Selected Physicochemical, Thermal, and Rheological Properties of Barley Starch Depending on the Type of Soil and Fertilization with Ash from Biomass Combustion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Material
Field Experiment
2.2. Methods
2.2.1. Selected Physicochemical Properties
2.2.2. Qualitative and Quantitative Analysis of Minerals in Starches
2.2.3. Thermodynamic Characteristics of Gelatinisation and Retrogradation by DSC
2.2.4. Pasting Characteristics by RVA
2.2.5. Flow and Viscosity Curves
2.2.6. Mechanical Spectra of Barley Starch Gels
2.2.7. Statistical Analysis of Research Results
3. Results and Discussions
3.1. Physicochemical Properties
3.2. Mineral Content in Starches
3.3. Thermodynamic Characteristics of Gelatinization and Retrogradiation by DSC of Barley Starches
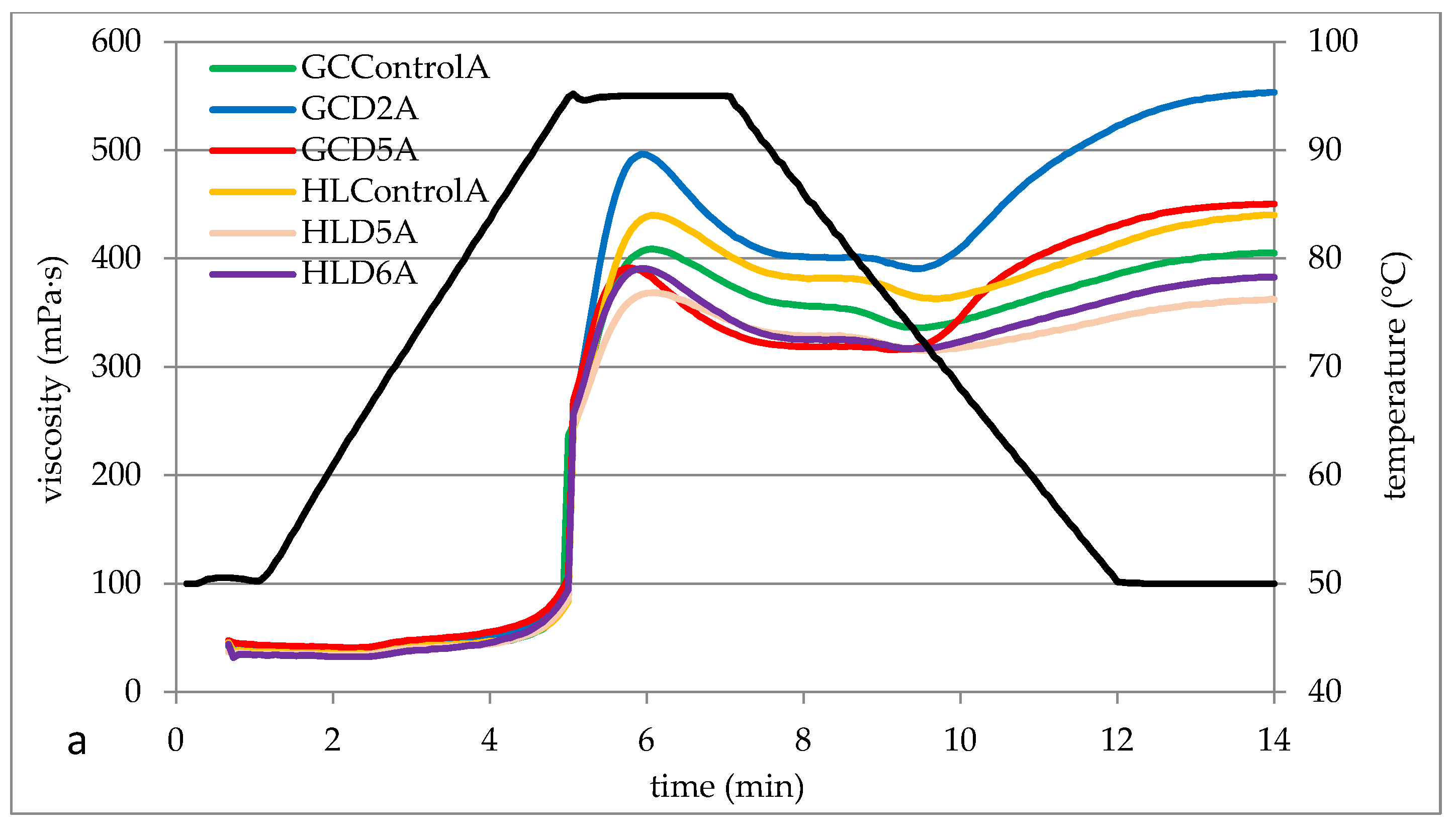
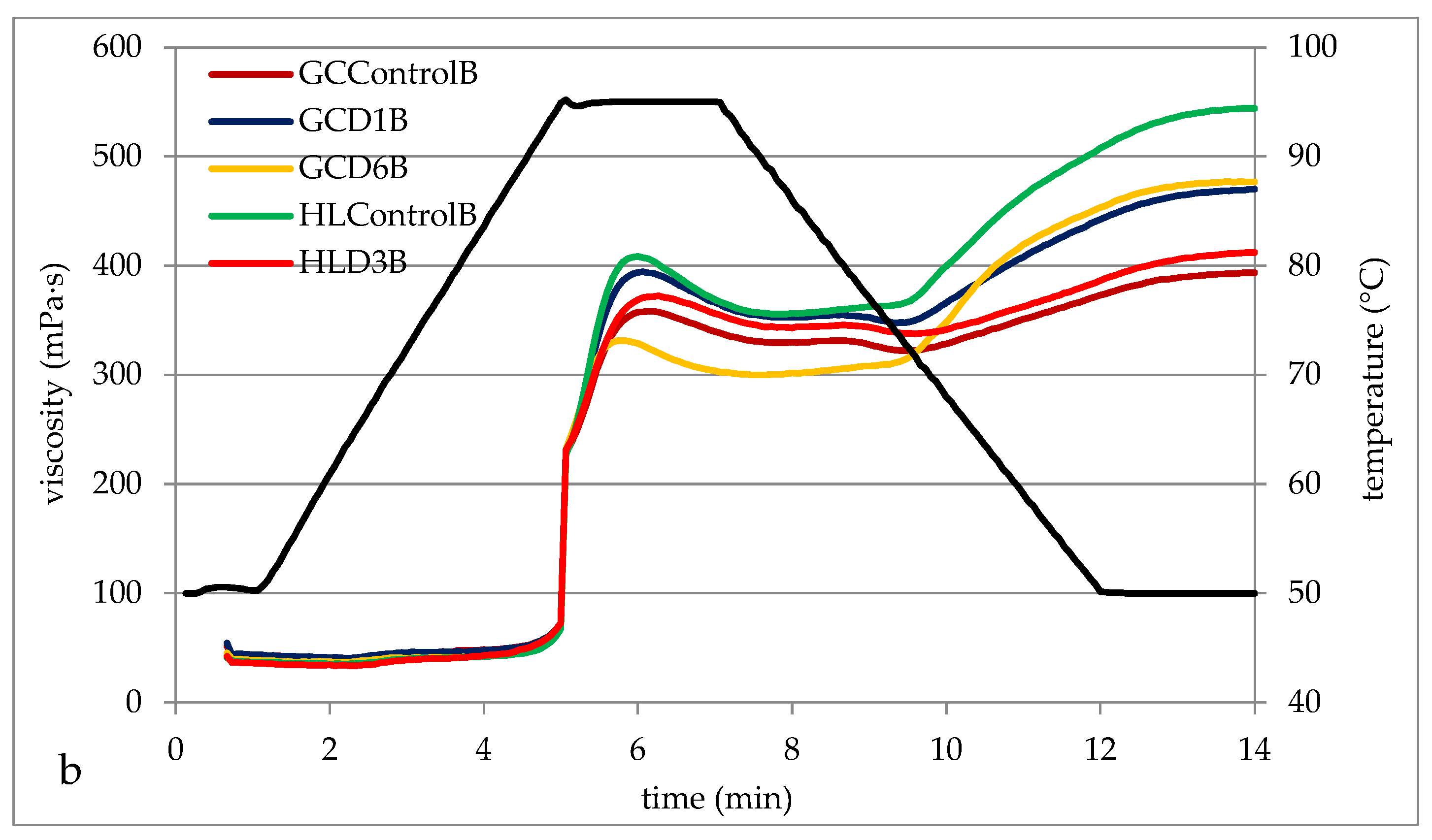
3.4. Pasting Properties of Barley Starches
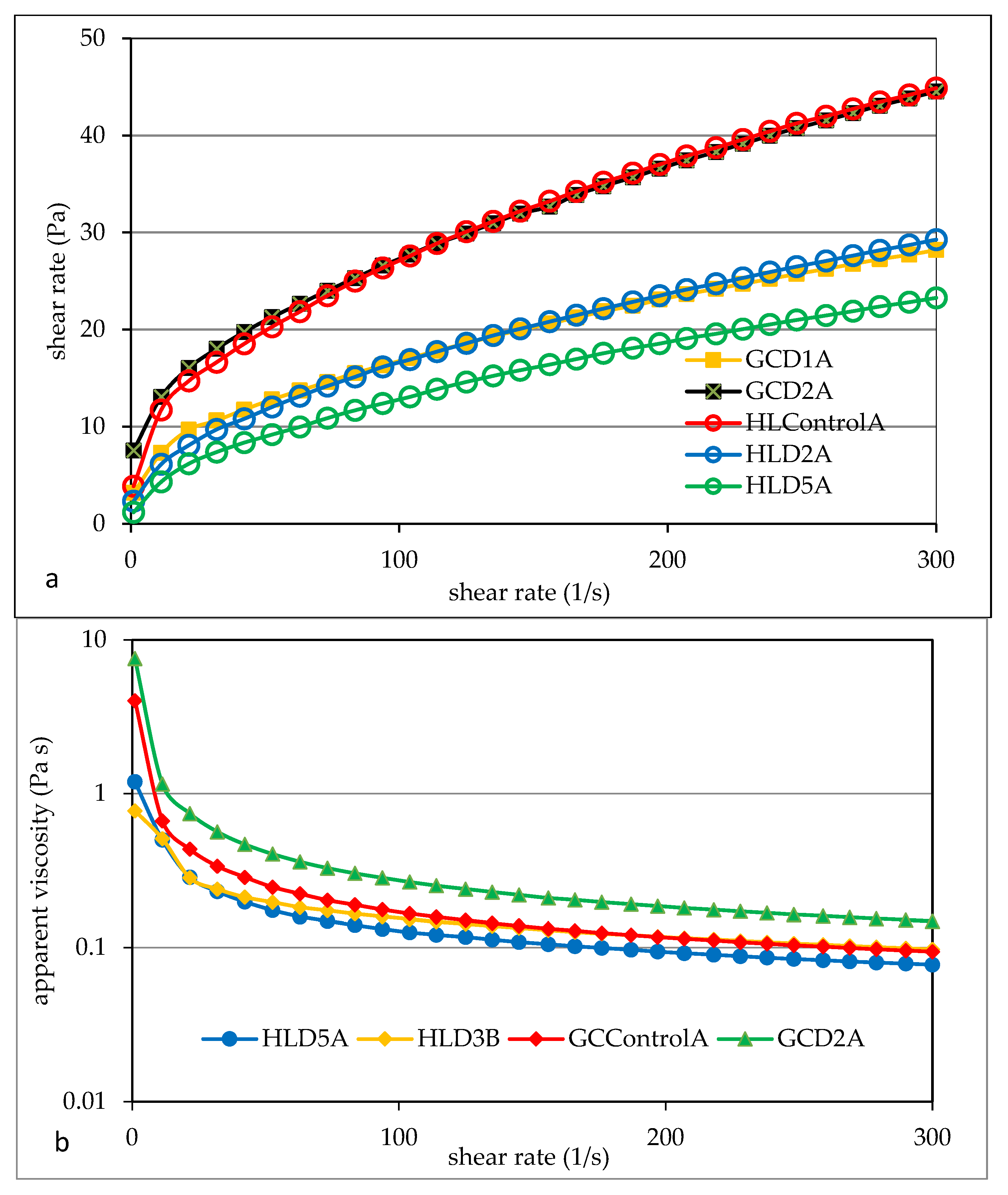
3.5. Flow Properties
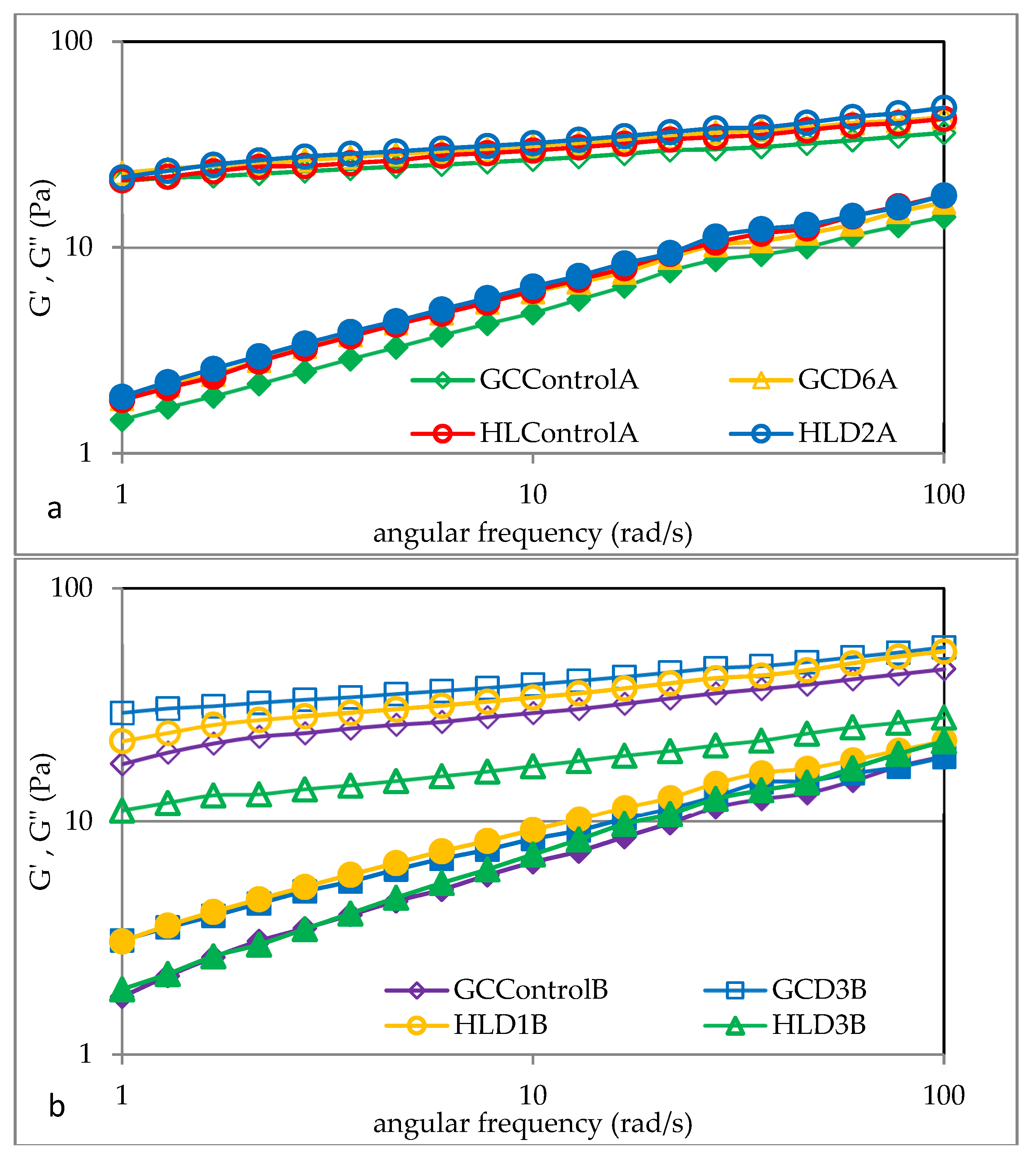
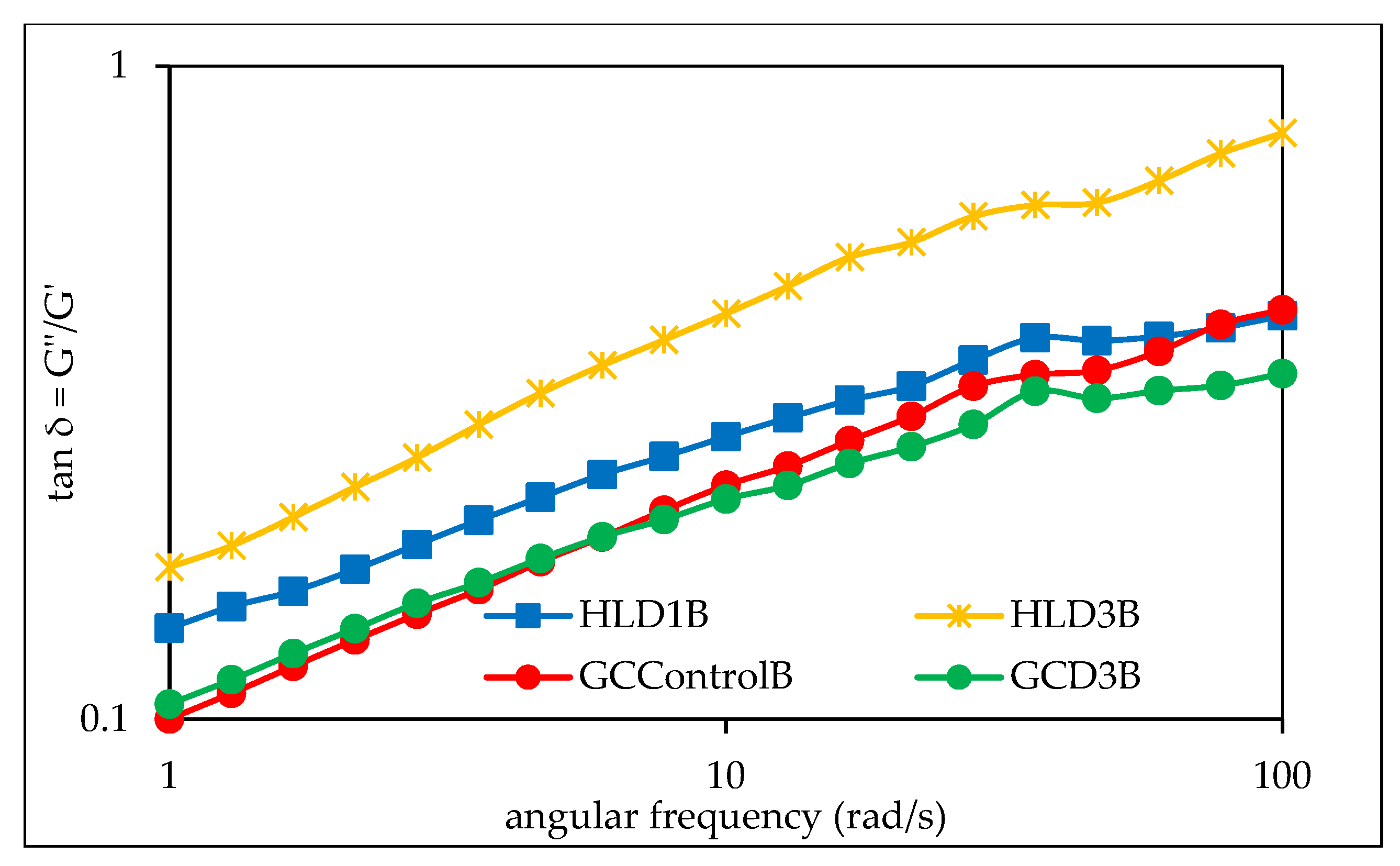
3.6. Viscoelastic Properties of Barley Starches
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Punia, S. Barley starch: Structure, properties and in vitro digestibility—A review. Int. J. Biol. Macromol. 2020, 155, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Lukinac, J.; Jukic, M. Barley in the production of cereal-based products. Plants 2022, 11, 3519. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, D.C. Barley; Agronomy Monographs; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2015. [Google Scholar] [CrossRef]
- Asare, E.K.; Jaiswal, S.; Maley, J.; Båga, M.; Sammynaiken, R.; Rossnagel, B.G.; Chibbar, R.N. Barley grain constituents, starch composition, and structure affect starch in vitro enzymatic hydrolysis. J. Agric. Food Chem. 2011, 5, 4743–4754. