Microencapsulates of Blue Maize Polyphenolics as a Promising Ingredient in the Food and Pharmaceutical Industry: Characterization, Antioxidant Properties, and In Vitro-Simulated Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Blue Popping Maize By-Product
2.1.2. Biopolymers (Carriers)
2.2. Preparation of Blue Maize By-Product Extract
2.3. Microencapsulation by the Spray-Drying Method
2.4. Analysis of the Technological Characteristics of SMEs and Microencapsulates
2.4.1. Powder Yield
2.4.2. Moisture Content
2.4.3. Bulk Density
2.4.4. Rehydration
2.5. Physical Characterization of SME and Microencapsulates
2.5.1. Particle Size Distribution
2.5.2. FTIR Spectroscopy
2.5.3. Differential Scanning Calorimetry (DSC) Analysis
2.6. Chemical Analyses of SME and Microencapsulates
2.6.1. Extraction of Soluble Free Phenolic Compounds
2.6.2. Analysis of Total Phenolic Compounds
2.6.3. Analysis of Phenolic Acids by HPLC
2.6.4. Analysis of Total Anthocyanins
2.6.5. Analysis of Individual Anthocyanins by HPLC and HPLC-MS
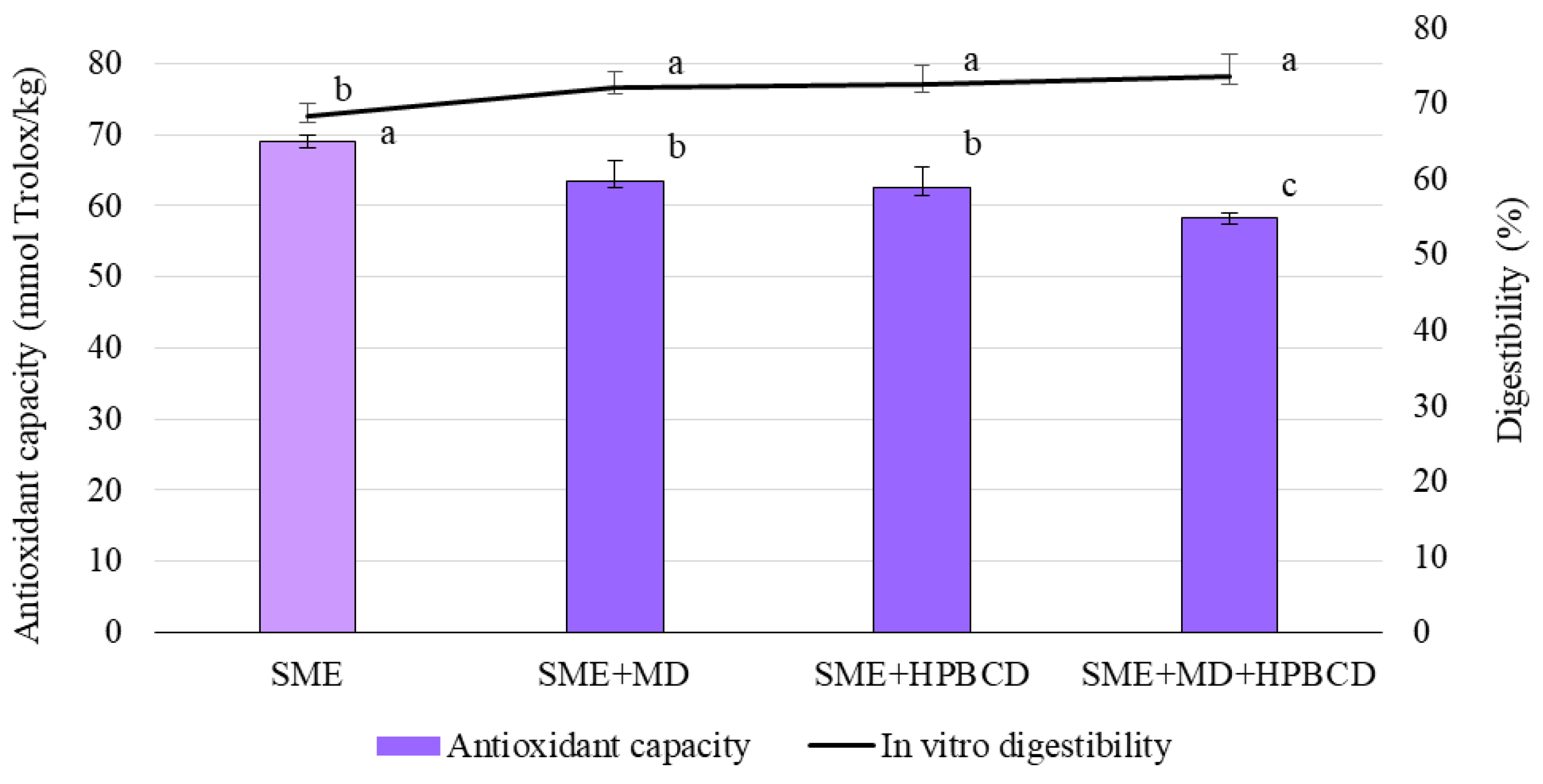
2.6.6. Analysis of the Total Antioxidant Capacity
2.6.7. In Vitro Multistep Enzymatic Digestion Protocol
2.7. Statistical Analysis
3. Results and Discussion
3.1. Technological Properties of SME and Microencapsulates
3.1.1. Powder Yield
3.1.2. Moisture Content
3.1.3. Bulk and Tapped Densities, Flow, and Cohesiveness of Powder Properties
3.1.4. Rehydration and pH
3.2. Physical Characterization of SME and Microencapsulates
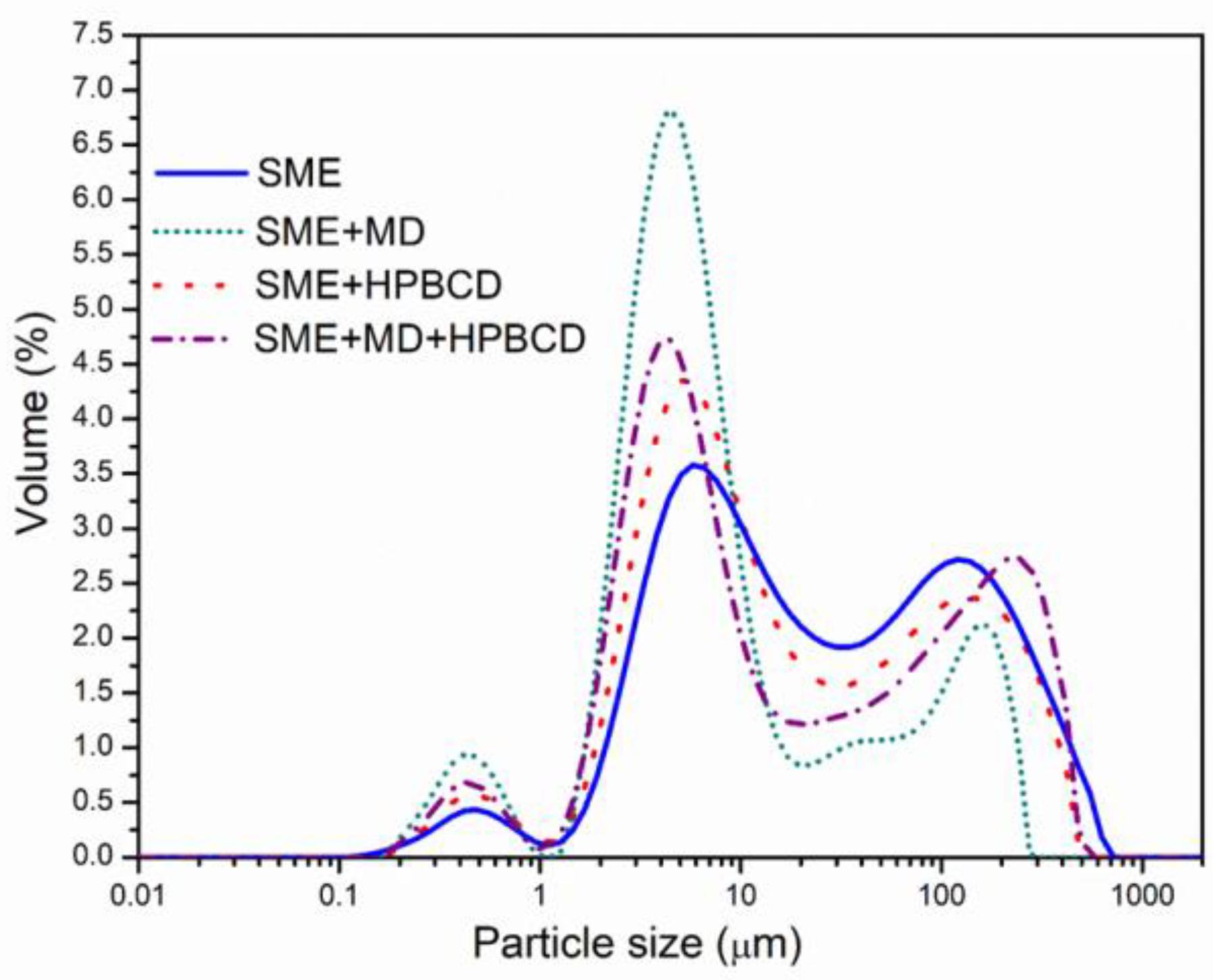
3.2.1. Particle Size Distribution
3.2.2. FTIR Spectroscopy
3.2.3. Thermal Characteristics based on DSC Analysis
3.3. Chemical Characteristics of SME and Microencapsulates
Content of Soluble Free Phenolic Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, X.; Beecher, R.G.; Holden, M.J.; Haytowitz, B.D.; Gebhardt, E.S.; Prior, L.R. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Konishi, T. Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar] [PubMed]
- DeFuria, J.; Bennett, G.; Strissel, K.J.; Perfield, J.W.; Milbury, P.E.; Greenberg, A.S.; Obin, M.S. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J. Nutr. 2009, 139, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Andriambeloson, E.; Kleschyov, A.L.; Muller, B.; Beretz, A.; Stoclet, J.C.; Andriantsitohaina, R. Nitric oxide production and endotheliumdependent vasorelaxation induced by wine polyphenols in rat aorta. Br. J. Nutr. 1997, 120, 1053–1058. [Google Scholar] [CrossRef]
- Astadi, I.R.; Astuti, M.; Santoso, U.; Nugraheni, P.S. In vitro antioxidant activity of anthocyanins of black soybean seed coat in human low density lipoprotein (LDL). Food Chem. 2009, 112, 659–663. [Google Scholar] [CrossRef]
- Kang, S.Y.; Seeram, N.P.; Nair, M.G.; Bourquin, L.D. Tart cherry anthocyanins inhibit tumor development in Apc (Min) mice and reduce proliferation of human colon cancer cells. Cancer Lett. 2003, 194, 13–19. [Google Scholar] [CrossRef]
- Fimognari, C.; Berti, F.; Nusse, M.; Cantelli-Forti, G.; Hrelia, P. Induction of apoptosis in two human leukemia cell lines as well as differentiation in human promyelocytic cells by cyanin-3-O-betaglucopyranoside. Biochem. Pharmacol. 2004, 67, 2047–2056. [Google Scholar] [CrossRef]
