Stabilization of Rice Bran: A Review
Abstract
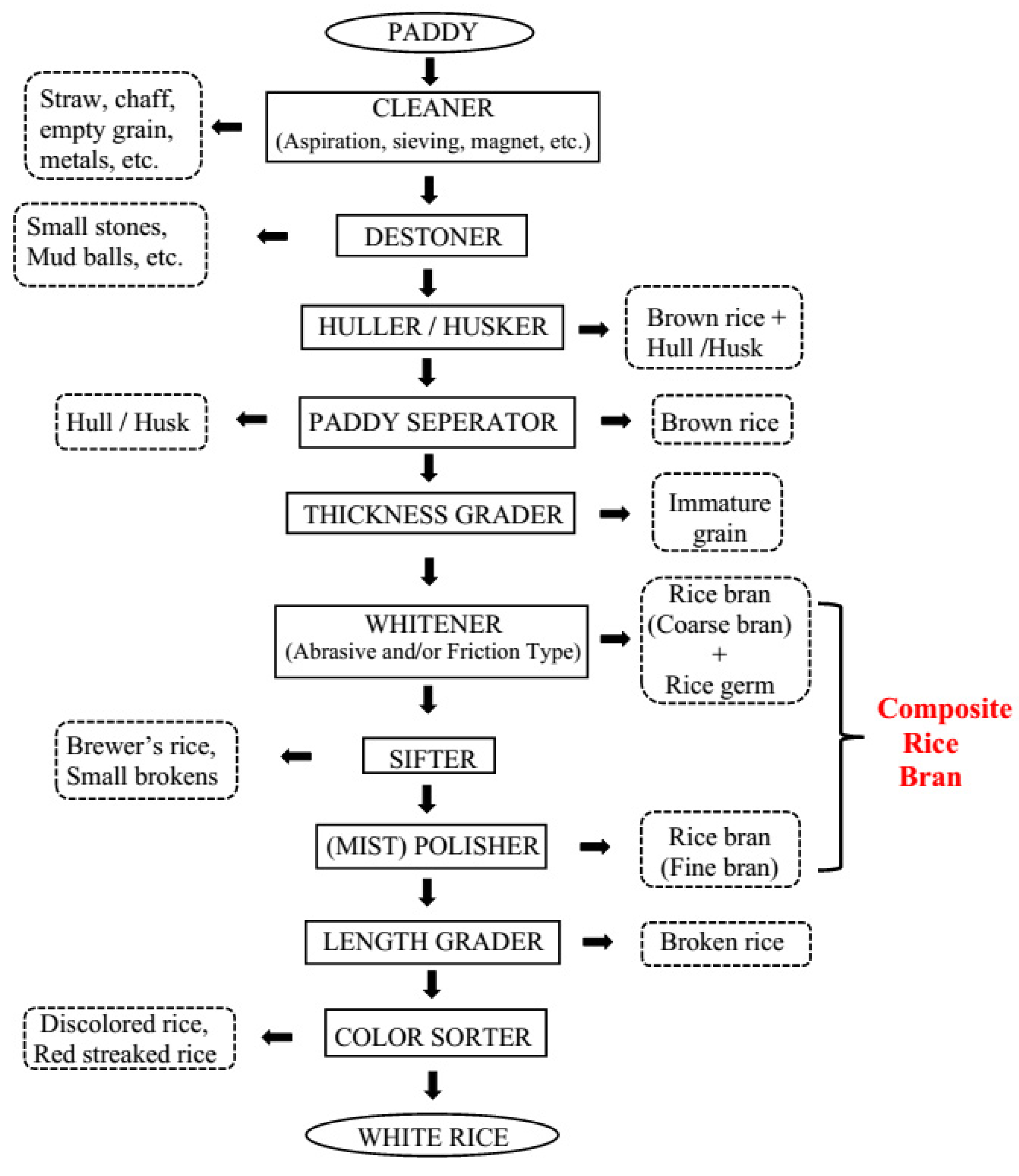
1. Introduction
2. Rice Bran Stabilization Methods
| Method and Conditions of Stabilization | Measure of Stabilization | Effect of Stabilization upon Storage | Co-Effect of Stabilization | Reference |
|---|---|---|---|---|
| Hot air drying at 110 °C for 2 h, Solar drying (8 h per day, max 42 °C), Steaming at 100 °C for 30 min | FFA | FFA levels of hot-air-dried, solar-dried, and steamed-RB were 9%, 39%, and 6%, respectively, while the FFA content of unstabilized RB was 78% at the end of 50 days. | Steaming showed the best results, with only 3% reduction in oil yield after 50 days in comparison with 89% oil reduction forunstabilized bran. | [49] |
| Extrusion at 125–130 °C for 30 s and held in the auger at 97–99 °C for 3 min | FFA | FFA levels of extruded rice bran increased from 2.8% to 3.2% and 3.3% in vacuum packs and zipper-top bags, respectively, at the end of 8 weeks when stored at 4–5 °C. | Water absorption capacity increased while fat absorption capacity decreased after the treatment compared with untreated raw bran. Emulsification and foaming capacities of extruded brans were significantly lower than that of raw bran. | [9] |
| Extrusion with a co-rotating twin-screw extruder (screw speed 140 rpm) at 130 °C for 20 s | Acid value | No significant change in the acid value of extruded RB for at least 20 days of storage at 25 °C. | Extrusion reduced the extractability of phytic acid. | [50] |
| Dry heat stabilization at 120 °C for 30 min Extrusion under the following conditions: Water flow rate: 0.000038 m3/s, feed rate: 27 kg/h, steam supply: 275.80 kPa, die opening: 0.0078 m, temperature: 135–140 °C. Extrudates were air dried at 50 °C for 24 h and then ground | FFA | FFA content of raw bran increased from 4.05% to 64.60%, while FFA content of dry-heated RB increased from 3.66% to 9.15%. Variation in FFA content (from 3.85% to 4.10%) of extruded bran during storage at ambient temperature in polyethylene bags for 60 days was insignificant (p > 0.05). | Slight increase in crude fat content, no significant change in protein content and calorific value, reducing, non-reducing, and total sugar contents, significant loss of lysine bioavailability, significant reduction in phytic acid content, significant increase in bulk density, water absorption, damaged starch content, significant decrease in fat absorption, protein solubility, and darker color were observed in stabilized brans compared with raw bran. | [51] |
| Extrusion (at 135 °C, 6% moisture addition, 5 s) Roasting (RB heated to 70, 90, 100, and 105 °C in a steam jacket roaster with four chambers) Pelleting (RB was conditioned with steam to increase the temperature to 90 °C, passed through a die of 5 mm, and cooled) Antioxidant addition (at 125, 250, and 375 ppm levels) | FFA Peroxide value Iodine value | Raw and pelleted RB behaved similarly in terms of storage stability. Addition of antioxidant to rice bran was not effective for stabilizing FFA, peroxide, and iodine values at any level. Extrusion was partly effective, resulting in 49.5% FFA as an average over 345 days of storage under ambient conditions. | [52] | |
| Extrusion with pH modification (addition of HCI or Ca(OH)2 at the levels of 1, 5, and 10%; extrusion temperature 110–140 °C; feed moisture 20, 30, and 40% | Spectrophotometric determination of FFA and copper acetate complex | 10% of HCI addition provided the lowest FFA increase at the end of 98 days of storage at room temperature (25 ± 3 °C). | [53] | |
| Extrusion (four zones: 70 °C, 90 °C, 110 °C and 130 °C, with screw speed set at 300 rpm) Microwave heating (800 W, 75 s) Dry heating (130 °C, 120 min) Steaming (30 min) High-pressure steam (121 °C, 0.1 MPa, 15 min) | Lipase activity Lipoxygenase activity Acid value Peroxide value | After 28 days of accelerated storage at 37 °C, the acid value of untreated RB was about 6 mg KOH/100 g, while the acid values of stabilized samples were around 1.7 KOH/100 g, except for atmospheric steam-treated RB. | High-pressure steam-treated, steam-treated, and extruded RB samples were more suitable for long-term storage and better maintained the stability of flavor. | [54] |
| Microwave heating at 850 W for 3 min (moisture content was adjusted to 21% before the treatment) | FFA | No significant change in FFA content of MW-treated RB stored at 4–5 °C at the end of 16 weeks. | No dramatic change in proximate composition and fatty acid composition as a result of MW stabilization. | [55,56] |
| Microwave heating at 550 W, 2450 MHz for 3 min (moisture content was adjusted to 21% before the treatment). The temperature of the bran was 107 °C after the process. | FFA | FFA content of MW-treated RB increased from 3.2% to 3.9% in both vacuum and zipper-top packs at the end of 8 weeks when stored at 4–5 °C. | Fat absorption, emulsification, and foaming capacities of MW-treated RB were significantly lower than that of raw bran. However, MW treatment increased water absorption capacity. | [9] |