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qui, C.; Dong, L.; Zhang, X.; Wu, Z.; Liu, L.; Yang, J.; Liu, L. Whole grain benefit: Synergistic effect of oat phenolic compounds and β-glucan on hyperlipidemia via gut microbiota in high-fat-diet mice. Food Funct. 2022, 13, 12686–12696. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, N.; Ezekiel, R.; Guraya, H.S. Physicochemical, thermal and pasting properties of starches separated from different potato cultivars grown at different locations. Food Chem. 2007, 101, 643–651. [Google Scholar] [CrossRef]
- Liszka-Skoczylas, M. Wpływ nawożenia roślin ziemniaka (Solanum tuberosum L.) na zawartość i jakość skrobi w bulwach/Effect of potato plants (Solanum tuberosum L.) fertilisation on content and quality of starch in tubers. Żywn. Nauka Technol. Jakość 2020, 27, 31–46. (In Polish) [Google Scholar] [CrossRef]
- Hlisnikovský, L.; Křížová, K.; Menšík, L.; Kunzová, E. How mineral fertilisation and soil-climate conditions affect spring barley grain yield and soil chemical properties. Agronomy 2021, 11, 1843. [Google Scholar] [CrossRef]
- Tester, R.F. Influence of growth conditions on barley starch properties. Int. J. Biol. Macromol. 1997, 21, 37–45. [Google Scholar] [CrossRef]
- Pycia, K.; Juszczak, L.; Fortuna, T.; Gałkowska, D.; Witczak, T. Physicochemical, thermal and rheological properties of starches isolated from malting barley varieties. J. Food Sci. Technol. 2015, 52, 4797–4807. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Szostek, M.; Pawlak, R.; Gorzelany, J.; Migut, D. Effect of fertilisation with ash from biomass combustion on the mechanical properties of potato tubers (Solanum tuberosum L.) grown in two types of soil. Agronomy 2022, 12, 379. [Google Scholar] [CrossRef]
- Iderawumi, A.M. Effects of ash on soil properties and yield of crops. Agric. Obs. 2020, 1, 8. Available online: https://www.researchgate.net/publication/344000712_Effects_of_Ash_on_Soil_Properties_and_Yield_of_Crops (accessed on 10 November 2023).
