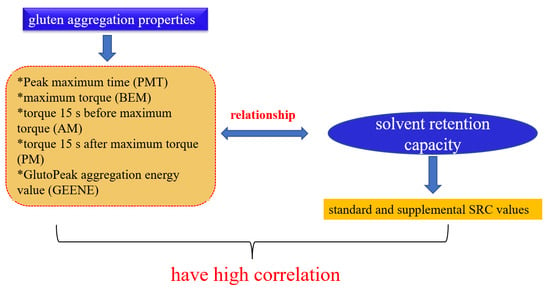
Correlation Analysis between Wheat Flour Solvent Retention Capacity and Gluten Aggregation Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Flour Test
2.3. Swelling Index of Glutenin Test
2.4. Disulfide-Sulfhydryl Analysis
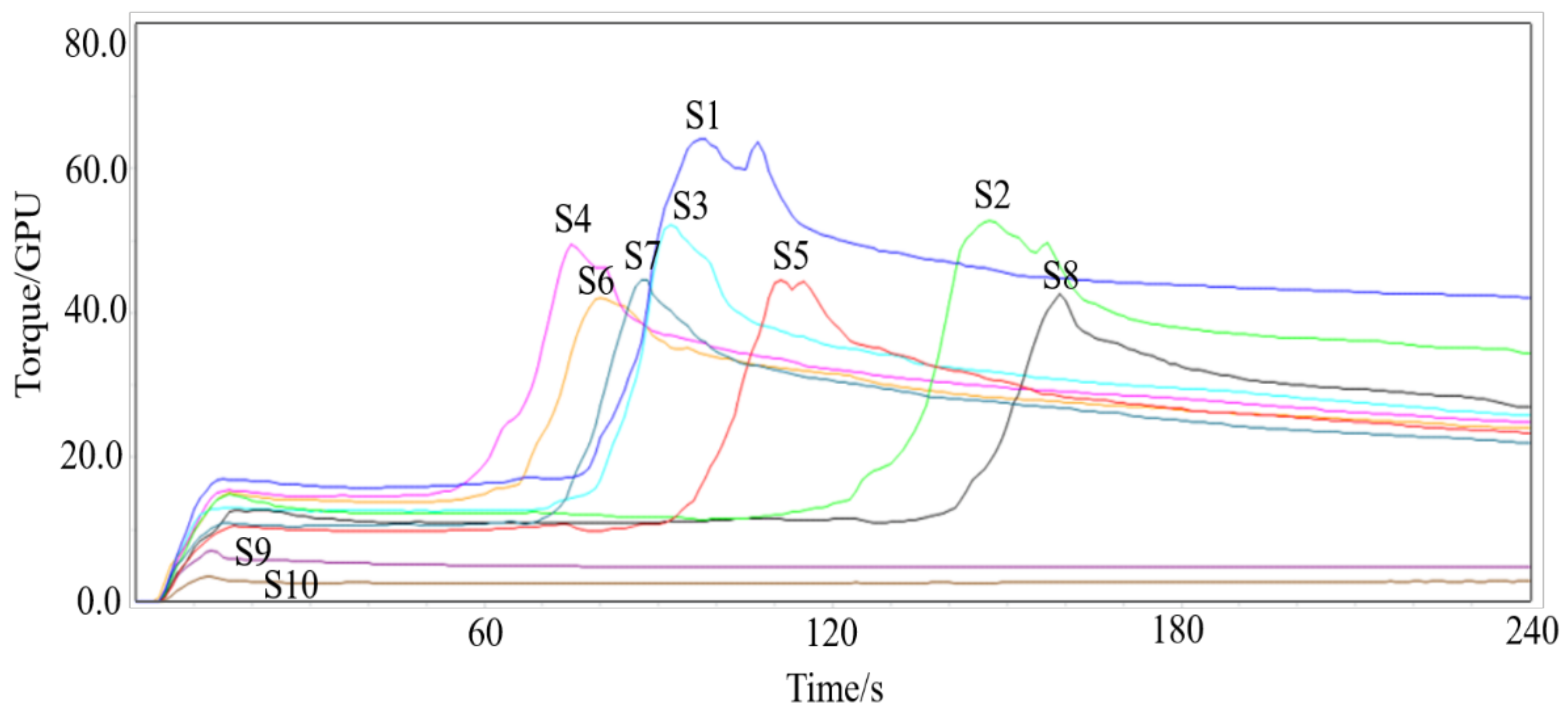
2.5. Gluten Aggregation Testing in GlutoPeak
2.6. Solvent Retention Capacity
2.7. Statistical Analysis
3. Results
3.1. Flour Physical Properties
3.2. SH and SS Content
3.3. SRC Assessment
3.4. Relationship between SRC Solvent and Flour Aggregation Properties
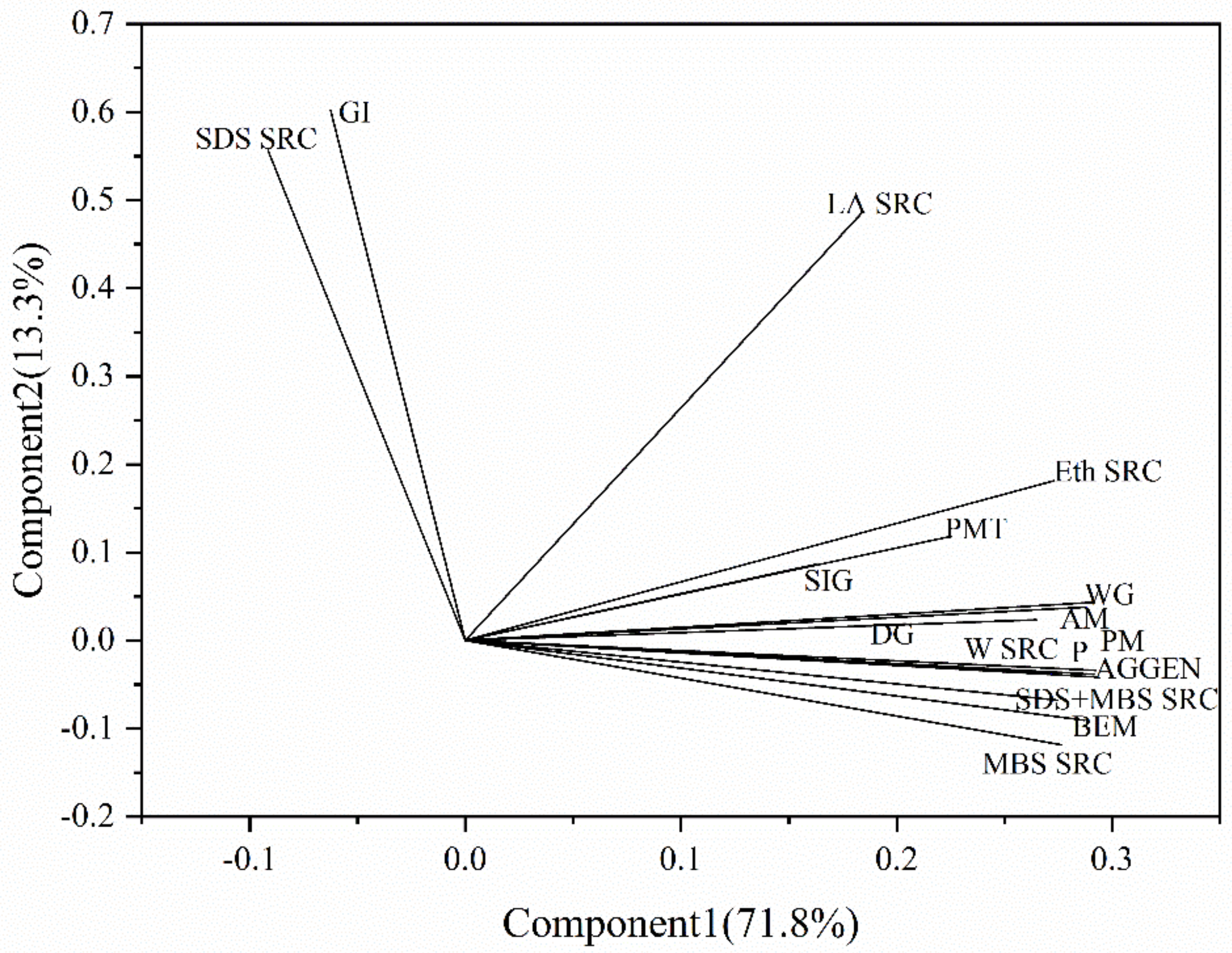
3.5. PCA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bonilla, J.C.; Erturk, M.Y.; Schaber, J.A.; Kokini, J.L. Distribution and function of LMW glutenins, HMW glutenins, and gliadins in wheat doughs analyzed with ‘in situ’ detection and quantitative imaging techniques. J. Cereal Sci. 2020, 93, 102931. [Google Scholar] [CrossRef]
- López, A.M.M.; Simsek, S. Solvent retention capacity: Supplemental solvents for evaluation of gluten quality. J. Cereal Sci. 2021, 102, 103339. [Google Scholar] [CrossRef]
- Chavoushi, M.; Kadivar, M.; Arzani, A.; Sabzalian, M.R. Relationships between grain, flour, and dough quality characteristics and solvent retention capacity tests of twelve triticale cultivars and parental species. Food Chem. 2022, 371, 131283. [Google Scholar] [CrossRef] [PubMed]
- Duyvejonck, A.E.; Lagrain, B.; Dornez, E.; Delcour, J.A.; Courtin, C.M. Suitability of solvent retention capacity tests to assess the cookie and bread making quality of European wheat flours. LWT Food Sci. Technol. 2012, 47, 56–63. [Google Scholar] [CrossRef]
- Hammed, A.M.; Ozsisli, B.; Ohm, J.-B.; Simsek, S. Relationship Between Solvent Retention Capacity and Protein Molecular Weight Distribution, Quality Characteristics, and Breadmaking Functionality of Hard Red Spring Wheat Flour. Cereal Chem. 2015, 92, 466–474. [Google Scholar] [CrossRef]
- Duyvejonck, A.E.; Lagrain, B.; Pareyt, B.; Courtin, C.M.; Delcour, J.A. Relative contribution of wheat flour constituents to Solvent Retention Capacity profiles of European wheats. J. Cereal Sci. 2011, 53, 312–318. [Google Scholar] [CrossRef]