- Kalt, W.; Blumberg, J.B.; McDonald, J.E.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A.; Graf, B.A.; O’Leary, J.M.; Milbury, P.E. Identification of anthocyanins in the liver, eye and brain of blueberry-fed pigs. J. Agric. Food Chem. 2008, 56, 705–712. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef]
- Žilić, S.; Simić, M.; Nikolić, V. Colored cereals: Food applications. In Functionality and Application of Colored Cereals: Nutritional, Bioactive and Health Aspects; Punia, B.S., Kumar, M., Eds.; Elsevier Inc.: London, UK, 2023. [Google Scholar] [CrossRef]
- Žilić, S.; Serpen, A.; Akillioglu, G.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem 2012, 60, 1224–1231. [Google Scholar] [CrossRef]
- Žilić, S.; Kocadağlı, T.; Vančetović, J.; Gökmen, V. The effects of baking conditions and dough formulations on phenolic compounds stability, antioxidant capacity and color of cookies made from anthocyanin-rich corn flour. LWT—Food Sci. Technol. 2016, 65, 597–603. [Google Scholar] [CrossRef]
- Simić, M.; Žilić, S.; Šimurina, O.; Filipčev, B.; Škrobot, D.; Vančetović, J. Effects of anthocyanin-rich popping maize flour on the phenolic profile and the antioxidant capacity of mix-bread and its physical and sensory properties. Polish J. Food Nutr. Sci. 2018, 68, 289–297. [Google Scholar] [CrossRef]
- Žilić, S.; Dodig, D.; Vančetović, J.; Grčić, N.; Perić, V.; Titan, P.; Maksimović, V. Composition of anthocyanins in colored grains and the relationship of their non-acylated and acylated derivatives. Polish J. Food Nutr. Sci. 2019, 69, 137–146. [Google Scholar] [CrossRef]
- Bakowska-Barczak, A.M.; Kolodziejczyk, P.P. Black currant polyphenols: Their storage stability and microencapsulation. Ind. Crops Prod. 2011, 34, 1301–1309. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Effect of spray drying and storage on the stability of bayberry polyphenols. Food Chem. 2011, 129, 1139–1147. [Google Scholar] [CrossRef]
- Barbosa, M.; Borsarelli, C.D.; Mercadante, A.Z. Light stability of spray-dried bixin encapsulated with different edible polysaccharide preparations. Food Res. Int. 2005, 38, 989–994. [Google Scholar] [CrossRef]
- Baranauskienė, R.; Venskutonis, P.R.; Dewettinck, K.; Verhé, R. Properties of oregano (Origanum vulgare L.), citronella (Cymbopogon nardus G.) and marjoram (Majorana hortensis L.) flavors encapsulated into milk protein-based matrices. Food Res. Int. 2006, 39, 413–425. [Google Scholar] [CrossRef]
- Castro-López, C.; Espinoza-González, C.; Ramos-González, R.; Boone-Villa, V.D.; Aguilar-González, M.A.; Martínez-Ávila, G.C.G.; Aguilar, C.N.; Ventura-Sobrevilla, J.M. Spray-drying encapsulation of microwave-assisted extracted polyphenols from Moringa oleifera: Influence of tragacanth, locust bean, and carboxymethyl-cellulose formulations. Food Res. Int. 2021, 144, 110291. [Google Scholar] [CrossRef]