| Microwave heating at 700 W, 2450 MHz for 1, 3, and 5 min (moisture content was adjusted to 21% before the treatment). MW power densities were 2, 4, and 6 W/g | FFA Peroxide value | FFA content of RB treated with MW at a power density of 6 W/g for 5 min was 1.12%, while FFA of untreated control was 58.5% at the end of 28 days of storage at ambient temperature. | Protein content and oil yield significantly decreased under the noted effective MW stabilization condition. | [57] |
| Microwave heating Flowing MW drum heater with a max power of 6 kW (6 magnetrons × 1000 W, 2450 MHz) | Lipase activity Lipoxygenase activity FFA Peroxide value | Residual lipase activity was 13.21 U/kg for untreated control and only 1.25 U/kg for MW-treated RB at 5 kW for 13 min. Lipoxygenase activity was completely destroyed after 7 min of MW processing. |
No significant decrease in fatty acid, tocopherols, and γ-oryzanol content of bran treated under optimum conditions (4 kW, 10 min). | [58] |
| Microwave heating at 850, 925, and 1000 W for 3, 4.5, and 6 min | FFA Acid value Peroxide value | Stabilization was investigated in 3 RB milling fractions and composite RB. Stabilization at 925 W for 3 min was recommended as the suitable condition for stabilization of RB milling fractions. | – | [59] |
| Infrared heating Short-wave IR radiation at 200, 300, 400, 500, 600, 700, 800, and 900 W for 1, 2, 3, 4, 5.2, 6.3, 7.2, 8.3, and 10 min | FFA | No significant increase was observed in FFA content of RB treated at IR powers of 600 W (for 4 and 5 min) and 700 W (for 2 and 3 min), while FFA content of raw RB increased from 4.32% to 43.08% at the end of 6 months at 25 °C. | The effect of IR stabilization was insignificant on γ-oryzanol content and fatty acid composition of RB. However, a significant decrease in tocopherol content of RB of up to 50% was determined. | [60] |
| Infrared heating Medium wave IR radiation at 500, 600, and 700 W IR power for 3.0, 5.5, and 7.0 min | FFA | Stabilization was investigated in 3 RB milling fractions. Stabilization at 700 W IR power for 7.0 min provided 90 days of shelf life without a notable change in FFA content of RB fraction obtained from the first whitening step. | Total tocopherol and γ-oryzanol contents of stabilized RB fractions were higher than in their crude counterparts. | [61] |
| Infrared heating (Simultaneous drying and stabilization) RB was heated for 55s to reach a surface temperature of 60 °C during each drying pass and tempered in an incubator set at 60 °C for various durations (1, 2, 3, 4, and 5 h). | FFA | It took 7 days for the FFA level of control RB to reach 10%. This period has been extended to 38 days for IR-treated RB. | IR heating at the employed conditions did not have a negative effect on milling quality. | [62] |
| Infrared heating with a max power of 2400 W, unspecified radiation intensity at 100, 120, and 140 °C for 5, 10, 15, and 20 min. | FFA Peroxide value | FFA content of raw RB increased from 4.4% to 62.8% after 6 months of storage at 25–30 °C, while RB treated with IR at 140 °C for 15 and 20 min maintained FFA content at around 7%. | IR stabilization at 140 °C for 15 min did not cause a significant decrease in γ-oryzanol content and fatty acid composition, but significantly decreased E vitamins. | [63] |
| Infrared heating with a laboratory-scale ceramic infrared drying device until the surface temperature of the rice bran reached 85 °C. | Lipase activity Lipoxygenase activity Peroxidase activity FFA Peroxide value | FFA content of untreated RB increased from 5.41% to 49.87%, and from 5.21% to 14.11% for IR-treated RB at the end of 20 days of storage at 20 °C. Lipase, lipoxygenase, and peroxidase activities in IR-treated samples were significantly higher at the end of the storage. | The contents of palmitic acid (C16:0), oleic acid (C18:1), and linoleic acid (C18:2) were well maintained by IR treatment during the whole storage period. The aroma of fresh RB was preserved. | [64] |
| Infrared heating either with medium or shortwave IR emitters at 700 W for 3 min | FFA | Hydrolytic lipid degradation occurred more likely in samples stored in bran form; however, samples stored in oil form were more prone to oxidative degradation. Medium-wave IR radiation was more effective in terms of retarding FFA increase compared with short-wave IR radiation. | Peroxide values of either raw or IR stabilized samples were <10 meq/kg at the end of 6 months of storage at 25 °C. Conjugated dienoic acids and p-anisidine values increased in all samples during storage. Storage in oil form resulted in a higher loss of total tocopherol and γ-oryzanol compared with storage in bran form. | [43] |
