Obtaining an Oily Ingredient Rich in PUFAS and Tocopherols and a High-Nutritional-Value Flour from Beans (Phaseolus vulgaris L.) by Supercritical CO2 Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Beans Samples
2.2. Obtaining Lipid Extract from Beans by Means of Supercritical CO2
2.2.1. RSM Optimization of Oil Extraction by sc-CO2
- Coded pressure, X1 (Bar): 380, 400, 420, (levels: −1, 0, 1)
- Coded temperature, X2 (°C): 35, 40, 45 (levels: −1, 0, 1)
2.2.2. Modeling of the Extraction Kinetics with sc-CO2
2.3. Characterization of Lipids Obtained by sc-CO2
2.3.1. Fatty Acid and Tocopherol Analysis
2.3.2. Tocopherol Content
2.4. Chemical and Physical Analysis Post Extraction with sc-CO2
2.4.1. Nutritional Analysis
2.4.2. Amino Acid Analysis
2.5. Physical Properties
Flow Properties of Bean Flour
2.6. Statistical Analysis
3. Results and Discussion
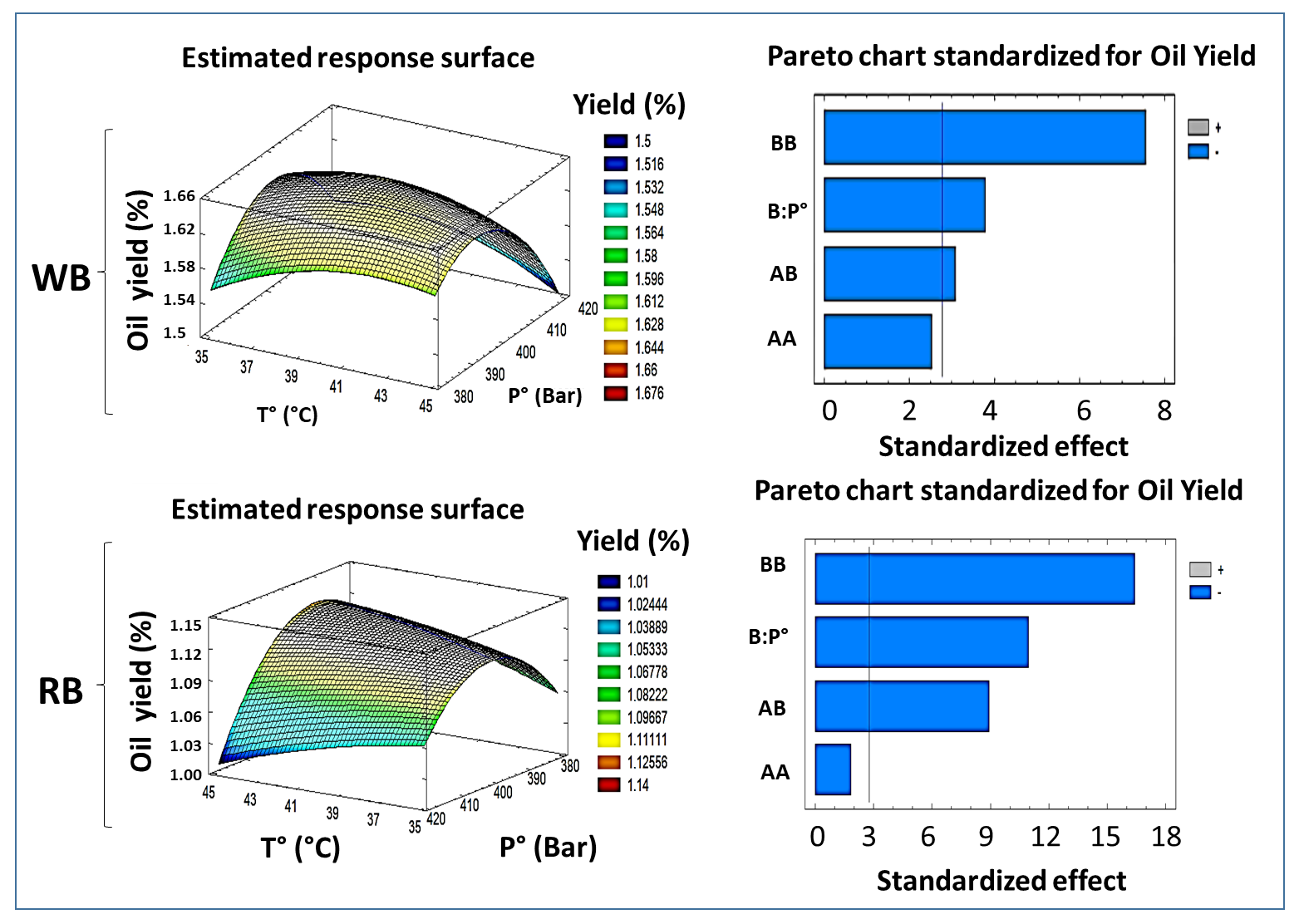
3.1. Oil Extraction Yield Optimization
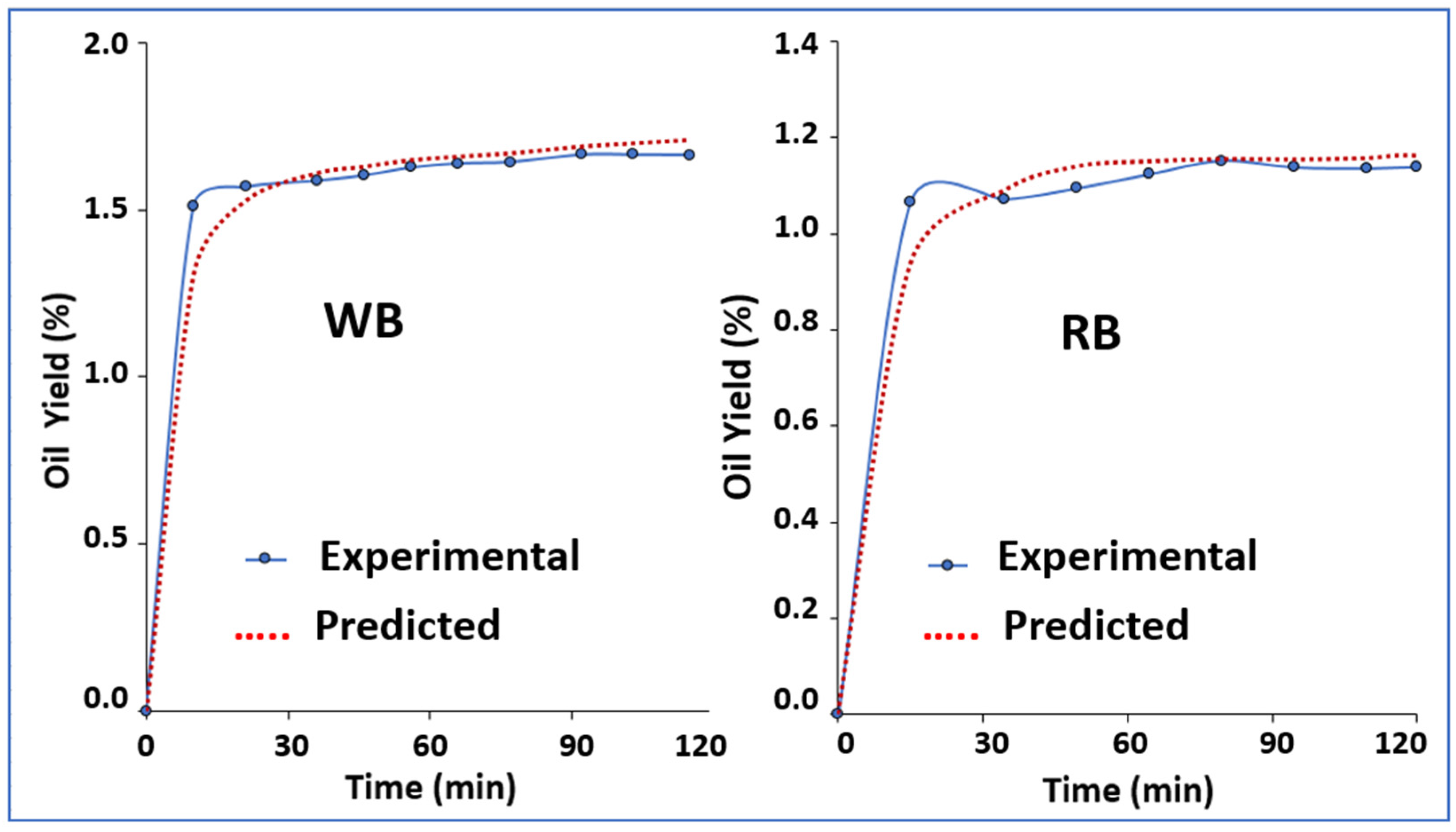
3.2. Kinetic Modeling of sc-CO2 Extraction
- WB; tCER = 15.90, tFER = 70.00, b0 = 0.1, b1 = 0.07, b2 = −0.06, b3 = −0.003 (r = 0.99)
- RB; tCER = 1.80, tFER = 12.99, b0 = 0.01, b1 = 0.42, b2 = −0.35, b3 = −0.007 (r = 0.99)
3.3. Composition of the Oil Extracted by sc-CO2
Fatty Acids and Tocopherols
3.4. Chemical Analysis of Residual Bean Flour Post Extraction with sc-CO2
3.4.1. Nutritional Analysis
3.4.2. Amino Acids Analysis
Total Amino Acids
Essential Amino Acids
Comparison with the FAO Standard
Non-Essential Amino Acids
3.5. Physical Properties of Residual Bean Flours
Residual Bean Flow Properties
| Rheological Model | Coefficients | Bean Flour Suspensions (10%) | |
|---|---|---|---|
| WB | RB | ||
| Cross–Willianson | η o(107) | 3.3 ± 0.3 | 2.1 ± 0.0 |
| η ꝏ | 35.86 ± 2.8 | 3442 ± 67 | |
| γo (10−2) | 5.2 ± 0.0 | 4.8 ± 0.1 | |
| η (10−2) | 95 ± 2 | 91 ± 1 | |
| r | 0.892 | 0.980 | |
| Herschel–Bulkley | τ0 | 317.9 ± 21.2 | −177.6 ± 0.0 |
| K | 453 ± 33 | 1588 ± 87 | |
| η (10−2) | 5.2 ± 0.1 | 22.1 ± 0.1 | |
| r | 0.855 | 0.882 | |
| Ostwald de Waele | K | 129 ± 8.1 | 1480 ± 97 |
| η (10−2) | 21.0 ± 0.1 | 29.1 ± 0.0 | |
| r | 0.936 | 0.988 | |
| Bingham | γ | 81.7 ± 5.6 | 500.4 ± 33.4 |
| η | 21.2 ± 1.1 | 504.2 ± 36.2 | |
| r | 0.7173 | 0.833 | |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, E.; Marques, G.; Lino-Neto, T. Phaseolus vulgaris L. as a functional food for aging protection. In Aging: Oxidative Stress and Dietary Antioxidants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 289–295. [Google Scholar] [CrossRef]
- Hayat, I.; Ahmad, A.; Masud, T.; Ahmed, A.; Bashir, S. Nutritional and Health Perspectives of Beans (Phaseolus vulgaris L.): An Overview. Crit. Rev. Food Sci. Nutr. 2014, 54, 580–592. [Google Scholar] [CrossRef]