- Ćujić, N.; Trifković, K.; Bugarski, B.; Ibrić, S.; Pljevljakušić, D.; Šavikin, K. Chokeberry (Aronia melanocarpa L.) extract loaded in alginate and alginate/inulin system. Ind. Crops Prod. 2016, 86, 120–131. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Emadzadeh, B.; Shahidi-Noghabi, M.; Abbaspour, M.; Akbari, E. Physico-chemical and antioxidant properties of barberry juice powder and its effervescent tablets. Chem. Biol. Technol. Agric. 2011, 8, 23. [Google Scholar] [CrossRef]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Green biopolymers from by-products as wall materials for spray drying microencapsulation of phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Šavikin, K.; Nastić, N.; Janković, T.; Bigović, D.; Miličević, B.; Vidović, S.; Menković, N.; Vladić, J. Effect of type and concentration of carrier material on the encapsulation of pomegranate peel using spray drying method. Foods 2021, 24, 1968. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Drinić, Z.; Bigović, D.; Zdunić, G.; Mudrić, J.; Šavikin, K. Effect of carrier type on the spray-dried willowherb (Epilobium angustifolium L.) leaves extract powder properties and bioactive compounds encapsulation. Lek. Sirovine 2021, 41, 41–45. [Google Scholar] [CrossRef]
- Connors, K.A. The stability of cyclodextrin complexes in solution. Cheml. Rev. 1997, 97, 1325–1358. [Google Scholar] [CrossRef]
- Chew, S.C.; Tan, C.P.; Nyam, K.L. Microencapsulation of refined kenaf (Hibiscus cannabinus L.) seed oil by spray drying using β-cyclodextrin/gum arabic/sodium caseinate. J. Food Eng. 2018, 237, 78–85. [Google Scholar] [CrossRef]
- Sharif, N.; Golmakani, M.T.; Hajjari, M.M.; Aghaee, E.; Ghasemi, J.B. Antibacterial cuminaldehyde/hydroxypropyl-β-cyclodextrin inclusion complex electrospun fibers mat: Fabrication and characterization. Food Packag. Shelf 2021, 29, 100738. [Google Scholar] [CrossRef]
- Ćujić-Nikolić, N.; Stanisavljević, N.; Šavikin, K.; Kalušević, A.; Nedović, V.; Samardžić, J.; Janković, T. Chokeberry polyphenols preservation using spray drying: Effect of encapsulation using maltodextrin and skimmed milk on their recovery following in vitro digestion. J. Microencapsul. 2019, 36, 693–703. [Google Scholar] [CrossRef]
- Wilkowska, A.; Ambroziak, W.; Adamiec, J.; Czyżowska, A. Preservation of antioxidant activity and polyphenols in chokeberry juice and wine with the use of microencapsulation. J. Food Process. Preserv. 2017, 41, e12924. [Google Scholar] [CrossRef]
- Wilkowska, A.; Ambroziak, W.; Czyzowska, A.; Adamiec, J. Effect of microencapsulation by spray-drying and freeze-drying technique on the antioxidant properties of blueberry (Vaccinium myrtillus) juice polyphenolic compounds. Pol. J. Food Nutr. Sci. 2016, 66, 11. [Google Scholar] [CrossRef]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Pegg, R.B.; Kong, F. Total phenolics content and antioxidant capacities of microencapsulated blueberry anthocyanins during in vitro digestion. Food Chem. 2014, 153, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Avello, D.; Avendaño-Godoy, J.; Santos, J.; Lozano-Castellón, J.; Mardones, C.; von Baer, D.; Luengo, J.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Gómez-Gaete, C. Encapsulation of phenolic compounds from a grape cane pilot-plant extract in hydroxypropyl beta-cyclodextrin and maltodextrin by spray drying. Antioxidants 2021, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Vidović, S.; Vladić, J.; Vaštag, Ž.; Zeković, Z.; Popović, L. Maltodextrin as a carrier of health benefit compounds in Satureja montana dry powder extract obtained by spray drying technique. Powder Technol. 2014, 258, 209–215. [Google Scholar] [CrossRef]