- Kweon, M.; Slade, L.; Levine, H.; Gannon, D. Cookie- versus cracker-baking—What’s the difference? Flour functionality requirements explored by SRC and alveography. Crit. Rev. Food Sci. Nutr. 2014, 54, 115–138. [Google Scholar] [CrossRef]
- Wessels, R.; Wentzel, B.; Labuschagne, M. Solvent retention capacity and swelling index of glutenin in hard red wheat flour as possible indicators of rheological and baking quality characteristics. J. Cereal Sci. 2020, 93, 102983. [Google Scholar] [CrossRef]
- Wang, C.; Kovacs, M.I.P. Swelling Index of Glutenin Test. I. Method and Comparison with Sedimentation, Gel-Protein, and Insoluble Glutenin Tests. Cereal Chem. 2002, 79, 183–189. [Google Scholar] [CrossRef]
- Cereals & Grains Association. AACC Approved Methods of Analysis, 11th ed.; Method 44-15.02: Moisture—Air-Oven Methods; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar]
- Cereals & Grains Association. AACC Approved Methods of Analysis, 11th ed.; Method 46-12.01: Crude Protein—Kjeldahl Method, Boric Acid Modification; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar]
- Cereals & Grains Association. AACC Approved Methods of Analysis, 11th ed.; Method 38-12.02: Wet Gluten, Dry Gluten, Water-Binding Capacity, and Gluten Index; Cereals & Grains Association: St. Paul, MN, USA, 2000. [Google Scholar]
- Cereals & Grains Association. AACC Approved Methods of Analysis, 11th ed.; Method 56-60.01: Sedimentation Test for Flour; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar]
- Wang, X.; Liang, Y.; Wang, Q.; Zhang, X.; Wang, J. Effect of low-sodium salt on the physicochemical and rheological properties of wheat flour doughs and their respective gluten. J. Cereal Sci. 2021, 102, 103371. [Google Scholar] [CrossRef]
- Tuhumury, H.; Small, D.; Day, L. The effect of sodium chloride on gluten network formation and rheology. J. Cereal Sci. 2014, 60, 229–237. [Google Scholar] [CrossRef]
- Wang, J.; Hou, G.G.; Liu, T.; Wang, N.; Bock, J. GlutoPeak method improvement for gluten aggregation measurement of whole wheat flour. LWT Food Sci. Technol. 2018, 90, 8–14. [Google Scholar] [CrossRef]
