Abstract
The increasing threat posed by antibiotic-resistant pathogens has prompted a shift to the use of naturally-derived antimicrobial peptides (AMPs) in place of chemical preservatives in controlling foodborne pathogens. In this study, ten peptides were identified from salt-fermented shrimps (Penaeus vannamei) using ultra-performance liquid chromatography-mass spectrometry. One of the peptides, designated PV-Q5 (QVRNFPRGSAASPSALASPR), with most features of an AMP, was further explored and found to possess strong antibacterial activity against Vibrio parahaemolyticus and Escherichia coli, with a minimum inhibitory concentration of 31.25 μg/mL. Moreover, PV-Q5 increased bacterial cell membrane permeability and ruptured bacteria cell membranes, as revealed by transmission electron microscopy. Circular dichroism analysis showed that the conformation of PV-Q5 was a random coil in phosphate-buffered saline and α-helical in sodium dodecyl sulfate, which is conducive for interaction with bacteria cell membranes. These findings indicated that PV-Q5 could find potential use in food preservation to control foodborne pathogenic bacteria.
1. Introduction
Generally, most foodborne diseases are caused by pathogenic microorganisms, which account for nearly 70% of global foodborne infections yearly [1]. Among these pathogenic foodborne microbes, bacteria are the most common pathogens associated with serious foodborne disease outbreaks and are responsible for the highest number of hospitalizations (63.9%) and deaths (63.7%) globally [2]. The halophilic Gram-negative bacterium Vibrio parahaemolyticus is an important foodborne pathogen that causes food poisoning and infections, characterized by abdominal pain, nausea, and vomiting [3]. Escherichia coli are bacteria species that are commonly found in animal and human gut. Although most E. coli strains are harmless, some pathogenic strains cause diseases in the gastrointestinal tract and respiratory pneumonia [4]. As a rich nutrient source, food provides a breeding ground for all types of E. coli, some of which have caused foodborne disease outbreaks globally [5]. Thus, these two foodborne pathogens are of public health concern in the food sector [6,7].
Chemical-based preservatives are mostly used in the control of foodborne pathogenic microorganisms [8]. Unfortunately, some chemical-based preservatives are perceived as “unhealthy”, such as the potential harm of long-term consumption to the human body and the emergence of some drug-resistant strains [9]. For this reason, alternative methods and agents that can preserve foods but limit the evolution of resistant strains are being explored. Some of the agents being explored include naturally produced bioactive substances, such as antimicrobial peptides (AMPs), that are produced as part of the innate immune response in many organisms and possess specific antibacterial activity, low resistance, and few toxic side effects [10,11]. In addition to the extraction of naturally-produced AMPs from organisms, salting has been a common traditional food processing and preservation method because it induces the production of peptides, some of which have antibacterial activity [12]. For instance, the peptide RHGYM was identified from Spanish dry-cured hams and found to possess strong antibacterial activity against Listeria monocytogenes (MIC of 4.14 mg/mL), indicating that AMPs could be potentially produced in cured meat products [13].
In the current study, we screened for peptides with antibacterial activity from salt-cured Penaeus vannamei using ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) and in silico analysis. Among the low molecular weight peptides identified, the antibacterial activity of one of the peptides (designated PV-Q5) with antibacterial potential was explored against four pathogenic bacteria, Gram-negative (V. parahaemolyticus and E. coli) and Gram-positive (S. aureus and S. iniae).
2. Materials and Methods
2.1. Materials and Reagents
The bacteria strains used (i.e., Vibrio parahaemolyticus ATCC 17802, Escherichia coli ATCC 15224, Staphylococcus aureus ATCC 27217, and Streptococcus iniae ATCC 29178) were obtained from the China Center of Industrial Culture Collection (Beijing, China). All bacteria were cultured in nutrient broth (NB) medium at 37 °C. The human liver LO2 cell line was obtained from the Chinese Academy of Science Type Culture Collection (Shanghai, China) and cultured in Dulbecco’s modified eagle’s medium (DMEM) with 15% fetal bovine serum (FBS) at 37 °C in 5% CO2. DMEM and FBS were obtained from Gibco Co., Ltd. (Carlsbad, CA, USA). Shrimp (Penaeus vannamei) were purchased from a local supermarket (Xinhuadu Super Center, Xiamen, China). O-nitrophenyl-β-D-galactopyranose (ONPG), sodium dodecylbenzene sulfonate (SDS), sodium chloride (NaCl), M9 medium, and nutrient broth (NB) were purchased from LABLEAD Inc. (Beijing, China). Other analytical grade reagents (i.e., methanol, acetonitrile, lead citrate, uranyl acetate, and glutaraldehyde) were purchased from Xilong Technology Co., Ltd. (Shantou, China).
