Seasonal Variation in Raw Milk VOC Profile within Intensive Feeding Systems
Abstract
1. Introduction
2. Materials and Methods
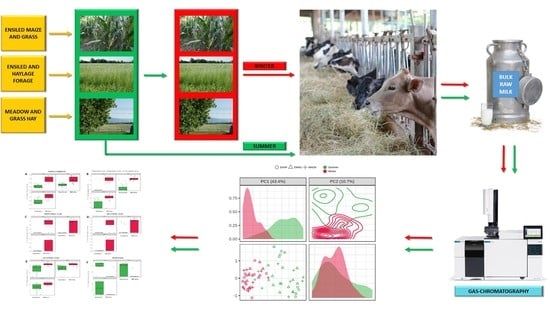
2.1. Experimental Design, Sample Collection and Chemical Analysis
2.2. HS-SPME and GC-FID Procedures
2.3. Statistical Analysis
3. Results
3.1. Feed and Chemical Composition of Dietary Feeding Systems
3.2. Milk Composition
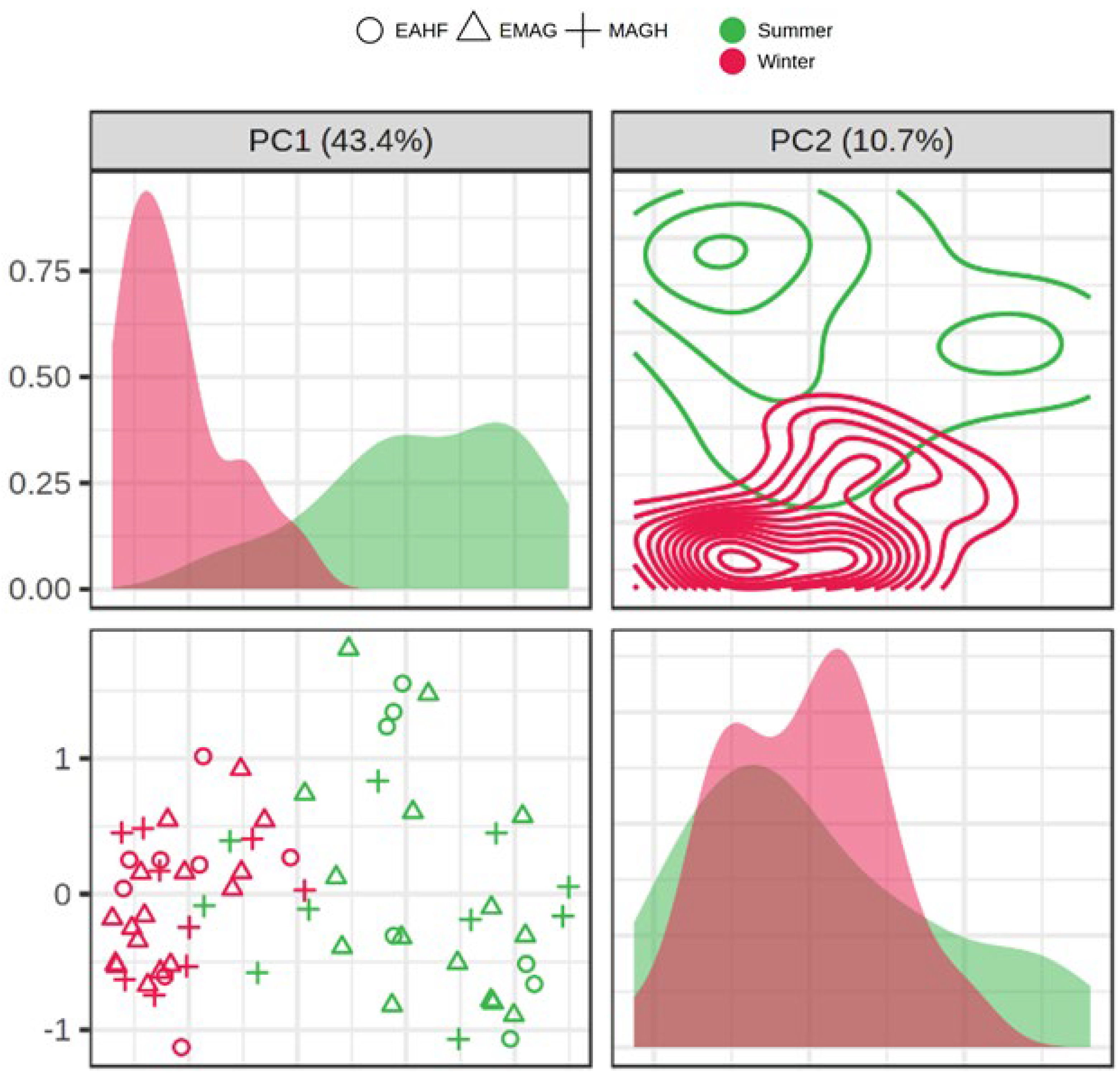
3.3. Milk Volatile Organic Compounds
4. Discussion
4.1. Milk Composition and Chemical Traits
4.2. Milk Volatile Organic Compounds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADF | acid detergent fiber |
| ADL | acid detergent lignin |
| ANOVA | analysis of variance |
| AOAC | association of official analytical chemists |
| EHAF | ensiled and haylage forage |
| EMAG | ensiled maize and grass |
| DIM | days in milk |
| DM | dry matter |
| DVB/CAR/PDMS | divinylbenzene/carboxen/polydimethylsiloxane |
| FA | fatty acid |
| FID | flame ionization detector |
| FPCM | fat protein corrected milk |
| FS | feeding system |
| FT-MIR | Fourier-transform mid infrared |
| GC | gas chromatography |
| GC-FID | gas chromatography-flame ionization detection |
| β-HB | β-hydroxybutyrate |
| HS-SPME | headspace solid-phase microextraction |
| iPCA | interactive principal component analysis |
| IRMS | isotope ratio mass spectrometry |
| LC/MS | liquid chromatography/mass spectrometry |
| MAGH | meadow and grass hay |
| aNDF | (amylase) neutral detergent fiber |
| NFC | non-fiber carbohydrates |
| NIST | National Institute of Standards and Technology |
| NMR | nuclear magnetic resonance |
| PC | principal component |
| PDO | protected designation of origin |
| PTFE | polytetrafluoroethylene |
| RMSE | root mean square error |
| RTL | retention time locked |
| S | season |
| SCC | somatic cell count |
| SCS | somatic cell score |
| TMR | total mixed ration |
| VOC | volatile organic compound |
References
- Riuzzi, G.; Tata, A.; Massaro, A.; Bisutti, V.; Lanza, I.; Contiero, B.; Bragolusi, M.; Miano, B.; Negro, A.; Gottardo, F.; et al. Authentication of forage-based milk by mid-level data fusion of (+/−) DART-HRMS signatures. Int. Dairy J. 2021, 112, 104859. [Google Scholar] [CrossRef]
- Rocchetti, G.; O’Callaghan, T.F. Application of metabolomics to assess milk quality and traceability. Curr. Opin. Food Sci. 2021, 40, 168–178. [Google Scholar] [CrossRef]
- Goldansaz, S.A.; Guo, A.C.; Sajed, T.; Steele, M.A.; Plastow, S.; Wishart, D.S. Livestock metabolomics and the livestock metabolome: A systematic review. PLoS ONE 2017, 12, e0177675. [Google Scholar] [CrossRef] [PubMed]