- Caliskan, G.; Dirim, S.N. The effect of different drying processes and the amounts of maltodextrin addition on the powder properties of sumac extract powders. Powder Technol. 2016, 287, 308–314. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. A new technique for spray drying orange juice concentrate. Innov. Food Sci. Emerg. Technol. 2010, 11, 342–351. [Google Scholar] [CrossRef]
- Jovanović, M.; Ćujić-Nikolić, N.; Drinić, Z.; Janković, T.; Marković, S.; Petrović, P.; Šavikin, K. Spray drying of Gentiana asclepiadea L. root extract: Successful encapsulation into powders with preserved stability of bioactive compounds. Ind. Crops Prod. 2021, 172, 114044. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Lević, S.M.; Pavlović, V.B.; Marković, S.B.; Pjanović, R.V.; Đorđević, V.B.; Nedović, V.; Bugarski, B.M. Freeze vs. Spray drying for dry wild thyme (Thymus serpyllum L.) extract formulations: The impact of gelatin as a coating material. Molecules 2021, 26, 3933. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Pellegrini, N.; Fogliano, V. Direct measurement of the total antioxidant capacity of cereal products. J. Cereal. Sci. 2008, 48, 816–820. [Google Scholar] [CrossRef]
- Papillo, V.A.; Vitaglione, P.; Graziani, G.; Gokmen, V.; Fogliano, V. Release of antioxidant capacity from five plant foods during a multistep enzymatic digestion protocol. J. Agric. Food Chem. 2014, 62, 4119–4126. [Google Scholar] [CrossRef] [PubMed]
- Hamzalıoğlu, A.; Gökmen, V. Formation and elimination reactions of 5-hydroxymethylfurfural during in vitro digestion of biscuits. Food Res. Int. 2017, 99, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Almansour, K.; Ali, R.; Alheibshy, F.; Almutairi, T.J.; Alshammari, R.F.; Alhajj, N.; Arpagaus, C.; Elsayed, M.M.A. Particle Engineering by Nano Spray Drying: Optimization of Process Parameters with Hydroethanolic versus Aqueous Solutions. Pharmaceutics 2022, 14, 800. [Google Scholar] [CrossRef]
- Şahin-Nadeem, H.; Torun, M.; Özdemir, F. Spray drying of the mountain tea (Sideritis stricta) water extract by using different hydrocolloid carriers. LWT—Food Sci. Technol. 2011, 44, 1626–1635. [Google Scholar] [CrossRef]
- Şahin-Nadeem, H.; Dinçer, C.; Torun, M.; Topuz, A.; Özdemir, F. Influence of inlet air temperature and carrier material on the production of instant soluble sage (Salvia fruticosa Miller) by spray drying. LWT—Food Sci. Technol. 2013, 52, 31–38. [Google Scholar] [CrossRef]