2.2. Screening and Identification of Peptides with Antimicrobial Peptides
P. vannamei were washed three times with cool distilled water and chopped into small pieces before being mixed with NaCl (30%, w/w), followed by fermentation in a laboratory fermenter (HB-EU 210, Holves Technology Co., Ltd., Beijing, China) at 37 °C for 15 days. Following this, the fermentation broth was centrifuged at 10,000 rpm/min for 10 min and desalted by solid phase extraction using a C18 cartridge (Sep-Pak, Waters, Milford, MA, USA), after which the desalted samples were digested overnight with mass-grade trypsin before being filtered through a 3 kDa ultrafiltration membrane. Next, samples were analyzed by ultra-performance liquid chromatography (UPLC, Nano Aquity UPLC system, Waters Corp, Milford, UT, USA) connected to a Thermo Q-Exactive mass spectrometer (Thermo Fisher Scientific, Milford, MA, USA) as previously described [14]. Briefly, 5.0 μL samples were injected onto a C18 column (Thermo Scientific Easy Column, 75 µm × 10 cm, 3 µm) used at a flow rate of 0.25 mL/min. Mobile phase A was 0.1% formic acid, while mobile phase B was acetonitrile. The program for the elution gradient was 0–1.0 min, 98% A, 1.0–55.0 min, 98% A–70% A, 55.0–60 min, 70% A–10% A. The mass spectrometry conditions were set as gas flow rate: 40 mL/min, auxiliary gas rate: 10 mL/min, spray voltage: 3.0 kV, capillary temperature: 300 °C, S-lens. 50%, HCD: 27%. Scan mode was positive ion, Full ms-ddms2, primary scan: resolution 70,000, range 350–1600 m/z, secondary scan: resolution 17,500, fixed first mass 120 m/z. Dynamic exclusion:10.0 s. The data obtained were searched against the P. vannamei protein database using the MAXQUANT (v1.6.5.0) software.
The identified peptides with antibacterial potential were predicted using in silico tools. The charge and hydrophobicity of peptides were calculated using the antimicrobial peptide calculator and predictor 3 (APD3) server (https://aps.unmc.edu/AP/prediction/prediction_main.php) (accessed on date 2 July 2020), while the antimicrobial characteristics of peptides were identified using the collection of anti-microbial peptides (CAMP) database (http://www.camp.bicnirrh.res.in/predict/) (accessed on date 5 August 2020). The 3D structure of peptides was predicted by ab initio calculations using the I-TASSER server (https://seq2fun.dcmb.med.umich.edu//I-TASSER/) (accessed on date 12 October 2021) [15].
2.3. Peptide Synthesis and Antibacterial Activity Determination
To determine the antibacterial activity of the peptides, PV-Q5 (QVRNFPRGSAASPSALASPR), we synthesized it by a commercial company (Scilight Biotechnology Co, Beijing, China) using the Fmoc solid-phase synthesis method as previously described [16]. The purity of the synthetic peptide determined by high-performance liquid chromatography (HPLC) was found to be 99%.
Different bacteria strains, i.e., V. parahaemolyticus, E. coli, S. aureus, and S. iniae were cultured in NB medium (8 g/L) at 37 °C for 24 h in a shaking incubator (New Brunswick, NY, USA). Bacteria cultures at the logarithmic growth phase, measured in terms of optical density at 600 nm (OD600), were used to determine the antibacterial activity of the peptides as previously described [17]. Briefly, 103 CFU/mL of the bacteria were individually incubated with different concentrations of the peptides (i.e., 500–7.8 μg/mL) for 2 h at 37 °C. Next, 20 μL of the samples were spread onto NB agar plates before incubating at 37 °C for 24 h. Bacteria colonies were counted, and the minimum inhibitory concentration (MIC) was determined, defined as the peptide concentration that inhibited 80% of bacterial growth.
The bactericidal activity of PV-Q5 was analyzed using the time–kill curve, as previously described [18], with some modifications. Briefly, PV-Q5 was diluted to 1× MIC before being mixed with diluted V. parahaemolyticus and E. coli (103 CFU/mL) and incubated at 37 °C. At different time points (i.e., 0, 1, 2, 3, 4, and 5 h), 20 μL of each sample was spread uniformly onto a glass culture dish and incubated at 37 °C for 24 h. The total number of bacteria on each plate was counted and recorded. Bacteria treated with sterile PBS (10 mM pH 7.4) were used as a control.
2.4. Bacteria Intracellular Membrane Permeability Determination
The ability of PV-Q5 to permeabilize the intracellular membrane of bacteria was determined using the level of o-nitrophenol produced as previously described [19]. Briefly, logarithmic growth phase bacteria (V. parahaemolyticus and E. coli) were collected by centrifugation (2700× g for 10 min), washed three times with PBS (10 mM pH 7.4) before being resuspended in 10 mL of sterile M9 lactose induction medium and incubated at 37 °C until the OD600nm was greater than 0.4. Next, bacteria suspensions were mixed with PV-Q5 (final concentration at the MIC value) and 30 μL of 0.5 mg/mL ONPG before being incubated at 37 °C for 2 h with mixing followed by determining the OD420nm at 1 h intervals for 8 h using a multimode plate reader (PerkinElmer, Shelton, CT, USA).
