influence of conventional and recent extraction technologies on physicochemical properties of bioactive macromolecules from natural sources: A review. Food Res. Int. 2019, 116, 827–839. [Google Scholar] [CrossRef]
- Renard, C.M.G.C. Extraction of bioactives from fruit and vegetables: State of the art and perspectives. LWT 2018, 93, 390–395. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Dranca, F.; Oroian, M. Optimization of ultrasound-assisted extraction of total monomeric anthocyanin (TMA) and total phenolic content (TPC) from eggplant (Solanum melongena L.) peel. Ultrason. Sonochem. 2016, 31, 637–646. [Google Scholar] [CrossRef]
- Espada-bellido, E.; Ferreiro-gonzález, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef]
- Teixeira, B.A.; Vidigal, M.C.T.R.; de Castro Leite Júnior, B.; Vieira, É.N.R.; Martins, E.M.F.; Stringheta, P.C. Optimization, Kinetic and Phenomenological Modeling of Ultrasound-Assisted Extraction Process of Bioactive Compounds from Raspberries (Rubus idaeus L.). Food Anal. Methods 2023, 16, 759–770. [Google Scholar] [CrossRef]
- Kitanović, S.; Milenović, D.; Veljković, V.B. Empirical kinetic models for the resinoid extraction from aerial parts of St. John’s wort (Hypericum perforatum L.). Biochem. Eng. J. 2008, 41, 1–11. [Google Scholar] [CrossRef]
- Boateng, I.D. Application of Graphical Optimization, Desirability, and Multiple Response Functions in the Extraction of Food Bioactive Compounds. Food Eng. Rev. 2023, 15, 309–328. [Google Scholar] [CrossRef]
- Ding, Y.; Zheng, J.; Xia, X.; Ren, T.; Kan, J. Box-Behnken design for the optimization of nanoscale retrograded starch formation by high-power ultrasonication. LWT-Food Sci. Technol. 2016, 67, 206–213. [Google Scholar] [CrossRef]
- Baran, A.; Goud, V.V.; Das, C. Extraction of phenolic compounds and anthocyanin from black and purple rice bran (Oryza sativa L.) using ultrasound: A comparative analysis and phytochemical profiling. Ind. Crop. Prod. 2017, 95, 332–341. [Google Scholar]
- Rocha, J.C.G.; Procópio, F.R.; Mendonça, A.C.; Vieira, L.M.; Perrone, Í.T.; Barros, F.A.R.; Stringheta, P.C. Optimization of ultrasound-assisted extraction of phenolic compounds from jussara (Euterpe edulis M.) and blueberry (Vaccinium myrtillus) fruits. Food Sci. Technol. 2017, 38, 45–53. [Google Scholar] [CrossRef]
- Teixeira, B.A.; Vidigal, M.C.T.R.; Stringheta, P.C. Optimization of ultrasound-assisted extraction of anthocyanins from purple tomatoes. Ciência Rural 2024, 54, e20220604. [Google Scholar] [CrossRef]
- Veličković, D.T.; Milenović, D.M.; Ristić, M.S.; Veljković, V.B. Ultrasonic extraction of waste solid residues from the Salvia sp. essential oil hydrodistillation. Biochem. Eng. J. 2008, 42, 97–104. [Google Scholar] [CrossRef]
- Dias, A.L.B.; Arroio Sergio, C.S.; Santos, P.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Ultrasound-assisted extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L.): Effects on the vegetable matrix and mathematical modeling. J. Food Eng. 2017, 198, 36–44. [Google Scholar] [CrossRef]
- Veličković, D.T.; Milenović, D.M.; Ristić, M.S.; Veljković, V.B. Kinetics of ultrasonic extraction of extractive substances from garden (Salvia officinalis L.) and glutinous (Salvia glutinosa L.) sage. Ultrason. Sonochem. 2006, 13, 150–156. [Google Scholar] [CrossRef]
- Fuleki, T.; Francis, F.J. Quantitative methods foranthocyanins: 1. Extraction and determination of totalanthocyanin in cranberries. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef]
- Liao, J.; Guo, Z.; Yu, G. Process intensification and kinetic studies of ultrasound-assisted extraction of flavonoids from peanut shells. Ultrason. Sonochem. 2021, 76, 105661. [Google Scholar] [CrossRef]