| Ohmic heating using an alternating current of 1 or 60 Hz and an electric field strength of 100 V/cm. | FFA | FFA content of RB (moisture content adjusted to 21%) stabilized with ohmic heating at 60 Hz increased from 3.25% to 5.47%, while FFA content of raw RB increased from 3.96% to 18.03% when stored in Ziploc bags at 4 °C for 6 weeks. The moisture content of the sample had a very decisive effect. | Increase in rice bran oil extraction yield. | [65] |
| Ohmic heating Using an alternating current of 50 Hz and an electric field strength of 75, 150, and 225 V.cm–1. Moisture content of RB was adjusted to 20, 30, and 40%. | FFA Lipase activity | Lower FFA levels and lipase activity were observed in ohmically treated RB compared with raw RB stored at 4 °C for 21 days. The moisture content of the sample had a very decisive effect. | A very slight increase was observed in total phenolic, α-tocopherol, and γ-oryzanol levels of ohmic-heated RB compared with raw RB. | [66] |
| Ohmic heating at 20, 30, and 40% initial moisture content, 132, 150, 168, 189, 216 V, and 50 Hz. Electrical field strengths were 44, 50, 56, 63, and 72 V/cm. | FFA Peroxide value | FFA of ohmically heated bran was 4.77% after 75 days of storage, whereas it was 41.84% for raw RB. | The peroxide value of ohmically heated samples after 75 days of storage was 4.7 meq/kg. | [67] |
| Hot air-assisted radio frequency (HA-RF) heating using a 6 kW, 27.12 MHz pilot-scale free-running oscillator RF system combined with a hot air oven | FFA Peroxide value Lipase activity Lipoxygenase activity | RB treated by HA-RF heating to 100 °C with 15 min holding can be stored at 35 °C and remain below acceptable thresholds for a period of 60 days without adverse effects on product quality. | Optimum HA-RF heating treatment led to a significant increase in tocopherol content, but had no significant effect on γ-oryzanol. | [68] |
| Radiofrequency heating at 5 kW, 40.68 MHz for 2 min | FFA Acid value Peroxide value Lipase activity | Lipase activity retention was close to zero after 2 min of RF heating. FFA content of untreated bran stored at 37 °C was almost 4-fold higher than that of RF-treated bran after 8 weeks. Peroxide values of either raw or processed brans were below 10 meq O2/kg for 8 weeks and at all storage temperatures (4, 25, 37 °C). | No significant difference in total phenolic and flavonoid contents, DPPH scavenging activity, reducing power, and color between the untreated control and radio frequency-treated RB (p > 0.05) | [69] |
2.1. Extrusion
2.2. Microwave Heating
2.3. Dry or Moist Heat Treatments
2.4. Infrared Heating
2.5. Ohmic Heating
2.6. Radio Frequency Heating
2.7. Irradiation
2.8. Other Stabilization Approaches
3. Stabilization of Individual Rice Bran Fractions
4. Stabilization of Other Rice Milling by–Products Comprising Rice Bran
5. Factors Affecting Hydrolytic Stability of Unprocessed Rice Bran
6. Nutritive Value of Rice Bran and the Effect of Stabilization
| Component | Average Range | Reference |
|---|---|---|
| Crude fat (%) | 18–23 | [50,68,73,98,99,101] |
| Crude protein (%) | 11–16 | [50,51,53,68,73,98,99] |
| Ash (%) | 8–12 | [50,68,73,98,99] |
| Soluble dietary fiber (%) | 2–5 | [50,73,98,99] |
| Insoluble dietary fiber (%) | 20–27 | |
| Total dietary fiber (%) | 22–32 | |
| γ-oryzanol (g/kg) | 0.5–5.5 | [46,50,60,82,95] |
| Vitamins | ||
| Total Tocopherols (mg/kg) | 100–150 | [60,82,101] |
| α-T (mg/kg) | 50–130 | |
| β-T (mg/kg) | 2–10 | |
| γ-T (mg/kg) | 10–50 | |
| δ-T (mg/kg) | 0–2 | |
| Total Tocotrienols (mg/kg) | 130–170 | [82,101] |
| α-T3 (mg/kg) | 38 | |
| β-T3 (mg/kg) | – | |
| γ-T3 (mg/kg) | 120–140 | |
| δ-T3 (mg/kg) | 0–10 | |
| Vitamin B1 (Thiamin) (mg/kg) | 12–40 | [5,50,99,102] |
| Vitamin B2 (Riboflavin) (mg/kg) | 1–4 | [5,50,99,102] |
| Vitamin B3 (Niacin) (mg/kg) | 300–800 | [5,50,99,102] |
| Vitamin B5 (Pantothenic acid) (mg/kg) | 74 | [102] |
| Vitamin B6 (mg/kg) (Pyridoxamine, pyridoxal, pyridoxine) | 20–40 | [99,102] |
| Minerals | ||
| Ca (mg/kg) | 300–1200 | [5,16] |
| K (mg/kg) | 5992 | [16] |
| Fe (mg/kg) | 86–430 | [5,16] |
| Zn (mg/kg) | 50–250 | [5,16] |
| P (mg/kg) | 6278 | [16] |
7. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kahlon, T.S. Rice bran: Production, composition, functionality and food applications, physiological benefits. In Fiber Ingredients: Food Applications and Health Benefits; Cho, S., Samuel, P., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 305–321. [Google Scholar]
- Basri, M.S.M.; Mustapha, F.; Mazlan, N.; Ishak, M.R. Optimization of rice husk ash based geopolymers coating composite for enhancement in flexural properties and microstructure using response surface methodology. Coatings 2020, 10, 165. [Google Scholar] [CrossRef]
- Rice Knowledge Bank, IRRIa. Available online: http://www.knowledgebank.irri.org/step-by-step-production/postharvest/rice-by-products/rice-husk (accessed on 16 February 2023).