2.5. Transmission Electron Microscopy (TEM)
The effect of PV-Q5 on the ultrastructure of V. parahaemolyticus and E. coli was examined using TEM. First, logarithmic growth phase bacteria (103 CFU/mL) were mixed with PV-Q5 (MIC value) and incubated at 37 °C for 2 h. Next, samples were collected by centrifugation (2700× g for 2 min) and washed three times with PBS (10 mM pH 7.4) before being fixed with 0.27 M glutaraldehyde at 37 °C for 12 h. Samples were then dehydrated with ethanol (30%, 50%, 70%, 80%, 90%, and 100%) before being treated with acetone for 20 min, followed by baking at 70 °C for 24 h. Thin slices (70–90 nm) were prepared on a copper grid and stained with lead citrate and uranyl acetate, after which samples were observed with an H-7650 transmission electron microscope (Hitachi, Tokyo, Japan).
2.6. Circular Dichroism (CD) Spectrophotometry
The secondary structure of PV-Q5 was determined using a Chirascan V100 circular dichroism chromatograph (Applied Photophysics Inc., London, UK) as previously described [20]. Briefly, PV-Q5 was dissolved in PBS (10 mM pH 7.4) or 25 mM SDS to a final concentration of 50 μg/mL. Next, CD spectra of samples were collected from 190 to 280 nm using a bandwidth of 1 nm and scanning speed of 100 nm/min at 25 °C.
2.7. Cytotoxicity of PV-Q5
The cytotoxicity of PV-Q5 on human cells was determined by the (4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) method. Briefly, normal human hepatocytes (LO2 cells) were seeded into 96-well plates (5000 cells/well) and incubated at 37 °C for 24 h. After the media was aspirated, 100 μL of PV-Q5 (at different concentrations of 1×–16× MIC) was added to each well before being incubated at 37 °C for 24 h. Next, 20 μL of MTT (5 mg/mL) was added to each well and incubated at 37 °C for 4 h, after which the media was aspirated and 150 μL of dimethyl sulfoxide was added to each well. The absorbance at 490 nm was read on a Synergy H1 multimode plate reader (Biotech Instruments Inc., Winooski, VT, USA). Samples treated with PBS were used as control, while wells containing only the media and MTT reagent were used as blank. Cell viability was calculated using the following equation:
2.8. Statistical Analysis
All experiments were performed in triplicate, and data were presented as mean ± standard deviation. One-way ANOVA was performed using the SPSS software (version 22.0) and GraphPad Prism software (version 8.3). Differences were considered statistically significant at p < 0.05.
3. Results and Discussion
3.1. Screening and Prediction of Peptides with Antibacterial Activity
Ten peptides of molecular weights (MW) < 3 kDa were identified from salt-fermented P. vannamei (Table 1). In silico analysis revealed that identified peptides had an average of 14.5 amino acids, molecular weights of 973 to 2116 Da, hydrophobicity of 20 to 44%, and a net charge of −3 to +3. All the sequences of the ten peptides were present in the proteomes of P. vannamei.

Table 1.
Predicted antibacterial potential of peptides isolated from salt-fermented P. vannamei.
During food fermentation, bioactive peptides are accumulated due to the activities of endogenous hydrolases and the metabolic activity of salt-tolerant or halophilic microorganisms that contribute to protein hydrolysis [21]. Although the efficiency of peptide production through natural salt fermentation is lower than that of hydrolysis by proteases, it indicates that salt fermentation could generate AMPs [22]. Generally, peptides with net charges of +2 to +9 and hydrophobicity between 30 and 60% have excellent antimicrobial properties [23]. In addition, most short-chain Pro-rich peptides exhibit potent activity against Gram-negative bacteria, while Gly-rich peptides have increased selectivity while retaining high antimicrobial activity [24]. In the current study, one of the identified, designated PV-Q5 (QVRNFPRGSAASPSALASPR) had a +3 net charge, 35% hydrophobicity, 20% glycine and proline ratio, and 0.800 antimicrobial peptide prediction score. This indicates that PV-Q5 possesses most of the characteristic properties of AMPs; hence, it was chosen for further evaluation.
3.2. Antibacterial Activity of PV-Q5
To explore the antibacterial activity of PV-Q5 against a selection of pathogenic Gram-negative (V. parahaemolyticus and E. coli) and Gram-positive (S. aureus and S. iniae) bacteria, the MIC was determined. As shown in Table 2, PV-Q5 had MICs > 500 μg/mL against S. aureus and S. iniae, while that against V. parahaemolyticus and E. coli was 31.25 μg/mL. These results indicate that PV-Q5 is more efficient against having a strong antibacterial effect on Gram-negative bacteria (V. parahaemolyticus and E. coli) compared with Gram-positive bacteria (S. aureus and S. iniae). Given the highest MIC of PV-Q5 against V. parahaemolyticus and E. coli, we further analyzed the antibacterial activity using a time–kill curve. The results from the time–kill kinetic analysis revealed that when V. parahaemolyticus was treated at the MIC of PV-Q5, the bacterial numbers reduced by 64.83% within 2 h and up to 87.36% in 5 h, compared with the control (Figure 1A). Similarly, 1× MIC of PV-Q5 was able to reduce the number of E. coli by 70.53% in 2 h and by 87.42% in 5 h, compared with the control (Figure 1B). These results further show that PV-Q5 has a strong antibacterial effect on V. parahaemolyticus and E. coli.

