The Influence of Whey Protein Isolate on the Quality Indicators of Acidophilic Ice Cream Based on Liquid Concentrates of Demineralized Whey
Abstract
1. Introduction
- -
- To study the influence of whey protein isolate on the physicochemical and rheological properties of mixes and ice cream;
- -
- To compare the dynamics of changes in the freezing process in ice cream with whey protein isolate of free water during freezing and subsequent storage of the product at sub-zero temperatures;
- -
- To study the microstructure of soft ice cream and determine the main physical and chemical parameters;
- -
- To measure the microbiological indicators of ice cream during storage.
2. Materials and Methods
2.1. Raw Materials
2.2. Ice Cream Production
2.2.1. Activated Starter
2.2.2. Hydrolyzed Concentrates
2.2.3. Ice Cream
2.3. Methods
2.3.1. Chemical Composition
2.3.2. Rheological and Thermophysical Characteristics of Ice Cream Mixes
2.3.3. Color
2.3.4. Microstructure
2.3.5. Analysis of Ice Crystals
2.3.6. Quality Indicators of Ice Cream
2.3.7. Microbiological Analysis
2.3.8. Statistical Processing
3. Results and Discussion
3.1. Chemical Composition
3.2. Physical Characteristics of Ice Cream Mixes
3.3. Color
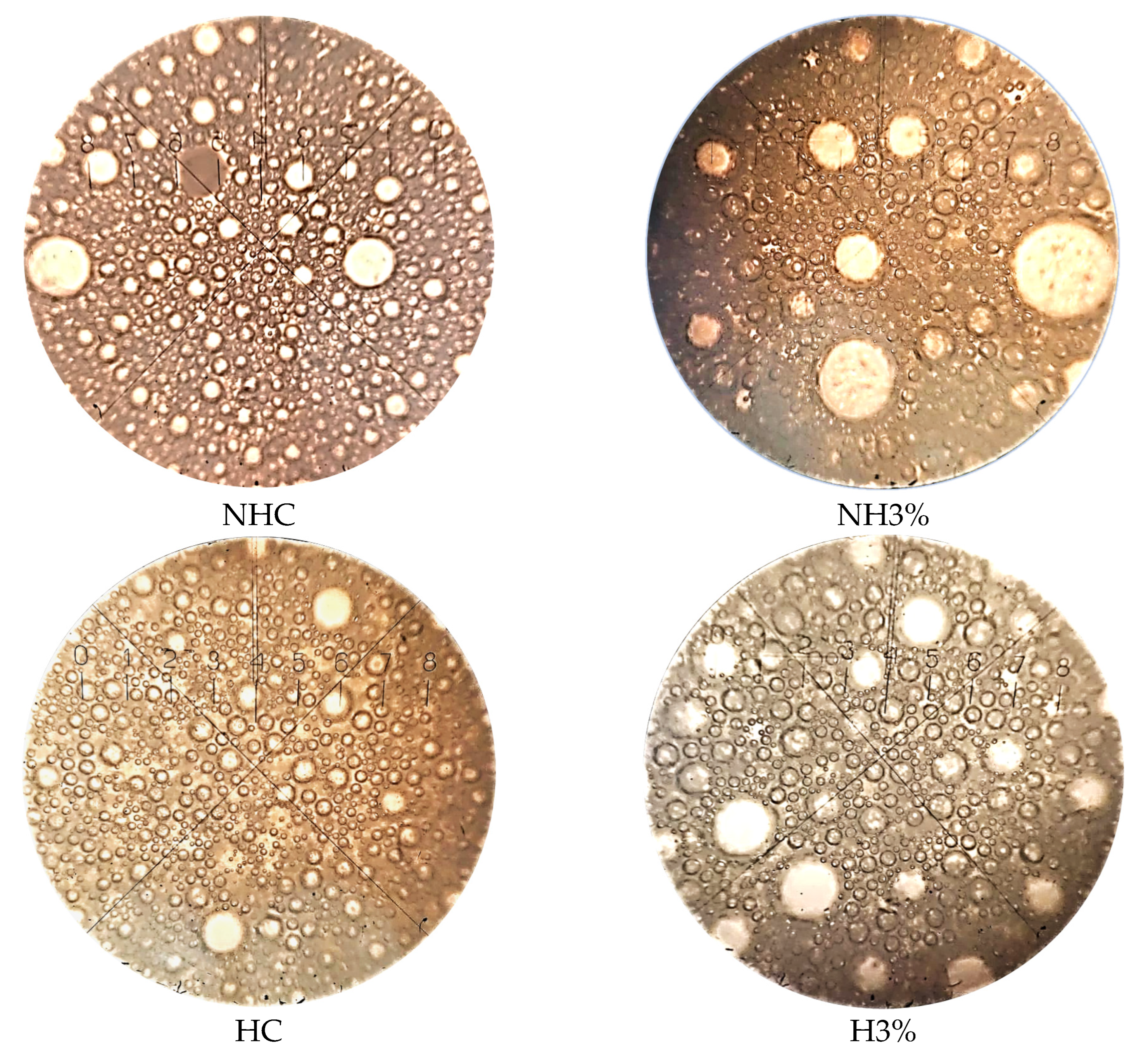
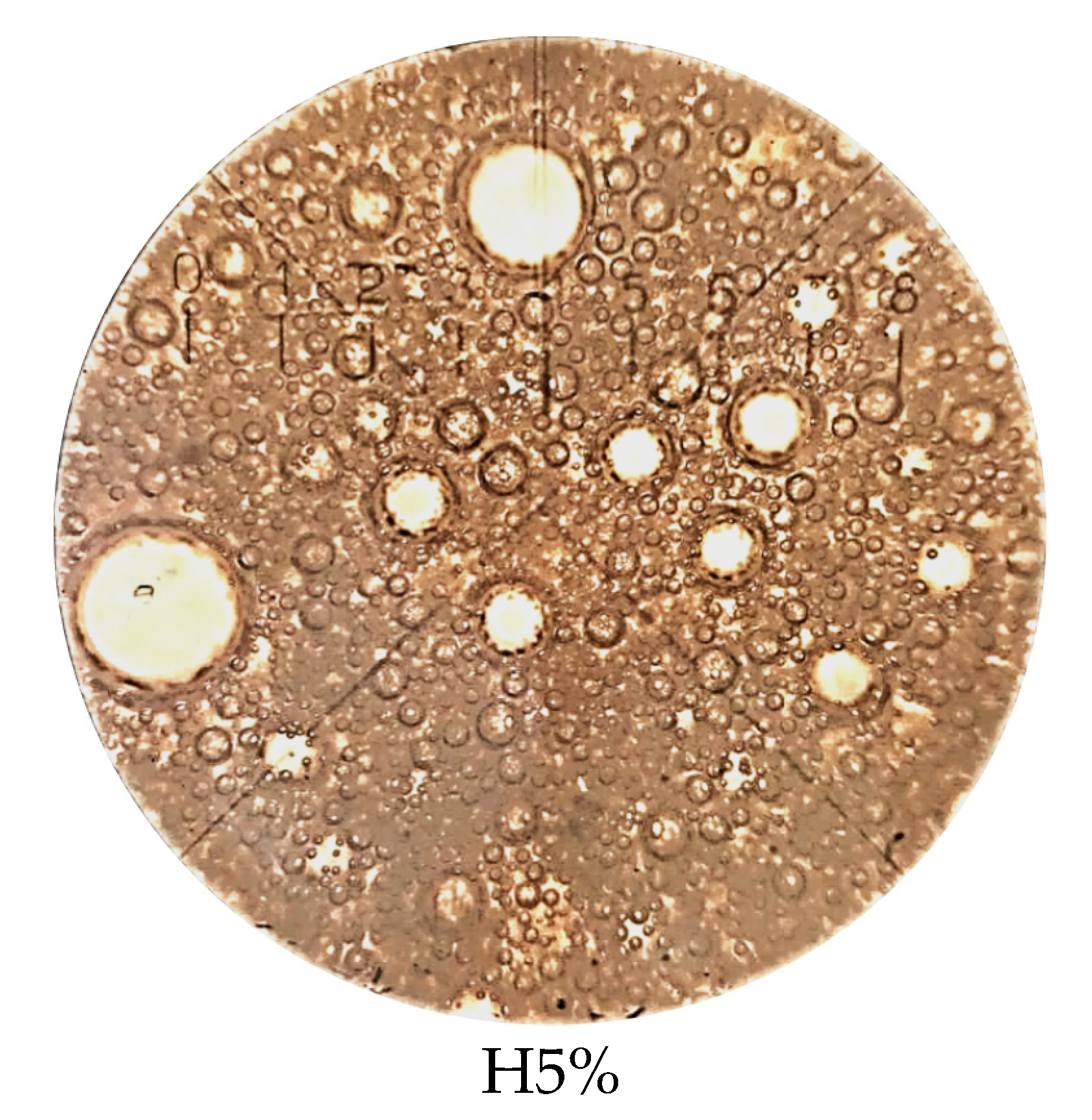
3.4. Microstructure
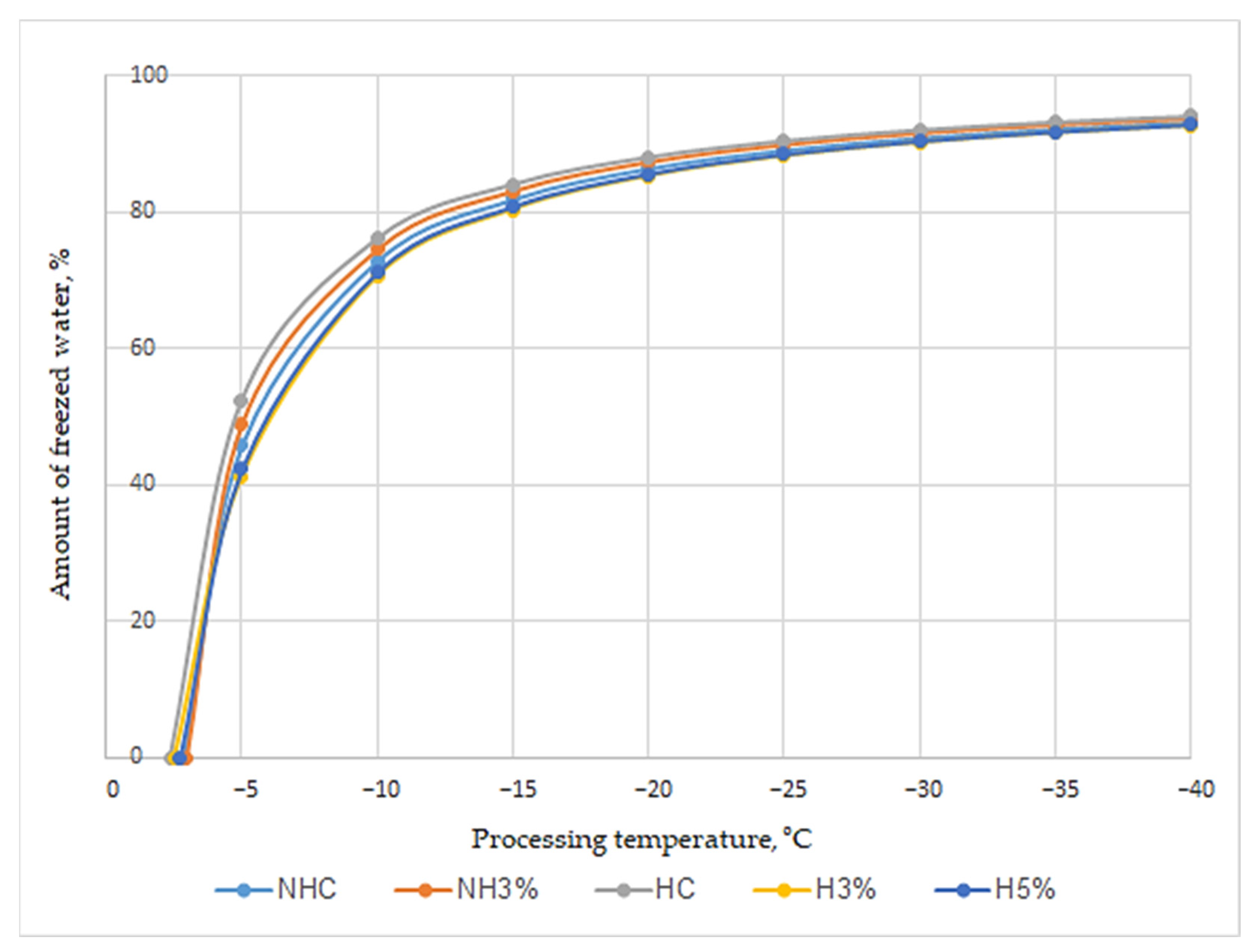
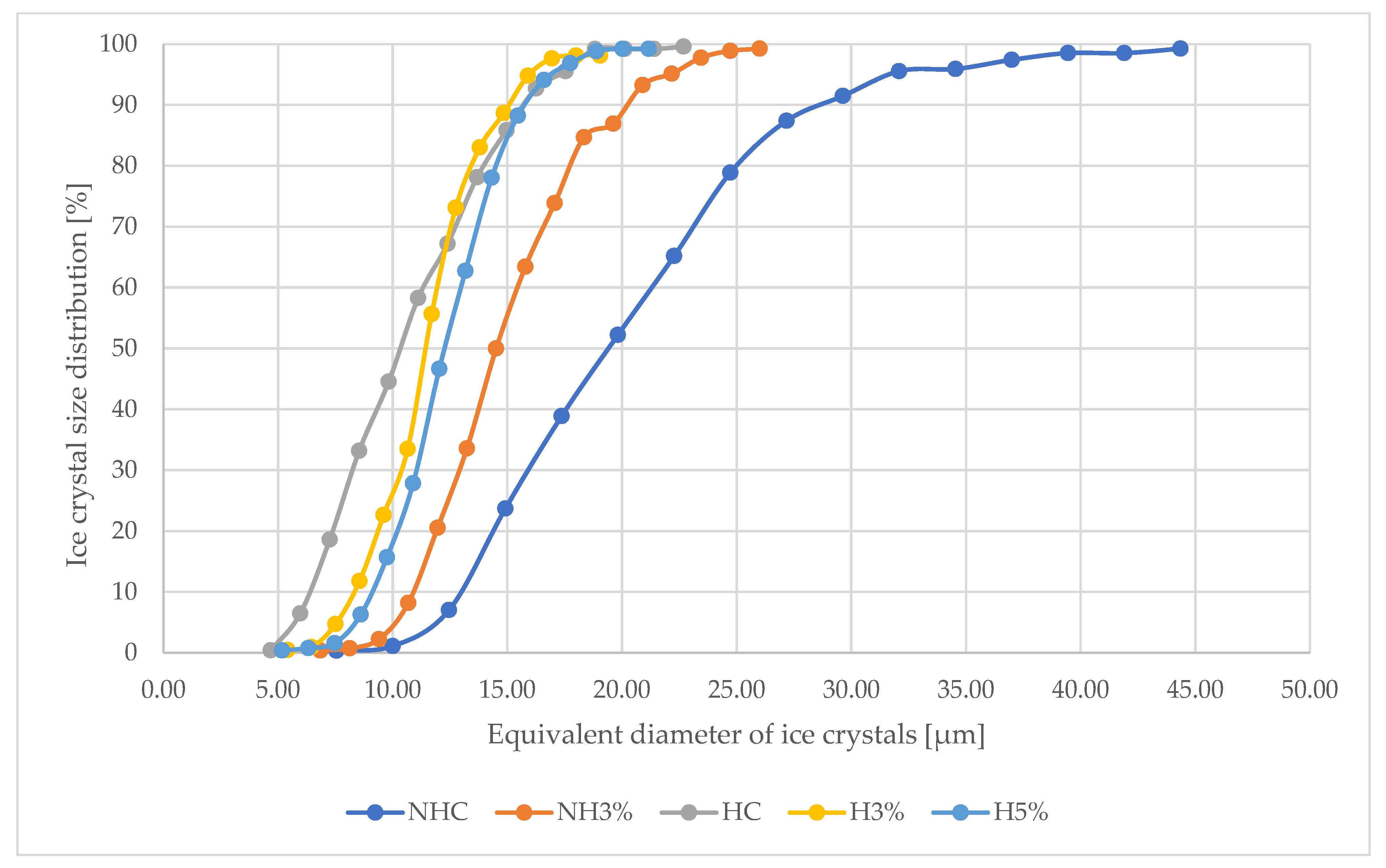
3.5. Microscopy Analysis
3.6. Quality Indicators
3.7. Microbiological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, J.S.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Functional food relevance of whey protein: A review of recent findings and scopes ahead. J. Funct. Foods 2015, 19, 308–319. [Google Scholar] [CrossRef]
- Henriques, M.H.F.; Gomes, D.M.G.S.; Borges, A.R.; Pereira, C.J.D. Liquid whey protein concentrates as primary raw material for acid dairy gels. Food Sci. Technol. 2019, 40, 361–369. [Google Scholar] [CrossRef]
- Henriques, M.H.F.; Gomes, D.M.G.S.; Pereira, C.J.D.; Gil, M.H.M. Effects of Liquid Whey Protein Concentrate on Functional and Sensorial Properties of Set Yogurts and Fresh Cheese. Food Bioprocess Technol. 2013, 6, 952–963. [Google Scholar] [CrossRef]
- Henriques, M.; Gomes, D.; Rodrigues, D.; Pereira, C.; Gil, M. Performance of bovine and ovine liquid whey protein concentrate on functional properties of set yoghurts. Procedia Food Sci. 2011, 1, 2007–2014. [Google Scholar] [CrossRef]
- Pereira, C.; Henriques, M.; Gomes, D.; Gomez-Zavaglia, A.; de Antoni, G. Novel functional whey-based drinks with great potential in the dairy industry. Food Technol. Biotechnol. 2015, 53, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Mykhalevych, A.; Kostenko, O.; Polishchuk, G.; Bandura, U. Application of milk protein concentrates in preparation of reduced fat sour cream. Ukr. Food J. 2022, 11, 429–447. [Google Scholar] [CrossRef]
- Polishchuk, G.; Sharakhmatova, T.; Shevchenko, I.; Manduk, O.; Mykhalevych, A.; Pukhlyak, A. Scientific substantiation of cream heating duration in the technology of sour cream, enriched with protein. Food Sci. Technol. 2023, 17, 3–10. [Google Scholar] [CrossRef]
- Shevchenko, O.; Mykhalevych, A.; Polischuk, G.; Buniowska-Olejnik, M.; Bass, O.; Bandura, U. Technological functions of hydrolyzed whey concentrate in ice cream. Ukr. Food J. 2022, 11, 498–517. [Google Scholar] [CrossRef]
- Dekker, P.; Koenders, D.; Bruins, M. Lactose-Free Dairy Products: Market Developments, Production, Nutrition and Health Benefits. Nutrients 2019, 11, 551. [Google Scholar] [CrossRef]
- Wang, G.; Guo, M. Manufacturing Technologies of Whey Protein Products. In Whey Protein Production, Chemistry, Functionality, and Applications; Guo, M., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2019; pp. 13–37. [Google Scholar] [CrossRef]
- Sofjan, R.P.; Hartel, R.W. Effects of overrun on structural and physical characteristics of ice cream. Int. Dairy J. 2004, 14, 255–262. [Google Scholar] [CrossRef]
- Panghal, A.; Patidar, R.; Jaglan, S.; Chhikara, N.; Khatkar, S.K.; Gat, Y.; Sindhu, N. Whey valorization: Current options and future scenario–a critical review. Nutr. Food Sci. 2018, 48, 520–535. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Mykhalevych, A.; Polishchuk, G.; Buniowska-Olejnik, M.; Tomczyńska-Mleko, M.; Mleko, S. Functional and technological properties of protein ingredients in whey ice cream. Ukr. J. Food Sci. 2022, 10, 125–135. [Google Scholar] [CrossRef]