- Li, X.; Rubæk, G.H.; Sørensen, P. High plant availability of phosphorus and low availability of cadmium in four biomass combustion ashes. Sci. Total Environ. 2016, 557–558, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Paredes, C.; López-García, Á.; Rubæk, G.H.; Hovmand, M.F.; Sørensen, P.; Kjøller, R. Risk assessment of replacing conventional P fertilizers with biomass ash: Residual effects on plant yield, nutrition, cadmium accumulation and mycorrhizal status. Sci. Total Environ. 2017, 575, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Pycia, K.; Szpunar-Krok, E.; Szostek, M.; Pawlak, R.; Juszczak, L. Effect of soil type and application of ecological fertilizer composed of ash from biomass combustion on selected physicochemical, thermal, and rheological properties of potato starch. Molecules 2022, 27, 4318. [Google Scholar] [CrossRef] [PubMed]
- Fertilization Recommendations. Part I. Limit Numbers for the Evaluation of the Content Macro-Soils and Micronutrients in Soils; Series P; IUNG-PIB: Puławy, Poland, 1990; Volume 44, pp. 1–26. (In Polish) [Google Scholar]
- Szostek, M.; Szpunar-Krok, E.; Ilek, A. Chemical speciation of trace elements in soil fertilized with biomass combustion ash and their accumulation in winter oilseed rape plants. Agronomy 2023, 13, 942. [Google Scholar] [CrossRef]
- AOAC: Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2006.
- Morrison, W.R.; Laignelet, B. An improved colorimetric procedure for determining apparent and total amylose in cereal and other starches. J. Cereal Sci. 1983, 1, 9–20. [Google Scholar] [CrossRef]
- Pycia, K.; Juszczak, L.; Gałkowska, D.; Witczak, M. Physicochemical properties of starches obtained from Polish potato cultivars. Starch/Stärke 2012, 64, 105–144. [Google Scholar] [CrossRef]
- Wieczorek, D.; Żyszka-Haberecht, B.; Kafka, A.; Lipok, J. Determination of phosphorus compounds in plant tissues: From colourimetry to advanced instrumental analytical chemistry. Plant Methods 2022, 18, 22. [Google Scholar] [CrossRef]
- PN-EN ISO 3946:2000; Starch and Derived Products—Determination of Total Phosphorus Content—Spectrophotometric Method. Polish Committee for Standardization: Warsaw, Poland, 1982.
- Zhang, W.; Liu, X.; Wang, Q.; Zhang, H.; Li, M.; Song, B.; Zhao, Z. Effects of potassium fertilisation on potato starch physicochemical properties. Int. J. Biol. Macromol. 2018, 117, 467–472. [Google Scholar] [CrossRef]
- Kandi, M.A.S.; Tobe, A.; Gholipoor, A.; Jahanbakhsh, S.; Hassanpanah, D.; Sofalian, O. Effects of different N fertilizer rate on starch percentage, soluble sugar, dry matter, yield and yield components of potato cultivars. Aust. J. Basic Appl. Sci. 2011, 5, 1846–1851. Available online: https://ajbasweb.com/old/ajbas/2011/September-2011/1846-1851.pdf (accessed on 9 November 2023).
- Källman, A.; Bertoft, E.; Koch, K.; Sun, C.; Åman, P.; Andersson, R. Starch structure in developing barley endosperm. Int. J. Biol. Macromol. 2015, 81, 730–735. [Google Scholar] [CrossRef]
- Obadi, M.; Qi, Y.J.; Xu, B. Highland barley starch (Qingke): Structures, properties, modifications, and applications. Int. J. Biol. Macromol. 2021, 185, 725–738. [Google Scholar] [CrossRef]
- Xie, J.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C.; Ban, X. Highland Barley Starch: Structures, Properties, and Applications. Foods 2023, 12, 387. [Google Scholar] [CrossRef]
- Singh, J.; Singh, N. Studies on the morphological, thermal and rheological properties of starch separated from some Indiana potato cultivars. Food Chem. 2001, 75, 67–77. [Google Scholar] [CrossRef]
- Wang, L.; Xie, B.; Shi, J.; Xue, S.J.; Deng, Q.; Wei, Y.; Tian, B. Physicochemical properties and structure of starches from Chinese rice cultivars. Food Hydrocoll. 2010, 24, 208–216. [Google Scholar] [CrossRef]
- Sobczyk, A.; Pycia, K.; Jaworska, G.; Kaszuba, J. Comparison of fermentation strength of the flours obtained from the grain of old varieties and modern breeding of spelt (Triticum aestivum ssp. spelta). J. Food Process. Preserv. 2017, 41, e13293. [Google Scholar] [CrossRef]
- Sobczyk, A.; Pycia, K.; Stankowski, S.; Jaworska, G.; Kuźniar, P. Evaluation of the rheological properties of dough and quality of bread made with the flour obtained from old cultivars and modern breeding lines of spelt (Triticum aestivum ssp. spelta). J. Cereal Sci. 2017, 77, 35–41. [Google Scholar] [CrossRef]
- Li, W.; Shan, Y.; Xiao, X.; Zheng, J.; Luo, Q.; Ouyang, S.; Zhang, G. Effect of nitrogen and sulfur fertilisation on accumulation characteristics and physicochemical properties of A- and B-wheat starch. J. Agric. Food Chem. 2013, 61, 2418–2425. [Google Scholar] [CrossRef]
- Tong, J.; Wang, S.; He, Z.; Zhang, Y. Effects of reduced nitrogen fertilisation and irrigation on structure and physicochemical properties of starch in two bread wheat cultivars. Agriculture 2021, 11, 26. [Google Scholar] [CrossRef]
- Leonel, M.; Carmo, E.L.; Fernandes, A.M.; Franco, C.M.; Soratto, R.P. Physico-chemical properties of starches isolated from potato cultivars grown in soils with different phosphorus availability. J. Sci. Food Agric. 2016, 96, 1900–1905. [Google Scholar] [CrossRef]
- Azzawi, W.A.; Gill, M.B.; Fatehi, F.; Zhou, M.; Acuña, T.; Shabala, L.; Yu, M.; Shabala, S. Effects of potassium availability on growth and development of barley cultivars. Agronomy 2021, 11, 2269. [Google Scholar] [CrossRef]
- Peña, R.J.; Trethowan, R.; Dfeiffer, W.H.; van Ginkel, M. Quality (End Use) improvement in wheat: Compositional, genetic, and environmental factors. J. Crop Prod. 2002, 5, 1–37. [Google Scholar] [CrossRef]
- Rakita, S.; Torbica, A.; Pezo, L.; Nikolić, I. Effect of Climatic Conditions on Wheat Starch Granule Size Distribution, Gelatinization and Flour Pasting Properties. Agronomy 2023, 13, 1551. [Google Scholar] [CrossRef]
- Haase, N.U.; Plate, J. Properties of potato starch in relation to varieties and environmental factors. Starch–Stärke 1996, 48, 167–171. [Google Scholar] [CrossRef]