- Tenori, L.; Santucci, C.; Meoni, G.; Morrocchi, V.; Matteucci, G.; Luchinat, C. NMR metabolomic fingerprinting distinguishes milk from different farms. Food Res. Int. 2018, 113, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Segato, S.; Caligiani, A.; Contiero, B.; Galaverna, G.; Bisutti, V.; Cozzi, G. 1H NMR metabolic profile to discriminate pasture based alpine asiago PDO cheeses. Animals 2019, 9, 722. [Google Scholar] [CrossRef] [PubMed]
- O’ Sullivan, R.; Schmidt, O.; Monahan, F.J. Stable isotope ratio analysis for the authentication of milk and dairy ingredients: A review. Int. Dairy J. 2022, 126, 105268. [Google Scholar] [CrossRef]
- Coppa, M.; Martin, B.; Pradel, P.; Leotta, B.; Priolo, A.; Vasta, V. Effect of a hay-based diet or different upland grazing systems on milk volatile compounds. J. Agric. Food Chem. 2011, 59, 4947–4954. [Google Scholar] [CrossRef]
- Liu, N.; Koot, A.; Hettinga, K.; de Jong, J.; van Ruth, S.M. Portraying and tracing the impact of different production systems on the volatile organic compound composition of milk by PTR-(Quad)MS and PTR-(ToF)MS. Food Chem. 2018, 239, 201–207. [Google Scholar] [CrossRef]
- Villeneuve, M.P.; Lebeuf, Y.; Gervais, R.; Tremblay, G.F.; Vuillemard, J.C.; Fortin, J.; Chouinard, P.Y. Milk volatile organic compounds and fatty acid profile in cows fed timothy as hay, pasture, or silage. J. Dairy Sci. 2013, 96, 7181–7194. [Google Scholar] [CrossRef]
- Faulkner, H.; O’Callaghan, T.F.; McAuliffe, S.; Hennessy, D.; Stanton, C.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Effect of different forage types on the volatile and sensory properties of bovine milk. J. Dairy Sci. 2018, 101, 1034–1047. [Google Scholar] [CrossRef]
- Niero, G.; Meoni, G.; Tenori, L.; Luchinat, C.; Visentin, G.; Cassandro, M.; De Marchi, M.; Penasa, M. Grazing affects metabolic pattern of individual cow milk. J. Dairy Sci. 2022, 105, 9702–9712. [Google Scholar] [CrossRef]
- Clarke, H.J.; Griffin, C.; Rai, D.K.; O’Callaghan, T.F.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Dietary compounds influencing the sensorial, volatile and phytochemical properties of bovine milk. Molecules 2020, 25, 26. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Asakuma, S.; Miyaji, M.; Akiyama, F. Effect of time at pasture and herbage intake on profile of volatile organic compounds of dairy cow milk. Anim. Sci. J. 2016, 87, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Lutz, N.L.; Tangwan, E.C.; Hajazimi, E.; Vetter, W. Phytanic acid concentrations and diastereomer ratios in milk fat during changes in the cow’s feed from concentrate to hay and back. Eur. Food Res. Technol. 2012, 234, 955–962. [Google Scholar] [CrossRef]