- Cruz Balarezo, J.; Camarena Mayta, F.; Pierre Baudoin, J.; Huaringa Joaquín, A.; Blas Sevillano, R. Evaluación Agromorfológica y Caracterización Molecular de la Ñuña (Phaseolus vulgaris L.). Idesia 2009, 27, 29–40. [Google Scholar] [CrossRef]
- Soba, D.; Arrese-Igor, C.; Aranjuelo, I. Additive effects of heatwave and water stresses on soybean seed yield is caused by impaired carbon assimilation at pod formation but not at flowering. Plant 2022, 321, 111320. [Google Scholar] [CrossRef]
- Nina, N.; Theoduloz, C.; Tapia, G.; Jimenéz-Aspee, F.; Márquez, K.; Schmeda-Hirschmann, G. Changes in polyphenol composition, antioxidant capacity and enzyme inhibition in Phaseolus vulgaris L. submitted to hydric stress. Sci. Hortic. 2023, 317, 112070. [Google Scholar] [CrossRef]
- Los, F.G.B.; Zielinski, A.A.F.; Wojeicchowski, J.P.; Nogueira, A.; Demiate, I.M. Beans (Phaseolus vulgaris L.): Whole seeds with complex chemical composition. Curr. Opin. Food Sci. 2018, 19, 63–71. [Google Scholar] [CrossRef]
- Chen, P.X.; Tang, Y.; Marcone, M.F.; Pauls, P.K.; Zhang, B.; Liu, R.; Tsao, R. Characterization of free, conjugated and bound phenolics and lipophilic antioxidants in regular- and non-darkening cranberry beans (Phaseolus vulgaris L.). Food Chem. 2015, 185, 298–308. [Google Scholar] [CrossRef]
- Coelho, R.C.; Faria, M.A.; Rocha, J.; Reis, A.; Oliveira, M.B.P.P.; Nunes, E. Assessing genetic variability in germplasm of Phaseolus vulgaris L. collected in Northern Portugal. Sci. Hortic. 2009, 122, 333–338. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Bai, Z.; Wang, J.; Zhou, Y.; Jiang, J.; Zeng, Q.; Song, K. Comparative study on the chemical composition, anthocyanins, tocopherols and carotenoids of selected legumes. Food Chem. 2018, 260, 317–326. [Google Scholar] [CrossRef]
- Arachis, L.; Plukenetia, L.; Thummajitsakul, S.; Piyaphan, P.; Khamthong, S.; Unkam, M.; Silprasit, K. Comparison of FTIR fingerprint, phenolic content, antioxidant and anti-glucosidase activities among Phaseolus vulgaris L. Electron. J. Biotechnol. 2023, 61, 14–23. [Google Scholar] [CrossRef]
- Fuentes, E.; Rodríguez, L.; Diego, M.; Burgos-Edwards, A.; Carrasco, B.; Schmeda-Hirschmann, G. Inhibition of platelet aggregation by extracts and compounds from the leaves of Chilean bean landraces (Phaseolus vulgaris L.). J. Funct. Foods 2023, 100, 105388. [Google Scholar] [CrossRef]
- Burgos-Edwards, A.; Theoduloz, C.; Katherine, M.; Carrasco, B.; Schmeda-Hirschmann, G. Leaf development in Chilean bean landraces (Phaseolus vulgaris) affects phenolic composition and α -glucosidase inhibition. Sci. Hortic. 2023, 309, 111613. [Google Scholar] [CrossRef]