- Axentii, M.; Stroe, S.-G.; Codină, G.G. Development and Quality Evaluation of Rigatoni Pasta Enriched with Hemp Seed Meal. Foods 2023, 12, 1774. [Google Scholar] [CrossRef] [PubMed]
- Arranz, E.; Segat, A.; Velayos, G.; Flynn, C.; Brodkorb, A.; Giblin, L. Dairy and plant based protein beverages: In vitro digestion behaviour and effect on intestinal barrier biomarkers. Food Res. Int. 2023, 169, 112815. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, A.; Liu, L.; Kan, Z.; Wang, W. The relationship between water-holding capacities of soybean–whey mixed protein and ice crystal size for ice cream. J. Food Process Eng. 2021, 44, e13723. [Google Scholar] [CrossRef]
- Tvorogova, A.A.; Gurskiy, I.A.; Shobanova, T.V.; Smykov, I.T. Effect of Protein Concentrates and Isolates on the Rheological, Structural, Thermal and Sensory Properties of Ice Cream. Curr. Res. Nutr. Food Sci. J. 2023, 11, 294–306. [Google Scholar] [CrossRef]
- El-Zeini, H.M.; El-Abd, M.M.; Mostafa, A.Z.; El-Ghany, F.H.Y. Effect of incorporating whey protein concentrate on chemical, rheological and textural properties of ice cream. J. Food Process. Technol. 2016, 7, 1000546. [Google Scholar]
- Hossain, M.K.; Petrov, M.; Hensel, O.; Diakité, M. Microstructure and Physicochemical Properties of Light Ice Cream: Effects of Extruded Microparticulated Whey Proteins and Process Design. Foods 2021, 10, 1433. [Google Scholar] [CrossRef]
- Das, N.; Hooda, A. Chemistry and Different Aspects of Ice Cream. In The Chemistry of Milk and Milk Products; Apple Academic Press: New York, NY, USA, 2023; pp. 65–86. [Google Scholar]
- Saentaweesuk, S.; Chaikham, P. Effect of whey protein isolate incorporated with various carbohydratebased fat replacers on physicochemical and sensorial properties of low-fat chocolate ice cream. Food Res. 2023, 7, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Saentaweesuk, S.; Aukkanit, N. Effects of Whey Protein Isolate and Soy Protein Isolate as Fat Replacers on the Physicochemical and Sensory Properties of Low-Fat Chocolate Ice Cream. Burapha Sci. J. 2022, 27, 686–701. [Google Scholar]
- Roy, S.; Hussain, S.A.; Prasad, W.G.; Khetra, Y. Quality attributes of high protein ice cream prepared by incorporation of whey protein isolate. Appl. Food Res. 2022, 2, 100029. [Google Scholar] [CrossRef]
- Song, X.; Perez-Cueto, F.; Bredie, W. Sensory-Driven Development of Protein-Enriched Rye Bread and Cream Cheese for the Nutritional Demands of Older Adults. Nutrients 2018, 10, 1006. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.A.; Hamad, E.M.; Ashoush, I.S. Effect of partial fat replacement by whey protein, oat, wheat germ and modified starch on sensory properties, viscosity and antioxidant activity of reduced fat ice cream. Food Nutr. Sci. 2016, 7, 397–404. [Google Scholar] [CrossRef][Green Version]
- Zhang, A.Q.; Xu, D.; Liu, B.H.; Shi, B.M.; Zhang, Y.H. Low-fat ice cream model system: Impact of incorporation of alcalase hydrolyzed zein. Food Funct. 2023, 14, 4430–4439. [Google Scholar] [CrossRef]
- Dhingra, M.; Singh, J. Enzymes in Food Industry and Their Regulatory Oversight. In Microbes Food Ind.; Scrivener Publishing LLC: Beverly, MA, USA, 2023; pp. 249–274. [Google Scholar] [CrossRef]
- Dadan, M.; Nowacka, M.; Czyzewski, J.; Witrowa-Rajchert, D. Modification of food structure and improvement of freezing processes by pulsed electric field treatment. In Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow; Academic Press: Cambridge, MA, USA, 2020; pp. 203–226. [Google Scholar] [CrossRef]
- Queiroz, E.S.; Rezende, A.L.L.; Perrone, Í.T.; Francisquini, J.D.A.; de Carvalho, A.F.; Alves, N.M.G.; de Oliveira, L.F.C.; Stephani, R. Spray drying and characterization of lactose-free goat milk. LWT 2021, 147, 111516. [Google Scholar] [CrossRef]
- Pertsevoy, F.; Ladyka, V.; Smetanska, I.; Bienias, D.; Ianchyk, M.; Grynchenko, N.; Omelchenko, S.; Hrynchenko, O. Technology of Thermostable and Frozen Fillings Using Dairy Raw Materials and Sesame Seeds Concentrate; Dissa+: Kharkiv, Ukraine, 2022; 192p. [Google Scholar]