- Cabiddu, A.; Peratoner, G.; Valenti, B.; Monteils, V.; Martin, B.; Coppa, M. A quantitative review of on-farm feeding practices to enhance the quality of grassland-based ruminant dairy and meat products. Animal 2022, 16, 100375. [Google Scholar] [CrossRef]
- Manzocchi, E.; Martin, B.; Bord, C.; Verdier-Metz, I.; Bouchon, M.; De Marchi, M.; Constant, I.; Giller, K.; Kreuzer, M.; Berard, J.; et al. Feeding cows with hay, silage, or fresh herbage on pasture or indoors affects sensory properties and chemical composition of milk and cheese. J. Dairy Sci. 2021, 104, 5285–5302. [Google Scholar] [CrossRef] [PubMed]
- Lanza, I.; Lolli, V.; Segato, S.; Caligiani, A.; Contiero, B.; Lotto, A.; Galaverna, G.; Magrin, L.; Cozzi, G. Use of GC-MS and 1H NMR low-level data fusion as an advanced and comprehensive metabolomic approach to discriminate milk from dairy chains based on different types of forage. Int. Dairy J. 2021, 123, 105174. [Google Scholar] [CrossRef]
- Nalepa, B.; Olszewska, M.A.; Markiewicz, L.H. Seasonal variances in bacterial microbiota and volatile organic compounds in raw milk. Int. J. Food Microbiol. 2018, 267, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.J.; Fitzpatrick, E.; Hennessy, D.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. The Influence of Pasture and Non-pasture-Based Feeding Systems on the Aroma of Raw Bovine Milk. Front. Nutr. 2022, 9, 841454. [Google Scholar] [CrossRef] [PubMed]
- De Nardi, R.; Marchesini, G.; Stefani, A.L.; Barberio, A.; Andrighetto, I.; Segato, S. Effect of feeding fine maize particles on the reticular pH, milk yield and composition of dairy cows. J. Anim. Physiol. Anim. Nutr. 2014, 98, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Segato, S.; Marchesini, G.; Serva, L.; Contiero, B.; Magrin, L.; Andrighetto, I. Assessment of Fermentative Quality of Ensiled High-Moisture Maize Grains by a Multivariate Modelling Approach. Agronomy 2022, 12, 429. [Google Scholar] [CrossRef]
- Vogel, K.P.; Pedersen, J.F.; Masterson, S.D.; Toy, J.J. Evaluation of a filter bag system for NDF, ADF, and IVDMD forage analysis. Crop Sci. 1999, 279, 276–279. [Google Scholar] [CrossRef]
- Ankom-Technology. Determining Acid Detergent Lignin in Beakers. Method_8_Lignin_in_beakers. 2020. Available online: https://www.ankom.com/sites/default/files/document-files/Method_8_Lignin_in_beakers_120922.pdf (accessed on 11 May 2020).