Table 2.
Antibacterial activity of peptide PV-Q5 against Gram-negative and Gram-positive bacteria.
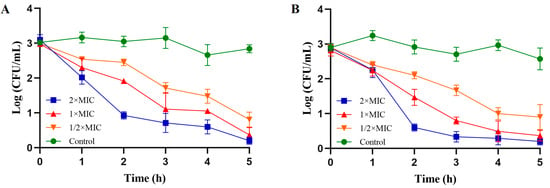
Figure 1.
Time–kill kinetics of PV-Q5 against V. parahaemolyticus and E. coli. Samples were treated with PBS (control) or different concentrations (1× MIC, 2× MIC, and ½× MIC) of PV-Q5 and (A) V. parahaemolyticus or (B) E. coli. The MIC used was 31.25 μg/mL.
Not all AMPs have broad-spectrum activity against Gram-negative and Gram-positive bacteria. Thus, the observed selective antibacterial activity of PV-Q5 against V. parahaemolyticus and E. coli (Gram-negative bacteria) is consistent with other AMPs. For example, Nisin, the naturally-derived cationic AMP produced by S. lactis and widely used as a food preservative, only has strong activity against Gram-positive bacteria (e.g., S. aureus and S. pneumoniae) and not against Gram-negative bacteria [25]. Similarly, rBPI 21, an AMP derived from the human neutrophil BPI protein, displays strong bactericidal activity against Gram-negative bacteria (e.g., E. coli) but weak activity against Gram-positive bacteria (e.g., S. aureus) [26]. Interestingly, PV-Q5 and one of our previously characterized peptides, PvHS9 [27], also isolated from the same shrimp species (P. vannamei), have different MIC against V. parahaemolyticus, i.e., the MIC of PV-Q5 (31.25 μg/mL) is lower than PvHS9 (62.5 μg/mL). The antibacterial activity of PV-Q5 and its mechanism of action against V. parahaemolyticus warrants further investigation.
3.3. Effect of PV-Q5 on Bacteria Membrane Permeability
The ability of PV-Q5 to permeabilize bacteria membranes was examined in terms of the OD420nm of O-nitrophenyl produced from O-nitrophenyl β-D-galactopyranoside after hydrolysis by bacteria membrane β-galactosidase [28]. When V. parahaemolyticus was treated with 1× MIC of PV-Q5, the OD420nm was 0.68, while that of the control was 0.57 at 1 h (Figure 2A). Similarly, treatment of E. coli with 1× MIC for 1 h produced an OD420nm of 0.65 compared to 0.53 of control (Figure 2B). Moreover, the level of O-nitrophenyl produced in terms of OD420nm upon treatment of both V. parahaemolyticus and E. coli was higher at 2× MIC of PV-Q5 compared with ½ × MIC, indicating that bacteria membrane permeability increases concentration dependently (Figure 2A,B). Thus, PV-Q5 can damage the intracellular membrane of V. parahaemolyticus and E. coli.
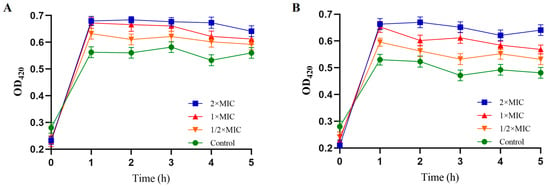
Figure 2.
Inner membrane permeability of V. parahaemolyticus and E. coli. After treatment with PBS (control) or different concentrations (1× MIC, 2× MIC, and ½× MIC) of PV-Q5 and (A) V. parahaemolyticus or (B) E.coli. The MIC of PV-Q5 used was 31.25 μg/mL.
Some AMPs can replace Mg2+ in the outer lipopolysaccharide membrane of Gram-negative bacteria to destabilize bacteria’s outer surface, which enhances the penetration of AMPs to disrupt the intracellular membrane [29]. Cationic AMPs can interfere with the formation and accumulation of negatively charged extracellular polymers between bacteria cells to change their inner membrane permeability, hence resulting in bacteria death [30].