- Cereals & Grains Association. AACC Approved Methods of Analysis, 11th ed.; Method 56-11.02: Solvent Retention Capacity Profile; Cereals & Grains Association: St. Paul, MN, USA, 2000. [Google Scholar]
- Melnyk, J.P.; Dreisoerner, J.; Bonomi, F.; Marcone, M.F.; Seetharaman, K. Effect of the Hofmeister series on gluten aggregation measured using a high shear-based technique. Food Res. Int. 2011, 44, 893–896. [Google Scholar] [CrossRef]
- Popa, C.N.; Berehoiu, R.M.T.; Lambrache, N. Assessment of Gluten Index Component Wet Gluten Remaining on the Sieve as Predictor of Wheat Bakery Potential. Rev. de Chim. 2019, 70, 3994–3999. [Google Scholar] [CrossRef]
- Guzmán, C.; Mondal, S.; Govindan, V.; Autrique, J.E.; Posadas-Romano, G.; Cervantes, F.; Crossa, J.; Vargas, M.; Singh, R.P.; Peña, R.J. Use of rapid tests to predict quality traits of CIMMYT bread wheat genotypes grown under different environments. LWT Food Sci. Technol. 2016, 69, 327–333. [Google Scholar] [CrossRef]
- Fu, B.X.; Wang, K.; Dupuis, B. Predicting water absorption of wheat flour using high shear-based GlutoPeak test. J. Cereal Sci. 2017, 76, 116–121. [Google Scholar] [CrossRef]
- Mu, M.; Geng, R.; Yue, Y.; Jia, F.; Zhang, X.; Wang, J. Quality prediction of freshly-harvested wheat using GlutoPeak during postharvest maturation. Grain Oil Sci. Technol. 2021, 4, 174–181. [Google Scholar] [CrossRef]
- Güçbilmez, Ç.M.; Şahin, M.; Akçacık, A.G.; Aydoğan, S.; Demir, B.; Hamzaoğlu, S.; Gür, S.; Yakışır, E. Evaluation of GlutoPeak test for prediction of bread wheat flour quality, rheological properties and baking performance. J. Cereal Sci. 2019, 90, 102827. [Google Scholar] [CrossRef]
- Zhang, X.; Mu, M.; Tian, Y.; Fu, J.; Jia, F.; Wang, Q.; Liang, Y.; Wang, J. Aggregative and structural properties of wheat gluten during post-harvest maturation. J. Stored Prod. Res. 2021, 94, 101897. [Google Scholar] [CrossRef]
- Karaduman, Y.; Sayaslan, A.; Akın, A. GlutoPeak parameters of whole wheat flours for gluten quality evaluation in soft wheat breeding programs. J. Cereal Sci. 2020, 95, 103031. [Google Scholar] [CrossRef]