- Arellano, M.; Benkhelifa, H.; Flick, D.; Alvarez, G. Online ice crystal size measurements during sorbet freezing by means of the focused beam reflectance measurement (FBRM) technology. Influence of operating conditions. J. Food Eng. 2012, 113, 351–359. [Google Scholar] [CrossRef]
- Buyck, J.R.; Baer, R.J.; Choi, J. Effect of storage temperature on quality of light and full-fat ice cream. J. Dairy Sci. 2011, 94, 2213–2219. [Google Scholar] [CrossRef]
- Tay, R.R.E.; Agatha, T.; Somang, G.; Yuliarti, O.; Tan, E.L.L. Structuring wheat flour-based crackers using whey protein isolate. Int. Dairy J. 2022, 128, 105314. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Korish, M.A.; Shiboob, M.H. Protein and Amino Acid Content in Four Brands of Commercial Table Eggs in Retail Markets in Relation to Human Requirements. Animals 2020, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Loveday, S.M. Food proteins: Technological, nutritional, and sustainability attributes of traditional and emerging proteins. Annu. Rev. Food Sci. Technol. 2019, 10, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Graulus, G.J.; Keshari Samal, S.; Van Nieuwenhove, I.; Dubruel, P. Porous hydrogel biomedical foam scaffolds for tissue repair. In Biomedical Foams for Tissue Engineering Applications; Netti, P.A., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 335–390. [Google Scholar] [CrossRef]
- de Castro, R.J.S.; Domingues, M.A.F.; Ohara, A.; Okuro, P.K.; dos Santos, J.G.; Brexó, R.P.; Sato, H.H. Whey protein as a key component in food systems: Physicochemical properties, production technologies and applications. Food Struct. 2017, 14, 17–29. [Google Scholar] [CrossRef]
- Khem, S.; Small, D.M.; May, B.K. The behaviour of whey protein isolate in protecting Lactobacillus plantarum. Food Chem. 2016, 190, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Maleki, O.; Khosrowshahi Asl, A.; Alizadeh Khaledabad, M.; Amiri, S. Production and characterization of synbiotic ice cream using microencapsulation and cryopreservation of Lactobacillus rhamnosus in whey protein/bio-cellulose/inulin composite microcapsules. J. Food Meas. Charact. 2023, 17, 3909–3917. [Google Scholar] [CrossRef]
- Osmak, T.; Mleko, S.; Bass, O.; Mykhalevych, A.; Kuzmyk, U. Enzymatic hydrolysis of lactose in concentrates of reconstituted demineralized whey, intended for ice cream production. Ukr. Food J. 2021, 10, 277–288. [Google Scholar] [CrossRef]
- Instituto Português da Qualidade. Milk—Fat Content Determination, Gerber Method (NP Standard No. 469 in Portuguese); IPQ: Monte de Caparica, Portugal, 2002. [Google Scholar]
- Slashcheva, A.; Nykyforov, R.; Popova, S.; Korenets, Y. Rationale for the use of protein-carbohydrate mix in the technology of disperse products. East.-Eur. J. Enterp. Technol. 2016, 2, 64–71. [Google Scholar] [CrossRef][Green Version]
- Romanchuk, I.; Minorova, A.; Krushelnytska, N. Physical-chemical composition and technological properties of demineralized milk whey received by membrane methods. Agric. Sci. Pract. 2018, 5, 33–39. [Google Scholar] [CrossRef]
- Frenzel, M.; Zerge, K.; Clawin-Rädecker, I.; Lorenzen, P.C. Comparison of the galacto-oligosaccharide forming activity of different β-galactosidases. LWT-Food Sci. Technol. 2015, 60, 1068–1071. [Google Scholar] [CrossRef]
- Lim, S.Y.; Swanson, B.G.; Clark, S. High hydrostatic pressure modification of whey protein concentrate for improved functional properties. J. Dairy Sci. 2008, 91, 1299–1307. [Google Scholar] [CrossRef]
- Nazarewicz, S.; Kozłowicz, K.; Kobus, Z.; Gładyszewska, B.; Matwijczuk, A.; Ślusarczyk, L.; Skrzypek, T.; Sujka, M.; Kozłowicz, N. The Use of Ultrasound in Shaping the Properties of Ice Cream with Oleogel Based on Oil Extracted from Tomato Seeds. Appl. Sci. 2022, 12, 9165. [Google Scholar] [CrossRef]
- Kuzmyk, U.; Marynin, A.; Svyatnenko, R.; Zheludenko, Y.; Kurmach, M.; Shvaiko, R. Prospects of use of vegetable raw materials in the technology of sour-milk dessert. EUREKA Life Sci. 2021, 3, 29–35. [Google Scholar] [CrossRef]