- Matsuki, J.; Yasui, T.; Kohyama, K.; Sasaki, T. Effects of environmental temperature on structure and gelatinization properties of wheat starch. Cereal Chem. 2003, 80, 476–480. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, L.; McCarthy, O.J. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—A review. Food Hydrocoll. 2007, 21, 1–22. [Google Scholar] [CrossRef]
- Krueger, B.R.; Knutson, C.A.; Inglett, G.E.; Walker, C.E. A differential scanning calorimetry study on the effect of annealing on gelatinization behaviour of corn starch. J. Food Sci. 1987, 52, 715–718. [Google Scholar] [CrossRef]
- Rittenauer, M.; Gladis, S.; Gastl, M.; Becker, T. Gelatinization or Pasting? The impact of different temperature levels on the saccharification efficiency of barley malt starch. Foods 2021, 10, 1733. [Google Scholar] [CrossRef]
- Tester, R.F.; Morrison, W.R. Swelling and gelatinization of cereal starches. II. Waxy rice starches. Cereal Chem. 1990, 67, 558–563. [Google Scholar]
- Li, W.; Xiao, X.; Zhang, W.; Zheng, J.; Luo, Q.; Ouyang, S.; Zhang, G. Compositional, morphological, structural and physicochemical properties of starches from seven naked barley cultivars grown in China. Food Res. Int. 2014, 58, 7–14. [Google Scholar] [CrossRef]
- Li, J.H.; Vasanthan, T.; Rossnagel, B.; Hoover, R. Starch from hull-less barley: I. Granule morphology, composition and amylopectin structure. Food Chem. 2001, 74, 95–405. [Google Scholar] [CrossRef]
- Ebúrneo, J.M.; Garcia, E.L.; dos Santos, T.P.R.; de Freitas Cordova de Souza, E.; Soratto, R.P.; Fernandes, A.M. Influence of nitrogen fertilisation on the characteristics of potato starch. Aust. J. Crop. Sci. 2018, 12, 365–373. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, H.; Guo, B.; Xu, K.; Dai, Q.; Wei, C.; Zhou, G.; Huo, Z. Effects of nitrogen level on structure and physicochemical properties of rice starch. Food Hydrocoll. 2017, 63, 525–532. [Google Scholar] [CrossRef]
- Leszczyński, W. Wpływ czynników działających w okresie wegetacji ziemniaka na jego jakość. Postępy Nauk Rol. 1994, 41, 55–68. (In Polish) [Google Scholar]
- Gani, A.; Wani, S.M.; Masoodi, F.A.; Salim, R. Characterization of rice starches extracted from Indian cultivars. Food Sci. Technol. Int. 2013, 19, 143–152. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.D.; Wang, T.Q.; Wang, B.; Zhang, W.H.; Li, G.H.; Guo, Z.L.; Zhang, Y.X.; Xue, B.; Luo, Z. Starch isolated from different hulless barley cultivars differs in their chemical and structural characteristics. Food Sci. Nutr. 2019, 7, 2374–2380. [Google Scholar] [CrossRef]
- Pietrzyk, S.; Juszczak, L.; Fortuna, T.; Ciemniewska, A. Effect of the oxidation level of corn starch on its acetylation and physicochemical and rheological properties. J. Food Eng. 2014, 120, 50–56. [Google Scholar] [CrossRef]
- Jekle, M.; Muehlberger, K.; Becker, T. Starch-gluten interactions during gelatinization and its functionality in dough like model systems. Food Hydrocoll. 2016, 54, 196–201. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Rayas-Duarte, P. The effect of mixing and wheat protein/gluten on the gelatinization of wheat starch. Food Chem. 2003, 81, 533–545. [Google Scholar] [CrossRef]
- Noda, T.; Tsuda, S.; Mori, M.; Takigawa, S.; Matsuura-Endo, C.; Saito, K.; Arachichige Mangalika, W.H.; Hanaoka, A.; Suzuki, Y.; Yamauchi, H. The effect of harvest dates on the starch properties of various potato cultivars. Food Chem. 2004, 86, 119–125. [Google Scholar] [CrossRef]
- Gu, J.; Chen, J.; Chen, L.; Wang, Z.; Zhang, H.; Yang, J. Grain quality changes and responses to nitrogen fertilizer of japonica rice cultivars released in the Yangtze River Basin from the 1950s to 2000s. Crop J. 2015, 3, 285–297. [Google Scholar] [CrossRef]
- Gao, L.; Bai, W.; Xia, M.; Wang, M.; Wang, P.; Gao, X.; Gao, J. Diverse effects of nitrogen fertilizer on the structural, pasting, and thermal properties of common buckwheat starch. Int. J. Biol. Macromol. 2021, 179, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Lagarrigue, S.; Alvarez, G. The rheology of starch dispersions at high temperatures and high shear rates: A review. J. Food Eng. 2001, 50, 189–202. [Google Scholar] [CrossRef]
| Type of Soil | Fertilization | Amylose Content (%) | Paste Clarity (%) |
|---|---|---|---|
| 2019 (A) | |||
| Gleyic Chernozem | Control | 16.9 ± 2.2 abA | 13.2 ± 0.56 deA |
| D1 | 16.4 ± 0.2 aA | 12.5 ± 0.4 cdA | |
| D2 | 18.9 ± 1.1 abcA | 10.4 ± 0.2 bA | |
| D3 | 22.0 ± 0.4 cdeA | 10.2 ± 0.1 abA | |
| D4 | 18.1 ± 0.1 abA | 23.8 ± 0.4 hB | |
| D5 | 20.4 ± 0.0 bcdA | 6.2 ± 0.1 abA | |
| D6 | 28.2 ± 0.2 gA | 11.5 ± 0.2 cA | |
| Haplic Luvisol | Control | 26.1 ± 1.5 fgA | 12.4 ± 0.3 cdA |
| D1 | 23.3 ± 0.4 defA | 14.6 ± 0.3 fA | |
| D2 | 17.8 ± 1.0 abA | 15.8 ± 0.2 gA | |
| D3 | 20.1 ± 0.8 bcdA | 13.9 ± 0.3 efA | |
| D4 | 22.9 ± 0.6 defA | 14.5 ± 0.7 fA | |
| D5 | 24.6 ± 0.5 efA | 16.5 ± 0.4 gA | |
| D6 | 20.2 ± 0.2 bcdA | 14.6 ± 0.1 fA | |
| Two-Way ANOVA (p-value) | |||
| Type of Soil | <0.001 | <0.001 | |
| Fertilization | <0.001 | <0.001 | |
| Type of Soil × Fertilization | <0.001 | <0.001 | |
| 2020 (B) | |||
| Gleyic Chernozem | Control | 24.3 ± 1.5 cdA | 18.9 ± 0.5 ghB |
| D1 | 18.9 ± 1.2 abA | 17.1 ± 0.3 cdB | |
| D2 | 23.4 ± 1.5 cdA | 16.2 ± 0.2 bcB | |
| D3 | 19.0 ± 0.0 abA | 15.6 ± 0.2 abB | |
| D4 | 19.7 ± 0.3 abA | 17.2 ± 0.7 cdeA | |
| D5 | 17.9 ± 1.0 Aa | 15.6 ± 1.2 abB | |
| D6 | 19.3 ± 0.3 abA | 14.8 ± 0.1 aB | |
| Haplic Luvisol | Control | 19.4 ± 0.5 abA | 17.4 ± 0.3 cdefB |
| D1 | 21.4 ± 0.7 bcA | 18.5 ± 0.1 efghB | |
| D2 | 25.1 ± 1.4 dA | 18.6 ± 0.2 fghB | |
| D3 | 19.2 ± 0.5 abA | 21.8 ± 0.6 iB | |
| D4 | 21.8 ± 0.4 bcdA | 17.5 ± 0.2 cdefB | |
| D5 | 18.6 ± 0.2 abA | 17.7 ± 0.4 defgA | |
| D6 | 17.5 ± 0.2 aA | 19.7 ± 0.2 hB | |
| Two-Way ANOVA (p-value) | |||
| Type of Soil | 0.824 | <0.001 | |
| Fertilization | <0.001 | <0.001 | |