- Marti, A.; Augst, E.; Cox, S.; Koehler, P. Correlations between gluten aggregation properties defined by the GlutoPeak test and content of quality-related protein fractions of winter wheat flour. J. Cereal Sci. 2015, 66, 89–95. [Google Scholar] [CrossRef]
- Chandi, G.K.; Lok, C.W.; Jie, N.Y.; Seetharaman, K. Functionality of Kamut and Millet flours in macro wire cut cookie systems. J. Food Sci. Technol. 2013, 52, 556–561. [Google Scholar] [CrossRef]
- Rakita, S.; Dokić, L.; Hadnađev, T.D.; Hadnađev, M.; Torbica, A. Predicting rheological behavior and baking quality of wheat flour using a GlutoPeak test. J. Texture Stud. 2018, 49, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Guttieri, M.J.; Souza, E. Sources of Variation in the Solvent Retention Capacity Test of Wheat Flour. Crop Sci. 2003, 43, 1628–1633. [Google Scholar] [CrossRef]
- Lindgren, A.; Simsek, S. Evaluation of Hard Red Spring Wheat Mill Stream Fractions Using Solvent Retention Capacity Test. J. Food Process. Preserv. 2016, 40, 131–139. [Google Scholar] [CrossRef]
- Ram, S.; Dawar, V.; Singh, R.; Shoran, J. Application of solvent retention capacity tests for the prediction of mixing properties of wheat flour. J. Cereal Sci. 2005, 42, 261–266. [Google Scholar] [CrossRef]
- Labuschagne, M.; Guzmán, C.; Phakela, K.; Wentzel, B.; van Biljon, A. Solvent Retention Capacity and Gluten Protein Composition of Durum Wheat Flour as Influenced by Drought and Heat Stress. Plants 2021, 10, 1000. [Google Scholar] [CrossRef]
- Kweon, M.; Slade, L.; Levine, H. Solvent Retention Capacity (SRC) Testing of Wheat Flour: Principles and Value in Predicting Flour Functionality in Different Wheat-Based Food Processes and in Wheat Breeding—A Review. Cereal Chem. 2011, 88, 537–552. [Google Scholar] [CrossRef]
- Urade, R.; Sato, N.; Sugiyama, M. Gliadins from wheat grain: An overview, from primary structure to nanostructures of aggregates. Biophys. Rev. 2018, 10, 435–443. [Google Scholar] [CrossRef]