- Corzo-Rios, L.; Sánchez-Chino, X.M.; Cardador-Martínez, A.; Martínez-Herrera, J.; Jiménez-Martínez, C. Effect of cooking on nutritional and non-nutritional compounds in two species of Phaseolus (P. vulgaris and P. coccineus) cultivated in Mexico. Int. J. Gastron. Food Sci. 2020, 20, 100206. [Google Scholar] [CrossRef]
- Halmos, E.P.; Christophersen, C.T.; Bird, A.R.; Shepherd, J.; Gibson, R.; Muir, J.-G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef]
- Martínez-Ávila, M.; Rodríguez-Rodríguez, J.; Gutiérrez Uribe, J.A.; Guajardo-Flores, D. Selective supercritical fluid extraction of non-polar phytochemicals from black beans (Phaseolus vulgaris L.) by-products. J. Supercrit. Fluids 2022, 189, 105730. [Google Scholar] [CrossRef]
- Hoper Hooper, S.D.; Glahn, R.P.; Cichy, K.A. Single Varietal Dry Bean (Phaseolus vulgaris L.) Pastas: Nutritional Profile and Consumer Acceptability. Plant Foods Hum. Nutr. 2019, 74, 342–349. [Google Scholar] [CrossRef]
- Dorado, D.J.; Hurtado-Benavides, A.M.; Martínez-Correa, H.A. Extracción con CO2 Supercrítico de aceite de semillas de guanábana (Annona muricata): Cinética, perfil de ácidos grasos y esteroles. Inf. Tecnol. 2016, 27, 37–48. [Google Scholar] [CrossRef]
- Mostert, M.E.; Botha, B.M.; Du Plessis, L.M.; Duodu, K.G. Effect of fruit ripeness and method of fruit drying on the extractability of avocado oil with hexane and supercritical carbon dioxide. J. Sci. Food Agric. 2007, 87, 2880–2885. [Google Scholar] [CrossRef]
- Fraguela-Meissimilly, H.; Bastías-Monte, J.M.; Vergara, C.; Ortiz-Viedma, J.; Lemus-Mondaca, R.; Flores, M.; Toledo-Merma, P.; Alcázar-Alay, S.; Gallón-Bedoya, M. New Trends in Supercritical Fluid Technology and Pressurized Liquids for the Extraction and Recovery of Bioactive Compounds from Agro-Industrial and Marine Food Waste. Molecules 2023, 28, 4421. [Google Scholar] [CrossRef]
- Ortiz-Viedma, J.; Bastias-Montes, J.M.; Char, C.; Vega, C.; Quintriqueo, A.; Gallón-Bedoya, M.; Flores, M.; Aguilera, J.M.; Miranda, J.M.; Barros-Velázquez, J. Sequential Biorefining of Bioactive Compounds of High Functional Value from Calafate Pomace (Berberis microphylla) Using Supercritical CO2 and Pressurized Liquids. Antioxidants 2023, 12, 323. [Google Scholar] [CrossRef]
- Esquivel, M.M.; Bernardo-Gil, G. Extraction of Olive Husk Oil with Compressed Carbon Dioxide. J. Supercrit. Fluids 1993, 6, 91–94. [Google Scholar] [CrossRef]
- Liu, G.; Xu, X.; Gong, Y.; He, L.; Gao, Y. Effects of supercritical CO2 extraction parameters on chemical composition and free radical-scavenging activity of pomegranate (Punica granatum L.) seed oil. Food Bioprod. Process. 2012, 90, 573–578. [Google Scholar] [CrossRef]
- Liu, S.; Yang, F.; Zhang, C.; Ji, H.; Hong, P.; Deng, C. Optimization of process parameters for supercritical carbon dioxide extraction of Passiflora seed oil by response surface methodology. J. Supercrit. Fluids 2009, 48, 9–14. [Google Scholar] [CrossRef]