3.4. Effect of PV-Q5 on Bacteria Membrane Ultrastructure
Transmission electron microscopy (TEM) was used to examine the effect of PV-Q5 on the membrane ultrastructure of V. parahaemolyticus and E. coli. When bacteria were treated with 1× MIC of PV-Q5 or PBS for 2 h followed by TEM examination, the ultrastructures of the cell membranes of V. parahaemolyticus and E. coli were smooth and fully dense in the PBS control group (Figure 3A,C). On the other hand, treatment with PV-Q5 caused severe destruction and deformation of V. parahaemolyticus, changing their spherical shape, into irregular bulged shapes, with the middle cell layer becoming loose compared with the control (Figure 3B). Similar structural destruction was observed in E. coli cells treated with PV-Q5, where the cytoplasmic cell density was reduced, and the cell membranes were damaged and deformed (Figure 3D). Damage to the bacterial cell membranes is manifested by the loss of lipid layer structure and a decrease in electron density [31], which was a similar phenomenon observed when V. parahaemolyticus and E. coli were treated with PV-Q5. The destruction of bacteria ultrastructure by PV-Q5 could be due to changes in bacterial membrane permeability, leading to their disruption and breakdown [32].
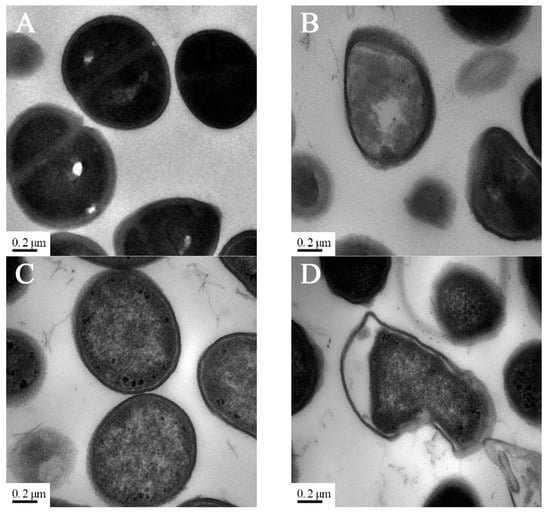
Figure 3.
TEM images of bacteria after treatment with PV-Q5. V. parahaemolyticus (103 CFU/mL) treated with (A) PBS (control) or (B) 2× MIC of PV-Q5 for 2 h. E. coli (103 CFU/mL) treated with (C) PBS (control) or (D) 2× MIC of PV-Q5 for 2 h. The MIC used was 31.25 μg/mL.
3.5. Changes in the Secondary Structure of PV-Q5
To observe the changes in the secondary structure of PV-Q5 under different conditions, we dissolved it in PBS (10 mM, pH 7.4) or SDS (25 mM) and examined it by circular dichroism (CD) spectroscopy. As shown in Figure 4A, the CD spectrum of PV-Q5 in PBS shows a negative absorption peak near the 200 nm wavelength with a random coil secondary structure. On the other hand, when PV-Q5 was in an SDS solution, it exhibited a positive absorption peak near 185 nm and negative absorption peaks near 200 nm and 220 nm, which are features of an α-helical structure (Figure 4A). These results indicated that the secondary structure of PV-Q5 undergoes transformation under different fluid conditions.
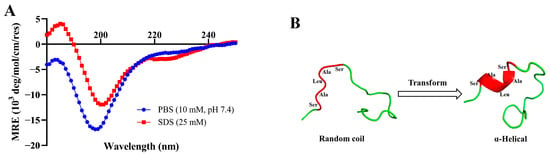
Figure 4.
Structural changes of PV-Q5 under different conditions. Changes in the structure of PV-Q5 after treatment with (A) PBS (10 mM pH 7.4) or SDS (25 mM). (B) Predicted changes in the tertiary structure of PV-Q5.
To facilitate the interaction between AMPs and bacterial cell membranes, the secondary structure of AMPs undergoes a rapid transformation when in contact with membranes [33]. The secondary structure of most AMPs exhibits random coil in PBS solution, but when in SDS solution, the hydrogen or hydrophobic bonds of AMPs unfold, changing the interaction between nonpolar side chains to form aggregates, which affects the formation of hydrogen bonds between polar side chains, and therefore induces helical structure formation [34]. In PV-Q5, the SALAS sequence contains serine (S) in the head and tail, which is an amino acid with a polar group, while the alanine (A) and leucine (L) in the middle are amino acids with nonpolar hydrophobic side chains. Thus, SDS can cause the serine-containing polar group to form a hydrogen bond, whereas the nonpolar hydrophobic side chains of alanine and leucine can aggregate with each other to curl and fold into an α-helical structure [35]. For this reason, PV-Q5 can adapt structurally from a random coil to α-helical when it binds to the liposomes of bacterial cell membranes to exert its antibacterial effect. Three-dimensional structural modeling predictions confirmed the topology for PV-Q5 (Figure 4B). PV-Q5 was predicted to have an α-helix motif, the site where it curls and folds into an α-helix is at the exact location of the five amino acids in the sequence (i.e., SALAS), which is essential for antibacterial activity.