- Uthayakumaran, S.; Gras, P.W.; Stoddard, F.L.; Bekes, F. Effect of Varying Protein Content and Glutenin-to-Gliadin Ratio on the Functional Properties of Wheat Dough. Cereal Chem. 1999, 76, 389–394. [Google Scholar] [CrossRef]
- Sissons, M. GlutoPeak: A Breeding Tool for Screening Dough Properties of Durum Wheat Semolina. Cereal Chem. 2016, 93, 550–556. [Google Scholar] [CrossRef]
| Samples | Protein (%) | Wet Gluten (%) | Dry Gluten (%) | Gluten Index (%) | SIG (%) | SDS-Sedi (mL) | SH-Groups (umol/g) | SS-Bonds (umol/g) |
|---|---|---|---|---|---|---|---|---|
| 1 | 13.08 ± 0.15 a | 38.57 ± 0.02 a | 14.01 ± 0.03 a | 92.69 ± 0.52 a | 6.49 ± 0.06 a | 53.05 ± 0.98 a | 4.38 ± 0.07 b | 2.58 ± 0.04 e |
| 2 | 11.48 ± 0.04 b | 30.84 ± 0.45 b | 11.49 ± 0.06 b | 95.70 ± 0.06 a | 6.09 ± 0.01 b | 51.10 ± 1.02 b | 4.33 ± 0.14 b | 3.64 ± 0.13 b |
| 3 | 11.03 ± 0.04 c | 26.66 ± 0.17 d | 10.43 ± 0.15 c | 81.27 ± 0.47 b | 5.73 ± 0.11 c | 46.87 ± 0.36 c | 2.94 ± 0.06 e | 3.66 ± 0.18 b |
| 4 | 10.63 ± 0.13 d | 26.47 ± 1.09 d | 10.52 ± 0.23 c | 82.29 ± 0.12 b | 5.23 ± 0.05 d | 39.38 ± 0.85 e | 3.46 ± 0.11 d | 4.04 ± 0.12 a |
| 5 | 10.23 ± 0.08 e | 29.42 ± 0.48 bc | 9.92 ± 0.01 de | 70.92 ± 2.19 d | 4.97 ± 0.01 e | 45.66 ± 0.11 c | 4.50 ± 0.33 ab | 3.00 ± 0.20 d |
| 6 | 10.08 ± 0.08 ef | 27.71 ± 0.05 cd | 10.23 ± 0.03 cd | 65.40 ± 2.52 e | 4.88 ± 0.00 e | 37.38 ± 0.12 f | 4.68 ± 0.03 a | 2.60 ± 0.39 d |
| 7 | 10.05 ± 0.05 f | 30.21 ± 0.93 b | 10.25 ± 0.19 cd | 76.06 ± 1.15 c | 4.90 ± 0.03 e | 41.43 ± 0.07 d | 4.47 ± 0.04 ab | 3.07 ± 0.13 d |
| 8 | 10.03 ± 0.13 f | 24.48 ± 0.07 e | 9.60 ± 0.06 e | 94.03 ± 0.65 a | 5.28 ± 0.07 d | 51.08 ± 0.58 b | 4.28 ± 0.15 bc | 3.33 ± 0.07 c |
| 9 | 5.73 ± 0.08 g | 21.50 ± 0.61 f | 7.44 ± 0.05 f | 94.28 ± 0.53 a | 4.34 ± 0.01 f | 41.98 ± 0.25 d | 4.07 ± 0.14 c | 3.23 ± 0.14 cd |
| 10 | 5.40 ± 0.00 h | 19.56 ± 0.33 g | 6.58 ± 0.31 g | 96.46 ± 0.90 a | 3.92 ± 0.04 g | 34.60 ± 0.25 g | 4.65 ± 0.13 a | 3.06 ± 0.19 e |
| Samples | PMT (S) | BEM (GPU) | AM (GPU) | PM (GPU) | AGGEN (cm2) |
|---|---|---|---|---|---|
| 1 | 95.25 ± 3.03 d | 64.50 ± 0.87 a | 27.25 ± 0.83 a | 54.25 ± 1.48 a | 1689.25 ± 16.60 a |
| 2 | 147.25 ± 1.09 b | 53.25 ± 0.83 b | 20.75 ± 0.43 b | 42.75 ± 0.43 b | 1351.06 ± 8.09 b |