- Polischuk, G.; Sharahmatova, T.; Breus, N.; Bass, O.; Shevchenko, I. Studies of water freezing features in ice cream with starch syrup. Food Sci. Technol. 2019, 13, 71–77. [Google Scholar] [CrossRef]
- Goff, H.D.; Hartel, R.W. Ice cream structure. In Ice Cream; Springer: Boston, MA, USA, 2013; pp. 313–352. [Google Scholar]
- Herrera, M.L.; M’Cann, J.I.; Ferrero, C.; Hagiwara, T.; Zaritzky, N.E.; Hartel, R.W. Thermal, mechanical, and molecular relaxation properties of frozen sucrose and fructose solutions containing hydrocolloids. Food Biophys. 2007, 2, 20–28. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A.; Łaba, S.; Jakubczyk, E. The effects of selected stabilizers addition on physical properties and changes in crystal structure of whey ice cream. LWT 2022, 154, 112841. [Google Scholar] [CrossRef]
- Muse, M.R.; Hartel, R.W. Ice cream structural elements that affect melting rate and hardness. J. Dairy Sci. 2004, 87, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Henriques, M.; Gomes, D.; Pereira, C. Liquid whey protein concentrates produced by ultrafiltration as primary raw materials for thermal dairy gels. Food Technol. Biotechnol. 2017, 55, 454. [Google Scholar] [CrossRef] [PubMed]
- Romulo, A.; Meindrawan, B. Effect of Dairy and Non-Dairy Ingredients on the Physical Characteristic of Ice Cream. IOP Conf. Ser. Earth Environ. Sci. 2021, 794, 012145. [Google Scholar] [CrossRef]
- Arbuckle, W.S. Ice Cream; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Özdemir, C.; Dağdemir, E.U.; Özdemir, S.; Sağdıç, O. The Effects of Using Alternative Sweeteners to Sucrose on Ice Cream Quality. J. Food Qual. 2008, 31, 415–428. [Google Scholar] [CrossRef]
- Özdemir, C.; Arslaner, A.; Özdemir, S.; Özdemir, G.U.C. Ice-Cream Production from Lactose-Free UHT Milk. J. Food Sci. Eng. 2018, 8, 210–214. [Google Scholar] [CrossRef]
- Lee, J.; Duggan, E. Whey protein microgels for stabilisation of foams. Int. Dairy J. 2022, 132, 105399. [Google Scholar] [CrossRef]
- Puangmanee, S.; Hayakawa, S.; Sun, Y.; Ogawa, M. Application of whey protein isolate glycated with rare sugars to ice cream. Food Sci. Technol. Res. 2008, 14, 457. [Google Scholar] [CrossRef][Green Version]
- Akalın, A.S.; Karagözlü, C.; Ünal, G. Rheological properties of reduced-fat and low-fat ice cream containing whey protein isolate and inulin. Eur. Food Res. Technol. 2008, 227, 889–895. [Google Scholar] [CrossRef]
- Koretska, I.; Polyovyk, V.; Maslikov, M.; Kuzmin, O. Thermophysical characteristics of frozen semifinished products for restaurant technology. Ukr. J. Food Sci. 2020, 8, 231–240. [Google Scholar] [CrossRef]
- Kozłowicz, K.; Nazarewicz, S.; Różyło, R.; Nastaj, M.; Parafiniuk, S.; Szmigielski, M.; Bieńczak, A.; Kozłowicz, N. The Use of Moldavian Dragonhead Bagasse in Shaping the Thermophysical and Physicochemical Properties of Ice Cream. Appl. Sci. 2021, 11, 8598. [Google Scholar] [CrossRef]
- Kot, A.; Jakubczyk, E.; Kamińska-Dwórznicka, A. The Effectiveness of Combination Stabilizers and Ultrasound Homogenization in Milk Ice Cream Production. Appl. Sci. 2023, 13, 7561. [Google Scholar] [CrossRef]
- Landikhovskaya, A.V.; Tvorogova, A.A. Ice cream and frozen desserts nutrient compositions: Current trends of researches. Food Syst. 2021, 4, 74–81. [Google Scholar] [CrossRef]
- Fernández-Garía, E.; McGregor, J.U.; Traylor, S. The addition of oat fiber and natural alternative sweeteners in the manufacture of plain yogurt. J. Dairy Sci. 1998, 81, 655–663. [Google Scholar] [CrossRef]
- Belokurova, E.S.; Pankina, I.A.; Sevastianova, A.D.; Asfondiarova, I.V.; Katkova, N.M. The effect of functional additives on the indicator “water activity” of biscuit semi-finished products. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 022022. [Google Scholar] [CrossRef]
- de Souza Fernandes, D.; Leonel, M.; Del Bem, M.S.; Mischan, M.M.; Garcia, É.L.; Dos Santos, T.P.R. Cassava derivatives in ice cream formulations: Effects on physicochemical, physical and sensory properties. J. Food Sci. Technol. 2017, 54, 1357–1367. [Google Scholar] [CrossRef][Green Version]
- Cook, K.L.K.; Hartel, R.W. Mechanisms of ice crystallization in ice cream production. Compr. Rev. Food Sci. Food Saf. 2010, 9, 213–222. [Google Scholar] [CrossRef]