Most of the reported AMPs are α-helical with hydrophilic and hydrophobic side chains on both sides of the α-helix that promotes interaction with bacteria cell membranes [36]. When α-helical AMPs bind to bacteria membranes, they aggregate to form pores on the membrane surface, which results in the outflow of cellular contents, a decrease in cytoplasmic density, and cell death [37]. PV-Q5 also contains three arginine residues (positively charged) and several other hydrophobic amino acid residues, which could also contribute to its antibacterial activity. The positively charged arginine residues promote electrostatic interactions, whereas the hydrophobic amino acid residues (i.e., phenylalanine, alanine, and proline) enhance hydrophobic interactions between PV-Q5 and bacterial membranes to facilitate the entry of PV-Q5 into the phospholipid bilayer of bacteria, to depolarize and disrupt the membranes and cause cell death.
3.6. Cytotoxicity Analysis of PV-Q5
To explore the cytotoxic effect of PV-Q5 on human cells, we examined the cytotoxicity of PV-Q5 on normal human cells (LO2 cells) using the MTT assay [38]. When LO2 cells were treated with various concentrations of PV-Q5 (MIC to 8× MIC), the cell viability was 95.3 ± 0.82% to 89.3 ± 1.7% (Figure 5). Even at a very high concentration of PV-Q5 (i.e., 16× MIC), the cell viability was still 88.3%, indicating that PV-Q5 has very low toxicity on LO2 cells. Moreover, the effect of PV-Q5 on cell viability was not significantly different from that of Nisin (Figure 5).
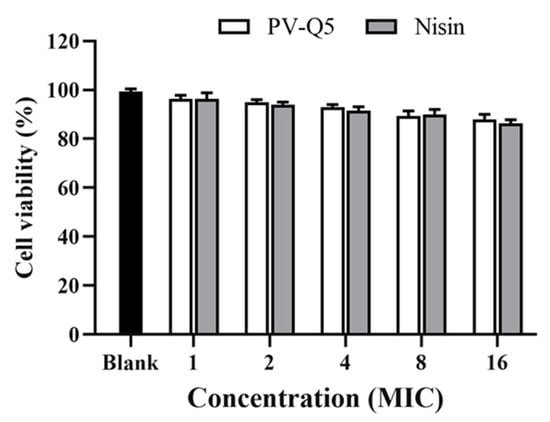
Figure 5.
Effect of PV-Q5 on the viability of normal human cells (LO2 cells). The effect of different concentrations (1× MIC–16× MIC) of PV-Q5 or Nisin on cell viability was determined using the MTT assay. The MIC of PV-Q5 was 31.25 μg/mL. Nisin was used as positive control and PBS as negative control.
Nisin is one of the AMP used in food preservation that has GRAS (generally considered as safe) status from both WHO and FDA [39]. Thus, given the strong antibacterial activity of PV-Q5 and its low toxicity on human cells (LO2 cells), it indicates that PV-Q5 could potentially be an important natural AMP (for Gram-negative bacteria) with the possibility of GRAS status since many bacteria have become more resistant to traditional antibiotics [40].
4. Conclusions
In this study, ten low molecular weight peptides (less than 3 kDa) were identified in salt-fermented shrimp (P. vannamei), among which one peptide designated PV-Q5 with most features of an AMP was further explored. PV-Q5 had strong antibacterial activity against V. parahaemolyticus and E. coli, both with MIC of 31.25 μg/mL. Structurally, PV-Q5 exhibited random coil in PBS and α-helical in SDS solution to facilitate its interaction with bacterial cell membranes. PV-Q5 could permeabilize bacteria membranes, destroying them and decreasing cytoplasmic density, hence causing bacteria death. These findings provided new insights into the potential screening for naturally-derived AMPs from salt-fermented food products for application in food preservation. Future studies would further explore the use of PV-Q5 in preserving some specific foods and also the reason for the selective action of PV-Q5 on Gram-negative bacteria and not Gram-positive bacteria.
Author Contributions
J.D.: experiments, data analysis, writing—original draft. R.J.: data analysis, writing—review and editing. J.G.: methodology. J.J.A.: software, validation. R.L.: methodology. G.L.: validation. S.Y.: supervision, resources, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Xiamen, China (3502Z20227202), Fund of Guangdong Provincial Key Laboratory of Aquatic Product Processing and Safety (GDPKLAPPS2015).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declared that they have no conflict of interest in this work.