- Mir, S.A.; Shah, M.A.; Bosco, S.J.D.; Sunooj, K.V.; Farooq, S. A review on nutritional properties, shelf life, health aspects, and consumption of brown rice in comparison with white rice. Cereal Chem. 2020, 97, 895–903. [Google Scholar] [CrossRef]
- Saleh, A.S.M.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z.G. Brown rice versus white rice: Nutritional quality, potential health benefits, development of food products, and preservation technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1070–1096. [Google Scholar] [CrossRef] [PubMed]
- Selvam, S.; Masilamani, P.; Umashankar, P.T.; Albert, V.A. Opportunities and challenges in marketing of brown rice. In Brown Rice; Monickavasagan, A., Santhakumar, C., Venkatachalapathy, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 296–305. [Google Scholar]
- Rice Knowledge Bank, IRRIb. Available online: http://www.knowledgebank.irri.org/step-by-step-production/postharvest/milling/milling-systems?tmpl=component&print=1 (accessed on 17 February 2023).
- Saunders, R.M. Rice bran: Composition and potential food uses. Food Rev. Int. 1985, 1, 465–495. [Google Scholar] [CrossRef]
- Malekian, F.; Rao, R.M.; Prinyawiwatkul, W.; Marshall, W.E.; Windhauser, M.; Ahmedna, M. Lipase and lipoxygenase activity, functionality, and nutrient losses in rice bran during storage. Bull. La. Agric. Exp. Stn. 2000, 870, 1–68. [Google Scholar]
- Bhardwaj, K.; Raju, A.; Rajasekharan, R. Identification, purification, and characterization of a thermally stable lipase from rice bran. A new member of the (phospho) lipase family. Plant Physiol. 2001, 127, 1728–1738. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations, FAOSTAT. 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 11 February 2023).
- Espinales, C.; Cuesta, A.; Tapia, J.; Palacios-Ponce, S.; Penas, E.; Martinez-Villaluenga, C.; Espinoza, A.; Caceres, P.J. The effect of stabilized rice bran addition on physicochemical, sensory, and techno-functional properties of bread. Foods 2022, 11, 3328. [Google Scholar] [CrossRef]
- Doan, N.T.T.; Lai, Q.D.; Vo, H.V.; Nguyen, H.D. Influence of adding rice bran on physio-chemical and sensory properties of bread. J. Food Meas. Charact. 2021, 15, 5369–5378. [Google Scholar] [CrossRef]
- Gul, K.; Yousuf, B.; Singh, A.K.; Singh, P.; Wani, A.B. Rice bran: Nutritional values and its emerging potential for development of functional food—A review. Bioact. Carbohydr. Diet. Fibre 2015, 6, 24–30. [Google Scholar] [CrossRef]
- Tuncel, N.B.; Yılmaz, N.; Kocabıyık, H.; Uygur, A. The effect of infrared stabilized rice bran substitution on physicochemical and sensory properties of pan breads: Part I. J. Cereal Sci. 2014, 59, 155–161. [Google Scholar] [CrossRef]
- Tuncel, N.B.; Yılmaz, N.; Kocabıyık, H.; Uygur, A. The effect of infrared stabilized rice bran substitution on B vitamins, minerals and phytic acid content of pan breads: Part II. J. Cereal Sci. 2014, 59, 162–166. [Google Scholar] [CrossRef]
- Yılmaz Tuncel, N.; Kaya, E.; Karaman, M. Rice bran substituted Turkish noodles (Erişte): Textural, sensorial, and nutritional properties. Cereal Chem. 2017, 94, 903–908. [Google Scholar] [CrossRef]
- Yılmaz, N.; Tuncel, N.B.; Kocabıyık, H. The effect of infrared stabilized rice bran substitution on nutritional, sensory, and textural properties of cracker. Eur. Food Res. Technol. 2014, 239, 259–265. [Google Scholar] [CrossRef]
- Ayoub, W.S.; Zahoor, I.; Dar, A.H.; Anjum, N.; Pandiselvam, R.; Farooq, S.; Rusu, A.V.; Rocha, J.M.; Trif, M.; Jeevarathinam, G. Effect of incorporation of wheat bran, rice bran and banana peel powder on the mesostructure and physicochemical characteristics of biscuits. Front. Nutr. 2022, 9, 1016717. [Google Scholar] [CrossRef] [PubMed]
- Akter, D.; Begum, R.; Rahman, M.N.; Talukder, N.; Alam, M.J. Optimization of extraction process parameter for rice bran protein concentrate and its utilization in high protein biscuit formulation. Curr. Res. Nutr. Food Sci. 2020, 8, 596–608. [Google Scholar] [CrossRef]
- Albayrak, B.B.; Tuncel, N.B.; Yılmaz Tuncel, N.; Masatcioglu, M.T. Extrusion cooking of immature rice grain: Under-utilized by-product of rice milling process. J. Food Sci. Technol. 2020, 57, 2905–2915. [Google Scholar] [CrossRef]
- Prakash, J. Rice bran proteins: Properties and food uses. Crit. Rev. Food Sci. Nutr. 1996, 36, 537–552. [Google Scholar] [CrossRef]
- Echeverria, L.; da Silva, C.; Danesi, E.D.G.; Porciuncula, B.D.A.; Barros, B.C.B. Characterization of okara and rice bran and their application as fat substitutes in chicken nugget formulations. LWT—Food Sci. Technol. 2022, 161, 113383. [Google Scholar] [CrossRef]
- Jiang, R.S.; Xiao, Z.G.; Huo, J.J.; Wang, H.G.; Li, H.; Su, S.; Duan, Y.M.; Gao, Y.Z. Effects of rice bran content on plant-based simulated meat: From the aspects of apparent properties and structural characteristics. Food Chem. 2022, 380, 131842. [Google Scholar] [CrossRef]
- Chakraborty, M.; Budhwar, S.; Kumar, S. Potential of milling byproducts for the formulation of health drink and detox tea-substitute. J. Food Meas. Charact. 2022, 16, 3153–3165. [Google Scholar] [CrossRef]
- Tran, K.N.; Gidley, M.J.; Fitzgerald, M. Opportunities and challenges in processing of by-product of rice milling protein as a food ingredient. Cereal Chem. 2017, 94, 369–376. [Google Scholar] [CrossRef]