- Kumar, P.K.; Rasco, B.A.; Tang, J.; Sablani, S.S. State/phase transitions, ice recrystallization, and quality changes in frozen foods subjected to temperature fluctuations. Food Eng. Rev. 2020, 12, 421–451. [Google Scholar] [CrossRef]
- Goff, H.D. Ice cream. In Advanced Dairy Chemistry; Springer: Boston, MA, USA, 2013; pp. 441–450. [Google Scholar]
- Syed, Q.A.; Anwar, S.; Shukat, R.; Zahoor, T. Effects of different ingredients on texture of ice cream. J. Nutr. Health Food Eng. 2018, 8, 422–435. [Google Scholar]
- Leducq, D.; Ndoye, F.T.; Alvarez, G. Phase change material for the thermal protection of ice cream during storage and transportation. Int. J. Refrig. 2015, 52, 133–139. [Google Scholar] [CrossRef]
- Barros, E.L.D.S.; Silva, C.C.; Canella, M.H.M.; Verruck, S.; Prestes, A.A.; Vargas, M.O.; Maran, B.M.; Esmerino, E.A.; Silva, R.; Balthazar, C.F.; et al. Effect of replacement of milk by block freeze concentrated whey in physicochemical and rheological properties of ice cream. Food Sci. Technol. 2021, 42, 1–9. [Google Scholar] [CrossRef]
- de Meneses, R.B.; Moura, D.C.C.; de Almeida, D.T.; da Silva Bispo, E.; Maciel, L.F.; da Rocha-Leão, M.H.M.; Conte-Junior, C.A. Impact of different dairy wheys on quality parameters of ice cream. Rev. Bras. Ciências Agrárias 2021, 16, 1–10. [Google Scholar] [CrossRef]
- García-Pérez, F.J.; Lario, Y.; Fernández-López, J.; Sayas, E.; Pérez-Alvarez, J.A.; Sendra, E. Effect of orange fiber addition on yogurt color during fermentation and cold storage. Color Res. Appl. 2005, 30, 457–463. [Google Scholar] [CrossRef]
- Skryplonek, K.; Gomes, D.; Viegas, J.; Pereira, C.; Henriques, M. Lactose-free frozen yogurt: Production and characteristics. Acta Sci. Pol. Technol. Aliment. 2017, 16, 171–179. [Google Scholar] [CrossRef]
- Meneses, R.B.; Silva, M.S.; Monteiro, M.L.G.; Rocha-Leão, M.H.M.; Conte-Junior, C.A. Effect of dairy by-products as milk replacers on quality attributes of ice cream. J. Dairy Sci. 2020, 103, 10022–10035. [Google Scholar] [CrossRef]
- Moschopoulou, E.; Dernikos, D.; Zoidou, E. Ovine ice cream made with addition of whey protein concentrates of ovine-caprine origin. Int. Dairy J. 2021, 122, 105146. [Google Scholar] [CrossRef]
- Buniowska-Olejnik, M.; Mykhalevych, A.; Polishchuk, G.; Sapiga, V.; Znamirowska-Piotrowska, A.; Kot, A.; Kamińska-Dwórznicka, A. Study of Water Freezing in Low-Fat Milky Ice Cream with Oat β-Glucan and Its Influence on Quality Indicators. Molecules 2023, 28, 2924. [Google Scholar] [CrossRef] [PubMed]
- Mykhalevych, A.; Sapiga, V.; Polischuk, G.; Osmak, T. Functional and technological properties of oat beta-glucan in acidophilic-whey ice cream. Food Environ. Saf. J. 2022, 21, 116–128. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Li, J.; Wei, C.; Ye, X.; Shi, J.; Chen, S. Pectin from citrus canning wastewater as potential fat replacer in ice cream. Molecules 2018, 23, 925. [Google Scholar] [CrossRef] [PubMed]
- El-Hadad, S.S.; Tikhomirova, N.A.; Tvorogova, A.A.; Shobanova, T.V.; El-Aziz, M.A. Physical properties and microstructure of ice cream supplemented with minor components of wheat germ oil. Int. J. Dairy Sci. 2020, 15, 189–199. [Google Scholar] [CrossRef]
- Arief, M.F.; Andini, R.D.; Rosyidi, D.; Radiati, L.E. Effect of Goat Kefir Utilization on Physicochemical Quality and Sensory Attributes of Ice Cream Probiotic. In Proceedings of the 3rd International Conference on Environmentally Sustainable Animal Industry 2022 (ICESAI 2022); Atlantis Press: Dordrecht, The Netherlands, 2022. [Google Scholar]
- Liu, X.; Sala, G.; Scholten, E. Structural and functional differences between ice crystal-dominated and fat network-dominated ice cream. Food Hydrocoll. 2023, 138, 108466. [Google Scholar] [CrossRef]
- Drewett, E.M.; Hartel, R.W. Ice crystallization in a scraped surface freezer. J. Food Eng. 2007, 78, 1060–1066. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A. Wpływ stabilizatorów na ograniczenie rekrystalizacji w lodach typu sorbet. Przemysł Spożywczy 2016, 1, 34–37. [Google Scholar] [CrossRef]
- Arellano, M.; Gonzalez, J.E.; Alvarez, G.; Benkhelifa, H.; Flick, D.; Leducq, D. Online ice crystal size measurements by the focused beam reflectance method (FBRM) during sorbet freezing. Procedia Food Sci. 2011, 1, 1256–1264. [Google Scholar] [CrossRef]