- Sang, J.; Sang, J.; Ma, Q.; Hou, X.F.; Li, C.Q. Extraction optimization and identification of anthocyanins from Nitraria tangutorun Bobr. seed meal and establishment of a green analytical method of anthocyanins. Food Chem. 2017, 218, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Das, P.R.; Eun, J.B. A comparative study of ultra-sonication and agitation extraction techniques on bioactive metabolites of green tea extract. Food Chem. 2018, 253, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.S.; Wahab, N.S.A.; Soo, J. Water soluble phenolics, flavonoids and anthocyanins extracted from jaboticaba berries using maceration with ultrasonic pretreatment. Food Chem. Adv. 2023, 3, 100387. [Google Scholar] [CrossRef]
- Tan, J.; Han, Y.; Han, B.; Qi, X.; Cai, X.; Ge, S.; Xue, H. Extraction and purification of anthocyanins: A review. J. Agric. Food Res. 2022, 8, 100306. [Google Scholar] [CrossRef]
- Torres, A.; Aguilar-Osorio, G.; Camacho, M.; Basurto, F.; Navarro-Ocana, A. Characterization of polyphenol oxidase from purple sweet potato (Ipomoea batatas L. Lam) and its affinity towards acylated anthocyanins and caffeoylquinic acid derivatives. Food Chem. 2021, 356, 129709. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, S.; Cueva-Mestanza, R.; de Pascual-Teresa, S. Effect of spray drying on the polyphenolic compounds present in purple sweet potato roots: Identification of new cinnamoylquinic acids. Food Chem. 2021, 345, 128679. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhou, J.; Liu, W.; Tao, W.; He, J.; Jin, W.; Guo, H.; Yang, N.; Li, Y. The anti-inflammatory potential of protein-bound anthocyanin compounds from purple sweet potato in LPS-induced RAW264.7 macrophages. Food Res. Int. 2020, 137, 109647. [Google Scholar] [CrossRef]
- Qin, Y.; Yun, D.; Xu, F.; Chen, D.; Kan, J.; Liu, J. Smart packaging films based on starch/polyvinyl alcohol and Lycium ruthenicum anthocyanins-loaded nano-complexes: Functionality, stability and application. Food Hydrocoll. 2021, 119, 106850. [Google Scholar] [CrossRef]
- Tutunchi, P.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Extraction of red beet extract with β-cyclodextrin-enhanced ultrasound assisted extraction: A strategy for enhancing the extraction efficacy of bioactive compounds and their stability in food models. Food Chem. 2019, 297, 124994. [Google Scholar] [CrossRef]
- Audilakshmi, S.; Swarnalatha, M. Sorghum for starch and grain ethanol. In Breeding Sorghum for Diverse End Uses; Woodhead Publishing: Sawston, UK, 2018; pp. 239–254. [Google Scholar]
- Chemat, S.; Lagha, A.; AitAmar, H.; Bartels, P.V.; Chemat, F. Comparison of conventional and ultrasound-assissted extraction of carvone and limonene from caraway seeds. Flavour Fragr. J. 2004, 19, 188–195. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Mat Taher, Z.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef] [PubMed]
- Khadhraoui, B.; Turk, M.; Fabiano-Tixier, A.S.; Petitcolas, E.; Robinet, P.; Imbert, R.; El Maâtaoui, M.; Chemat, F. Histo-cytochemistry and scanning electron microscopy for studying spatial and temporal extraction of metabolites induced by ultrasound. Towards chain detexturation mechanism. Ultrason. Sonochem. 2018, 42, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.C.; Siu, K.C.; Wu, J.Y. Kinetic Models for Ultrasound-Assisted Extraction of Water-Soluble Components and Polysaccharides from Medicinal Fungi. Food Bioprocess Technol. 2013, 6, 2659–2665. [Google Scholar] [CrossRef]
- Veljković, V.B.; Milenović, D.M. Extraction of resinoids from St. John’s wort (Hypericum perforatum L.). II. Modeling of extraction kinects. Hem. Ind 2002, 56, 60–67. (In Serbian) [Google Scholar] [CrossRef]


| Standard Order | Time, X1 (min) | Temperature, X2 (°C) | Solid-to-Liquid Ratio, X3 (g/mL) | TA (mg/100 g) | TPC (mg GAE/100 g) |
|---|---|---|---|---|---|
| 1 | 5 (−1) | 30 (−1) | 1:10 (0) | 6.765 ± 0.233 | 81.416 ± 0.559 |
| 2 | 75 (+1) | 30 (−1) | 1:10 (0) | 11.767 ± 0.529 | 107.151 ± 1.688 |