- Wang, N.; Cui, X.; Duan, Y.; Yang, S.; Wang, P.; Saleh, A.S.M.; Xiao, Z. Potential health benefits and food applications of rice bran protein: Research advances and challenges. Food Rev. Int. 2021, in press. [Google Scholar] [CrossRef]
- Tu, Y.; Zhang, X.X.; Wang, L. Effect of salt treatment on the stabilization of Pickering emulsions prepared with rice bran protein. Food Res. Int. 2023, 166, 112537. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Regenstein, J.M.; Wang, Z.J.; Zhang, H.J.; Zhou, L.Y. Reconstituted rice protein: The raw materials, techniques and challenges. Trends Food Sci. Technol. 2023, 133, 267–276. [Google Scholar] [CrossRef]
- Magnaye, M.J.F.A.; Mopera, L.E.; Flores, F.P. Effect of rice bran protein concentrate as wall material adjunct on selected physicochemical and release properties of microencapsulated beta-carotene. Food Sci. Technol. Int. 2022, 28, 653–662. [Google Scholar] [CrossRef]
- Bonifacino, C.; Palazolo, G.G.; Panizzolo, L.A.; Abirached, C. Study of emulsifying properties of soluble proteins obtained from defatted rice bran concentrate. J. Am. Oil Chem. Soc. 2021, 98, 851–860. [Google Scholar] [CrossRef]
- Pang, M.; Kang, S.M.; Liu, L.; Ma, T.F.; Zheng, Z.; Cao, L.L. Physicochemical properties and cookie-making performance as fat replacer of wax-based rice bran oil oleogels. Gels 2023, 9, 13. [Google Scholar] [CrossRef]
- Wang, Z.H.; Li, S.Z.; Zhang, M.; Yang, H.Y.; Li, G.; Ren, X.; Liang, S. Optimization of oil extraction from rice bran with mixed solvent using response surface methodology. Foods 2022, 11, 3849. [Google Scholar] [CrossRef]
- Chen, H.S.; He, S.D.; Sun, H.J.; Li, Q.Y.; Gao, K.; Miao, X.Y.; Xiang, J.; Wu, X.J.; Gao, L.M.; Zhang, Y. A comparative study on extraction and physicochemical properties of soluble dietary fiber from glutinous rice bran using different methods. Separations 2023, 10, 90. [Google Scholar] [CrossRef]
- Ismail, N.A.; Zhao, J. Effects of ultrasound and steam explosion treatments on the physicochemical properties of rice bran fibre. Pertenika J. Trop. Agric. Sci. 2022, 45, 893–918. [Google Scholar] [CrossRef]
- Wang, L.; Duan, W.; Zhou, S.; Qian, H.F.; Zhang, H.; Qi, X.G. Effect of rice bran fibre on the quality of rice pasta. Int. J. Food Sci. Technol. 2018, 53, 81–87. [Google Scholar] [CrossRef]
- Gu, X.Y.; Du, L.Y.; Meng, Z. Comparative study of natural wax-based W/O emulsion gels: Microstructure and macroscopic properties. Food Res. Int. 2023, 165, 112509. [Google Scholar] [CrossRef] [PubMed]
- Dent, T.; Hallinan, R.; Chitchumroonchokchai, C.; Maleky, F. Rice bran wax structured oleogels and in vitro bioaccessibility of curcumin. J. Am. Oil Chem. Soc. 2022, 99, 299–311. [Google Scholar] [CrossRef]
- Ghosh, S.; Bollinedi, H.; Krishnan, S.G.; Kundu, A.; Singh, A.; Bhowmick, P.K.; Singh, A.; Nagarajan, M.; Vinod, K.K.; Ellur, R.K.; et al. From farm to plate: Spatio-temporal characterization revealed compositional changes and reduced retention of gamma-oryzanol upon processing in rice. Front. Nutr. 2022, 9, 1040362. [Google Scholar] [CrossRef]
- Zaky, A.A.; Chen, Z.; Qin, M.; Wang, M.Z.; Jia, Y.M. Assessment of antioxidant activity, amino acids, phenolic acids and functional attributes in defatted rice bran and rice bran protein concentrate. Prog. Nutr. 2020, 22, e2020069. [Google Scholar]
- Cosmetic Ingredient Review Expert Panel. Amended final report on the safety assessment of Oryza Sativa (rice) bran oil, Oryza Sativa (rice) germ oil, rice bran acid, Oryza Sativa (rice) bran wax, hydrogenated rice bran wax, Oryza Sativa (rice) bran extract, Oryza Sativa (rice) extract, Oryza Sativa (rice) germ powder, Oryza Sativa (rice) starch, Oryza Sativa (rice) bran, hydrolyzed rice bran extract, hydrolyzed rice bran protein, hydrolyzed rice extract, and hydrolyzed rice protein. Int. J. Toxicol. 2006, 2, 91–120. [Google Scholar]
- Tao, J.; Rao, R.; Liuzzo, J. Microwave heating for rice bran stabilization. J. Microw. Power Electromagn. Energy 1993, 28, 156–164. [Google Scholar] [CrossRef]
- Yılmaz Tuncel, N.; Yılmaz Korkmaz, F. Comparison of lipid degradation in raw and infrared stabilized bran and rice bran oil: Matrix effect. J. Food Meas. Charact. 2021, 15, 1057–1067. [Google Scholar] [CrossRef]
- Ju, Y.H.; Vali, S.R. Rice bran oil as a potential resource for biodiesel: A review. J. Sci. Ind. Res. 2005, 64, 866–882. [Google Scholar]
- Saji, N.; Schwarz, L.J.; Santhakumar, A.B.; Blanchard, C.L. Stabilization treatment of rice bran alters phenolic content and antioxidant activity. Cereal Chem. 2020, 97, 281–292. [Google Scholar] [CrossRef]
- Pokkanta, P.; Yuenyong, J.; Mahatheeranont, S.; Jiamyangyuen, S.; Sookwong, P. Microwave treatment of rice bran and its effect on phytochemical content and antioxidant activity. Sci. Rep. 2022, 12, 7708. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.T.; Liu, K.; Hen, S.; Jatoi, M.A.; Sarpong, F. Nutritional composition and volatile compounds stability in dry-heat and extruded stabilised rice bran during storage. Int. J. Food Sci. Technol. 2023, in press. [Google Scholar] [CrossRef]
- Chen, Y.X.; Ma, Y.X.; Dong, L.H.; Jia, X.C.; Liu, L.; Huang, F.; Chi, J.W.; Xiao, J.; Zhang, M.W.; Zhang, R.F. Extrusion and fungal fermentation change the profile and antioxidant activity of free and bound phenolics in rice bran together with the phenolic bioaccessibility. LWT—Food Sci. Technol. 2019, 115, 108461. [Google Scholar] [CrossRef]
- Amarasinghe, B.M.W.P.K.; Kumarasiri, N.C.; Gongodavilage, N.G. Effect of method of stabilization on aqueous extraction of rice bran oil. Food Bioprod. Process. 2009, 87, 108–114. [Google Scholar] [CrossRef]