- Kamińska-Dwórznicka, A.; Matusiak, M.; Samborska, K.; Witrowa-Rajchert, D.; Gondek, E.; Jakubczyk, E.; Antczak, A. The influence of kappa carrageenan and its hydrolysates on the recrystallization process in sorbet. J. Food Eng. 2015, 167, 162–165. [Google Scholar] [CrossRef]
- Lomolino, G.; Zannoni, S.; Zabara, A.; Da Lio, M.; De Iseppi, A. Ice recrystallisation and melting in ice cream with different proteins levels and subjected to thermal fluctuation. Int. Dairy J. 2020, 100, 104557. [Google Scholar] [CrossRef]
- Regand, A.; Goff, H.D. Structure and ice recrystallization in frozen stabilized ice cream model systems. Food Hydrocoll. 2003, 17, 95–102. [Google Scholar] [CrossRef]
- Gruneberg, A.K.; Graham, L.A.; Eves, R.; Agrawal, P.; Oleschuk, R.D.; Davies, P.L. Ice recrystallization inhibition activity varies with ice-binding protein type and does not correlate with thermal hysteresis. Cryobiology 2021, 99, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Kaleda, A.; Tsanev, R.; Klesment, T.; Vilu, R.; Laos, K. Ice cream structure modification by ice-binding proteins. Food Chem. 2018, 246, 164–171. [Google Scholar] [CrossRef]
- Keim, S.; Hinrichs, J. Influence of stabilizing bonds on the texture properties of high-pressure-induced whey protein gels. Int. Dairy J. 2004, 14, 355–363. [Google Scholar] [CrossRef]
- O’Chiu, E.; Vardhanabhuti, B. Utilizing whey protein isolate and polysaccha-ride complexes to stabilize aerated dairy gels. J. Dairy Sci. 2017, 100, 3404–3412. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sala, G.; Scholten, E. Effect of fat aggregate size and percentage on the melting properties of ice cream. Food Res. Int. 2022, 160, 111709. [Google Scholar] [CrossRef] [PubMed]
- Azari-Anpar, M.; Khomeiri, M.; Daraei Garmakhany, A.; Lotfi-Shirazi, S. Development of camel and cow’s milk, low-fat frozen yoghurt incorporated with Qodume Shahri (Lepidium perfoliatum) and cress seeds (Lepidium sativum) gum: Flow behavior, textural, and sensory attributes’ assessment. Food Sci. Nutr. 2021, 9, 1640–1650. [Google Scholar] [CrossRef]
- Warren, M.M.; Hartel, R.W. Effects of emulsifier, overrun and dasher speed on ice cream microstructure and melting properties. J. Food Sci. 2018, 83, 639–647. [Google Scholar] [CrossRef]
- Wu, B.; Freire, D.O.; Hartel, R.W. The effect of overrun, fat destabilization, and ice cream mix viscosity on entire meltdown behavior. J. Food Sci. 2019, 84, 2562–2571. [Google Scholar] [CrossRef]
- Lindamood, J.B.; Grooms, D.J.; Hansen, P.M.T. Effect of hydrolysis of lac-tose and sucrose on firmness of ice cream. Food Hydrocoll. 1989, 3, 379–388. [Google Scholar] [CrossRef]
- Patel, M.R.; Baer, R.J.; Acharya, M.R. Increasing the protein content of ice cream. J. Dairy Sci. 2006, 89, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Alfaifi, M.S.; Stathopoulos, C.E. Effect of egg yolk substitution by sweet whey protein isolate on texture, stability and colour of Gelato-style vanilla ice cream. Int. J. Dairy Technol. 2010, 63, 593–598. [Google Scholar] [CrossRef]
- Danesh, E.; Goudarzi, M.; Jooyandeh, H. Effect of whey protein addition and transglutaminase treatment on the physical and sensory properties of reduced-fat ice cream. J. Dairy Sci. 2017, 100, 5206–5211. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, T.C.; de Oliveira, L.I.G.; de Souza, R.C.; Magnani, M. Probiotic ice cream: A literature overview of the technological and sensory aspects and health properties. Int. J. Dairy Technol. 2022, 75, 59–76. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Hussain, M.; Ismail, Z.; Siddeeg, A.; Ammar, A.F.; Aljobair, M.O. Influence of encapsulation on the survival of probiotics in food matrix under simulated stress conditions. Saudi J. Biol. Sci. 2022, 29, 103394. [Google Scholar] [CrossRef]
- Burgain, J.; Scher, J.; Lebeer, S.; Vanderleyden, J.; Cailliez-Grimal, C.; Corgneau, M.; Francius, G.; Gaiani, C. Significance of bacterial surface molecules interactions with milk proteins to enhance microencapsulation of Lactobacillus rhamnosus GG. Food Hydrocoll. 2014, 41, 60–70. [Google Scholar] [CrossRef]