- Santiago, E.R. Profiling Flavors and Fragrances in Complex Matrices Using Linear Retention Indices without Sample Preparation; Application note Agilent Technologies: Santa Clara, CA, USA, 2019. [Google Scholar]
- David, F.; Scanlan, F.; Sandra, P.; Szelewski, M. Analysis of Essential Oil Compounds Using Retention Time Locked Methods and Retetion Time Databases; Application note Agilent Technologies: Santa Clara, CA, USA, 2002. [Google Scholar]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Borreani, G.; Coppa, M.; Revello-Chion, A.; Comino, L.; Giaccone, D.; Ferlay, A.; Tabacco, E. Effect of different feeding strategies in intensive dairy farming systems on milk fatty acid profiles, and implications on feeding costs in Italy. J. Dairy Sci. 2013, 96, 6840–6855. [Google Scholar] [CrossRef] [PubMed]
- Tata, A.; Massaro, A.; Riuzzi, G.; Lanza, I.; Bragolusi, M.; Negro, A.; Novelli, E.; Piro, R.; Gottardo, F.; Segato, S. Ambient mass spectrometry for rapid authentication of milk from Alpine or lowland forage. Sci. Rep. 2022, 12, 7360. [Google Scholar] [CrossRef] [PubMed]
- Benedet, A.; Manuelian, C.L.; Penasa, M.; Cassandro, M.; Righi, F.; Sternieri, M.; Galimberti, P.; Zambrini, A.V.; De Marchi, M. Factors associated with herd bulk milk composition and technological traits in the Italian dairy industry. J. Dairy Sci. 2018, 101, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Sailo, L.; Verma, N.; Bharti, P.; Saikia, J.; Imtiwati; Kumar, R. Impact of heat stress on health and performance of dairy animals: A review. Vet. World 2016, 9, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Cowley, F.C.; Barber, D.G.; Houlihan, A.V.; Poppi, D.P. Immediate and residual effects of heat stress and restricted intake on milk protein and casein composition and energy metabolism. J. Dairy Sci. 2015, 98, 2356–2368. [Google Scholar] [CrossRef]
- Hao, L.; Wang, J.; Sun, P.; Bu, D. The Effect of Heat Stress on the Metabolism of Dairy Cows: Updates & Review. Austin J. Nutr. Metab. 2016, 3, 1036. [Google Scholar]