- Ekinci, M.S.; Gürü, M. Extraction of oil and β-sitosterol from peach (Prunus persica) seeds using supercritical carbon dioxide. J. Supercrit. Fluids 2014, 92, 319–323. [Google Scholar] [CrossRef]
- Sovová, H. Modeling the supercritical fluid extraction of essential oils from plant materials. J. Chromatogr. A 2012, 1250, 27–33. [Google Scholar] [CrossRef] [PubMed]
- AOCS. Official Methods and Recommended Practices of American Oil Chemists’ Society, 5th ed.; AOCS Press: Champaign, IL, USA, 1998; Volume 1. [Google Scholar]
- American Oil Chemists’ Society. Official Methods and Recommended Practices of the American Oil Chemists’ Society; American Oil Chemists’ Society (AOCS Press): Champaign, IL, USA, 1993. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Alaiz, M.; Navarro, J.L.; Girón, J.; Vioque, E. Amino acid analysis by high-performance liquid chromatography after derivatization with diethyl ethoxymethylenemalonate. J. Chromatogr. 1992, 591, 181–186. [Google Scholar] [CrossRef]
- Gaitonde, M.K.; Dovey, T. A Rapid and Direct Method for the Quantitative Determination of Tryptophan in the Intact Protein. Biochem. J. 1970, 117, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Sodek, L.; Vecchia, P.T.D.; Lima, M.L.G.P. Rapid Determination of Tryptophan in Beans (Phaseolus vulgaris) by the Acid Ninhydrin Method. J. Agric. Food Chem. 1975, 23, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, E.; Raofie, F.; Najafi, N.M. Application of response surface methodology and central composite design for the optimisation of supercritical fluid extraction of essential oils from Myrtus communis L. leaves. Food Chem. 2011, 126, 1449–1453. [Google Scholar] [CrossRef]
- Jokić, S.; Nagy, B.; Zeković, Z.; Vidović, S.; Bilić, M.; Velić, D.; Simándi, B. Effects of supercritical CO 2 extraction parameters on soybean oil yield. Food Bioprod. Process. 2012, 90, 693–699. [Google Scholar] [CrossRef]
- Duba, K.S.; Fiori, L. Supercritical CO2 extraction of grape seed oil: Effect of process parameters on the extraction kinetics. J. Supercrit. Fluids 2015, 98, 33–43. [Google Scholar] [CrossRef]
- Uquiche, E.L.; Toro, M.T.; Quevedo, R.A. Supercritical extraction with carbon dioxide and co-solvent from Leptocarpha rivularis. J. Appl. Res. Med. Aromat. Plants 2019, 14, 100210. [Google Scholar] [CrossRef]
- Barros, H.D.F.Q.; Coutinho, J.P.; Grimaldi, R.; Godoy, H.T.; Cabral, F.A. Simultaneous extraction of edible oil from avocado and capsanthin from red bell pepper using supercritical carbon dioxide as solvent. J. Supercrit. Fluids 2016, 107, 315–320. [Google Scholar] [CrossRef]