- Tontul, I.; Topuz, A. Spray-drying of fruit and vegetable juices: Effect of drying conditions on the product yield and physical properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Datta, N.; Crooks, R.; Howes, T.; Rigby, S. A semi-empirical approach to optimize the quantity of drying aids required to spray dry sugar-rich foods. Dry Technol. 1997, 15, 2509–2525. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. Effect of maltodextrin addition during spray drying of tomato pulp in dehumidified air: II. Powder properties. Dry Technol. 2008, 26, 726–737. [Google Scholar] [CrossRef]
- Cassol, L.; Noreña, C.P.Z. Microencapsulation and accelerated stability testing of bioactive compounds of Hibiscus sabdariffa. J. Food Meas. Charact. 2021, 15, 1599–1610. [Google Scholar] [CrossRef]
- Pasrija, D.; Ezhilarasi, P.N.; Indrani, D.; Anandharamakrishnan, C. Microencapsulation of green tea polyphenols and its effect on incorporated bread quality. LWT—Food Sci. Technol. 2015, 64, 289–296. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Marksa, M.; Jakstas, V.; Ivanauskas, L.; Kopustinskiene, D.M.; Bernatoniene, J. Microencapsulation of Elsholtzia ciliata herb ethanolic extract by spray-drying: Impact of resistant-maltodextrin complemented with sodium caseinate, skim milk, and beta-cyclodextrin on the quality of spray-dried powders. Molecules 2019, 24, 1461. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, J.A.; Santos, E.H.; Hill, L.E.; Gomes, C.L. Antimicrobial and antioxidant activities of carvacrol microencapsulated in hydroxypropyl-beta-cyclodextrin. LW—Food Sci. Technol. 2014, 57, 701–709. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, Y.; Liu, Y.; Cui, B. Physicochemical characterization and antibacterial activity assessment of lavender essential oil encapsulated in hydroxypropyl-beta-cyclodextrin. Ind. Crops Prod. 2019, 130, 104–110. [Google Scholar] [CrossRef]
- Pandya, H.V. Formulation and evaluation of microcapsules of trihexyphenidyle hydrochloride by solvent evaporation method. Int. J. Pharm. Sci. Res. 2012, 3, 539–544. [Google Scholar]
- Seo, E.J.; Min, S.G.; Choi, M.J. Release characteristics of freeze-dried eugenol encapsulated with β-cyclodextrin by molecular inclusion method. J. Microencapsul. 2010, 27, 496–505. [Google Scholar] [CrossRef]
- Duran-Barón, R.; Valle-Vargas, M.F.; Quintero-Gamero, G.; Quintanilla-Carvajal, M.X.; Alean, J. Encapsulation of citrulline extract from watermelon (Citrullus lanatus) by-product using spray drying. Powder Technol. 2021, 385, 455–465. [Google Scholar] [CrossRef]
- Espinosa-Andrews, H.; Sandoval-Castilla, O.; Vazquez-Torres, H.; Vernon-Carter, E.J.; Lobato-Calleros, C. Determination of the gum Arabic–chitosan interactions by Fourier transform infrared spectroscopy and characterization of the microstructure and rheological features of their coacervates. Carbohydr. Polym. 2010, 79, 541–546. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Santiago-Adame, R.; Calderas, F.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Núñez-Ramírez, D.M.; Bernad-Bernad, M.J.; Manero, O. Microencapsulation by spray drying of laurel infusions (Litsea glaucescens) with maltodextrin. Ind. Crops Prod. 2016, 90, 1–8. [Google Scholar] [CrossRef]
- de Barros Fernandes, R.V.; Borges, S.V.; Botrel, D.A. Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydr. Polym. 2014, 101, 524–532. [Google Scholar] [CrossRef]
