LC-MS/MS Method Minimizing Matrix Effect for the Analysis of Bifenthrin and Butachlor in Chinese Chives and Its Application for Residual Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Standard Solutions and Calibration Curves
2.3. LC–MS/MS Analytical Conditions
2.4. Comparison of the Sample Extraction and Partitioning
2.5. Comparison of the Sample Purification
2.6. The Established Method
2.7. Method Validation
2.8. Field Experiment and Residue Analysis
2.9. Pesticide Residue Characteristics
3. Results and Discussion
3.1. The Established MRM Conditions
3.2. Optimization of the Sample Extraction and Partitioning
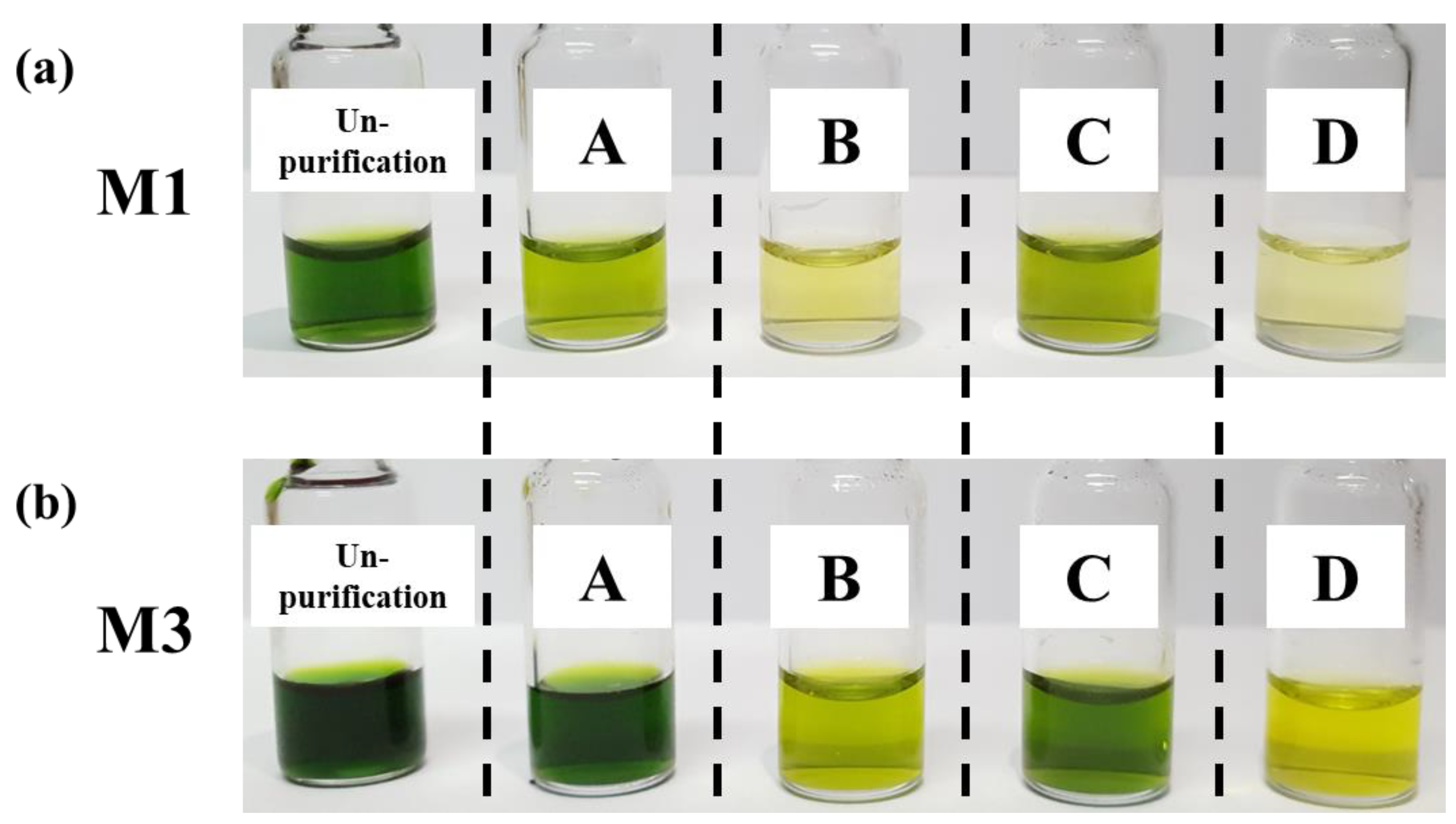
3.3. Optimization of Sample Purification
3.4. Method Validation
3.5. Determination of the Pesticide Residues from the Field Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trufelli, H.; Palma, P.; Famiglini, G.; Cappiello, A. An overview of matrix effects in liquid chromatography–mass spectrometry. Mass Spectrom. Rev. 2011, 30, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.; Wagrowski-Diehl, D.M.; Lu, Z.; Mazzeo, J.R. Systematic and comprehensive strategy for reducing matrix effects in LC/MS/MS analyses. J. Chromatogr. B 2007, 852, 22–34. [Google Scholar] [CrossRef]
- Hajšlová, J.; Zrostlíková, J. Matrix effects in (ultra)trace analysis of pesticide residues in food and biotic matrices. J. Chromatogr. A 2003, 1000, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Alder, L.; Greulich, K.; Kempe, G.; Vieth, B. Residue analysis of 500 high priority pesticides: Better by GC–MS or LC–MS/MS? Mass Spectrom. Rev. 2006, 25, 838–865. [Google Scholar] [CrossRef]
- Choi, S.; Kim, S.; Shin, J.Y.; Kim, M.; Kim, J.-H. Development and verification for analysis of pesticides in eggs and egg products using QuEChERS and LC–MS/MS. Food Chem. 2015, 173, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.S.; Utture, S.; Banerjee, K.; Ahammed Shabeer, T.P.; Kamble, N.; Mathew, S.; Ashok Kumar, K. Multiresidue analysis of multiclass pesticides and polyaromatic hydrocarbons in fatty fish by gas chromatography tandem mass spectrometry and evaluation of matrix effect. Food Chem. 2016, 196, 1–8. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, J.; Park, E.; Lee, J.; Lee, H.S.; Kim, J.-H. A Quantitative tandem mass spectrometry and scaled-down QuEChERS approach for simultaneous analysis of pesticide multiresidues in human urine. Molecules 2019, 24, 1330. [Google Scholar] [CrossRef]