- Salinas, F.; Vardanega, R.; Espinosa-Álvarez, C.; Jimenez, D.; Munoz, W.B.; Ruiz-Domínguez, M.C.; Meireles, M.A.A.; Cerezal-Mezquita, P. Supercritical fluid extraction of chañar (Geoffroea decorticans) almond oil: Global yield, kinetics and oil characterization. J. Supercrit. Fluids 2020, 161, 104824. [Google Scholar] [CrossRef]
- Chañi-Paucar, L.O.; Johner, J.C.F.; Zabot, G.L.; Meireles, M.A.A. Technical and economic evaluation of supercritical CO2 extraction of oil from sucupira branca seeds. J. Supercrit. Fluids 2022, 181, 105494. [Google Scholar] [CrossRef]
- Dos Santos, P.; De Aguiar, A.C.; Viganó, J.; Boeing, J.S.; Visentainer, J.V.; Martínez, J. Supercritical CO2 extraction of cumbaru oil (Dipteryx alata Vogel) assisted by ultrasound: Global yield, kinetics and fatty acid composition. J. Supercrit. Fluids 2016, 107, 75–83. [Google Scholar] [CrossRef]
- Fornereto Soldan, A.C.; Arvelos, S.; Watanabe, É.O.; Hori, C.E. Supercritical fluid extraction of oleoresin from Capsicum annuum industrial waste. J. Cleaner Prod. 2021, 297, 126593. [Google Scholar] [CrossRef]
- Sutivisedsak, N.; Moser, B.R.; Sharma, B.K.; Evangelista, R.L.; Cheng, H.N.; Lesch, W.C.; Tangsrud, R.R.; Biswas, A. Physical properties and fatty acid profiles of oils from black, kidney, great northern, and pinto beans. J. Am. Oil Chem. Soc. 2011, 88, 193–200. [Google Scholar] [CrossRef]
- Pirman, T.; Stibilj, V. An influence of cooking on fatty acid composition in three varieties of common beans and in lentil. Eur. Food Res. Technol. 2003, 217, 498–503. [Google Scholar] [CrossRef]
- Grelaap, E.R.; Giinterb, K.D. Animal Feed Fatty acid composition and tocopherol content of some legume seeds. Anim. Feed. Sci. Technol. 1995, 52, 325–331. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Tang, Y.; Chen, P.; Liu, R.; Ramdath, D.D.; Liu, Q.; Hernandez, M.; Tsao, R. Fatty acid, carotenoid and tocopherol compositions of 20 Canadian lentil cultivars and synergistic contribution to antioxidant activities. Food Chem. 2014, 161, 296–304. [Google Scholar] [CrossRef]
- Yoshida, H.; Tomiyama, Y.; Yoshida, N.; Shibata, K.; Mizushina, Y. Regiospecific profiles of fatty acids in triacylglycerols and phospholipids from adzuki beans (Vigna angularis). Nutrients 2010, 2, 49–59. [Google Scholar] [CrossRef]
- Padhi, E.M.T.; Liu, R.; Hernandez, M.; Tsao, R.; Ramdath, D.D. Total polyphenol content, carotenoid, tocopherol and fatty acid composition of commonly consumed Canadian pulses and their contribution to antioxidant activity. J. Funct. Foods 2017, 38, 602–611. [Google Scholar] [CrossRef]
- Shimelis, E.A.; Rakshit, S.K. Proximate composition and physico-chemical properties of improved dry bean (Phaseolus vulgaris L.) varieties grown in Ethiopia. LWT 2005, 38, 331–338. [Google Scholar] [CrossRef]