- Nikoloudaki, O.; Junior, W.J.F.L.; Borruso, L.; Campanaro, S.; De Angelis, M.; Vogel, R.F.; Di Cagno, R.; Gobbetti, M. How multiple farming conditions correlate with the composition of the raw cow’s milk lactic microbiome. Environ. Microbiol. 2021, 23, 1702–1716. [Google Scholar] [CrossRef]
- McGrath, T.F.; Haughey, S.A.; Patterson, J.; Fauhl-Hassek, C.; Donarski, J.; Alewijn, M.; van Ruth, S.; Elliott, C.T. What are the scientific challenges in moving from targeted to non-targeted methods for food fraud testing and how can they be addressed? Spectroscopy case study. Trends Food Sci. Technol. 2018, 76, 38–55. [Google Scholar] [CrossRef]
- Katz, L.; Tata, A.; Woolman, M.; Zarrine-Afsar, A. Lipid profiling in cancer diagnosis with hand-held ambient mass spectrometry probes: Addressing the late-stage performance concerns. Metabolites 2021, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.K.; Nielsen, J.H.; Butler, G.; Leifert, C.; Slots, T.; Kristiansen, G.H.; Gustafsson, A.H. Milk quality as affected by feeding regimens in a country with climatic variation. J. Dairy Sci. 2010, 93, 2863–2873. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; Hennessy, D.; Mcauliffe, S.; Kilcawley, K.N.; Donovan, M.O.; Dillon, P.; Ross, R.P.; Stanton, C. Effect of pasture versus indoor feeding systems on raw milk composition and quality over an entire lactation. J. Dairy Sci. 2016, 99, 9424–9440. [Google Scholar] [CrossRef] [PubMed]
- Gottardo, P.; Penasa, M.; Righi, F.; Lopez-Villalobos, N.; Cassandro, M.; De Marchi, M. Fatty acid composition of milk from holstein-friesian, brown swiss, simmental and alpine grey cows predicted by mid-infrared spectroscopy. Ital. J. Anim. Sci. 2017, 16, 380–389. [Google Scholar] [CrossRef]
- Pacheco-Pappenheim, S.; Yener, S.; Heck, J.M.L.; Dijkstra, J.; van Valenberg, H.J.F. Seasonal variation in fatty acid and triacylglycerol composition of bovine milk fat. J. Dairy Sci. 2021, 104, 8479–8492. [Google Scholar] [CrossRef] [PubMed]
- Wölk, M.; Milkovska-Stamenova, S.; Fedorova, M.; Hoffmann, R. Variations in the milk lipidomes of two dairy cow herds fed hay- or silage-based diets over a full year. Food Chem. 2022, 390, 133091. [Google Scholar] [CrossRef] [PubMed]