- Heidebach, T.; Först, P.; Kulozik, U. Microencapsulation of probiotic cells for food applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 291–311. [Google Scholar] [CrossRef]
- Tavares, G.M.; Croguennec, T.; Carvalho, A.F.; Bouhallab, S. Milk proteins as encapsulation devices and delivery vehicles: Applications and trends. Trends Food Sci. Technol. 2014, 37, 5–20. [Google Scholar] [CrossRef]
| Ingredients, % | Ice Cream Samples | ||||
|---|---|---|---|---|---|
| NHC | NH3% | HC | H3% | H5% | |
| Non-hydrolyzed concentrate of demineralized whey | 75.0 | 75.0 | – | – | – |
| Hydrolyzed concentrate of demineralized whey | – | – | 75.0 | 75.0 | 75.0 |
| White sugar | 11.0 | 11.0 | 9.0 | 9.0 | 9.0 |
| Whey protein isolate 90% | – | 3.0 | – | 3.0 | 5.0 |
| Stabilization system | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Activated starter | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Vanilla | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Water | 10.3 | 7.3 | 12.3 | 9.3 | 7.3 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Sample | Total Solids, % | Protein, % | Fat, % | Carbohydrates, % | Lactose, % | The Degree of Lactose Hydrolysis, % | Level of Protein Supply, % | Ice Cream Category/ Nutritional Status |
|---|---|---|---|---|---|---|---|---|
| Whey concentrates | ||||||||
| NHCDW | 39.92 a ± 0.30 | 4.45 a ± 0.12 | 0.43 b ± 0.01 | 30.71 a ± 0.68 | 30.50 b ± 1.19 | – | – | – |
| HCDW | 40.01 a ± 0.84 | 4.41 a ± 0.55 | 0.40 a ± 0.01 | 30.61 a ± 0.51 | 1.29 a ± 0.04 | 98.71 a ± 0.04 | – | – |
| Whey ice cream | ||||||||
| NHC | 41.61 ab ± 1.24 | 3.31 a ± 0.11 | 0.74 abc ± 0.02 | 33.05 a ± 1.52 | 22.90 b ± 0.53 | – | 8.92 a ± 0.52 | premium |
| NH3% | 44.55 b ± 0.87 | 6.10 b ± 0.25 | 0.72 a ± 0.01 | 33.46 a ± 0.79 | 22.87 b ± 0.12 | – | 15.11 b ± 0.35 | super premium, enriched with protein |
| HC | 39.58 a ± 1.53 | 3.30 a ± 0.14 | 0.72 a ± 0.03 | 33.10 a ± 0.65 | 0.97 a ± 0.01 | 99.03 a ± 0.01 | 8.89 a ± 0.27 | super premium |
| H3% | 42.54 ab ± 1.14 | 6.02 b ± 0.11 | 0.78 bc ± 0.01 | 33.28 a ± 0.47 | 0.98 a ± 0.03 | 99.02 a ± 0.03 | 15.02 b ± 0.19 | super premium, enriched with protein |
| H5% | 44.63 b ± 1.01 | 7.84 c ± 0.05 | 0.79 b ± 0.01 | 33.53 a ± 0.34 | 0.98 a ± 0.01 | 99.02 a ± 0.01 | 18.59 c ± 0.13 | super premium, enriched with protein |
| Indicator | Sample | |||||
|---|---|---|---|---|---|---|
| NHC | NH3% | HC | H3% | H5% | ||
| Foam overrun, % | 5 | 144.58 a ± 1.67 | 158.74 c ± 2.10 | 150.08 b ± 1.5 | 203.33 d ± 1.7 | 192.14 e ± 2.0 |
| 10 | 177.51 a ± 1.59 | 184.96 a ± 1.47 | 201.42 b ± 4.2 | 236.78 d ± 2.5 | 220.43 c ± 3.2 | |
| 15 | 164.23 a ± 2.54 | 179.7 b ± 1.89 | 187.54 c ± 1.6 | 246.75 e ± 4.7 | 221.59 d ± 1.2 | |
| Foam resistance, хв | 5 | 31.57 a ± 0.87 | 33.89 b ± 0.27 | 44.82 c ± 0.67 | 48.41 d ± 0.74 | 45.90 c ± 0.81 |
| 10 | 32.46 a ± 0.54 | 39.94 b ± 0.71 | 49.55 c ± 0.56 | 58.45 d ± 0.25 | 57.63 d ± 0.90 | |
| 15 | 32.11 a ± 0.68 | 38.43 b ± 0.44 | 48.13 c ± 1.36 | 61.94 e ± 0.49 | 59.33 d ± 1.01 | |
| Viscosity, Mpa × s | 254.42 b ± 1.86 | 384.55 d ± 1.12 | 228.61 a ± 2.54 | 339.47 c ± 1.07 | 511.05 e ± 1.24 | |
| Cryoscopic temperature, °C | −2.88 a ± 0.01 | −2.95 a ± 0.08 | −2.39 d ± 0.01 | −2.55 c ± 0.02 | −2.71 b ± 0.05 | |
| Water activity, units | 0.955 e ± 0.02 | 0.941 d ± 0.03 | 0.912 c ± 0.01 | 0.905 b ± 0.01 | 0.896 a ± 0.02 | |
| Sample | Color Parameters | ||||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h° | |||
| NHC | mix | 72.80 a ± 1.08 | −4.38 b ± 0.12 | 20.50 a ± 0.88 | 20.96 a ± 0.66 | 102.07 a ± 2.28 | |
| ice cream | 1st day | 81.66 a ± 2.54 | −4.09 b ± 0.04 | 20.16 a ± 0.50 | 14.73 a ± 0.05 | 101.50 a ± 2.05 | |
| 14th day | 76.81 a ± 1.27 | −4.11 b ± 0.17 | 21.84 ab ± 0.17 | 13.52 a ± 0.64 | 103.17 b ± 1.84 | ||
| NH3% | mix | 69.56 a ± 1.59 | −3.61 a ± 0.09 | 20.18 a ± 0.01 | 20.37 a ± 0.95 | 100.24 a ± 1.10 | |
| ice cream | 1st day | 82.78 a ± 2.22 | −2.98 a ± 0.13 | 21.03 a ± 0.67 | 21.26 b ± 0.54 | 98.08 a ± 2.89 | |
| 14th day | 76.07 a ± 2.18 | −3.05 a ± 0.01 | 21.56 ab ± 0.94 | 20.90 b ± 0.96 | 98.99 a ± 2.17 | ||
| HC | mix | 79.03 a ± 2.01 | −3.94 a ± 0.11 | 24.07 a ± 0.58 | 24.41 a ± 1.14 | 99.19 a ± 2.74 | |
| ice cream | 1st day | 82.31 a ± 0.87 | −2.42 a ± 0.10 | 14.55 b ± 0.69 | 20.57 a ± 0.71 | 99.11 a ± 2.54 | |