- Tarone, A.G.; Cazarin, C.B.B.; Junior, M.R.M. Anthocyanins: New techniques and challenges in microencapsulation. Food Res. Int. 2020, 133, 109092. [Google Scholar] [CrossRef]
- Žilić, S.; Basić, Z.; Hadži-Tašković Šukalović, V.; Maksimović, V.; Janković, M.; Filipović, M. Can the sprouting process applied to wheat improve the contents of vitamins and phenolic compounds and antioxidant capacity of the flour? Int. J. Food Sci. Technol. 2014, 49, 1040–1047. [Google Scholar] [CrossRef]
- Žilić, S.; Delić, N.; Basić, Z.; Ignjatović-Micić, D.; Janković, M.; Vančetović, J. Effects of alkaline cooking and sprouting on bioactive compounds, their bioavailability and relation to antioxidant capacity of maize flour. J. Food Nutr. Res. 2015, 54, 155–164. Available online: https://www.vup.sk/en/index.php?mainID=2&navID=34&version=2&volume=54&article=1968 (accessed on 29 April 2023).
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhu, M.; Liu, X.; Wang, Y.; Cheng, J. Insight into the effect of microcapsule technology on the processing stability of mulberry polyphenols. LWT 2020, 126, 109144. [Google Scholar] [CrossRef]
- Kalušević, A.; Lević, S.; Čalija, B.; Pantić, M.; Belović, M.; Pavlović, V.; Bugarski, B.; Milić, J.; Žilić, S.; Nedović, V. Microencapsulation of anthocyanin-rich black soybean coat extract by spray drying with maltodextrin, gum Arabic and skimmed milk powder. J. Microencapsul. 2017, 34, 5–475. [Google Scholar] [CrossRef]
- Nthimole, C.T.; Kaseke, T.; Fawole, O.A. Micro-encapsulation and characterization of anthocyanin-rich raspberry juice powder for potential applications in the food industry. Processes 2022, 10, 1038. [Google Scholar] [CrossRef]
- Tan, C.; Sun, Y.; Yao, X.; Zhu, Y.; Jafari, S.M.; Sun, B.; Wang, J. Stabilization of anthocyanins by simultaneous encapsulation-copigmentaation via protein-polysaccharide polyelectrolyte complexes. Food Chem. 2023, 416, 135732. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Ponce-Alquicira, E. The Role of Microencapsulation in Food Application. Molecules 2022, 27, 1499. [Google Scholar] [CrossRef]
- Zhao, X.; Corrales, M.; Zhang, C.; Hu, X.; Ma, Y.; Tausher, B. Composition and thermal stability of anthocyanins from Chinese purple corn (Zea mays L.). J. Agric. Food Chem. 2008, 56, 10761–10766. [Google Scholar] [CrossRef]
- Mora-Rochín, S.; Gaxiola-Cuevas, N.; Gutiérrez-Uribe, J.A.; Milán-Carrillo, J.; Milán-Noris, E.M.; Reyes-Moreno, C.; Serna-Saldivar, S.O.; Cuevas-Rodriguez, E.O. Effect of traditional nixtamalization on anthocyanin content and profile in Mexican blue maize (Zea mays L.) landraces. LWT—Food Sci. Technol. 2016, 68, 563–569. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Ramaswamy, S.H. Effect of microencapsulation on antioxidant and antifungal properties of aqueous extract of pomegranate peel. J. Food Sci. Technol. 2020, 57, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kumar, H.V.; Rao, L.J.M. Influence of milk and sugar on antioxidant potential of black tea. Food Res. Int. 2008, 41, 124–129. [Google Scholar] [CrossRef]