- Park, E.; Lee, J.; Lee, J.; Lee, J.; Lee, H.S.; Shin, Y.; Kim, J.-H. Method for the simultaneous analysis of 300 pesticide residues in hair by LC-MS/MS and GC-MS/MS, and its application to biomonitoring of agricultural workers. Chemosphere 2021, 277, 130215. [Google Scholar] [CrossRef]
- Park, E.; Lee, J.; Lee, H.S.; Kim, J.-H.; Shin, Y. Simple and rapid method for 336 multiresidual pesticide analysis in saliva, determination of their chemical stabilities, and biomonitoring of farmers. Chemosphere 2022, 309, 136725. [Google Scholar] [CrossRef]
- Poole, C.F. Matrix-induced response enhancement in pesticide residue analysis by gas chromatography. J. Chromatogr. A 2007, 1158, 241–250. [Google Scholar] [CrossRef]
- Damale, R.D.; Dutta, A.; Shaikh, N.; Pardeshi, A.; Shinde, R.; Babu, K.D.; Gaikwad, N.N.; Banerjee, K. Multiresidue analysis of pesticides in four different pomegranate cultivars: Investigating matrix effect variability by GC-MS/MS and LC-MS/MS. Food Chem. 2023, 407, 135179. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.F. New trends in solid-phase extraction. TrAC Trends Anal. Chem. 2003, 22, 362–373. [Google Scholar] [CrossRef]
- Ścigalski, P.; Kosobucki, P. Recent Materials Developed for Dispersive Solid Phase Extraction. Molecules 2020, 25, 4869. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.-C.; Cheng, W.-S. Antioxidant Activity of Several Allium Members. J. Agric. Food Chem. 1998, 46, 4097–4101. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Z.; Wang, H.; Chen, X.; Yang, J.; Han, A.; Lin, D.-H.; Hong, J. Underlying action mechanism of a novel antioxidant peptide derived from Allium tuberosum Rottler protein hydrolysates and its protective effects on hydrogen peroxide induced cell injury. J. Funct. Foods 2018, 40, 606–613. [Google Scholar] [CrossRef]
- Park, H.S.; Choi, E.J.; Lee, J.-H.; Kim, G.-H. Evaluation of Allium Vegetables for Anti-Adipogenic, Anti-Cancer, and Anti-Inflammatory Activities In Vitro. J. Life Sci. 2013, 5, 127–132. [Google Scholar]
- Choudhary, R. Benificial effect of Allium sativum and Allium tuberosum on experimental hyperlipidemia and atherosclerosis. Pak. J. Physiol. 2008, 4, 7–9. [Google Scholar]
- Zhou, S.-M.; Chen, L.-M.; Liu, S.-Q.; Wang, X.-F.; Sun, X.-D. De Novo Assembly and Annotation of the Chinese Chive (Allium tuberosum Rottler ex Spr.) Transcriptome Using the Illumina Platform. PLoS ONE 2015, 10, e0133312. [Google Scholar] [CrossRef]