- Bendall, J.G.; Olney, S.D. Hept-cis-4-enal: Analysis and flavour contribution to fresh milk. Int. Dairy J. 2001, 11, 855–864. [Google Scholar] [CrossRef]
- Vazquez-Landaverde, P.A.; Velazquez, G.; Torres, J.A.; Qian, M.C. Quantitative determination of thermally derived off-flavor compounds in milk using solid-phase microextraction and gas chromatography. J. Dairy Sci. 2005, 88, 3764–3772. [Google Scholar] [CrossRef]
- Smit, B.A.; Engels, W.J.M.; Smit, G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999. [Google Scholar] [CrossRef]
- Segato, S.; Marchesini, G.; Magrin, L.; Contiero, B.; Andrighetto, I.; Serva, L. A Machine Learning-Based Assessment of Maize Silage Dry Matter Losses by Net-Bags Buried in Farm Bunker Silos. Agriculture 2022, 12, 785. [Google Scholar] [CrossRef]
- Zhou, Y.; Drouin, P.; Lafrenière, C. Effects on microbial diversity of fermentation temperature (10 °C and 20 °C), long-term storage at 5 °C, and subsequent warming of corn silage. Asian-Australas. J. Anim. Sci. 2019, 32, 1528–1539. [Google Scholar] [CrossRef] [PubMed]
- Buccioni, A.; Decandia, M.; Minieri, S.; Molle, G.; Cabiddu, A. Lipid metabolism in the rumen: New insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Anim. Feed Sci. Technol. 2012, 174, 1–25. [Google Scholar] [CrossRef]
- Hanuš, O.; Kučera, J.; Samková, E.; Němečková, I.; Čítek, J.; Kopec, T.; Falta, D.; Nejeschlebová, H.; Rysová, L.; Klimešová, M.; et al. Raw cow milk protein stability under natural and technological conditions of environment by analysis of variance. Foods 2021, 10, 2017. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C.J.; Gleeson, D.; Toole, P.W.O.; Cotter, D. Impacts of Seasonal Housing and Teat Preparation on Raw Milk Microbiota: A high-throughput sequencing study. Appl. Environ. Microbiol. 2017, 83, e02694-16. [Google Scholar] [CrossRef]
- Kilcawley, K.N.; Faulkner, H.; Clarke, H.J.; O’Sullivan, M.G.; Kerry, J.P. Factors influencing the flavour of bovine milk and cheese from grass based versus non-grass based milk production systems. Foods 2018, 7, 37. [Google Scholar] [CrossRef]