| Type of Soil × Fertilisation | <0.001 | <0.001 | |
| Type of Soil | Fertilization | P | K | Ca | Mg | Na |
|---|---|---|---|---|---|---|
| [mg kg−1] | ||||||
| 2019 (A) | ||||||
| Gleyic Chernozem | Control | 235.4 ± 4.36 abcA | 146 ± 0.4 bA | 69.7 ± 0.1 bcdA | 44.1 ± 0.0 abcA | 4.43 ± 0.07 aA |
| D1 | 218.0 ± 39.2 aA | 105 ± 1.2 aA | 71.8 ± 2.1 cdeA | 42.5 ± 3.0 abA | 4.90 ± 0.02 aA | |
| D2 | 222.4 ± 13.1 abA | 109 ± 0.4 aA | 62.2 ± 0.5 abcA | 37.6 ± 0.4 aA | 4.79 ± 0.65 aA | |
| D3 | 226.7 ± 8.7 abA | 131 ± 7.9 bA | 74.8 ± 0.2 defA | 43.8 ± 0.1 abcA | 7.36 ± 0.07 dA | |
| D4 | 266.0 ± 4.4 bcdA | 188 ± 4.5 cA | 77.7 ± 2.2 defgA | 54.2 ± 1.6 deA | 6.13 ± 0.08 bcA | |
| D5 | 287.8 ± 4.6 cdA | 172 ± 1.7 cA | 86.9 ± 3.4 ghA | 53.9 ± 1.8 deA | 7.27 ± 0.36 cdA | |
| D6 | 292.1 ± 8.7 dA | 177 ± 2.6 cA | 88.4 ± 1.2 hA | 56.5 ± 2.6 eB | 9.46 ± 0.00 eB | |
| Haplic Luvisol | Control | 244.2 ± 4.8 abcdA | 265 ± 1.9 eA | 71.8 ± 0.2 cdeA | 44.0 ± 1.6 abcA | 5.09 ± 0.23 abA |
| D1 | 252.9 ± 5.1 abcdA | 281 ± 4.0 efA | 61.0 ± 1.8 abA | 45.3 ± 1.7 bcA | 6.15 ± 0.04 bcA | |
| D2 | 213.6 ± 13.1 aA | 293 ± 15.9 fA | 70.9 ± 2.2 bcdeA | 49.5 ± 1.9 cdA | 6.16 ± 0.38 bcA | |
| D3 | 252.9 ± 4.9 abcdA | 243 ± 3.5 dA | 79.9 ± 1.1 efghA | 56.5 ± 1.5 eB | 5.16 ± 0.23 abA | |
| D4 | 261.6 ± 5.3 abcdA | 239 ± 3.5 dA | 83.5 ± 4.6 fghA | 59.2 ± 0.2 eA | 6.94 ± 0.17 cdA | |
| D5 | 257.2 ± 4.9 abcdA | 364 ± 2.6 gB | 56.9 ± 0.7 aA | 53.0 ± 2.4 deA | 6.74 ± 0.02 cdA | |
| D6 | 261.6 ± 4.6 abcdA | 233 ± 1.0 dB | 90.0 ± 6.1 hA | 54.7 ± 0.7 deB | 6.88 ± 0.55 cdA | |
| Two-Way ANOVA (p-value) | ||||||
| Type of soil | 0.879 | <0.001 | 0.021 | <0.001 | 0.123 | |
| Fertilization | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Type of soil × Fertilization | 0.009 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 2020 (B) | ||||||
| Gleyic Chernozem | Control | 235.4 ± 4.4 abcA | 276 ± 4 fgB | 69.4 ± 2.5 aA | 43.5 ± 1.2 abcA | 8.38 ± 1.13 deA |
| D1 | 257.2 ± 17.4 bcdeA | 223 ± 2 cB | 92.7 ± 1.2 bA | 40.3 ± 1.5 abA | 6.99 ± 0.09 abcdB | |
| D2 | 244.2 ± 13.1 abcdA | 228 ± 3 cdB | 86.3 ± 0.1 bB | 44.6 ± 1.2 abcA | 6.86 ± 0.06 abcdA | |
| D3 | 252.9 ± 8.7 bcdA | 272 ± 1 fgB | 92.7 ± 1.1 bB | 47.5 ± 0.6 cdA | 8.25 ± 0.52 deA | |
| D4 | 300.8 ± 43.6 eA | 237 ± 11 cdB | 94.6 ± 3.6 bA | 42.8 ± 1.0 abcA | 7.60 ± 0.57 cdeA | |
| D5 | 270.3 ± 13.1 cdeA | 293 ± 3 gB | 91.6 ± 0.4 bA | 48.9 ± 0.7 dA | 7.44 ± 0.23 bcdeA | |
| D6 | 283.4 ± 8.72 deA | 220 ± 3 bcA | 87.6 ± 2.2 bA | 39.9 ± 0.9 abA | 6.85 ± 0.13 abcdA | |
| Haplic Luvisol | Control | 235.4 ± 4.4 abcA | 270 ± 10 fA | 73.7 ± 5.7 aA | 42.9 ± 1.2 abcA | 9.20 ± 0.98 eA |
| D1 | 213.6 ± 4.4 abA | 200 ± 6 abA | 74.6 ± 1.1 aB | 38.5 ± 3.3 aA | 5.89 ± 0.34 abcA | |
| D2 | 204.9 ± 30.5 aA | 367 ± 1 hB | 75.7 ± 0.8 aA | 48.9 ± 1.7 dA | 8.70 ± 0.36 deB | |
| D3 | 257.2 ± 4.3 bcdeA | 376 ± 1 hB | 76.6 ± 1.1 aA | 46.6 ± 1.3 cdA | 8.66 ± 0.08 deB | |
| D4 | 257.2 ± 4.1 bcdeA | 262 ± 11 efA | 76.1 ± 0.8 aA | 43.3 ± 4.8 abcA | 5.64 ± 0.50 abcA | |
| D5 | 257.2 ± 4.0 bcdeA | 246 ± 2 deA | 74.4 ± 0.7 aB | 41.8 ± 1.6 abcA | 5.00 ± 0.27 aA | |
| D6 | 261.6 ± 4.0 cdeA | 198 ± 1 aA | 76.5 ± 0.5 aA | 45.0 ± 0.9 abcA | 5.53 ± 0.14 abA | |
| Two-Way ANOVA (p-value) | ||||||
| Type of soil | <0.001 | <0.001 | <0.001 | 0.925 | 0.013 | |
| Fertilization | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | |
| Type of soil × Fertilization | <0.001 | <0.001 | <0.001 | 0.010 | <0.001 | |
| Type of Soil | Fertilization | Fe | Mn | Cu | Zn |
|---|---|---|---|---|---|
| [mg kg−1] | |||||
| 2019 (A) | |||||
| Gleyic Chernozem | Control | 3.93 ± 0.98 abcA | 0.54 ± 0.01 abA | 0.67 ± 0.10 aA | 2.26 ± 0.42 abA |
| D1 | 5.41 ± 2.51 abcA | 0.48 ± 0.10 aA | 0.66 ± 0.20 aA | 2.03 ± 0.01 aA | |
| D2 | 7.03 ± 0.55 cA | 0.60 ± 0.01 abA | 0.98 ± 0.32 aA | 3.57 ± 0.16 cdeA | |
| D3 | 4.97 ± 0.83 abcA | 0.68 ± 0.07 abA | 0.88 ± 0.17 aA | 6.62 ± 0.06 gB | |
| D4 | 6.04 ± 0.05 abcA | 0.75 ± 0.04 bA | 0.64 ± 0.17 aA | 3.69 ± 0.29 cdefB | |
| D5 | 6.66 ± 0.88 bcB | 0.60 ± 0.11 abA | 1.05 ± 0.25 aA | 6.09 ± 0.01 gB | |
| D6 | 5.26 ± 0.65 abcB | 1.06 ± 0.00 cA | 0.75 ± 0.16 aA | 4.79 ± 0.32 fB | |
| Haplic Luvisol | Control | 3.26 ± 0.17 abA | 1.46 ± 0.07 deA | 0.71 ± 0.28 aA | 3.20 ± 0.08 bcdA |
| D1 | 3.02 ± 0.17 aA | 1.36 ± 0.03 dA | 0.74 ± 0.19 aA | 2.75 ± 0.16 abcA | |
| D2 | 3.38 ± 0.31 abA | 1.28 ± 0.00 cdA | 0.67 ± 0.14 aA | 2.64 ± 0.23 abcA | |
| D3 | 2.76 ± 0.57 aA | 1.66 ± 0.10 eB | 0.81 ± 0.01 aA | 4.13 ± 0.65 defB | |
| D4 | 5.80 ± 0.63 abcA | 1.52 ± 0.02 deA | 0.81 ± 0.04 aA | 3.75 ± 0.32 cdefB | |
| D5 | 2.67 ± 0.01 aA | 1.35 ± 0.07 dA | 0.79 ± 0.01 aB | 2.78 ± 0.34 abcA | |
| D6 | 4.75 ± 0.38 abcB | 1.30 ± 0.12 cdA | 0.79 ± 0.02 aA | 4.37 ± 0.15 efB | |
| Two-Way ANOVA (p-value) | |||||
| Type of soil | <0.001 | <0.001 | 0.527 | <0.001 | |
| Fertilization | 0.025 | <0.001 | 0.494 | <0.001 | |
| Type of soil × Fertilization | 0.038 | <0.001 | 0.429 | <0.001 | |
| 2020 (B) | |||||
| Gleyic Chernozem | Control | 5.63 ± 1.71 aA | 0.73 ± 0.01 abA | 0.76 ± 0.32 aA | 2.18 ± 0.02 aA |
| D1 | 3.37 ± 0.06 aA | 0.82 ± 0.13 abcdB | 0.96 ± 0.04 aA | 3.52 ± 0.22 bA | |
| D2 | 3.25 ± 0.87 aA | 0.86 ± 0.00 abcdB | 0.79 ± 0.05 aA | 2.67 ± 0.40 abA | |
| D3 | 3.53 ± 0.53 aA | 1.00 ± 0.03 bcdA | 0.77 ± 0.09 aA | 3.10 ± 0.13 abA | |
| D4 | 4.01 ± 1.30 aA | 0.79 ± 0.05 abcdA | 0.88 ± 0.10 aA | 3.08 ± 0.23 abA | |
| D5 | 2.38 ± 0.08 aA | 0.77 ± 0.02 abcA | 0.62 ± 0.06 aA | 1.93 ± 0.04 aA | |
| D6 | 1.85 ± 0.16 aA | 0.63 ± 0.12 aA | 0.47 ± 0.31 aA | 2.13 ± 0.03 aA | |
| Haplic Luvisol | Control | 3.34 ± 0.88 aA | 0.92 ± 0.15 abcAd | 0.79 ± 0.16 aA | 2.27 ± 0.41 abA |
| D1 | 3.38 ± 0.97 aA | 1.14 ± 0.01 dA | 0.55 ± 0.03 aA | 2.31 ± 0.52 abA | |
| D2 | 2.76 ± 0.33 aA | 1.12 ± 0.06 cdA | 0.57 ± 0.06 aA | 2.63 ± 0.58 abA | |
| D3 | 2.34 ± 0.00 aA | 0.88 ± 0.02 abcdA | 0.57 ± 0.02 aA | 2.03 ± 0.59 aA | |
| D4 | 3.38 ± 1.44 aA | 1.03 ± 0.21 bcdA | 0.57 ± 0.09 aA | 2.10 ± 0.18 aA | |