- Fuh, W.S.; Chiang, B.H. Dephytinisation of rice bran and manufacturing a new food ingredient. J. Sci. Food Agric. 2001, 81, 1419–1425. [Google Scholar] [CrossRef]
- Sharma, H.R.; Chauhan, G.S.; Agrawal, K. Physico-chemical characteristics of rice bran processed by dry heating and extrusion cooking. Int. J. Food Prop. 2004, 7, 603–614. [Google Scholar] [CrossRef]
- Mujahid, A.; Haq, I.; Asif, M.; Gilani, A.H. Effect of various processing techniques and different levels of antioxidant on stability of rice bran during storage. J. Sci. Food Agric. 2005, 85, 847–852. [Google Scholar] [CrossRef]
- Escamillo-Castillo, B.; Varela-Montellano, R.; Sanchez-Tovar, S.A.; Solis-Fuentes, J.A.; Duran-de-Bazua, C. Extrusion deactivation of rice bran enzymes by pH modification. Eur. J. Lipid Sci. Technol. 2005, 107, 871–876. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Wang, Y.; Fan, M.; Wang, L.; Qian, H. Analysis of the aroma volatile compounds in different stabilized rice bran during storage. Food Chem. 2023, 405, 134753. [Google Scholar] [CrossRef]
- Ramezanzadeh, F.M.; Rao, R.M.; Windhause, M.; Prinyawiwatkul, W.; Tulley, R.; Marshall, W.E. Prevention of hydrolytic rancidity in rice bran during storage. J. Agric. Food Chem. 1999, 47, 3050–3052. [Google Scholar] [CrossRef]
- Ramezanzadeh, F.M.; Rao, R.M.; Windhause, M.; Prinyawiwatkul, W.; Marshall, W.E.; Windhauser, M. Effects of microwave heat, packaging, and storage temperature on fatty acid and proximate compositions in rice bran. J. Agric. Food Chem. 2000, 48, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Kar, A.; Mohapatra, D. Stabilization of rice bran using microwave: Process optimization and storage studies. Food Bioprod. Process. 2016, 99, 204–211. [Google Scholar] [CrossRef]
- Li, B.; Zhao, L.; Xu, B.; Deng, B.; Liu, Y.; Dong, Y. Rice bran real-time stabilization technology with flowing microwave radiation: Its impact on rancidity and some bioactive compounds. Qual. Assur. Saf. Crops Foods 2018, 10, 25–34. [Google Scholar] [CrossRef]
- Lavanya, M.N.; Saikiran, K.C.H.; Venkatachalapathy, N. Stabilization of rice bran milling fractions using microwave heating and its effect on storage. J. Food Sci. Technol. 2019, 56, 889–895. [Google Scholar] [CrossRef]
- Yılmaz, N.; Tuncel, N.B.; Kocabıyık, H. Infrared stabilization of rice bran and its effects on γ-oryzanol content, tocopherols and fatty acid composition. J. Sci. Food Agric. 2014, 94, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, N. Middle infrared stabilization of individual rice bran milling fractions. Food Chem. 2016, 190, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Khir, R.; Pan, Z.; Yuan, Q. Simultaneous rough rice drying and rice bran stabilization using infrared radiation heating. LWT—Food Sci. Technol. 2017, 78, 281–288. [Google Scholar] [CrossRef]
- Irakli, M.; Kleisiaris, F.; Mygdalia, A.; Katsantonis, D. Stabilization of rice bran and its effect on bioactive compounds content, antioxidant activity and storage stability during infrared radiation heating. J. Cereal Sci. 2018, 80, 135–142. [Google Scholar] [CrossRef]
- Yan, W.; Liu, Q.; Wang, Y.; Tao, T.; Liu, B.; Liu, J.; Ding, C. Inhibition of lipid and aroma deterioration in rice bran by infrared heating. Food Bioprocess. Technol. 2020, 13, 1677–1687. [Google Scholar] [CrossRef]
- Lakkakula, N.R.; Lima, M.; Walker, T. Rice bran stabilization and rice bran oil extraction using ohmic heating. Biosour. Technol. 2004, 92, 157–161. [Google Scholar] [CrossRef]
- Loypimai, P.; Moonggarm, A.; Chottanom, P. Effects of ohmic heating on lipase activity, bioactive compounds and antioxidant activity of rice bran. Aust. J. Basic Appl. Sci. 2009, 3, 3642–3652. [Google Scholar]
- Dhingra, D.; Chopra, S.; Rai, D.R. Stabilization of raw rice bran using ohmic heating. Agric. Res. 2012, 1, 392–398. [Google Scholar] [CrossRef]
- Ling, B.; Lyng, J.G.; Wang, S. Effects of hot air-assisted radio frequency heating on enzyme inactivation, lipid stability and product quality of rice bran. LWT—Food Sci. Technol. 2018, 91, 453–459. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yen, Y.F.; Chen, S.D. Effects of radio frequency heating on the stability and antioxidant properties of rice bran. Foods 2021, 10, 810. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.M.; Sayre, R.N.; Schultz, W.G.; Fong, R.Y.; Mossman, A.P.; Tribelhorn, R.E.; Saunders, R.M. Rice bran stabilization by extrusion cooking for extraction of edible oil. J. Food Sci. 1985, 50, 361–364. [Google Scholar] [CrossRef]
- Kim, C.J.; Byun, S.M.; Cheigh, H.S.; Kwon, T.W. Optimization of extrusion rice bran stabilization process. J. Food Sci. 1987, 52, 1355. [Google Scholar] [CrossRef]
- Shin, T.S.; Godber, J.S.; Martin, D.E.; Wells, J.H. Hydrolytic stability and changes in E vitamers and oryzanol of extruded rice bran during storage. J. Food Sci. 1997, 62, 704–728. [Google Scholar] [CrossRef]
- Rafe, A.; Sadeghian, A. Stabilization of Tarom and Domesiah cultivars rice bran: Physicochemical, functional and nutritional properties. J. Cereal Sci. 2017, 74, 64–71. [Google Scholar] [CrossRef]
- Abdul-Hamid, A.; Sulaiman, R.R.R.; Osman, A.; Saari, N. Preliminary study of the chemical composition of rice milling fractions stabilized by microwave heating. J. Food Compos. Anal. 2007, 20, 627–637. [Google Scholar] [CrossRef]