| 14th day | 80.98 a ± 1.85 | −2.55 a ± 0.02 | 14.69 a ± 0.54 | 18.09 b ± 0.05 | 99.54 a ± 1.71 | ||
| H3% | mix | 81.36 a ± 1.54 | −3.17 a ± 0.13 | 24.43 a ± 0.47 | 24.66 a ± 1.19 | 97.46 a ± 0.86 | |
| ice cream | 1st day | 83.25 a ± 1.73 | −2.84 a ± 0.08 | 18.84 a ± 0.54 | 18.57 a ± 0.55 | 98.23 a ± 3.57 | |
| 14th day | 78.56 a ± 1.25 | −2.91 a ± 0.11 | 19.63 ab ± 0.07 | 18.08 b ± 0.76 | 99.07 a ± 2.60 | ||
| H5% | mix | 77.20 a ± 1.89 | −2.71 a ± 0.01 | 23.39 a ± 1.05 | 23.08 a ± 1.00 | 96.54 a ± 0.97 | |
| ice cream | 1st day | 80.01 a ± 2.12 | −3.30 a ± 0.13 | 21.58 a ± 0.68 | 21.84 a ± 0.58 | 98.33 a ± 1.18 | |
| 14th day | 77.88 a ± 2.57 | −3.35 a ± 0.05 | 22.42 b ± 0.84 | 20.05 b ± 1.13 | 98.74 a ± 1.36 | ||
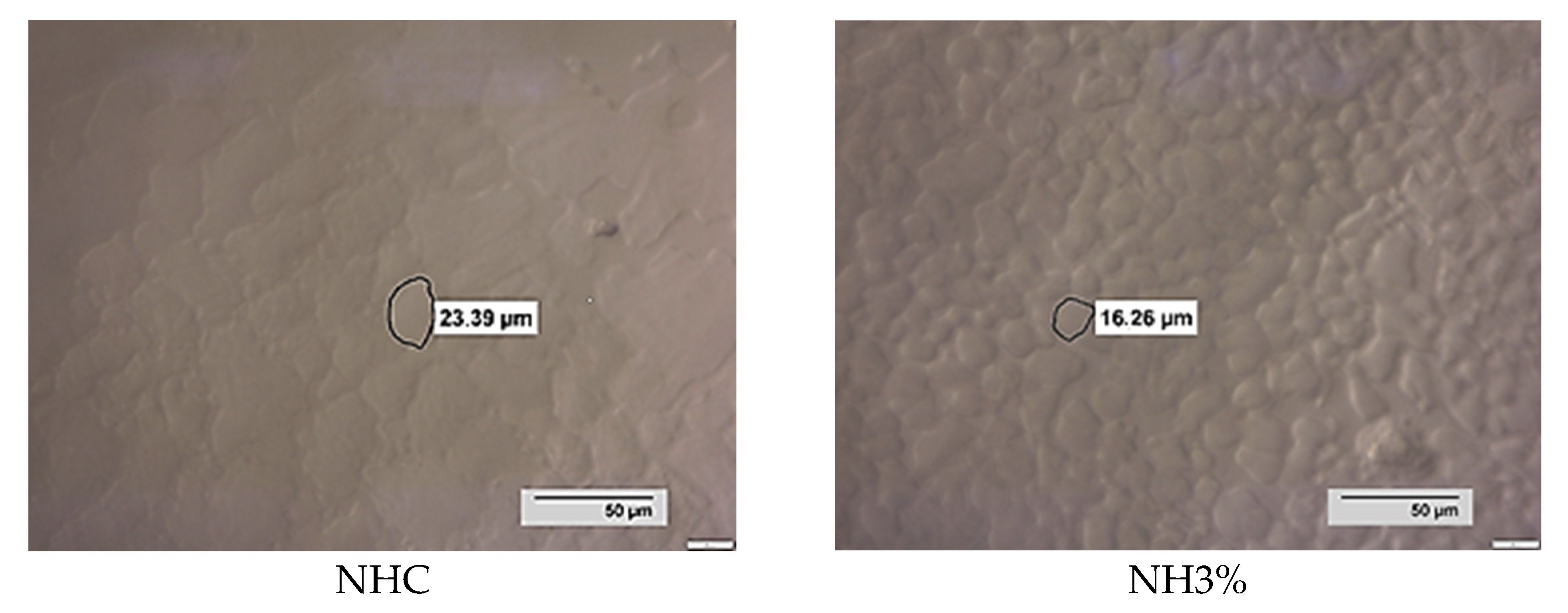
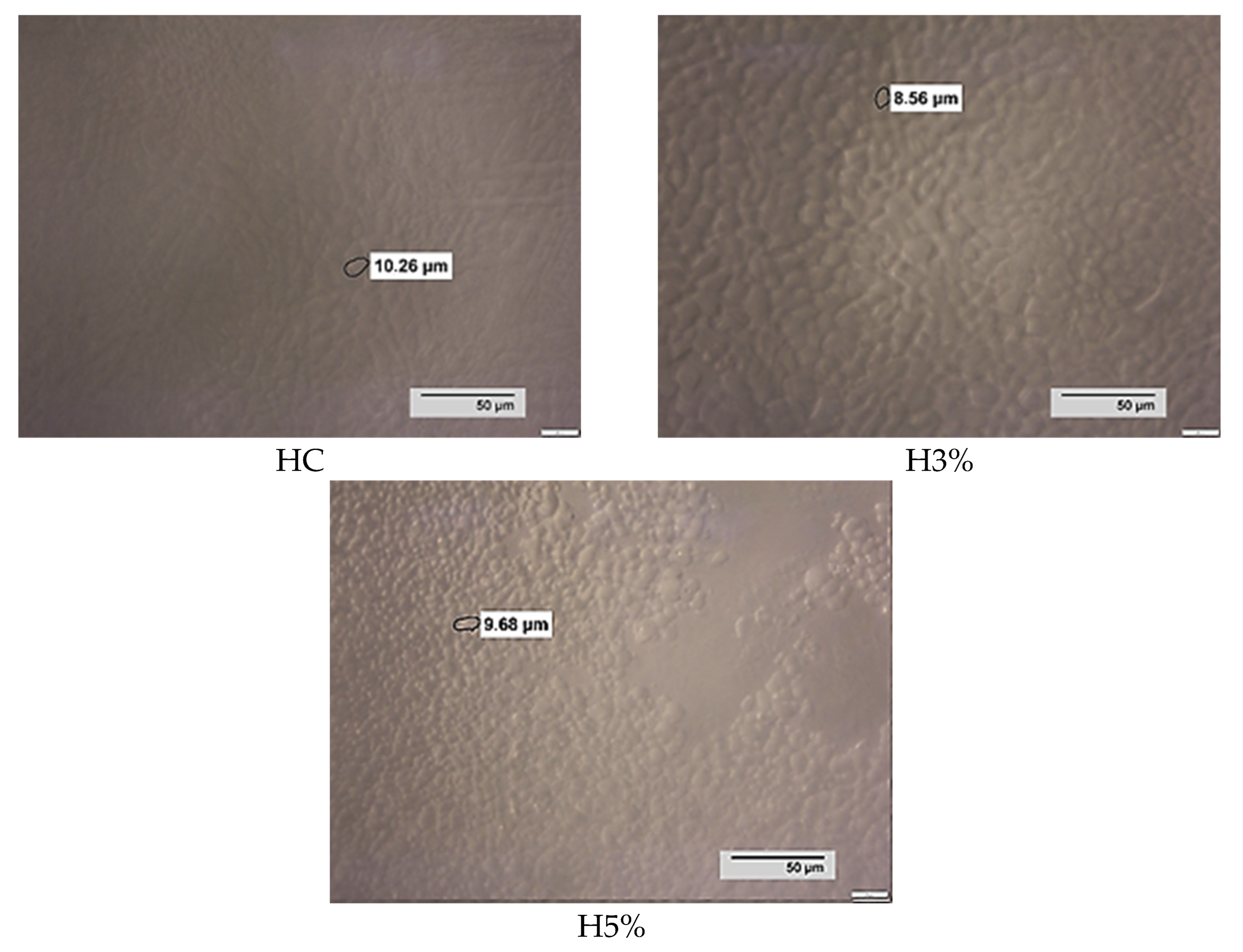
| Sample | Minimum Diameter of Ice Crystals (μm) | Maximum Diameter of Ice Crystals (μm) | The Average Value of the Diameter of Ice Crystals (μm) |
|---|---|---|---|
| NHC | 7.55 d ± 0.12 | 44.36 d ± 2.03 | 25.96 c ± 1.04 |
| NH3% | 6.85 c ± 0.09 | 26.00 c ± 0.52 | 16.43 b ± 1.17 |
| HC | 4.68 a ± 0.10 | 22.68 b ± 0.16 | 13.68 a ± 0.02 |
| H3% | 5.41 b ± 0.24 | 19.04 a ± 0.12 | 12.23 a ± 0.18 |
| H5% | 5.18 a ± 0.02 | 21.17 ab ± 0.95 | 13.18 a ± 0.56 |
| Indicator | Ice Cream Samples | ||||||
|---|---|---|---|---|---|---|---|
| NHC | NH3% | HC | H3% | H5% | |||
| Overrun, % | 71.84 b ± 1.45 | 59.3 a ± 0.86 | 79.18 c ± 2.55 | 76.55 bc ± 3.08 | 71.98 b ± 2.72 | ||
| pH | 1st day | 5.25 a ± 0.01 | 5.22 a ± 0.05 | 5.23 a ± 0.08 | 5.19 a ± 0.03 | 5.17 a ± 0.01 | |
| 14th day | 5.20 b ± 0.10 | 5.13 ab ± 0.04 | 5.12 ab ± 0.01 | 5.09 ab ± 0.01 | 5.05 a ± 0.04 | ||
| Resistance to melting | 1 drop | 1st day | 29.81 b ± 0.53 | 34.58 c ± 1.04 | 24.85 a ± 0.95 | 26.07 a ± 1.17 | 29.11 b ± 1.50 |
| 14th day | 30.11 bc ± 1.08 | 35.87 d ± 1.55 | 25.04 a ± 0.37 | 28.54 b ± 1.20 | 32.42 c ± 1.16 | ||
| 10 cm3 | 1st day | 44.08 b ± 1.62 | 49.76 c ± 1.89 | 33.69 a ± 1.36 | 36.25 a ± 1.24 | 40.67 b ± 1.33 | |
| 14 th day | 46.11 c ± 1.91 | 52.32 d ± 1.01 | 34.78 a ± 0.88 | 39.82 b ± 1.45 | 43.83 c ± 1.57 | ||
| Hardness, g/cm3 | 1st day | 1734.88 b ± 44.37 | 2512.46 d ± 50.27 | 1580.27 a ± 41.86 | 1941.43 c ± 51.87 | 2409.74 d ± 47.08 | |
| 14th day | 1808.51 b ± 30.27 | 2567.09 d ± 14.90 | 1602.82 a ± 55.68 | 2154.37 c ± 44.20 | 2618.74 d ± 36.17 | ||
| Sample | Storage Time (Days) | Counting (log CFU/g) | |||
|---|---|---|---|---|---|
| Coliform | LA-5® | Yeasts | Fungi | ||
| NHC | 1 | ND | 6.6 | 5.1 | 6.0 |
| 14 | ND | 6.6 | 5.5 | 6.2 | |
| NH3% | 1 | ND | 6.3 | 5.4 | ND |
| 14 | ND | 6.2 | 5.7 | 5.1 | |
| HC | 1 | ND | >7.7 | 5.3 | 5.3 |
| 14 | ND | >7.7 | 5.5 | 5.5 | |
| H3% | 1 | ND | 7.6 | 5.0 | ND |
| 14 | ND | 7.6 | 5.3 | ND | |
| H5% | 1 | ND | 7.4 | 5.0 | ND |
| 14 | ND | 7.3 | 5.2 | ND | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mykhalevych, A.; Buniowska-Olejnik, M.; Polishchuk, G.; Puchalski, C.; Kamińska-Dwórznicka, A.; Berthold-Pluta, A. The Influence of Whey Protein Isolate on the Quality Indicators of Acidophilic Ice Cream Based on Liquid Concentrates of Demineralized Whey. Foods 2024, 13, 170. https://doi.org/10.3390/foods13010170
Mykhalevych A, Buniowska-Olejnik M, Polishchuk G, Puchalski C, Kamińska-Dwórznicka A, Berthold-Pluta A. The Influence of Whey Protein Isolate on the Quality Indicators of Acidophilic Ice Cream Based on Liquid Concentrates of Demineralized Whey. Foods. 2024; 13(1):170. https://doi.org/10.3390/foods13010170
Chicago/Turabian StyleMykhalevych, Artur, Magdalena Buniowska-Olejnik, Galyna Polishchuk, Czesław Puchalski, Anna Kamińska-Dwórznicka, and Anna Berthold-Pluta. 2024. "The Influence of Whey Protein Isolate on the Quality Indicators of Acidophilic Ice Cream Based on Liquid Concentrates of Demineralized Whey" Foods 13, no. 1: 170. https://doi.org/10.3390/foods13010170
APA StyleMykhalevych, A., Buniowska-Olejnik, M., Polishchuk, G., Puchalski, C., Kamińska-Dwórznicka, A., & Berthold-Pluta, A. (2024). The Influence of Whey Protein Isolate on the Quality Indicators of Acidophilic Ice Cream Based on Liquid Concentrates of Demineralized Whey. Foods, 13(1), 170. https://doi.org/10.3390/foods13010170