| D5 | 2.82 ± 0.03 aA | 0.97 ± 0.06 abcdA | 0.53 ± 0.00 aA | 2.03 ± 0.10 aA | |
| D6 | 1.92 ± 0.65 aA | 1.11 ± 0.06 cdA | 0.53 ± 0.04 aA | 1.97 ± 0.02 aA | |
| Two-Way ANOVA (p-value) | |||||
| Type of soil | 0.090 | <0.001 | 0.007 | 0.002 | |
| Fertilization | 0.018 | 0.168 | 0.123 | 0.002 | |
| Type of soil × Fertilization | 0.363 | 0.014 | 0.220 | 0.028 | |
| Type of Soil | Fertilization | TO (°C) | TP (°C) | TE (°C) | ΔT (°C) | ΔHG (J/g) |
|---|---|---|---|---|---|---|
| 2019 (A) | ||||||
| Gleyic Chernozem | Control | 60.8 ± 0.7 aB | 65.0 ± 0.1 abcB | 70.7 ± 0.8 aA | 9.9 ± 1.2 aA | 9.07 ± 0.31 abcdA |
| D1 | 59.8 ± 0.3 aA | 64.7 ± 0.1 aA | 70.3 ± 0.3 aA | 10.5 ± 0.6 aA | 11.38 ± 0.27 eB | |
| D2 | 60.7 ± 0.4 aB | 64.9 ± 0.2 abcB | 70.4 ± 0.3 aB | 9.7 ± 0.1 aA | 10.22 ± 0.80 cdeB | |
| D3 | 60.3 ± 0.7 aA | 64.9 ± 0.2 abcB | 70.4 ± 0.1 aA | 10.1 ± 0.7 aA | 10.45 ± 0.22 deB | |
| D4 | 61.0 ± 0.4 aB | 65.7 ± 0.8 cB | 71.5 ± 1.7 aA | 10.5 ± 1.3 aA | 10.11 ± 0.29 cdB | |
| D5 | 60.7 ± 0.0 aB | 65.0 ± 0.1 abcB | 70.4 ± 0.2 aA | 9.7 ± 0.1 aA | 8.58 ± 0.52 abcA | |
| D6 | 60.7 ± 0.5 aB | 65.6 ± 0.4 bcB | 71.6 ± 0.7 aA | 10.9 ± 1.2 aA | 9.32 ± 0.22 bcdB | |
| Haplic Luvisol | Control | 60.1 ± 0.0 aB | 64.6 ± 0.1 aB | 70.1 ± 0.2 aB | 9.9 ± 0.2 aA | 7.90 ± 0.46 abA |
| D1 | 59.8 ± 0.2 aB | 64.8 ± 0.0 abB | 70.6 ± 0.2 aB | 10.8 ± 0.4 aA | 7.86 ± 0.31 aA | |
| D2 | 59.6 ± 0.4 aB | 64.9 ± 0.2 abcB | 70.4 ± 0.3 aB | 10.8 ± 0.6 aA | 10.22 ± 0.80 cdeB | |
| D3 | 59.3 ± 0.2 aB | 64.6 ± 0.0 aB | 70.1 ± 0.4 aA | 10.8 ± 0.6 aA | 9.12 ± 0.09 bcdB | |
| D4 | 59.3 ± 0.6 aB | 64.5 ± 0.1 aB | 70.6 ± 0.5 aA | 11.3 ± 1.1 aA | 7.89 ± 0.14 abA | |
| D5 | 59.8 ± 0.5 aB | 64.6 ± 0.2 aB | 70.2 ± 0.7 aA | 10.4 ± 1.2 aA | 8.58 ± 0.16 abcA | |
| D6 | 60.1 ± 0.5 aB | 65.3 ± 0.3 abcB | 70.4 ± 1.0 aB | 10.3 ± 0.6 aA | 8.60 ± 0.44 abcB | |
| Two-Way ANOVA (p-value) | ||||||
| Type of soil | <0.001 | 0.001 | 0.058 | 0.114 | <0.001 | |
| Fertilization | 0.085 | 0.002 | 0.253 | 0.370 | <0.001 | |
| Type of soil × Fertilization | 0.075 | 0.037 | 0.448 | 0.613 | <0.000 | |
| 2020 (B) | ||||||
| Gleyic Chernozem | Control | 58.6 ± 0.4 aA | 63.2 ± 0.1 aA | 69.1 ± 0.3 aA | 10.5 ± 0.7 aA | 9.52 ± 0.09 eA |
| D1 | 59.2 ± 0.8 aA | 63.5 ± 1.1 aA | 69.7 ± 0.9 aA | 10.5 ± 0.1 aA | 7.64 ± 0.43 bcdA | |
| D2 | 58.8 ± 0.2 aA | 62.7 ± 0.2 aA | 68.1 ± 0.6 aA | 9.3 ± 0.2 aA | 6.39 ± 1.19 abA | |
| D3 | 59.6 ± 1.2 aA | 63.3 ± 0.2 aA | 69.7 ± 1.2 aA | 10.1 ± 0.4 aA | 9.04 ± 0.51 deA | |
| D4 | 58.6 ± 0.1 aA | 62.9 ± 0.1 aA | 68.3 ± 0.2 aA | 9.7 ± 0.2 aA | 8.11 ± 0.56 cdeA | |
| D5 | 58.4 ± 0.0 aA | 63.2 ± 0.5 aA | 68.6 ± 0.7 aA | 10.2 ± 0.7 aA | 8.90 ± 0.28 deA | |
| D6 | 58.1 ± 0.4 aA | 62.5 ± 0.7 aA | 67.9 ± 1.1 aA | 9.8 ± 1.0 aA | 6.87 ± 0.61 abcA | |
| Haplic Luvisol | Control | 58.4 ± 0.1 aA | 63.2 ± 0.2 aA | 68.4 ± 0.4 aA | 10.1 ± 0.4 aA | 7.25 ± 0.15 abcA |
| D1 | 57.8 ± 0.4 aA | 62.8 ± 0.3 aA | 68.5 ± 0.1 aA | 10.7 ± 0.3 aA | 6.53 ± 0.60 abA | |
| D2 | 57.9 ± 0.2 aA | 62.8 ± 0.1 aA | 68.4 ± 0.4 aA | 10.6 ± 0.3 aA | 7.02 ± 0.54 abcA | |
| D3 | 58.4 ± 0.2 aA | 63.3 ± 0.1 aA | 68.9 ± 0.9 aA | 10.5 ± 1.0 aA | 7.05 ± 0.30 abcA | |
| D4 | 57.9 ± 0.3 aA | 63.1 ± 0.0 aA | 68.6 ± 0.4 aA | 10.8 ± 0.7 aA | 7.77 ± 0.24 bcdA | |
| D5 | 58.2 ± 0.3 aA | 63.1 ± 0.0 aA | 68.7 ± 0.3 aA | 10.5 ± 0.6 aA | 6.77 ± 0.20 abcA | |
| D6 | 56.5 ± 1.0 aA | 61.7 ± 0.2 aA | 67.5 ± 1.1 aA | 11.1 ± 2.1 aA | 5.85 ± 0.10 aA | |
| Two-Way ANOVA (p-value) | ||||||
| Type of soil | <0.001 | 0.166 | 0.154 | 0.021 | <0.001 | |
| Fertilization | 0.001 | <0.001 | 0.016 | 0.853 | <0.001 | |
| Type of soil × Fertilization | 0.191 | 0.214 | 0.409 | 0.448 | <0.001 | |
| Type of Soil | Fertilization | TP (°C) | TE (°C) | ΔT (°C) | ΔHR (J/g) | R (%) |
|---|---|---|---|---|---|---|
| 2019 (A) | ||||||
| Gleyic Chernozem | Control | 51.5 ± 2.5 abcA | 60.3 ± 1.1 abcdB | 18.1 ± 0.7 abA | 4.04 ± 0.37 abA | 44.5 ± 3.7 bA |
| D1 | 51.5 ± 1.2 abcA | 59.9 ± 0.5 abcA | 17.4 ± 0.4 abA | 3.57 ± 0.10 aA | 31.4 ± 1.0 aA | |
| D2 | 54.0 ± 0.4 abcdeA | 61.8 ± 0.2 defB | 18.1 ± 0.6 abA | 4.47 ± 0.24 bcA | 43.9 ± 3.0 bA | |
| D3 | 54.9 ± 0.0 bcdeB | 62.9 ± 0.2 fgB | 17.3 ± 0.1 aA | 4.77 ± 0.03 cB | 45.6 ± 1.2 bcA | |
| D4 | 55.8 ± 0.2 deA | 63.9 ± 0.4 gB | 21.8 ± 2.3 cdeA | 4.62 ± 0.08 bcB | 45.7 ± 0.6 bcA | |
| D5 | 56.7 ± 0.2 eB | 64.0 ± 0.3 gB | 20.3 ± 0.3 bcdA | 4.75 ± 0.17 cB | 55.4 ± 3.7 defB | |
| D6 | 55.4 ± 0.6 cdeB | 62.1 ± 0.7 efA | 19.8 ± 0.7 abcdB | 4.96 ± 0.14 cB | 53.3 ± 2.8 bcdeA | |
| Haplic Luvisol | Control | 51.0 ± 0.5 abA | 61.1 ± 0.5 bcdeA | 19.2 ± 1.0 abcdA | 4.07 ± 0.18 abA | 51.7 ± 4.6 bcdeA |
| D1 | 52.6 ± 0.3 abcdA | 60.4 ± 0.1 bcdA | 17.9 ± 0.4 abA | 4.48 ± 0.09 bcB | 57.0 ± 3.4 efA | |
| D2 | 52.8 ± 1.2 abcdeA | 58.8 ± 0.1 aA | 19.0 ± 0.3 abcA | 4.54 ± 0.27 bcA | 44.5 ± 2.5 bA | |
| D3 | 54.9 ± 0.6 bcdeA | 61.5 ± 0.5 cdefA | 20.3 ± 1.1 bcdA | 4.05 ± 0.04 abA | 44.4 ± 0.2 bA | |
| D4 | 56.6 ± 2.0 deA | 63.1 ± 0.1 fgA | 22.0 ± 2.0 deA | 4.98 ± 0.40 cA | 63.1 ± 6.0 fA | |
| D5 | 50.0 ± 3.4 aA | 59.5 ± 0.9 abA | 18.7 ± 0.4 abA | 4.69 ± 0.24 cA | 54.8 ± 3.6 cdefA | |
| D6 | 54.4 ± 0.2 bcdeA | 67.0 ± 0.4 hB | 24.0 ± 0.2 eA | 4.03 ± 0.05 abA | 46.9 ± 2.0 bcdA | |
| Two-Way ANOVA (p-value) | ||||||
| Type of Soil | 0.015 | 0.007 | 0.001 | 0.452 | <0.001 | |
| Fertilization | <0.001 | <0.001 | <0.000 | <0.001 | <0.001 | |
| Type of Soil × Fertilization | 0.001 | <0.001 | 0.001 | <0.001 | <0.001 | |
| 2020 (B) | ||||||
| Gleyic Chernozem | Control | 47.6 ± 0.8 aA | 57.6 ± 0.8 aA | 17.7 ± 0.8 abcA | 4.16 ± 0.05 abcA | 43.7 ± 0.7 aA |
| D1 | 50.4 ± 0.6 aA | 59.1 ± 0.4 abcA | 17.2 ± 0.4 abcA | 3.91 ± 0.16 abA | 51.2 ± 2.7 abcB | |
| D2 | 46.8 ± 4.7 aA | 58.5 ± 0.7 abA | 17.9 ± 1.8 abcA | 3.95 ± 0.22 abA | 62.9 ± 9.7 cdeB | |
| D3 | 51.7 ± 0.4 aA | 59.7 ± 0.4 abcdeA | 17.0 ± 0.4 abA | 4.08 ± 0.12 abcA | 45.2 ± 1.3 abA | |
| D4 | 53.5 ± 27.0 aA | 60.7 ± 1.2 bcdefA | 17.6 ± 1.2 abcA | 3.57 ± 0.11 aA | 44.1 ± 3.1 aA | |