| 3 | 5 (−1) | 70 (+1) | 1:10 (0) | 8.202 ± 0.058 | 85.710 ± 1.091 |
| 4 | 75 (+1) | 70 (+1) | 1:10 (0) | 14.558 ± 0.209 | 127.527 ± 1.820 |
| 5 | 5 (−1) | 50 (0) | 1:5 (−1) | 9.917 ± 0.029 | 53.366 ± 1.640 |
| 6 | 75 (+1) | 50 (0) | 1:5 (−1) | 11.334 ± 0.030 | 72.995 ± 1.090 |
| 7 | 5 (−1) | 50 (0) | 1:15 (+1) | 6.750 ± 0.177 | 79.971 ± 1.444 |
| 8 | 75 (+1) | 50 (0) | 1:15 (+1) | 16.424 ± 0.435 | 130.079 ± 1.354 |
| 9 | 40 (0) | 30 (−1) | 1:5 (−1) | 9.979 ± 0.088 | 74.622 ± 1.205 |
| 10 | 40 (0) | 70 (+1) | 1:5 (−1) | 11.020 ± 0.147 | 88.822 ± 1.860 |
| 11 | 40 (0) | 30 (−1) | 1:15 (+1) | 12.399 ± 0.177 | 124.780 ± 1.876 |
| 12 | 40 (0) | 70 (+1) | 1:15 (+1) | 13.833 ± 0.175 | 130.661 ± 1.757 |
| 13 | 40 (0) | 50 (0) | 1:10 (0) | 9.583 ± 0.354 | 93.094 ± 1.651 |
| 14 | 40 (0) | 50 (0) | 1:10 (0) | 9.124 ± 0.059 | 91.135 ± 0.195 |
| 15 | 40 (0) | 50 (0) | 1:10 (0) | 10.270 ± 0.059 | 95.236 ± 0.335 |
| 16 | 40 (0) | 50 (0) | 1:10 (0) | 9.589 ± 0.468 | 91.894 ± 1.178 |
| 17 | 40 (0) | 50 (0) | 1:10 (0) | 9.842 ± 0.058 | 95.074 ± 1.603 |
| Model | Kinetic Equation | Linear Transformation |
|---|---|---|
| Film theory [18] | ||
| Unsteady diffusion through plant material [18] | ||
| Empirical equation of Ponomaryov [18] | ||
| TA (mg/100 g) | TPC (mg GAE/100 g) | |||
|---|---|---|---|---|
| Variable | Coefficient | p-Value | Coefficient | p-Value |
| Constant | 11.079 | <0.0001 * | 96.425 | <0.0001 * |
| X1 | 5.613 | <0.0001 * | 34.323 | <0.0001 * |
| X2 | 1.961 | 0.008 * | 9.608 | 0.046 * |
| X3 | 0.809 | 0.159 | 39.118 | <0.0001 * |
| X12 | 0.030 | 0.923 | 6.727 | 0.026 * |
| X22 | −0.671 | 0.067 | −13.891 | 0.001 * |
| X32 | −1.455 | 0.003 * | 2.457 | 0.325 |
| X1X2 | 0.677 | 0.313 | 8.041 | 0.137 |
| X1X3 | 4.129 | 0.0005 * | 15.239 | 0.017 * |
| Model | Process | Total Anthocyanin—TA | Total Phenolic Content—TPC | ||||
|---|---|---|---|---|---|---|---|
| R2 | k × 10−3 (min−1) | b (1) | R2 | k × 10−3 (min−1) | b (1) | ||
| Film theory | UAE | 0.899 | 1.126 | 0.297 | 0.884 | 1.261 | 0.427 |
| Conventional | 0.592 | 0.225 | 0.177 | 0.501 | 0.397 | 0.325 | |
| Unsteady diffusion | UAE | 0.962 | 3.051 | 0.366 | 0.976 | 2.187 | 0.459 |
| Conventional | 0.889 | 0.349 | 0.135 | 0.811 | 0.487 | 0.231 | |
| Ponomaryov’s equation | UAE | 0.937 | 1.304 | 0.392 | 0.961 | 0.902 | 0.469 |
| Conventional | 0.882 | 0.291 | 0.135 | 0.799 | 0.355 | 0.231 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, B.A.; Gutiérrez, E.A.; de Souza, M.S.d.S.; Rigolon, T.C.B.; Martins, E.; Pessoa, F.L.P.; Vidigal, M.C.T.R.; Stringheta, P.C. Design, Optimization, and Modeling Study of Ultrasound-Assisted Extraction of Bioactive Compounds from Purple-Fleshed Sweet Potatoes. Foods 2024, 13, 1497. https://doi.org/10.3390/foods13101497
Teixeira BA, Gutiérrez EA, de Souza MSdS, Rigolon TCB, Martins E, Pessoa FLP, Vidigal MCTR, Stringheta PC. Design, Optimization, and Modeling Study of Ultrasound-Assisted Extraction of Bioactive Compounds from Purple-Fleshed Sweet Potatoes. Foods. 2024; 13(10):1497. https://doi.org/10.3390/foods13101497
Chicago/Turabian StyleTeixeira, Bárbara Avancini, Eliana Alviarez Gutiérrez, Mariane Sampaio da Silveira de Souza, Thaís Caroline Buttow Rigolon, Evandro Martins, Fernando Luiz Pellegrini Pessoa, Márcia Cristina Teixeira Ribeiro Vidigal, and Paulo Cesar Stringheta. 2024. "Design, Optimization, and Modeling Study of Ultrasound-Assisted Extraction of Bioactive Compounds from Purple-Fleshed Sweet Potatoes" Foods 13, no. 10: 1497. https://doi.org/10.3390/foods13101497
APA StyleTeixeira, B. A., Gutiérrez, E. A., de Souza, M. S. d. S., Rigolon, T. C. B., Martins, E., Pessoa, F. L. P., Vidigal, M. C. T. R., & Stringheta, P. C. (2024). Design, Optimization, and Modeling Study of Ultrasound-Assisted Extraction of Bioactive Compounds from Purple-Fleshed Sweet Potatoes. Foods, 13(10), 1497. https://doi.org/10.3390/foods13101497