- Ojij, N.; Kimura, T.; Koazel, H. Composition, Soaking and Softening Characteristics of Some Kenyan Beans (Phaseolus vulgaris L.). Food Sci. Technol. Res. 2000, 6, 12–18. [Google Scholar] [CrossRef]
- Amir, Y.; Haenni, A.L.; Youyou, A. Physical and biochemical differences in the composition of the seeds of Algerian leguminous crops. J. Food Compos. Anal. 2007, 20, 466–471. [Google Scholar] [CrossRef]
- Abdelwhab, N.M.; Nour, A.A.A.M.; Fageer, A.S.M. The Nutritive and Functional Properties of Dry Bean (Phaseolus vulgaris) as Affected by Gamma Irradiation. Pak. J. Nutr. 2009, 8, 1739–1742. [Google Scholar] [CrossRef]
- Güzel, D.; Sayar, S. Effect of cooking methods on selected physicochemical and nutritional properties of barlotto bean, chickpea, faba bean, and white kidney bean. J. Food Sci. Technol. 2012, 49, 89–95. [Google Scholar] [CrossRef]
- Fan, G.; Beta, T. Proximate composition, phenolic profiles and antioxidant capacity of three common bean varieties (Phaseolus vulgaris l.). J. Food Chem. Nanotechnol. 2016, 2, 147–152. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci. Technol. 2020, 97, 170–184. [Google Scholar] [CrossRef]
- Nielsen, K.; Kondrup, J.; Elsner, P.; Juul, A.; Jensen, E.S. Casein and soya-bean protein have different effects on whole body protein turnover at the same nitrogen balance. Br. J. Nutr. 1994, 72, 69–81. [Google Scholar] [CrossRef]
- Martino, H.S.D.; Bigonha, S.M.; Cardoso, L.D.M.; Rosa, C.D.O.B.; Costa, N.M.B.; Cárdenas, L.D.L.Á.R.; Ribeiro, S.M.R. Nutritional and bioactive compounds of bean: Benefits to human health. ACS Symp. Ser. 2012, 1109, 233–258. [Google Scholar] [CrossRef]
- Mbithi-Mwikya, S.; Ooghe, W.; Van Camp, J.; Ngundi, D.; Huyghebaert, A. Amino acid profiles after sprouting, autoclaving, and lactic acid fermentation of finger millet (Eleusine coracan) and kidney beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2000, 48, 3081–3085. [Google Scholar] [CrossRef] [PubMed]
- Sgarbieri, V.C. Composition and Nutritive Value of Beans (Phaseolus vulgaris L.). Nutr. Value Cereal Prod. Beans Starches 1989, 60, 132–198. [Google Scholar]
- Pelegrine, D.H.; Silva, F.C.; Gasparetto, C.A. Rheological behavior of pineapple and mango pulps. LWT 2002, 35, 645–648. [Google Scholar] [CrossRef]
- Ortega-Quintana, F.A.; Torres, R.; Pérez, O. Physicochemical and rheological characterization of guava pulp (Psidium guajava L.) varieties Hybrid Klom Sali, Puerto Rico, D14 and Red. Vitae 2009, 16, 13–18. [Google Scholar]
- Bezerra, T.S.; Fernandes, T.R.; Videla de Resende, J. Effects of added sucrose and pectin on the rheological behavior and freezing kinetics of passion fruit pulp Studied by response surface methodology. J. Food Sci. Technol. 2015, 52, 3350–3357. [Google Scholar] [CrossRef][Green Version]