- Ertürk, B.; Meral, R. The impact of stabilization on functional, molecular and thermal properties of rice bran. J. Cereal Sci. 2019, 88, 71–78. [Google Scholar] [CrossRef]
- Thanonkaew, A.; Wongyai, S.; McClements, D.J.; Decker, E.A. Effect of stabilization of rice bran by domestic heating on mechanical extraction yield, quality, and antioxidant properties of cold-pressed rice bran oil (Oryza saltiva L.). LWT—Food Sci. Tech. 2012, 48, 231–236. [Google Scholar] [CrossRef]
- Orthoefer, F.T.; Eastman, J. Rice bran and oil. In Rice: Chemistry and Technology; Champagne, E.T., Ed.; Agricultural Research Service, Southern Regional Research Center, U.S. Department of Agriculture: New Orleans, LA, USA, 2004; Chapter 19; pp. 569–593. [Google Scholar]
- Brunschwiler, C.; Heine, D.; Kappeler, S.; Conde-Petit, B.; Nyström, L. Direct measurement of rice bran lipase activity for inactivation kinetics and storage stability prediction. J. Cereal Sci. 2013, 58, 272–277. [Google Scholar] [CrossRef]
- Atungulu, G.G.; Pan, Z. Infrared radiative properties of food materials. In Infrared Heating for Food and Agricultural Processing; Pan, Z., Atungulu, G.G., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 29–35. [Google Scholar]
- Kubo, M.T.K.; Siguemoto, E.S.; Funcia, E.S.; Augusto, P.E.D.; Curet, S.; Boillereaux, L.; Sastry, S.K.; Gut, J.A.W. Non-thermal effects of microwave and ohmic processing on microbial and enzyme inactivation: A critical review. Curr. Opin. Food Sci. 2020, 35, 36–48. [Google Scholar] [CrossRef]
- Liao, M.; Damayanti, W.; Xu, Y.; Zhao, Y.; Xu, X.; Zheng, Y.; Jiao, S. Hot air-assisted radio frequency heating for stabilization of rice bran: Enzyme activity, phenolic content, antioxidant activity and microstructure. LWT-Food Sci. Technol. 2020, 131, 109754. [Google Scholar] [CrossRef]
- Shin, T.S.; Godber, J.S. Changes of endogenous antioxidants and fatty acid composition in irradiated rice bran during storage. J. Agric. Food Chem. 1996, 44, 567–573. [Google Scholar] [CrossRef]
- Masamran, S.; Chookaew, S.; Tepsongkroh, B.; Supawong, S. Impact of gamma irradiation pre-treatment before subcritical water extraction on recovery yields and antioxidant properties of rice bran extract. Radiat. Phys. Chem. 2023, 207, 110834. [Google Scholar] [CrossRef]
- Pourali, O.; Asghari, F.S.; Yoshida, H. Simultaneous rice bran oil stabilization and extraction using sub-critical water medium. J. Food Eng. 2009, 95, 510–516. [Google Scholar] [CrossRef]
- Raghavendra, M.P.; Kumar, P.R.; Prakash, V. Mechanism of inhibition of rice bran lipase by polyphenols: A case study with chlorogenic acid and caffeic acid. J. Food Sci. 2007, 72, 412–419. [Google Scholar] [CrossRef]
- Gopinger, E.; Ziegler, V.; Catalan, A.A.S.; Krabbe, E.L.; Elias, M.C.; Xavier, E.G. Whole rice bran stabilization using a short chain organic acid mixture. J. Stored Prod. Res. 2015, 61, 108–113. [Google Scholar] [CrossRef]
- Yu, C.W.; Hu, Q.R.; Wang, H.W.; Deng, Z.Y. Comparison of 11 rice bran stabilization methods by analyzing lipase activities. J. Food Process. Preserv. 2020, 44, e14370. [Google Scholar] [CrossRef]
- Yu, C.W.; Luo, T.; Xie, T.; Deng, Z.Y. Classified processing of different rice bran fractions according to their component distributions. Int. J. Food Sci. Technol. 2022, 57, 4052–4064. [Google Scholar] [CrossRef]
- Yılmaz, F.; Yılmaz Tuncel, N.; Tuncel, N.B. Stabilization of immature rice grain using infrared radiation. Food Chem. 2018, 253, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Wang, Y.; Xu, G.; Zhou, L.; Liu, C.; Luo, S. Peroxidase inactivation by cold plasma and its effects on the storage, physicochemical and bioactive properties of brown rice. Food Biosci. 2023, 52, 102383. [Google Scholar] [CrossRef]
- Qian, J.Y.; Gu, T.P.; Jiang, W.; Chen, W. Inactivating effect of pulse electric field on lipase in brown rice. Innov. Food Sci. Emerg. Technol. 2014, 22, 89–94. [Google Scholar] [CrossRef]
- Bergonio, K.B.; Lucatin, L.G.G.; Corpuz, G.A.; Ramos, N.C.; Bartolome, J.; Duldulao, J.B.A. Improved shelf life of brown rice by heat and microwave treatment. J. Microbiol. Biotechnol. Food Sci. 2016, 5, 378–385. [Google Scholar] [CrossRef]
- Goffman, F.D.; Bergman, C. Relationship between hydrolytic rancidity, oil concentration, and esterase activity in rice bran. Cereal Chem. 2003, 80, 689–692. [Google Scholar] [CrossRef]
- Goffman, F.D.; Bergman, C. Hydrolytic degradation of triacylglycerols and changes in fatty acid composition in rice bran during storage. Cereal Chem. 2003, 80, 459–461. [Google Scholar] [CrossRef]
- Rattanathanan, Y.; Kanha, N.; Osiriphun, S.; Rakariyatham, K.; Klangpetch, W.; Laokuldilok, T. Changes in content of antioxidants and hydrolytic stability of black rice bran after heat- and enzymatic stabilizations and degradation kinetics during storage. J. Food Process. Preserv. 2022, 46, e16795. [Google Scholar] [CrossRef]
- Andriani, R.; Subroto, T.; Ishmayana, S.; Kurnia, D. Enhancement methods of antioxidant capacity in rice bran: A review. Foods 2022, 11, 2994. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Strappe, P.; Zhou, Z.K.; Blanchard, C. Impact on the nutritional attributes of rice bran following various stabilization procedures. Crit. Rev. Food Sci. Nutr. 2019, 59, 2458–2466. [Google Scholar] [CrossRef]