| 3 | 94.00 ± 2.55 d | 52.25 ± 0.43 b | 15.00 ± 0.00 d | 38.75 ± 0.83 c | 1191.46 ± 16.60 c |
| 4 | 76.25 ± 1.79 g | 49.50 ± 0.87 c | 17.75 ± 1.30 c | 37.00 ± 0.71 d | 1144.93 ± 15.03 d |
| 5 | 107.00 ± 3.39 c | 44.25 ± 1.5 d | 15.00 ± 2.35 d | 35.25 ± 0.43 e | 1082.20 ± 42.34 e |
| 6 | 81.75 ± 1.48 f | 42.75 ± 0.8 d | 16.00 ± 1.22 cd | 34.25 ± 0.43 e | 1057.78 ± 9.29 ef |
| 7 | 88.50 ± 1.76 e | 43.25 ± 2.28 d | 12.25 ± 1.09 e | 34.00 ± 0.00 e | 1012.59 ± 50.00 g |
| 8 | 156.00 ± 2.12 a | 43.00 ± 0.71 d | 16.50 ± 1.12 cd | 34.50 ± 0.50 e | 1036.16 ± 21.31 fg |
| 9 | —— | —— | —— | —— | —— |
| 10 | —— | —— | —— | —— | —— |
| Samples | Main-SRCs | Supplementary SRCs | ||||
|---|---|---|---|---|---|---|
| WSRC (%) | LASRC (%) | EthSRC (%) | SDSSRC (%) | MBSSRC (%) | SDS + MBSSRC (%) | |
| 1 | 76.96 ± 0.46 a | 139.97 ± 1.02 a | 67.32 ± 0.72 a | 92.09 ± 3.58 cd | 73.73 ± 0.73 a | 74.94 ± 0.14 a |
| 2 | 66.74 ± 0.42 c | 120.56 ± 1.50 b | 62.07 ± 0.20 ab | 101.66 ± 2.65 b | 71.76 ± 2.24 a | 70.83 ± 1.06 b |
| 3 | 69.78 ± 0.20 b | 91.50 ± 0.27 e | 53.58 ± 0.32 c | 83.56 ± 0.81 d | 69.44 ± 0.19 ab | 66.87 ± 0.22 d |
| 4 | 68.84 ± 0.49 b | 86.48 ± 2.19 f | 61.86 ± 1.07 ab | 77.01 ± 1.15 e | 71.14 ± 0.17 a | 69.00 ± 0.50 c |
| 5 | 64.31 ± 0.58 d | 92.88 ± 0.12 e | 62.60 ± 1.00 a | 89.79 ± 2.39 c | 59.92 ± 9.28 bcd | 64.91 ± 0.29 e |
| 6 | 68.92 ± 0.52 b | 80.68 ± 1.68 g | 63.02 ± 0.77 a | 87.25 ± 2.33 cd | 66.70 ± 0.70 abc | 69.77 ± 1.08 ab |
| 7 | 63.77 ± 0.16 d | 88.44 ± 0.20 f | 61.07 ± 0.20 ab | 84.65 ± 1.33 d | 64.88 ± 0.10 abc | 63.95 ± 0.09 ef |
| 8 | 66.63 ± 0.95 c | 107.31 ± 1.03 c | 61.78 ± 0.09 ab | 106.76 ± 3.52 a | 61.09 ± 1.59 bcd | 63.03 ± 0.19 f |
| 9 | 54.56 ± 1.21 f | 96.68 ± 0.57 d | 53.50 ± 0.09 c | 96.98 ± 1.27 b | 57.70 ± 9.66 cd | 60.21 ± 1.41 g |
| 10 | 58.48 ± 0.26 e | 80.18 ± 0.65 g | 55.62 ± 9.20 bc | 100.18 ± 1.57 b | 52.13 ± 0.11 d | 53.75 ± 0.50 h |
| Samples | PMT | BEM | AM | PM | AGGEN | WSRC | LASRC | MBSSRC | MBS + SDSSRC | EthSRC | SDSSRC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PMT | 1 | ||||||||||
| BEM | −0.062 | 1 | |||||||||