- Izidoro, D.R.; Scheer, A.P.; Sierakowski, M.R.; Haminiuk, C.W.I. Influence of green banana pulp on the rheological behaviour and chemical characteristics of emulsions (mayonnaises). LWT 2008, 41, 1018–1028. [Google Scholar] [CrossRef]


| Fatty Acid (%) | WB | RB | Black Bean | Kidney Bean | Great Northern | Pinto Bean |
|---|---|---|---|---|---|---|
| C14:0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 | 0.1 | 0.1 | 0.1 |
| C16:0 | 13.2 ± 1.1 a | 18.2 ± 3.4 b | 10.7 | 12.3 | 11.5 | 12.7 |
| C18:0 | 2.4 ± 0.2 a | 6.0 ± 1.4 b | 1.8 | 1.4 | 2.0 | 1.7 |
| C20:0 | 0.5 ± 0.0 a | 1.6 ± 0.3 b | 0.5 | 0.5 | 0.5 | 0.3 |
| C22:0 | 1.2 ± 0.1 | 1.3 ± 0.1 | 0.5 | 0.7 | 0.5 | 0.4 |
| Sat | 17.5 | 27.2 | 13.8 | 15.0 | 14.6 | 15.2 |
| C16:1 ω9 | 0.2 ± 0.0 | _ | 0.3 | 0.3 | 0.2 | 0.2 |
| C18:1 ω9 | 15.7 ± 2.1 | 15.7 ± 2.5 | 9.3 | 9.5 | 5.2 | 5.9 |
| C18:1 ω11 | 2.1 ± 0.1 a | 4.0 ± 0.8 b | 1.9 | 2.6 | 1.8 | 1.7 |
| C20:1 ω11 | 0.3 ± 0.0 | _ | 0.2 | 0.2 | 0.1 | 0.1 |
| Monounsat | 18.3 | 19.7 | 11.7 | 12.6 | 7.3 | 7.9 |
| C18:2ω9c | 29.3 ± 2.7 | 24.8 ± 5.3 | 31.1 | 24.1 | 33.4 | 32.1 |
| C18:3 ω9 | 33.6 ± 4.1 | 25.7 ± 4.1 | 41.7 | 46.0 | 42.8 | 43.3 |
| C22:2 | 0.9 ± 0.1 a | 2.3 ± 0.3 b | 1.0 | 1.8 | 1.2 | 1.2 |
| C22:3 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.7 | 0.5 | 0.7 | 0.3 |
| Polyunsat | 63.6 | 52.8 | 73.6 | 71.0 | 77.1 | 75.8 |
| Tocopherols (ppm) | ||||||
| α-Tocopherol | –a | –a | 110 ± 4 | 151 ± 4 | 25 ± 2 | 29 ± 1 |
| β-Tocopherol | –a | –a | –a | –a | –a | –a |
| γ-Tocopherol | 19.50 ± 4.1 | 12.20 ± 2.4 | 2692 ± 21 | 2380 ± 19 | 2828 ± 24 | 2737 ± 32 |
| δ-Tocopherol | 3.2 ± 0.1 | 2.7 ± 0.2 | 157 ± 6 | 137 ± 3 | 116 ± 3 | 88 ± 3 |
| Total Tocopherols | 22.7 | 12.47 | 2959 | 2668 | 2969 | 2854 |
| Raw Beans | Cooked Beans | FAO ref. * | Raw Beans | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminoacid | WB | RB | Perola | Carioca | Ouro Blanco | Diamante Negro | RBS Radiante | Talisman | Aminoacid Score | WB | RB |
| (g/100 g) | |||||||||||
| Essential amino acid | |||||||||||
| Phen + Tyr | 3.328 ± 0.524 a | 3.885 ± 0.099 b | 1.29 | 0.94 | 1.19 | 1.57 | 1.42 | 1.62 | 6.3 | 5.3 * | 6.2 + |
| His | 0.795 ± 0.011 a | 1.041 ± 0.035 b | 0.58 | 0.44 | 0.39 | 0.36 | 0.37 | 0.44 | 1.9 | 4.4 + | 5.5 + |
| Isoleu | 1.273 ± 0.132 a | 1.436 ± 0.043 b | 1.16 | 0.81 | 0.43 | 0.55 | 0.56 | 0.62 | 2.8 | 4.5 + | 5.1 + |
| Leu | 2.941 ± 0.328 a | 3.378 ± 0.138 b | 1.77 | 1.30 | 0.84 | 1.15 | 1.16 | 1.20 | 6.6 | 4.3 * | 5.0 * |
| Lys | 0.325 ± 0.001 a | 0.379 ± 0.002 b | 1.78 | 1.25 | 0.67 | 0.97 | 1.05 | 0.94 | 5.8 | 4.5 * | 6.5 + |
| Met + Cys | 0.647 ± 0.008 a | 0.728 ± 0.005 b | 0.26 3 | 0.17 | 0.20 | 0.24 | 0.22 | 0.27 | 2.5 | 2.6 + | 2.9 + |
| Threonine | 1.665 ± 0.012 a | 1.945 ± 0.114 b | 0.98 | 0.75 | 0.48 | 0.59 | 0.51 | 0.53 | 3.4 | 3.9 + | 5.7 + |