| EMAG (Ensiled Forage) | EAHF (Ensiled/Haylage Forage) | MAGH (Dried Forage) 1 | ||||
|---|---|---|---|---|---|---|
| Winter (n = 16) | Summer (n = 15) | Winter (n = 9) | Summer (n = 10) | Winter (n = 8) | Summer (n = 8) | |
| Ingredients 2 | ||||||
| Maize silage (%) | 35 (±6) | 28 (±6) | 11 (±3) | 8 (±2) | 0 | 0 |
| Winter cereal silage (%) | 6 (±2) | 8 (±2) | 17 (±7) | 16 (±7) | 0 | 0 |
| Haylage (%) | 2 (±1) | 3 (±1) | 13 (±4) | 10 (±3) | 12 (±2) | 11 (±3) |
| Fresh herbage (%) | 0 | 0 | 0 | 9 (±2) | 0 | 0 |
| Hay (%) | 10 (±3) | 11 (±3) | 10 (±2) | 7 (±2) | 36 (±4) | 37 (±5) |
| Concentrates (%) | 43 (±4) | 45 (±4) | 45 (±6) | 45 (±6) | 47 (±3) | 45 (±4) |
| Residual (%) | 4 (±2) | 5 (±2) | 4 (±2) | 5 (±2) | 5 (±1) | 7 (±1) |
| Chemical traits 3 | ||||||
| Crude protein (%) | 14.6 (±0.5) | 14.5 (±0.6) | 14.3 (±0.7) | 14.5 (±0.5) | 13.9 (±0.8) | 14.1 (±0.8) |
| aNDF (%) | 35.8 (±2.7) | 35.6 (±2.6) | 35.9 (±2.7) | 36.8 (±3.5) | 38.2 (±4.4) | 38.6 (±4.2) |
| ADF | 22.1 (±1.4) | 21.9 (±1.4) | 21.8 (±1.7) | 22.6 (±2.0) | 23.4 (±2.2) | 23.8 (±2.4) |
| ADL | 3.3 (±0.3) | 3.5 (±0.3) | 3.6 (±0.4) | 3.5 (±0.5) | 3.8 (±0.3) | 4.1 (±0.5) |
| NFC (%) | 38.5 (±2.2) | 38.9 (±2.3) | 38.3 (±2.5) | 37.5 (±2.6) | 37.4 (±3.2) | 37.0 (±2.9) |
| Starch (%) | 24.4 (±2.2) | 24.6 (±2.1) | 23.3 (±2.5) | 23.1 (±2.4) | 20.9 (±2.4) | 20.5 (±2.5) |
| aNDF/starch | 1.47 | 1.45 | 1.54 | 1.59 | 1.83 | 1.88 |
| Item 1 | Feeding System 2 | Season | RMSE 3 | |||||
|---|---|---|---|---|---|---|---|---|
| EMAG | EAHF | MAGH | p-Value | Winter | Summer | p-Value | ||
| Protein, g/100 g | 3.39 b | 3.38 b | 3.48 a | 0.003 | 3.45 a | 3.38 b | 0.012 | 0.182 |
| Casein, g/100 g | 2.58 b | 2.60 b | 2.68 a | 0.008 | 2.70 a | 2.57 b | 0.009 | 0.159 |
| Fat, g/100 g | 4.00 | 3.96 | 3.95 | 0.737 | 4.07 a | 3.87 b | 0.003 | 0.223 |
| Lactose, g/100 g | 4.82 a | 4.80 a | 4.72 b | 0.001 | 4.79 | 4.76 | 0.123 | 0.073 |
| SCS, U | 3.6 | 3.7 | 4.1 | 0.103 | 3.8 | 3.8 | 0.874 | 0.573 |
| pH | 6.64 | 6.66 | 6.65 | 0.144 | 6.62 b | 6.68 a | 0.011 | 0.020 |
| β-HB, mmol/L | 0.070 | 0.067 | 0.068 | 0.924 | 0.067 | 0.070 | 0.692 | 0.031 |