- Oh, M.; Kim, S.-Y.; Park, S.; Kim, K.-N.; Kim, S.H. Phytochemicals in Chinese Chive (Allium tuberosum) Induce the Skeletal Muscle Cell Proliferation via PI3K/Akt/mTOR and Smad Pathways in C2C12 Cells. Int. J. Mol. Sci. 2021, 22, 2296. [Google Scholar] [CrossRef]
- Nath, R.; Singha, S.; Nath, D.; Das, G.; Patra, J.K.; Talukdar, A.D. Phytochemicals from Allium tuberosum Rottler ex Spreng Show Potent Inhibitory Activity against B-Raf, EGFR, K-Ras, and PI3K of Non-Small Cell Lung Cancer Targets. Appl. Sci. 2022, 12, 11749. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Tamura, M.; Takahashi, A.; Uyama, A.; Mochizuki, N. A Method for Multiple Mycotoxin Analysis in Wines by Solid Phase Extraction and Multifunctional Cartridge Purification, and Ultra-High-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Toxins 2012, 4, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Li, C.; Liu, X.; Zhou, S.; Ma, Y. Graphene as dispersive solidphase extraction materials for pesticides LC-MS/MS multi-residue analysis in leek, onion and garlic. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk. Assess. 2014, 31, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A. The Pesticide Manual: A World Compendium, 17th ed.; British Crop Production Council: Hampshire, UK, 2015. [Google Scholar]
- Gammon, D.W.; Liu, Z.; Chandrasekaran, A.; El-Naggar, S.F.; Kuryshev, Y.A.; Jackson, S. Pyrethroid neurotoxicity studies with bifenthrin indicate a mixed Type I/II mode of action. Pest Manage. Sci. 2019, 75, 1190–1197. [Google Scholar] [CrossRef]
- Götz, T.; Böger, P. The Very-Long-Chain Fatty Acid Synthase Is Inhibited by Chloroacetamides. Z. Für Nat. C 2004, 59, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Abigail, M.E.A.; Samuel, S.M.; Ramalingam, C. Addressing the environmental impacts of butachlor and the available remediation strategies: A systematic review. Int. J. Environ. Sci. Technol. 2015, 12, 4025–4036. [Google Scholar] [CrossRef]
- Kaur, P.; Randhawa, S.K.; Duhan, A.; Bhullar, M.S. Influence of Long Term Application of Butachlor on its Dissipation and Harvest Residues in Soil and Rice. Bull. Environ. Contam. Toxicol. 2017, 98, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Safety. Mrls in Pesticide. Available online: https://residue.foodsafetykorea.go.kr/prd/mrl (accessed on 5 February 2023).