| D5 | 52.3 ± 0.4 aA | 60.7 ± 1.2 bcdefA | 17.9 ± 1.8 abcA | 3.66 ± 0.16 aA | 41.2 ± 2.6 aA | |
| D6 | 52.2 ± 0.5 aA | 59.5 ± 0.4 abcdA | 16.4 ± 0.6 aA | 3.51 ± 0.20 aA | 51.3 ± 3.9 abcA | |
| Haplic Luvisol | Control | 51.4 ± 0.2 aA | 61.1 ± 0.3 cdefA | 18.3 ± 0.4 abcA | 4.08 ± 0.28 abcA | 56.4 ± 4.1 abcdA |
| D1 | 52.1 ± 1.1 aA | 61.1 ± 0.3 cdefA | 18.2 ± 1.0 abcA | 4.12 ± 0.14 abcA | 63.6 ± 7.3 cdeA | |
| D2 | 51.4 ± 0.1 aA | 59.7 ± 0.6 abcdeA | 18.2 ± 0.9 abcA | 4.62 ± 0.09 bcdA | 66.1 ± 5.1 cdeB | |
| D3 | 55.2 ± 0.1 aA | 60.9 ± 0.4 cdefA | 19.1 ± 1.1 abcA | 4.77 ± 0.08 cdB | 67.8 ± 4.0 deB | |
| D4 | 55.2 ± 0.5 aA | 61.6 ± 0.7 defA | 20.2 ± 0.7 bcdA | 4.77 ± 0.08 cdA | 61.4 ± 2.6 bcdeA | |
| D5 | 55.2 ± 0.7 aA | 61.8 ± 0.2 efA | 20.7 ± 1.0 cdA | 5.07 ± 0.08 dA | 74.9 ± 2.9 efB | |
| D6 | 55.8 ± 1.8 aA | 62.3 ± 1.4 fA | 23.5 ± 2.5 dA | 5.00 ± 0.76 dA | 85.4 ± 12.7 fB | |
| Two-Way ANOVA (p-value) | ||||||
| Type of Soil | 0.022 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Fertilization | 0.450 | <0.001 | 0.026 | 0.107 | <0.001 | |
| Type of Soil × Fertilization | 0.492 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Type of Soil | Fertilization | PT [°C] | PV [mPa·s] | HPV [mPa·s] | BD [mPa·s] | FV [mPa·s] | SB [mPa·s] |
|---|---|---|---|---|---|---|---|
| 2019 (A) | |||||||
| Gleyic Chernozem | Control | 90.7 ± 0.8 aA | 409 ± 2 bcB | 336 ± 1 cdB | 73 ± 1 bcB | 405 ± 2 bA | 69 ± 3 bA |
| D1 | 90.4 ± 0.0 aA | 424 ± 3 cdeB | 347 ± 1 deA | 77 ± 2 bcdB | 416 ± 4 bcA | 69 ± 3 bA | |
| D2 | 90.2 ± 0.5 aA | 497 ± 11 gB | 390 ± 8 hB | 106 ± 4 eB | 553 ± 11 hB | 163 ± 13 eB | |
| D3 | 89.6 ± 0.8 aA | 412 ± 2 cdB | 329 ± 2 bcA | 83 ± 1 Db | 462 ± 4 gA | 133 ± 4 dA | |
| D4 | 90.4 ± 0.8 aA | 455 ± 14 fB | 375 ± 10 gB | 80 ± 5 bcdB | 457 ± 11 fgA | 82 ± 5 bcA | |
| D5 | 89.4 ± 0.5 a | 391 ± 4 bB | 316 ± 4 aA | 76 ± 1 bcdB | 450 ± 3 efgA | 135 ± 2 dA | |
| D6 | 90.1 ± 0.5 aA | 420 ± 6 cdB | 342 ± 4 deB | 77 ± 2 bcdB | 431 ± 14 cdeA | 88 ± 10 cA | |
| Haplic Luvisol | Control | 90.6 ± 0.4 a | 440 ± 2 efA | 363 ± 2 fgA | 77 ± 1 bcdB | 440 ± 4 defA | 77 ± 4 bcA |
| D1 | 89.9 ± 2.2 aA | 430 ± 3 deB | 349 ± 2 deA | 81 ± 2 dB | 426 ± 3 bcdA | 77 ± 2 bcA | |
| D2 | 89.9 ± 0.5 a | 413 ± 8 cdB | 341 ± 7 cdeA | 72 ± 1 bB | 410 ± 5 bca | 69 ± 3 bA | |
| D3 | 90.6 ± 0.4 aA | 431 ± 8 deB | 351 ± 4 efB | 81 ± 4 cdB | 444 ± 6 defgB | 94 ± 5 cB | |
| D4 | 90.7 ± 0.4 aA | 415 ± 3 cdB | 336 ± 2 cdA | 79 ± 3 bcdB | 416 ± 7 bcA | 80 ± 8 bcA | |
| D5 | 90.1 ± 0.9 aA | 369 ± 9 aA | 315 ± 4 aA | 54 ± 5 aA | 362 ± 8 aA | 47 ± 4 aA | |
| D6 | 89.1 ± 0.4 aA | 391 ± 4 bA | 316 ± 2 abA | 75 ± 3 bcdB | 383 ± 4 aA | 67 ± 5 bA | |
| Two-Way ANOVA (p-value) | |||||||
| Type of Soil | 0.962 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Fertilization | 0.284 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Type of Soil × Fertilization | 0.363 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 2020 (B) | |||||||
| Gleyic Chernozem | Control | 92.2 ± 0.9 aA | 358 ± 2 bcA | 322 ± 1 bcA | 36.7 ± 1.5 bcdA | 394 ± 7 aA | 72 ± 7 aB |
| D1 | 92.0 ± 0.6 aB | 393 ± 4 ghA | 346 ± 3 efA | 47.3 ± 1.2 eA | 470 ± 17 bcB | 120 ± 12 bB | |
| D2 | 91.9 ± 0.7 aA | 375 ± 3 defA | 341 ± 2 eA | 33.7 ± 1.2 abA | 459 ± 8 bA | 118 ± 7 bA | |
| D3 | 92.2 ± 0.4 aA | 367 ± 1 bcdA | 327 ± 3 cdA | 39.7 ± 2.3 dA | 560 ± 4 fB | 233 ± 6 gB | |
| D4 | 91.2 ± 0.7 aA | 359 ± 3 bcA | 314 ± 3 bA | 45.3 ± 0.6 eA | 487 ± 4 bcA | 173 ± 7 defB | |
| D5 | 91.7 ± 0.4 aB | 357 ± 2 bA | 318 ± 2 bcA | 39.0 ± 0.0 cdA | 486 ± 3 bcB | 167 ± 5 cdeB | |
| D6 | 91.9 ± 0.8 aB | 332 ± 4 aA | 301 ± 3 aA | 30.7 ± 1.2 aA | 477 ± 7 bcB | 175 ± 6 efB | |
| Haplic Luvisol | Control | 92.4 ± 0.9 aA | 408 ± 13 iA | 356 ± 9 fA | 52.3 ± 4.2 fA | 544 ± 18 efB | 188 ± 10 efB |
| D1 | 92.1 ± 0.9 aA | 386 ± 5 efgA | 346 ± 5 efA | 40.0 ± 0.0 dA | 498 ± 7 cdB | 152 ± 2 cdB | |
| D2 | 91.6 ± 0.4 aA | 389 ± 2 fghA | 336 ± 2 deA | 52.3 ± 0.6 fA | 482 ± 6 bcB | 146 ± 7 cB | |
| D3 | 91.1 ± 0.7 aA | 372 ± 7 cdeA | 338 ± 6 dea | 34.7 ± 1.5 abcA | 412 ± 11 aA | 74 ± 8 aA | |
| D4 | 90.9 ± 1.1 aA | 400 ± 3 ghiA | 344 ± 3 eB | 56.7 ± 1.5 fA | 521 ± 11 deB | 177 ± 10 efB | |
| D5 | 91.4 ± 0.4 aA | 401 ± 5 hiB | 346 ± 4 efB | 54.7 ± 1.5 fA | 538 ± 6 efB | 192 ± 3 fB | |
| D6 | 91.9 ± 0.0 aA | 388 ± 2 fghA | 343 ± 2 eB | 45.7 ± 0.6 eA | 489 ± 4 cB | 147 ± 4 cB | |
| Two-Way ANOVA (p-value) | |||||||
| Type of Soil | 0.345 | <0.001 | <0.001 | <0.001 | <0.001 | 0.274 | |
| Fertilization | 0.087 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Type of Soil × Fertilization | 0.768 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Type of Soil | Fertilization | K [Pa sn] | n [-] | R2 | HA [Pa s−1] |
|---|---|---|---|---|---|
| 2019 (A) | |||||
| Gleyic Chernozem | Control | 3.1 ± 0.1 cdB | 0.366 ± 0.010 bcdA | 0.984 | 365 ± 52 bA |
| D1 | 2.9 ± 0.1 cA | 0.393 ± 0.010 defA | 0.989 | 328 ± 31 bA | |
| D2 | 6.1 ± 0.2 gB | 0.335 ± 0.002 abA | 0.973 | 1003 ± 96 efA | |
| D3 | 4.8 ± 0.1 fB | 0.323 ± 0.003 aA | 0.977 | 723 ± 11 cA | |
| D4 | 4.7 ± 0.2 fB | 0.373 ± 0.006 cdeA | 0.993 | 1003 ± 66 efA | |
| D5 | 4.6 ± 0.2 fB | 0.329 ± 0.006 aA | 0.991 | 793 ± 51 cdA | |
| D6 | 4.8 ± 0.1 fB | 0.346 ± 0.011 abcA | 0.990 | 948 ± 64 efA | |
| Haplic Luvisol | Control | 3.9 ± 0.1 eA | 0.427 ± 0.009 ghA | 0.998 | 1443 ± 70 gA |
| D1 | 3.8 ± 0.2 eB | 0.405 ± 0.014 efgA | 0.994 | 905 ± 34 defA | |
| D2 | 2.1 ± 0.1 bA | 0.458 ± 0.018 hA | 0.994 | 258 ± 22 abA | |
| D3 | 3.5 ± 0.1 deB | 0.407 ± 0.005 fgA | 0.997 | 1025 ± 32 fB | |
| D4 | 3.7 ± 0.3 deB | 0.380 ± 0.004 defA | 0.992 | 864 ± 13 cdeA | |
| D5 | 1.2 ± 0.1 aA | 0.516 ± 0.017 iB | 0.997 | 148 ± 16 aA | |
| D6 | 2.1 ± 0.2 bA | 0.435 ± 0.016 ghA | 0.991 | 259 ± 14 abA | |
| Two-Way ANOVA (p-value) | |||||
| Type of Soil | <0.000 | <0.000 | - | 0.018 | |
| Fertilization | <0.000 | <0.000 | - | <0.000 | |
| Type of Soil × Fertilization | <0.000 | <0.000 | - | <0.000 | |