| Urea (mg/dL) | 20.7 | 23.8 | 23.7 | 0.150 | 20.9 b | 24.6 a | 0.013 | 3.523 |
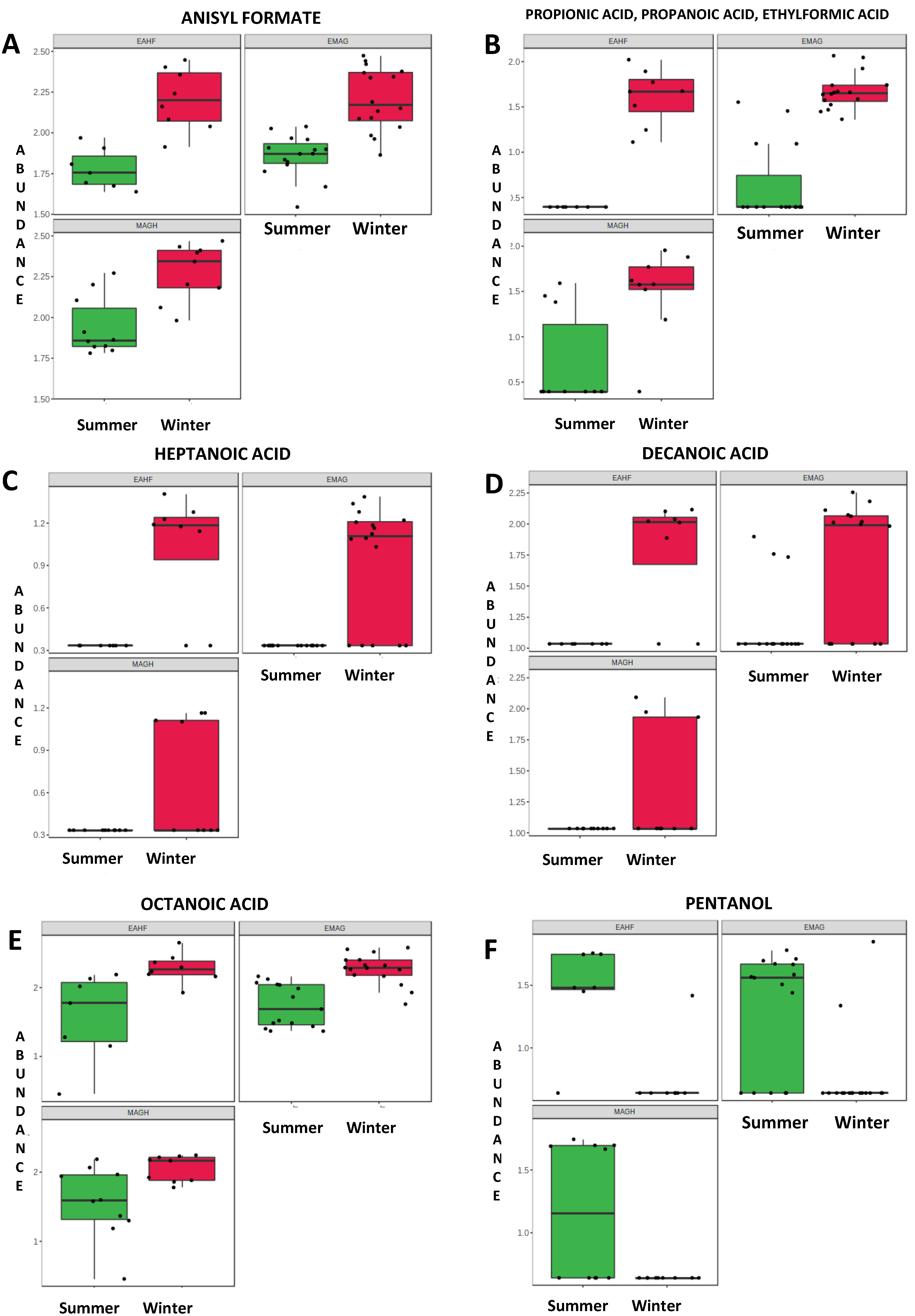
| Retention Time (min) | Name | Class | Formula | Mass | p-Value 1 |
| Winter | |||||
| 1.88 | Propionic acid | Carboxylic acid | C3H6O2 | 74.079 | 0.001 |
| 4.25 | cis-4-Heptenal | Aldehyde | C7H12O | 112.172 | 0.019 |
| 6.51 | 2-Octanone | Ketone | C8H16O | 128.215 | 0.008 |
| 9.48 | Heptanoic acid | Carboxylic acid | C7H14O2 | 130.187 | 0.001 |
| 13.63 | Octanoic acid | Carboxylic acid | C8H16O2 | 144.214 | 0.001 |
| 20.39 | Anisyl formate | Aldehyde | C9H10O3 | 166.176 | 0.001 |
| 22.06 | Decanoic acid | Carboxylic acid | C10H20O2 | 172.268 | 0.001 |
| 27.68 | Not assigned | ||||
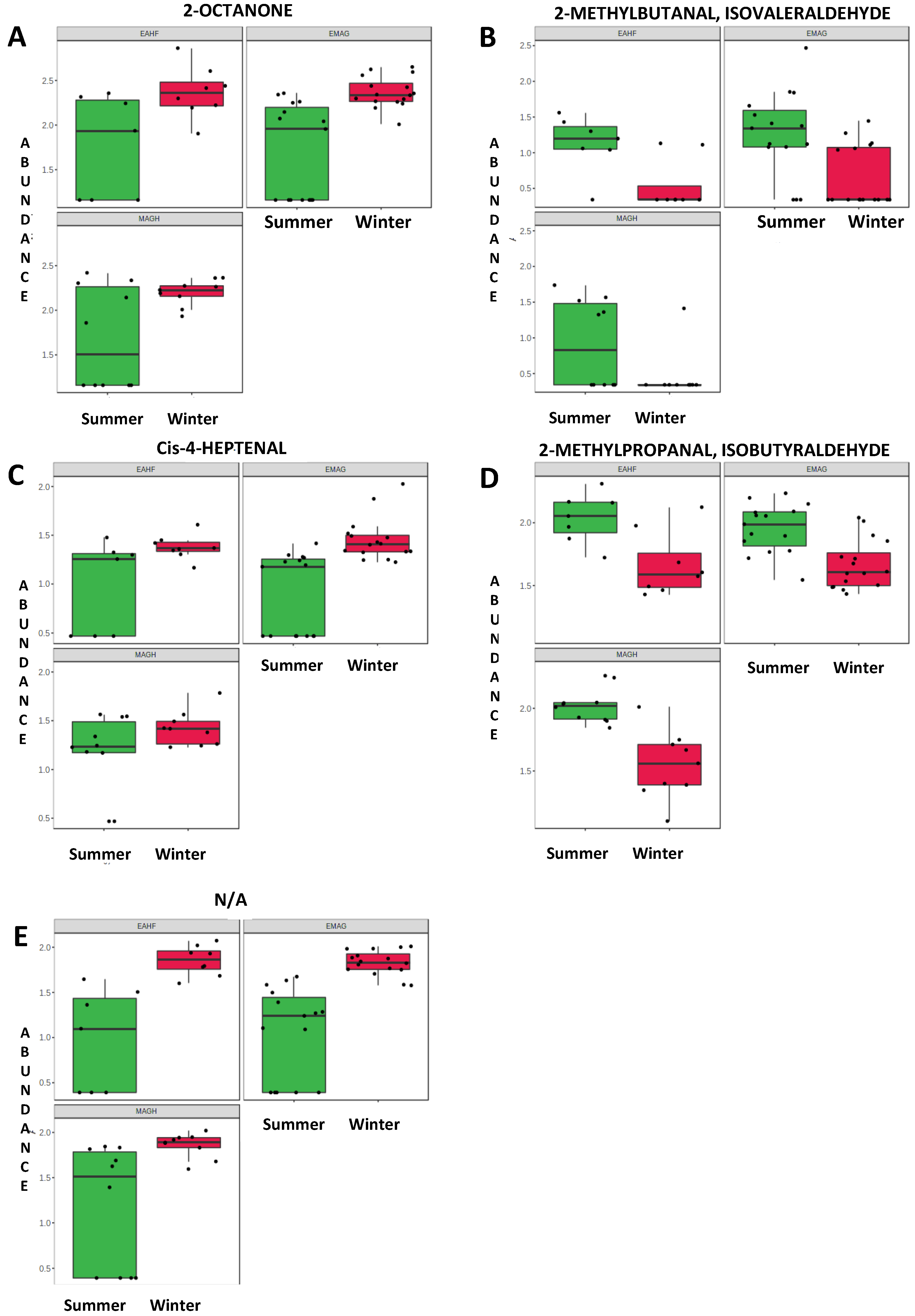
| Summer | |||||
| 1.43 | 2-methylpropanal | Aldehyde | C4H8O | 72.107 | 0.002 |
| 1.65 | 2-methylbutanal | Aldehyde | C5H10O | 86.134 | 0.022 |
| 1.96 | 2-Pentanol | Alcohol | C5H12O | 88.15 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zacometti, C.; Tata, A.; Massaro, A.; Riuzzi, G.; Bragolusi, M.; Cozzi, G.; Piro, R.; Khazzar, S.; Gerardi, G.; Gottardo, F.; et al. Seasonal Variation in Raw Milk VOC Profile within Intensive Feeding Systems. Foods 2023, 12, 1871. https://doi.org/10.3390/foods12091871
Zacometti C, Tata A, Massaro A, Riuzzi G, Bragolusi M, Cozzi G, Piro R, Khazzar S, Gerardi G, Gottardo F, et al. Seasonal Variation in Raw Milk VOC Profile within Intensive Feeding Systems. Foods. 2023; 12(9):1871. https://doi.org/10.3390/foods12091871
Chicago/Turabian StyleZacometti, Carmela, Alessandra Tata, Andrea Massaro, Giorgia Riuzzi, Marco Bragolusi, Giulio Cozzi, Roberto Piro, Sara Khazzar, Gabriele Gerardi, Flaviana Gottardo, and et al. 2023. "Seasonal Variation in Raw Milk VOC Profile within Intensive Feeding Systems" Foods 12, no. 9: 1871. https://doi.org/10.3390/foods12091871
APA StyleZacometti, C., Tata, A., Massaro, A., Riuzzi, G., Bragolusi, M., Cozzi, G., Piro, R., Khazzar, S., Gerardi, G., Gottardo, F., & Segato, S. (2023). Seasonal Variation in Raw Milk VOC Profile within Intensive Feeding Systems. Foods, 12(9), 1871. https://doi.org/10.3390/foods12091871