- Moura, A.C.M.; Lago, I.N.; Cardoso, C.F.; dos Reis Nascimento, A.; Pereira, I.; Vaz, B.G. Rapid monitoring of pesticides in tomatoes (Solanum lycopersicum L.) during pre-harvest intervals by paper spray ionization mass spectrometry. Food Chem. 2020, 310, 125938. [Google Scholar] [CrossRef]
- Fantke, P.; Juraske, R. Variability of Pesticide Dissipation Half-Lives in Plants. Environ. Sci. Technol. 2013, 47, 3548–3562. [Google Scholar] [CrossRef]
- Wang, J.; Chow, W.; Chang, J.; Wong, J.W. Development and Validation of a Qualitative Method for Target Screening of 448 Pesticide Residues in Fruits and Vegetables Using UHPLC/ESI Q-Orbitrap Based on Data-Independent Acquisition and Compound Database. J. Agric. Food Chem. 2017, 65, 473–493. [Google Scholar] [CrossRef]
- Zhou, H.; Cao, Y.-M.; Miao, S.; Lan, L.; Chen, M.; Li, W.-T.; Mao, X.-H.; Ji, S. Qualitative screening and quantitative determination of 569 pesticide residues in honeysuckle using ultrahigh-performance liquid chromatography coupled to quadrupole-Orbitrap high resolution mass spectrometry. J. Chromatogr. A 2019, 1606, 460374. [Google Scholar] [CrossRef]
- European Commission. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed (SANTE/11312/2021). Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11312_2021.pdf (accessed on 5 February 2023).
- Kecojević, I.; Đekić, S.; Lazović, M.; Mrkajić, D.; Baošić, R.; Lolić, A. Evaluation of LC-MS/MS methodology for determination of 179 multi-class pesticides in cabbage and rice by modified QuEChERS extraction. Food Control 2021, 123, 107693. [Google Scholar] [CrossRef]
- Ferrer, C.; Lozano, A.; Agüera, A.; Girón, A.J.; Fernández-Alba, A.R. Overcoming matrix effects using the dilution approach in multiresidue methods for fruits and vegetables. J. Chromatogr. A 2011, 1218, 7634–7639. [Google Scholar] [CrossRef]
- Kmellár, B.; Fodor, P.; Pareja, L.; Ferrer, C.; Martínez-Uroz, M.A.; Valverde, A.; Fernandez-Alba, A.R. Validation and uncertainty study of a comprehensive list of 160 pesticide residues in multi-class vegetables by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2008, 1215, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Rogatsky, E.; Stein, D. Evaluation of matrix effect and chromatography efficiency: New parameters for validation of method development. J. Am. Soc. Mass Spectrom. 2005, 16, 1757–1759. [Google Scholar] [CrossRef] [PubMed]
- Besil, N.; Pequeño, F.; Alonzo, N.; Hladki, R.; Cesio, M.V.; Heinzen, H. Evaluation of different QuEChERS procedures for pesticide residues determination in Calendula officinalis (L) inflorescences. J. Appl. Res. Med. Aromat. Plants 2017, 7, 143–148. [Google Scholar] [CrossRef]
- Varela-Martínez, D.A.; González-Curbelo, M.Á.; González-Sálamo, J.; Hernández-Borges, J. Analysis of multiclass pesticides in dried fruits using QuEChERS-gas chromatography tandem mass spectrometry. Food Chem. 2019, 297, 124961. [Google Scholar] [CrossRef]
- Kim, B.J.; Yang, S.-H.; Choi, H. Simultaneous Determination of Pyrethroid Insecticides in Foods of Animal Origins Using the Modified QuEChERS Method and Gas Chromatography-Mass Spectrometry. Foods 2022, 11, 3634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, G.; Zhang, L.; Chen, H.; Zhu, L.; Wang, C.; Liu, X. Chitosan-reduced graphene oxide composites with 3D structures as effective reverse dispersed solid phase extraction adsorbents for pesticides analysis. Analyst 2019, 144, 5164–5171. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Xu, J.; Dong, F.; Liang, X.; Li, M.; Duan, L.; Zheng, Y. Simultaneous determination of spirotetramat and its four metabolites in fruits and vegetables using a modified quick, easy, cheap, effective, rugged, and safe method and liquid chromatography/tandem mass spectrometry. J. Chromatogr. A 2013, 1299, 71–77. [Google Scholar] [CrossRef]
- Pinto, M.I.; Micaelo, C.; Vale, C.; Sontag, G.; Noronha, J.P. Screening of Priority Pesticides in Ulva sp. Seaweeds by Selective Pressurized Solvent Extraction Before Gas Chromatography with Electron Capture Detector Analysis. Arch. Environ. Contam. Toxicol. 2014, 67, 547–556. [Google Scholar] [CrossRef]
- Ferrer, C.; Martínez-Bueno, M.J.; Lozano, A.; Fernández-Alba, A.R. Pesticide residue analysis of fruit juices by LC–MS/MS direct injection. One year pilot survey. Talanta 2011, 83, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Rajski, Ł.; Lozano, A.; Uclés, A.; Ferrer, C.; Fernández-Alba, A.R. Determination of pesticide residues in high oil vegetal commodities by using various multi-residue methods and clean-ups followed by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2013, 1304, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Huang, B.; Zhang, J.; Han, Y.; Li, Y.; Zou, N.; Yang, J.; Pan, C. Analytical method for 44 pesticide residues in spinach using multi-plug-filtration cleanup based on multiwalled carbon nanotubes with liquid chromatography and tandem mass spectrometry detection. J. Sep. Sci. 2016, 39, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Yaqub, G.; Hafeez, T.; Tariq, M. Assessment of Health Risk due to Pesticide Residues in Fruits, Vegetables, Soil, and Water. J. Chem. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- European Commission. Pesticide Residue(s) and Maximum Residue Levels (mg/kg). Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/mrls/details?lg_code=EN&pest_res_id_list=28&product_id_list= (accessed on 5 February 2023).