- Gualberto, D.G.; Bergman, C.J.; Kazemzadeh, M.; Weber, C.W. Effect of extrusion processing on the soluble and insoluble fiber, and phytic acid contents of cereal brans. Plant Food Hum. Nutr. 1997, 51, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, N.; Tuncel, N.B. The effect of infrared stabilisation on B vitamins, phenolics and and antioxidants in rice bran. Int. J. Food Sci. Technol. 2015, 50, 84–91. [Google Scholar] [CrossRef]
- Wang, C.; Xu, F.; Li, D.; Zhang, M. Physico-chemical and structural properties of four rice bran protein fractions based on the multiple solvent extraction method. Czech J. Food Sci. 2015, 33, 283–291. [Google Scholar] [CrossRef]
- Pradeep, P.M.; Jayadeep, A.; Guha, M.; Singh, V. Hydrothermal and biotechnological treatments on nutraceutical content and antioxidant activity of rice bran. J. Cereal Sci. 2014, 60, 187–192. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, S.; Singh, B.; Dar, B.N. Effect of extrusion variables (temperature, moisture) on the antinutrient components of cereal brans. J. Food Sci. Technol. 2015, 52, 1670–1676. [Google Scholar] [CrossRef]
- Goffman, F.D.; Pinson, S.; Bergman, C. Genetic diversity for lipid content and fatty acid prodifle in rice bran. J. Am. Oil Chem. Soc. 2003, 80, 485–490. [Google Scholar] [CrossRef]
- Punia, S.; Kumar, M.; Siroha, A.K.; Purewal, S.S. Rice bran oil: Emerging trends in extraction, health benefit, and its industrial application. Rice Sci. 2021, 28, 217–232. [Google Scholar] [CrossRef]
- Lerma-Garcia, M.J.; Herrero-Martinez, J.M.; Simo-Alfonso, E.F.; Mendonça, C.R.B.; Ramis-Ramos, G. Composition, industrial processing and applications of rice bran γ-oryzanol. Food Chem. 2009, 115, 389–404. [Google Scholar] [CrossRef]
- Murase, Y.; Lishima, H. Clinical studies of oral administration of gamma-oryzanol on climacteric complaints and its syndrome. Obstet. Gynecol. Prac. 1963, 12, 147–149. [Google Scholar]
- Xu, Z.; Godber, J.S. Antioxidant activities of major components of γ-oryzanol from rice bran using a linoleic acid model. J. Am. Oil Chem. Soc. 2001, 78, 645–649. [Google Scholar] [CrossRef]
- Juliano, C.; Cossu, M.; Alamanni, M.C.; Piu, L. Antioxidant activity of γ-oryzanol: Mechanism of action and its effect on oxidative stability of pharmaceutical oils. Int. J. Pharm. 2005, 299, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, W.H. Plasma LDL cholesterol lowering by plant phytosterols in a hamster model. Trends Food Sci. Technol. 2004, 15, 528–531. [Google Scholar] [CrossRef]
- Kim, J.; Godber, J.S.; Prinyawiwatkul, W. Inhibition of cholesterol autoxidation by the nanosaponifiable fraction in rice bran in an aqueous model system. J. Am. Oil Chem. Soc. 2001, 78, 685–689. [Google Scholar] [CrossRef]
- Seetharamaiah, G.S.; Krishnakantha, T.P.; Chandrasekhara, N. Influence of oryzanol on platelet aggregation in rats. J. Nutr. Sci. Vitaminol. 1990, 36, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Bergman, C.J.; Xu, Z. Genotype and environment effects on tocopherols, tocotrienols and gamma-oryzanol contents of Southern US rice. Cereal Chem. 2003, 80, 446–449. [Google Scholar] [CrossRef]
- Irakli, M.; Lazaridou, A.; Biliaderis, C.G. Comparative evaluation of the nutritional, antinutritional, functional, and bioactivity attributes of rice bran stabilized by different heat treatments. Foods 2021, 10, 57. [Google Scholar] [CrossRef]
- Britz, S.J.; Prasad, P.V.V.; Moreau, R.A.; Allen, L.H.; Kremer, D.F.; Boote, K.J. Influence of growth temperature on the amounts of tocopherols, tocotrienols, and γ-oryzanol in brown rice. J. Agric. Food Chem. 2007, 55, 7559–7565. [Google Scholar] [CrossRef]
- Singanusong, R.; Garba, U. Micronutrients in rice bran oil. In Rice Bran and Rice Bran Oil. Chemistry, Processing and Utilization; Cheong, L.-Z., Xu, X., Eds.; AOAC Press: London, UK, 2019; Chapter 5; pp. 125–159. [Google Scholar]
- Peanparkdee, M.; Iwamoto, S. Bioactive compounds from by-products of rice cultivation and rice processing: Extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2019, 86, 109–117. [Google Scholar] [CrossRef]
- Jun, H.I.; Song, G.S.; Yang, E.I.; Youn, Y.; Kim, Y.S. Antioxidant activities and phenolic compounds of pigmented rice bran extracts. J. Food Sci. 2012, 77, 759–764. [Google Scholar] [CrossRef]
- Nagendra Prasad, M.N.; Sanjay, K.R.; Shravya Khatokar, M.; Vismaya, M.N.; Nanjunda Swamy, S. Health benefits of rice bran: A review. J. Nutr. Food Sci. 2011, 1, 1000108. [Google Scholar] [CrossRef]
- Kumar, A.; Sahu, C.; Panda, P.A.; Biswal, M.; Sah, R.P.; Lal, M.K.; Baig, M.J.; Swain, P.; Behera, L.; Chattopadhyay, K.; et al. Phytic acid content may affect starch digestibility and glycemic index value of rice (Oryza sativa L.). J. Sci. Food Agric. 2020, 100, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Sapwarobol, S.; Saphyakhajorn, W.; Astina, J. Biological functions and activities of rice bran as a functional ingredient: A review. Nutr. Metab. Insights 2021, 14, 11786388211058559. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yılmaz Tuncel, N. Stabilization of Rice Bran: A Review. Foods 2023, 12, 1924. https://doi.org/10.3390/foods12091924
Yılmaz Tuncel N. Stabilization of Rice Bran: A Review. Foods. 2023; 12(9):1924. https://doi.org/10.3390/foods12091924
Chicago/Turabian StyleYılmaz Tuncel, Neşe. 2023. "Stabilization of Rice Bran: A Review" Foods 12, no. 9: 1924. https://doi.org/10.3390/foods12091924
APA StyleYılmaz Tuncel, N. (2023). Stabilization of Rice Bran: A Review. Foods, 12(9), 1924. https://doi.org/10.3390/foods12091924