References
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef]
- Qiao, Z.; Fu, Y.; Lei, C.; Li, Y. Advances in antimicrobial peptides-based biosensing methods for detection of foodborne pathogens: A review. Food Control 2020, 112, 107116. [Google Scholar] [CrossRef]
- Pu, Y.; Sun, L.; Wang, Y.; Qi, D.; Chen, D.; Liu, H.; Xu, D.; Deng, C.; Li, J. Modeling inhibitory activity of a novel antimicrobial peptide AMPNT-6 from Bacillus subtilis against Vibrio parahaemolyticus in shrimp under various environmental conditions. Food Control 2013, 33, 249–253. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, S.; Fei, Y.; Liu, G.; Jang, H.; Fang, J. Antimicrobial activity of anthocyanins and catechins against foodborne pathogens Escherichia coli and Salmonella. Food Control 2019, 106, 106712. [Google Scholar] [CrossRef]
- Aijuka, M.; Buys, E.M. Persistence of food borne diarrheagenic Escherichia coli in the agricultural and food peoduction environment: Implications for food safety and public health. Food Microbiol. 2019, 82, 363–370. [Google Scholar] [CrossRef]
- Munekata, P.E.; Pateiro, M.; Rodríguez-Lázaro, D.; Domínguez, R.; Zhong, J.; Lorenzo, J.M. The role of essential oils against pathogenic Escherichia coli in food products. Microorganisms 2020, 8, 924. [Google Scholar] [CrossRef]
- Zarei, M.; Borujeni, M.P.; Jamnejad, A.; Khezrzadeh, M. Seasonal prevalence of vibrio species in retail shrimps with an emphasis on Vibrio parahaemolyticus. Food Control 2012, 25, 107–109. [Google Scholar] [CrossRef]
- Abdelhamid, A.G.; El-Dougdoug, N.K. Controlling foodborne pathogens with natural antimicrobials by biological control and antivirulence strategies. Heliyon 2020, 6, e05020. [Google Scholar] [CrossRef]
- Courvalin, P. Why is antibiotic resistance a deadly emerging disease? Clin. Microbiol. Infec. 2016, 22, 405–407. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms: My perspective. Adv. Exp. Med. Biol. 2019, 1117, 3–6. [Google Scholar]
- Jiang, Y.; Chen, Y.; Song, Z.; Tan, Z.; Cheng, J. Recent advances in design of antimicrobial peptides and polypeptides toward clinical translation. Adv. Drug Deliver Rev. 2021, 170, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Tkaczewska, J. Peptides and protein hydrolysates as food preservatives and bioactive components of edible films and coatings-A review. Trends Food Sci. Technol. 2020, 106, 298–311. [Google Scholar] [CrossRef]
- Castellano, P.; Mora, L.; Escudero, E.; Vignolo, G.; Aznar, R.; Toldrá, F. Antilisterial peptides from Spanish dry-cured hams: Purification and identification. Food Microbiol. 2016, 59, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Bingham, J.P.; Mitsunaga, E.; Bergeron, Z.L. Drugs from slugs-past, present and future perspectives of ω-Conotoxin research. Chem.-Biol. Interact. 2010, 183, 1–18. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, X.; Pearce, R.; Shen, H.B.; Zhang, Y. A new protocol for atomic-level protein structure modeling and refinement using low-to-medium resolution cryo-em density maps. J. Mol. Biol. 2020, 432, 5365–5377. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, Y.; Dong, M.; Hang, B.; Sun, Y.; Wang, L.; Wang, Y.; Hu, J.; Zhang, W. Hjh-1, a broad-spectrum antimicrobial activity and low cytotoxicity antimicrobial peptide. Molecules 2018, 23, 2026. [Google Scholar] [CrossRef]
- Xedzro, C.; Tano-Debrah, K.; Nakano, H. Antibacterial efficacies and time-kill kinetics of indigenous Ghanaian spice extracts against Listeria monocytogenes and some other food-borne pathogenic bacteria. Microbiol. Res. 2022, 258, 126980. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, J.S.; Kim, E.S.; Lee, H.G. Preparation, characterization, and food application of rosemary extract-loaded antimicrobial nanoparticle dispersions. LWT-Food Sci. Technol. 2019, 101, 138–144. [Google Scholar] [CrossRef]
- Yi, L.; Li, X.; Luo, L.; Lu, Y.; Yan, H.; Qiao, Z.; Lü, X. A novel bacteriocin BMP11 and its antibacterial mechanism on cell envelope of Listeria monocytogenes and Cronobacter sakazakii. Food Control 2018, 91, 160–169. [Google Scholar] [CrossRef]
- Lan, Y.; Ye, Y.; Kozlowska, J.; Lam, J.K.; Drake, A.F.; Mason, A.J. Structural contributions to the intracellular targeting strategies of antimicrobial peptides. Biochim. Biophys. Acta 2010, 1798, 1934–1943. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Jakobsen, L.M.; Geiker, N.R.; Bertram, H.C. Chemically acidified, live and heat-inactivated fermented dairy yoghurt show distinct bioactive peptides, free amino acids and small compounds profiles. Food Chem. 2021, 376, 131919. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Baffoe, D.K.; Zhan, Y.; Zhang, M.; Li, Y.; Zhang, G. Halophile, an essential platform for bioproduction. J. Microbiol. Methods 2019, 166, 105704. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Database-guided discovery of potent peptides to combat HIV-1 or superbugs. Pharmaceuticals 2013, 6, 728–758. [Google Scholar] [CrossRef]