| AM | 0.132 | 0.878 ** | 1 | ||||||||
| PM | 0.019 | 0.971 ** | 0.936 ** | 1 | |||||||
| GEENE | 0.024 | 0.972 ** | 0.944 ** | 0.997 ** | 1 | ||||||
| WSRC | −0.248 | 0.841 ** | 0.827 * | 0.838 ** | 0.919 ** | 1 | |||||
| LASRC | 0.461 | 0.791 * | 0.877 ** | 0.879 ** | 0.584 | 0.521 | 1 | ||||
| MBSSRC | -0.270 | 0.811 * | 0.679 | 0.725 * | 0.880 ** | 0.833 ** | 0.516 | 1 | |||
| MBS + SDSSRC | −0.236 | 0.818 ** | 0.861 ** | 0.831 ** | 0.912 ** | 0.856 ** | 0.586 | 0.941 ** | 1 | ||
| EthSRC | 0.040 | 0.264 | 0.615 | 0.447 | 0.735 * | 0.687 * | 0.517 | 0.526 | 0.690 | 1 | |
| SDSSRC | 0.942 ** | 0.005 | 0.276 | 0.146 | −0.292 | −0.325 | 0.375 | −0.441 | −0.354 | −0.061 | 1 |
| Protein | −0.012 | 0.989 ** | 0.914 ** | 0.994 ** | 0.999 ** | 0.919 ** | 0.569 | 0.711 * | −0.314 | 0.885 ** | 0.906 ** |
| Dry gluten | −0.105 | 0.934 ** | 0.915 ** | 0.978 ** | 0.960 ** | 0.908 ** | 0.709 * | 0.898 ** | 0.941 ** | 0.752 * | −0.270 |
| Wet gluten | −0.154 | 0.761 * | 0.744 * | 0.855 ** | 0.879 ** | 0.792 ** | 0.701 * | 0.767 ** | 0.854 ** | 0.778 * | −0.244 |
| SIG | 0.265 | 0.937 ** | 0.858 ** | 0.923 ** | 0.916 ** | 0.846 ** | 0.791 * | 0.881 ** | 0.874 ** | 0.557 | −0.086 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, W.; Wang, X.; Wang, F.; Wang, J. Correlation Analysis between Wheat Flour Solvent Retention Capacity and Gluten Aggregation Characteristics. Foods 2023, 12, 1879. https://doi.org/10.3390/foods12091879
Gong W, Wang X, Wang F, Wang J. Correlation Analysis between Wheat Flour Solvent Retention Capacity and Gluten Aggregation Characteristics. Foods. 2023; 12(9):1879. https://doi.org/10.3390/foods12091879
Chicago/Turabian StyleGong, Wei, Xiaohua Wang, Fengjiao Wang, and Jinshui Wang. 2023. "Correlation Analysis between Wheat Flour Solvent Retention Capacity and Gluten Aggregation Characteristics" Foods 12, no. 9: 1879. https://doi.org/10.3390/foods12091879
APA StyleGong, W., Wang, X., Wang, F., & Wang, J. (2023). Correlation Analysis between Wheat Flour Solvent Retention Capacity and Gluten Aggregation Characteristics. Foods, 12(9), 1879. https://doi.org/10.3390/foods12091879