- Gil, M.; Restrepo, A.; Millan, L.; Alzate, L.; Rojano, B. Microencapsulation of banana passion fruit (Passiflora tripartita var. mollissima): A new alternative as a natural additive as antioxidant. Food Nutr. Sci. 2014, 5, 671–682. [Google Scholar] [CrossRef]
- Hussain, S.A.; Hameed, A.; Nazir, Y.; Naz, T.; Wu, Y.; Suleria, H.A.R.; Song, Y. Microencapsulation and the Characterization of Polyherbal Formulation (PHF) Rich in Natural Polyphenolic Compounds. Nutrients 2018, 10, 843. [Google Scholar] [CrossRef] [PubMed]
- Mohammadalinejhad, S.; Kurek, A.M. Microencapsulation of anthocyanins—Critical review of techniques and wall materials. Appl. Sci. 2021, 11, 3936. [Google Scholar] [CrossRef]
- Zhang, X.; Leemhuis, H.; van der Maarel, M.J.E.C. Digestion kinetics of low, intermediate and highly branched maltodextrins produced from gelatinized starches with various microbial glycogen branching enzymes. Carbohydr. Polym. 2020, 247, 116729. [Google Scholar] [CrossRef]
- Flourié, B.; Molis, C.; Achour, L.; Dupas, H.; Hatat, C.; Rambaud, J.C. Fate of β-cyclodextrin in the human intestine. J. Nutr. 1993, 123, 676–680. [Google Scholar] [CrossRef]


| Samples | Yield | Moisture | Bulk Density | Tapped Density | CI | HR | Rehydration | pH |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (g/mL) | (g/mL) | (s) | ||||
| SME | 73.0 ± 6.1 a | 5.40 ± 0.2 a | 0.280 ± 0.01 c | 0.400 ± 0.02 a | 29.29 ± 1.0 a | 1.41 ± 0.02 a | 224.20 ± 30.7 a | 6.62 ± 0.1 a |
| SME + MD | 83.2 ± 6.8 a | 4.74 ± 0.4 ab | 0.334 ± 0.09 bc | 0.377 ± 0.02 a | 11.27 ± 3.1 b | 1.13 ± 0.04 ab | 217.29 ± 22.7 a | 6.47 ± 0.1 a |
| SME + HPBCD | 74.53 ± 4.2 a | 4.22 ± 0.2 b | 0.374 ± 0.02 ab | 0.454 ± 0.04 a | 17.51 ± 2.5 b | 1.21 ± 0.04 ab | 153.80 ± 14.5 a | 6.48 ± 0.1 a |
| SME + MD + HPBCD | 66.7 ± 1.6 a | 4.39 ± 0.1 b | 0.260 ± 0.01 cd | 0.433 ± 0.03 a | 9.17 ± 0.1 b | 1.01 ± 0.13 b | 133.25 ± 20.2 a | 6.55 ± 0.1 a |
| Samples | d10 * | d50 ** | d90 | Span *** | D [4.3] | D [3.2] | Uniformity |
|---|---|---|---|---|---|---|---|
| SME | 3.33± 0.31 a | 21.33 ± 3.43 a | 237.15 ± 17.9 a | 10.96 ± 1.05 c | 78.53 ± 8.59 a | 5.59 ± 0.36 a | 3.32 ± 0.17 b |
| SME + MD | 2.22 ± 0.39 b | 4.72 ± 0.47 c | 128.62 ± 8.2 b | 20.67 ± 2.69 ab | 32.34 ± 2.32 b | 3.11 ± 0.38 c | 4.72 ± 0.59 b |
| SME + HPBCD | 2.81 ± 0.49 ab | 12.28 ± 2.12 b | 206.50 ± 18.7 a | 16.01 ± 2.64 bc | 63.00 ± 11.28 a | 4.69 ± 0.68 ab | 4.52 ± 0.28 b |
| SME + MD + HPBCD | 2.45 ± 0.42 ab | 10.73 ± 1.21 bc | 257.14 ± 37.3 a | 23.73 ± 3.71 a | 75.02 ± 7.14 a | 4.06 ± 0.47 bc | 6.57 ± 1.02 a |
| Samples | T1 | T2 | T3 | T4 | ∆H1 | ∆H2 | ∆H3 | ∆H4 |
|---|---|---|---|---|---|---|---|---|
| SME | 86.18 ± 14.1 a | 150.55± 11.9 a | 197.06 ± 23.7 a | 261.11 ± 42.6 a | 70.68 | 145.91 | 6.51 | 60.73 |
| SME + MD | 86.26 ± 4.45 a | 154.18± 16.1 a | 241.00 ± 16.8 a | 275.00 ± 22.8 a | 41.85 | 140.29 | 5.66 | 12.67 |
| SME + HPBCD | 76.87 ± 11.9 a | 146.57± 14.7 a | 236.28 ± 39.4 a | 276.00 ± 43.9 a | 35.89 | 161.08 | 9.70 | 16.78 |
| SME + MD + HPBCD | 83.53 ± 4.9 a | 152.48± 20.2 a | 234.00 ± 40.9 a | 234.00 ± 31.2 a | 40.63 | 70.62 | 4.96 | 5.14 |
| Samples | TPC | DHBA | VA | CAFA | SYRA | p-CA | FA | TPA |
|---|---|---|---|---|---|---|---|---|