- Park, N.-I.; Lee, I.-Y.; Park, J.-H.; Lim, T.-K.; Chun, J.-C.; Kim, K.-H.; Park, J.-E. Residue Patterns of Butachlor and Pyrazosulfuron-ethyl in Soil, Water and Products of Transplanted Rice Paddy Field. Kor. J. Weed Sci. 2007, 27, 49–55. [Google Scholar]
- Lee, J.-K. Degradation of the herbicide, butachlor, in soil. Appl. Biol. Chem. 1983, 26, 53–57. [Google Scholar]
- Park, J.-W.; Son, K.-A.; Kim, T.-H.; Chae, S.; Sim, J.-R.; Bae, B.-J.; Lee, H.-K.; Im, G.-J.; Kim, J.-B.; Kim, J.-E. Comparision of the Residue Property of Insecticide Bifenthrin and Chlorfenapyr in Green Onion and Scallion under Greenhouse Condition. Korean J. Pestic. Sci. 2012, 16, 294–301. [Google Scholar] [CrossRef]
- Reddy, A.A.; Vemuri, S.; Cherukuri, S.R. Dissipation pattern of bifenthrin in cabbage (Brassica oleracea var. capitata). Indian J. Adv. Plant Res. 2014, 1, 51–56. [Google Scholar]
- Lee, J.; Jung, M.W.; Lee, J.; Lee, J.; Shin, Y.; Kim, J.-H. Dissipation of the Insecticide Cyantraniliprole and Its Metabolite IN-J9Z38 in Proso Millet during Cultivation. Sci. Rep. 2019, 9, 11648. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, E.; Zhang, W.; Baccarelli, A.A.; Gao, F. Predicting pesticide dissipation half-life intervals in plants with machine learning models. J. Hazard. Mater. 2022, 436, 129177. [Google Scholar] [CrossRef] [PubMed]


| Pesticide | tR (min) | Monoisotopic Mass | Ionization Type of Precursor Ion | Precursor Ion > Product Ion (CE, V) | |
|---|---|---|---|---|---|
| Quantifier | Qualifier | ||||
| Bifenthrin | 5.1 | 422.1 | [M + NH4]+ | 440.2 > 181.2 (−15) | 440.2 > 166.1 (−41) |
| Butachlor | 4.2 | 311.2 | [M + H]+ | 312.3 > 162.2 (−21) | 312.3 > 147.2 (−35) |
| Method | Extraction Solvent | Partitoning Salt Type | Recovery (n = 3) | ME (n = 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bifenthrin | Butachlor | Bifenthrin | Butachlor | |||||||
| Value (%) | RSD (%) | Value (%) | RSD (%) | Value (%) | RSD (%) | Value (%) | RSD (%) | |||
| M1 | MeCN | Unbuffered 1 | 93.7 | 4.7 | 100.0 | 0.2 | –41.7 | 1.3 | –20.4 | 4.8 |
| M2 | MeCN | Citrate-buffer 2 | 91.6 | 0.9 | 99.2 | 1.2 | –41.2 | 7.8 | –22.9 | 7.4 |
| M3 | MeCN/EA 3 | Unbuffered | 102.2 | 4.6 | 91.2 | 6.2 | –44.2 | 5.9 | –22.6 | 3.3 |