- Wang, J.; Dou, X.; Song, J.; Lyu, Y.; Zhu, X.; Xu, L.; Li, W.; Shan, A. Antimicrobial peptides: Promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019, 39, 831–859. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a food preservative: Part 1: Physicochemical properties, antimicrobial activity, and main uses. Crit. Rev. Food Sci. 2015, 56, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.; Patrícia, M.; Henri, G.; Filomena, A.; Miguel, A.; Nuno, N. Antimicrobial protein rBPI21-induced surface changes on gram-negative and gram-positive bacteria. Nanomedicine 2014, 10, 543–551. [Google Scholar]
- Yang, S.; Yuan, Z.; Aweya, J.J.; Huang, S.; Deng, S.; Shi, L.; Zheng, M.; Zhang, Y.; Liu, G. Low-intensity ultrasound enhances the antimicrobial activity of neutral peptide TGH2 against Escherichia coli. Ultrason. Sonochem. 2021, 77, 105676. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Wu, D.; Li, W.; Zheng, X.; Li, W.; Shan, A.; Chen, Z. High specific selectivity and membrane-active mechanism of synthetic cationic hybrid antimicrobial peptides based on the peptide fv7. Int. J. Mol. Sci. 2017, 18, 339. [Google Scholar] [CrossRef]
- Agbale, C.M.; Sarfo, J.K.; Galyuon, I.K.; Juliano, S.A.; Silva, G.; Buccini, D.F.; Buccini, D.F.; Cardoso, M.H.; Torres, M.D.; Angeles-Boza, A.M.; et al. Antimicrobial and antibiofilm activities of helical antimicrobial peptide sequences incorporating metal-binding motifs. Biochemistry 2019, 58, 3802–3812. [Google Scholar] [CrossRef]
- Martínez, M.; Polizzotto, A.; Flores, N.; Semoriel, L.; Maffía, P.C. Antibacterial, anti-biofilm and in vivo activities of the antimicrobial peptides P5 and P6.2. Microb. Pathog. 2020, 139, 103886. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, H.W.; Moon, H.; Rhee, M.S. Sodium chloride significantly enhances the bactericidal actions of carvacrol and thymol against the halotolerant species Escherichia coli O157:H7, Listeria monocytogenes, and Staphylococcus aureus. LWT-Food Sci. Technol. 2020, 122, 109015. [Google Scholar] [CrossRef]
- Amiri, E.O.; Farmani, J.; Amiri, Z.R.; Dehestani, A.; Mohseni, M. Antimicrobial activity, environmental sensitivity, mechanism of action, and food application of αs165-181 peptide. Int. J. Food Microbiol. 2021, 358, 109403. [Google Scholar] [CrossRef]
- Yang, S.; Huang, H.; Aweya, J.J.; Zheng, Z.; Liu, G.; Zhang, Y. PvHS9 is a novel in silico predicted antimicrobial peptide derived from hemocyanin of Penaeus vannamei. Aquaculture 2021, 530, 735926. [Google Scholar] [CrossRef]
- Duplantier, A.J.; Hoek, M.L. The human cathelicidin antimicrobial peptide LL-37 as a potential treatment for polymicrobial infected wounds. Front. Immunol. 2013, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Shelar, A.; Bansal, M. Helix perturbations in membrane proteins assist in inter-helical interactions and optimal helix positioning in the bilayer. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2804–2817. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Y.; Xue, Z.; Jia, Y.; Li, R.; He, C.; Chen, H. The structure-mechanism relationship and mode of actions of antimicrobial peptides: A review. Trends Food Sci. Technol. 2021, 109, 103–115. [Google Scholar] [CrossRef]
- Raja, Z.; André, S.; Piesse, C.; Sereno, D.; Nicolas, P.; Foulon, T.; Oury, B.; Ladram, A. Structure, antimicrobial activities and mode of interaction with membranes of novel Phylloseptins from the painted-belly leaf frog, Phyllomedusa sauvagii. PLoS ONE 2013, 8, e70782. [Google Scholar] [CrossRef]
- Armas, F.; Stasi, A.D.; Mardirossian, M.; Romani, A.A.; Benincasa, M.; Scocchi, M. Effects of lipidation on a proline-rich antibacterial peptide. Int. J. Mol. Sci. 2021, 22, 7959. [Google Scholar] [CrossRef]
- Mendes-Oliveira, G.; Gu, G.; Luo, Y.; Zografos, A.; Minas, I.; Nou, X. Edible and water-soluble corn zein coating impregnated with nisin for Listeria monocytogenes reduction on nectarines and apples. Postharvest Biol. Technol. 2022, 185, 111811. [Google Scholar] [CrossRef]
- Yu, X.; Lu, N.; Wang, J.; Chen, Z.; Chen, C.; Mac Regenstein, J.; Zhou, P. Effect of N-terminal modification on the antimicrobial activity of nisin. Food Control 2020, 114, 107227. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).