| SME | 35506 ± 1800 a | 96.47 ± 0.7 a | 14.89 ± 0.1 a | 117.64 ± 1.6 a | 25.91 ± 0.6 a | 105.32 ± 0.5 a | 29.18 ± 0.8 a | 389.4 a |
| SME + MD | 32211 ± 1085 bc | 78.04 ± 1.7 b | 12.38 ± 1.1 ab | 81.27 ± 1.1 b | 25.40 ± 0.6 a | 85.70 ± 0.9 b | 28.29 ± 0.8 a | 311.1 b |
| SME + HPBCD | 31308 ± 708 c | 81.29 ± 0.9 b | 10.53 ± 0.7 b | 79.02 ± 1.1 b | 22.98 ± 0.5 b | 86.22 ± 0.7 b | 27.70 ± 0.6 a | 307.6 b |
| SME + MD + HPBCD | 30622 ± 361 c | 82.29 ± 1.4 b | 10.35 ± 0.5 b | 79.2 ± 0.8 b | 18.93 ± 0.8 c | 77.96 ± 1.2 c | 17.23 ± 1.6 b | 285.9 b |
| Compounds * | tR | SME | SME + MD | SME + HPBCD | SME + MD + HPBCD |
|---|---|---|---|---|---|
| TANs | 12,846 ± 84 a | 12,182 ± 77 ab | 10,802 ± 152 bcd | 9642 ± 451 d | |
| Cy-3-Glu | 8.87 | 770.4 ± 6.1 a | 657.4 ± 29.7 ab | 742.6 ± 38.2 ab | 626.9 ± 38.2 b |
| Pg-3-Glu | 10.73 | 244.3 ± 1.1 a | 225.4 ± 4.9 b | 234.9 ± 5.1 ab | 184.9 ± 2.8 c |
| Pn-3-Glu | 11.70 | trace | trace | trace | trace |
| Cy-3-3Mal-Glu | 12.08 | 326.3 ± 6.9 a | 239.2 ± 4.1 bc | 238.4 ± 3.7 bc | 187.3 ± 22.7 c |
| Cy-3-6Mal-Glu | 13.61 | 2720.2 ± 25.9 a | 2258.1 ± 13.9 bc | 2188.1 ± 3.1 c | 1936.8 ± 33.4 d |
| Cy-3-diMal-Glu | 15.63 | 309.7 ± 0.4 a | 246.6 ± 1.1 bc | 242.9 ± 5.3 bc | 205.4 ± 9.4 c |
| Cy-3-diMal-Glu | 16.19 | 80.5 ± 8.1 a | 56.2 ± 7.1 b | 47.5 ± 5.4 bc | 34.4 ± 2.5 c |
| Cy-3-diMal-Glu | 16.77 | 786.5 ± 33.9 a | 716.5 ± 18.6 a | 733.9 ± 22.7 a | 734.5 ± 29.7 a |
| Total detected ANs | 5237.8 a | 4399.4 b | 4428.3 b | 3910.2 c | |
| Total cyanidin derivatives | |||||
| Non-acylated | 770.4 a | 657.6 ab | 742.7 ab | 627.0 b | |
| Acylated | 4223.2 a | 3516.5 bc | 3450.8 c | 3098.3 d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ćujić Nikolić, N.; Žilić, S.; Simić, M.; Nikolić, V.; Živković, J.; Marković, S.; Šavikin, K. Microencapsulates of Blue Maize Polyphenolics as a Promising Ingredient in the Food and Pharmaceutical Industry: Characterization, Antioxidant Properties, and In Vitro-Simulated Digestion. Foods 2023, 12, 1870. https://doi.org/10.3390/foods12091870
Ćujić Nikolić N, Žilić S, Simić M, Nikolić V, Živković J, Marković S, Šavikin K. Microencapsulates of Blue Maize Polyphenolics as a Promising Ingredient in the Food and Pharmaceutical Industry: Characterization, Antioxidant Properties, and In Vitro-Simulated Digestion. Foods. 2023; 12(9):1870. https://doi.org/10.3390/foods12091870
Chicago/Turabian StyleĆujić Nikolić, Nada, Slađana Žilić, Marijana Simić, Valentina Nikolić, Jelena Živković, Smilja Marković, and Katarina Šavikin. 2023. "Microencapsulates of Blue Maize Polyphenolics as a Promising Ingredient in the Food and Pharmaceutical Industry: Characterization, Antioxidant Properties, and In Vitro-Simulated Digestion" Foods 12, no. 9: 1870. https://doi.org/10.3390/foods12091870
APA StyleĆujić Nikolić, N., Žilić, S., Simić, M., Nikolić, V., Živković, J., Marković, S., & Šavikin, K. (2023). Microencapsulates of Blue Maize Polyphenolics as a Promising Ingredient in the Food and Pharmaceutical Industry: Characterization, Antioxidant Properties, and In Vitro-Simulated Digestion. Foods, 12(9), 1870. https://doi.org/10.3390/foods12091870