| 2020 (B) | |||||
| Gleyic Chernozem | Control | 2.0 ± 0.1 bA | 0.488 ± 0.009 deB | 0.996 | 910 ± 181 abcB |
| D1 | 3.3 ± 0.1 fgA | 0.426 ± 0.010 abcB | 0.997 | 1400 ± 67 efB | |
| D2 | 2.5 ± 0.2 cdeA | 0.475 ± 0.014 deB | 0.997 | 1462 ± 50 fB | |
| D3 | 3.8 ± 0.2 gA | 0.411 ± 0.008 abB | 0.996 | 1857 ± 94 gB | |
| D4 | 2.0 ± 0.2 bcA | 0.481 ± 0.019 deB | 0.998 | 1008 ± 48 bcdA | |
| D5 | 2.2 ± 0.1 bcdA | 0.485 ± 0.012 deB | 0.997 | 1161 ± 59 cdeB | |
| D6 | 2.6 ± 0.2 cdeA | 0.453 ± 0.008 abcdB | 0.998 | 1433 ± 96 efB | |
| Haplic Luvisol | Control | 4.6 ± 0.3 hA | 0.406 ± 0.018 aA | 0.993 | 2012 ± 80 gB |
| D1 | 2.6 ± 0.2 cdeA | 0.481 ± 0.017 deB | 0.994 | 1391 ± 146 eBf | |
| D2 | 2.3 ± 0.2 bcdA | 0.471 ± 0.010 cdeA | 0.996 | 638 ± 48 aB | |
| D3 | 0.9 ± 0.1 aA | 0.616 ± 0.036 eB | 0.995 | 783 ± 46 abB | |
| D4 | 2.6 ± 0.1 cdeA | 0.477 ± 0.004 deB | 0.993 | 1256 ± 119 defB | |
| D5 | 2.9 ± 0.1 efB | 0.455 ± 0.010 bcdA | 0.992 | 1344 ± 58 efB | |
| D6 | 1.9 ± 0.1 bA | 0.519 ± 0.023 deB | 0.996 | 1238 ± 107 defB | |
| Two-Way ANOVA (p-value) | |||||
| Type of Soil | 0.163 | <0.000 | - | 0.010 | |
| Fertilization | <0.000 | <0.000 | - | <0.000 | |
| Type of Soil × Fertilization | <0.000 | <0.000 | - | <0.000 | |
| Type of Soil | Fertilization | K′ [Pa sn′] | n′ [-] | R2 | K″ [Pa sn″] | n″ [-] | R2 |
|---|---|---|---|---|---|---|---|
| 2019 (A) | |||||||
| Gleyic Chernozem | Control | 20.8 ± 0.5 abA | 0.113 ± 0.005 aA | 0.992 | 1.5 ± 0.0 aA | 0.503 ± 0.008 eA | 0.996 |
| D1 | 21.1 ± 1.1 bA | 0.109 ± 0.007 aA | 0.974 | 1.6 ± 0.1 aA | 0.486 ± 0.008 deA | 0.997 | |
| D2 | 31.2 ± 0.2 fB | 0.118 ± 0.001 aA | 0.990 | 3.0 ± 0.1 gB | 0.407 ± 0.006 aA | 0.997 | |
| D3 | 24.2 ± 0.2 deA | 0.120 ± 0.005 aA | 0.988 | 2.1 ± 0.0 cdA | 0.426 ± 0.008 aB | 0.995 | |
| D4 | 22.8 ± 1.2 bcdA | 0.118 ± 0.07 aA | 0.987 | 1.9 ± 0.1 bA | 0.481 ± 0.004 cdeB | 0.995 | |
| D5 | 23.6 ± 0.5 cdA | 0.116 ± 0.008 aA | 0.993 | 2.1 ± 0.1 bcdA | 0.421 ± 0.008 aA | 0.996 | |
| D6 | 23.1 ± 1.1 bcdA | 0.133 ± 0.012 aA | 0.993 | 2.0 ± 0.1 bcdA | 0.472 ± 0.013 bcdB | 0.993 | |
| Haplic Luvisol | Control | 21.4 ± 0.7 bcA | 0.139 ± 0.015 aA | 0.991 | 1.9 ± 0.1 bcA | 0.498 ± 0.004 eB | 0.997 |
| D1 | 23.8 ± 0.6 deA | 0.161 ± 0.019 aA | 0.978 | 2.2 ± 0.1 deA | 0.480 ± 0.008 bcdBe | 0.995 | |
| D2 | 22.9 ± 0.8 bcdA | 0.152 ± 0.015 aA | 0.989 | 2.1 ± 0.1 bcdA | 0.486 ± 0.009 deB | 0.989 | |
| D3 | 24.9 ± 0.5 deB | 0.149 ± 0.011 aA | 0.988 | 2.4 ± 0.1 efB | 0.462 ± 0.009 bcA | 0.995 | |
| D4 | 26.0 ± 0.6 eB | 0.159 ± 0.006 aA | 0.990 | 2.5 ± 0.1 fA | 0.457 ± 0.010 bA | 0.994 | |
| D5 | 18.7 ± 0.7 aA | 0.154 ± 0.009 aA | 0.981 | 1.5 ± 0.0 aA | 0.526 ± 0.007 fB | 0.997 | |
| D6 | 23.9 ± 1.1 deB | 0.152 ± 0.008 aA | 0.985 | 2.0 ± 0.1 bcdA | 0.487 ± 0.007 deB | 0.998 | |
| Two-Way ANOVA (p-value) | |||||||
| Type of Soil | 0.005 | <0.001 | - | 0.039 | <0.001 | - | |
| Fertilization | <0.001 | 0.240 | - | <0.001 | <0.001 | - | |
| Type of Soil × Fertilization | <0.001 | 0.163 | - | <0.001 | <0.001 | - | |
| 2020 (B) | |||||||
| Gleyic Chernozem | Control | 19.4 ± 1.3 bA | 0.182 ± 0.011 deB | 0.992 | 2.1 ± 0.1 aB | 0.498 ± 0.014 eA | 0.995 |
| D1 | 25.6 ± 1.1 defB | 0.147 ± 0.012 abB | 0.992 | 2.5 ± 0.2 bB | 0.462 ± 0.012 dA | 0.996 | |
| D2 | 25.2 ± 1.2 defA | 0.150 ± 0.008 abcB | 0.993 | 2.5 ± 0.1 bA | 0.465 ± 0.008 dB | 0.995 | |
| D3 | 28.8 ± 0.4 gB | 0.136 ± 0.004 aB | 0.994 | 3.3 ± 0.1 deB | 0.396 ± 0.004 aA | 0.994 | |
| D4 | 27.9 ± 1.1 fgB | 0.156 ± 0.003 abcdB | 0.993 | 3.1 ± 0.1 cdeB | 0.428 ± 0.008 bcA | 0.996 | |
| D5 | 24.9 ± 0.7 cdeB | 0.162 ± 0.003 abcdB | 0.997 | 3.0 ± 0.1 cdB | 0.441 ± 0.008 cdB | 0.997 | |
| D6 | 25.0 ± 1.4 deB | 0.154 ± 0.013 abcB | 0.993 | 3.0 ± 0.2 cdB | 0.430 ± 0.007 cA | 0.997 | |
| Haplic Luvisol | Control | 29.3 ± 0.4 gB | 0.162 ± 0.003 abcdB | 0.997 | 3.7 ± 0.0 fB | 0.403 ± 0.005 abA | 0.995 |
| D1 | 23.1 ± 0.9 cdA | 0.176 ± 0.009 cdeA | 0.990 | 3.3 ± 0.1 eB | 0.428 ± 0.004 bcA | 0.996 | |
| D2 | 23.0 ± 0.7 cdA | 0.183 ± 0.008 deB | 0.994 | 3.4 ± 0.1 efB | 0.424 ± 0.005 bcA | 0.995 | |
| D3 | 11.2 ± 0.9 aA | 0.194 ± 0.017 eB | 0.992 | 2.0 ± 0.1 aA | 0.530 ± 0.011 fB | 0.995 | |
| D4 | 22.3 ± 0.6 cA | 0.163 ± 0.003 abcdA | 0.993 | 3.3 ± 0.1 deB | 0.423 ± 0.009 bcA | 0.995 | |
| D5 | 26.0 ± 0.1 efB | 0.145 ± 0.002 abA | 0.991 | 3.2 ± 0.0 deB | 0.423 ± 0.004 bcA | 0.995 | |
| D6 | 19.0 ± 0.6 bA | 0.171 ± 0.015 bcdeB | 0.991 | 2.8 ± 0.1 bcB | 0.457 ± 0.015 dA | 0.991 | |
| Two-Way ANOVA (p-value) | |||||||
| Type of Soil | <0.001 | <0.001 | - | <0.001 | 0.100 | - | |
| Fertilization | <0.001 | 0.054 | - | <0.001 | <0.001 | - | |
| Type of Soil × Fertilization | <0.001 | <0.001 | - | <0.001 | <0.001 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pycia, K.; Szpunar-Krok, E.; Szostek, M.; Pawlak, R.; Juszczak, L. Selected Physicochemical, Thermal, and Rheological Properties of Barley Starch Depending on the Type of Soil and Fertilization with Ash from Biomass Combustion. Foods 2024, 13, 49. https://doi.org/10.3390/foods13010049
Pycia K, Szpunar-Krok E, Szostek M, Pawlak R, Juszczak L. Selected Physicochemical, Thermal, and Rheological Properties of Barley Starch Depending on the Type of Soil and Fertilization with Ash from Biomass Combustion. Foods. 2024; 13(1):49. https://doi.org/10.3390/foods13010049
Chicago/Turabian StylePycia, Karolina, Ewa Szpunar-Krok, Małgorzata Szostek, Renata Pawlak, and Lesław Juszczak. 2024. "Selected Physicochemical, Thermal, and Rheological Properties of Barley Starch Depending on the Type of Soil and Fertilization with Ash from Biomass Combustion" Foods 13, no. 1: 49. https://doi.org/10.3390/foods13010049
APA StylePycia, K., Szpunar-Krok, E., Szostek, M., Pawlak, R., & Juszczak, L. (2024). Selected Physicochemical, Thermal, and Rheological Properties of Barley Starch Depending on the Type of Soil and Fertilization with Ash from Biomass Combustion. Foods, 13(1), 49. https://doi.org/10.3390/foods13010049