| Tryp | 1.102 ± 0.029 | 1.101 ± 0.018 | nd | nd. | nd | nd | Nd | nd | 1.1 | 1.1 | 1.1 |
| Val | 1.485 ± 0.033 a | 1.654 ± 0.289 | 1.30 | 0.91 | 0.56 | 0.64 | 0.64 | 0.72 | 3.5 | 4.2 + | 4.7 + |
| Subtotal | 11.794 ± 1.08 a | 15.547 ± 3.06 b | 9.12 | 6.58 | 4.76 | 6.07 | 5.93 | 6.34 | |||
| Non essential amino acid | |||||||||||
| Ala | 0.833 ± 0.013 | 0.821 ± 0.067 | 0.90 | 0.70 | 0.61 | 0.67 | 0.64 | 0.69 | |||
| Arg | 2.101 ± 0.043 a | 1.201 ± 0.088 b | 2.06 | 1.60 | 0.80 | 0.79 | 0.7 | 0.93 | |||
| Asp. acid | 1.678 ± 0.064 a | 1.116 ± 0.982 b | 3.80 | 2.77 | 1.70 | 1.75 | 1.66 | 1.78 | |||
| Glut. acid | 2.221 ± 0.005 | 2.101 ± 0.873 | 4.29 | 3.15 | 2.15 | 2.12 | 1.99 | 2.26 | |||
| Gly | 0.768 ± 0.041 a | 0.511 ± 0.211 b | 0.81 | 0.59 | 0.49 | 0.51 | 0.47 | 0.51 | |||
| Prol | 0.838 ± 0.025 a | 0.796 ± 0.013 b | 0.90 | 0.70 | 0.62 | 0.65 | 0.63 | 0.72 | |||
| Ser | 1.222 ± 0.055 a | 1.198 ± 0.343 b | 1.35 | 1.03 | 0.76 | 0.84 | 0.74 | 0.82 | |||
| Subtotal | 7.222 ± 0.026 a | 5.616 ± 0.998 b | 14.11 | 10.55 | 7.13 | 7.33 | 6.83 | 7.71 | |||
| Total | 23.222 ± 4.589 | 23.291 ± 4.598 | 23.22 | 17.13 | 11.89 | 13.40 | 12.76 | 14.05 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benites-Mena, J.; Vargas-De-La-Cruz, C.; Vergara-Valdés, C.; Jave-Nakayo, J.; Ortiz-Viedma, J.; Char, C.; Inga-Guevara, M.; Flores, M.; Cepeda, A. Obtaining an Oily Ingredient Rich in PUFAS and Tocopherols and a High-Nutritional-Value Flour from Beans (Phaseolus vulgaris L.) by Supercritical CO2 Extraction. Foods 2024, 13, 36. https://doi.org/10.3390/foods13010036
Benites-Mena J, Vargas-De-La-Cruz C, Vergara-Valdés C, Jave-Nakayo J, Ortiz-Viedma J, Char C, Inga-Guevara M, Flores M, Cepeda A. Obtaining an Oily Ingredient Rich in PUFAS and Tocopherols and a High-Nutritional-Value Flour from Beans (Phaseolus vulgaris L.) by Supercritical CO2 Extraction. Foods. 2024; 13(1):36. https://doi.org/10.3390/foods13010036
Chicago/Turabian StyleBenites-Mena, Jesus, Celia Vargas-De-La-Cruz, Claudia Vergara-Valdés, Jorge Jave-Nakayo, Jaime Ortiz-Viedma, Cielo Char, Marianela Inga-Guevara, Marcos Flores, and Alberto Cepeda. 2024. "Obtaining an Oily Ingredient Rich in PUFAS and Tocopherols and a High-Nutritional-Value Flour from Beans (Phaseolus vulgaris L.) by Supercritical CO2 Extraction" Foods 13, no. 1: 36. https://doi.org/10.3390/foods13010036
APA StyleBenites-Mena, J., Vargas-De-La-Cruz, C., Vergara-Valdés, C., Jave-Nakayo, J., Ortiz-Viedma, J., Char, C., Inga-Guevara, M., Flores, M., & Cepeda, A. (2024). Obtaining an Oily Ingredient Rich in PUFAS and Tocopherols and a High-Nutritional-Value Flour from Beans (Phaseolus vulgaris L.) by Supercritical CO2 Extraction. Foods, 13(1), 36. https://doi.org/10.3390/foods13010036