| M4 | MeCN/EA | Citrate-buffer | 100.4 | 6.8 | 94.5 | 7.4 | –47.9 | 14.6 | –33.0 | 12.5 |
| M5 | EA | Unbuffered | 95.4 | 18.3 | 91.1 | 8.7 | –50.5 | 24.5 | –34.6 | 17.1 |
| M6 | EA | Citrate-buffer | 83.8 | 26.9 | 76.4 | 20.6 | –45.1 | 30.1 | –29.5 | 20.2 |
| Method | PurificationMethod | Recovery | Matrix Effect | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bifenthrin | Butachlor | Bifenthrin | Butachlor | ||||||
| Value (%) | RSD (%) | Value (%) | RSD (%) | Value (%) | RSD (%) | Value (%) | RSD (%) | ||
| M1 | Untreated | 93.7 | 4.7 | 100.0 | 0.2 | –41.7 | 1.3 | –20.4 | 4.8 |
| M3 | Untreated | 102.2 | 4.6 | 91.2 | 6.2 | –44.2 | 5.9 | –22.6 | 3.3 |
| M1-A | PSA 1 | 98.2 | 3.5 | 104.0 | 4.2 | –3.2 | 2.5 | –16.5 | 3.0 |
| M3-A | PSA | 101.8 | 1.7 | 89.4 | 10.5 | –20.9 | 1.4 | –14.3 | 7.6 |
| M1-B | PSA + GCB 2 | 99.3 | 5.1 | 99.7 | 3.9 | –9.0 | 1.1 | 1.9 | 3.2 |
| M3-B | PSA + GCB | 96.2 | 4.5 | 90.1 | 6.1 | –7.6 | 3.2 | –1.5 | 4.6 |
| M1-C | HLB 3 | 102.6 | 3.5 | 103.5 | 2.9 | –31.8 | 3.4 | –24.2 | 4.6 |
| M3-C | HLB | 109.9 | 3.5 | 93.3 | 4.1 | –32.3 | 1.3 | –23.7 | 7.4 |
| M1-D | PSA + GCB/HLB 4 | 100.5 | 5.0 | 107.1 | 6.2 | –20.4 | 2.6 | –16.3 | 5.9 |
| M3-D | PSA + GCB/HLB | 109.7 | 5.2 | 92.6 | 4.5 | –29.2 | 1.1 | –21.5 | 11.0 |
| Pesticide | Study | Concentration (mg/kg) | Storage Period (days) | Accuracy (%) | RSD (n = 3) (%) |
|---|---|---|---|---|---|
| Bifenthrin | Recovery | 0.01 | - | 101.3 | 1.8 |
| 0.1 | - | 99.5 | 5.9 | ||
| 2 | - | 88.0 | 4.0 | ||
| Storage stability | 0.1 | 38 | 109.6 | 1.4 | |
| Butachlor | Recovery | 0.01 | - | 107.3 | 2.6 |
| 0.1 | - | 106.0 | 2.9 | ||
| Storage stability | 0.1 | 25 | 104.3 | 4.9 |
| Crop | Sample | Source | Matrix Effect (% ME) | |||
|---|---|---|---|---|---|---|
| Bifenthrin | Butachlor | |||||
| Value (%) | RSD (%) | Value (%) | RSD (%) | |||
| C1 | Korean chives 1 | Commercial market (used for method validation) | −5.2 | 3.2 | −5.1 | 4.4 |
| C2 | Korean chives 2 | Jeonju-si (harvested in the study) | −7.2 | 0.7 | 2.8 | 1.4 |
| C3 | Korean chives 3 | Jincheon-gun (harvested in the study) | −18.8 | 0.7 | 2.1 | 5.2 |
| C4 | Chinese chives | Commercial market | −0.1 | 4.7 | 3.0 | 3.1 |
| Sh | Shallot | Commercial market | 4.2 | 0.7 | 1.9 | 3.8 |
| Sp | Spinach | Commercial market | −11.0 | 1.9 | 7.2 | 0.9 |
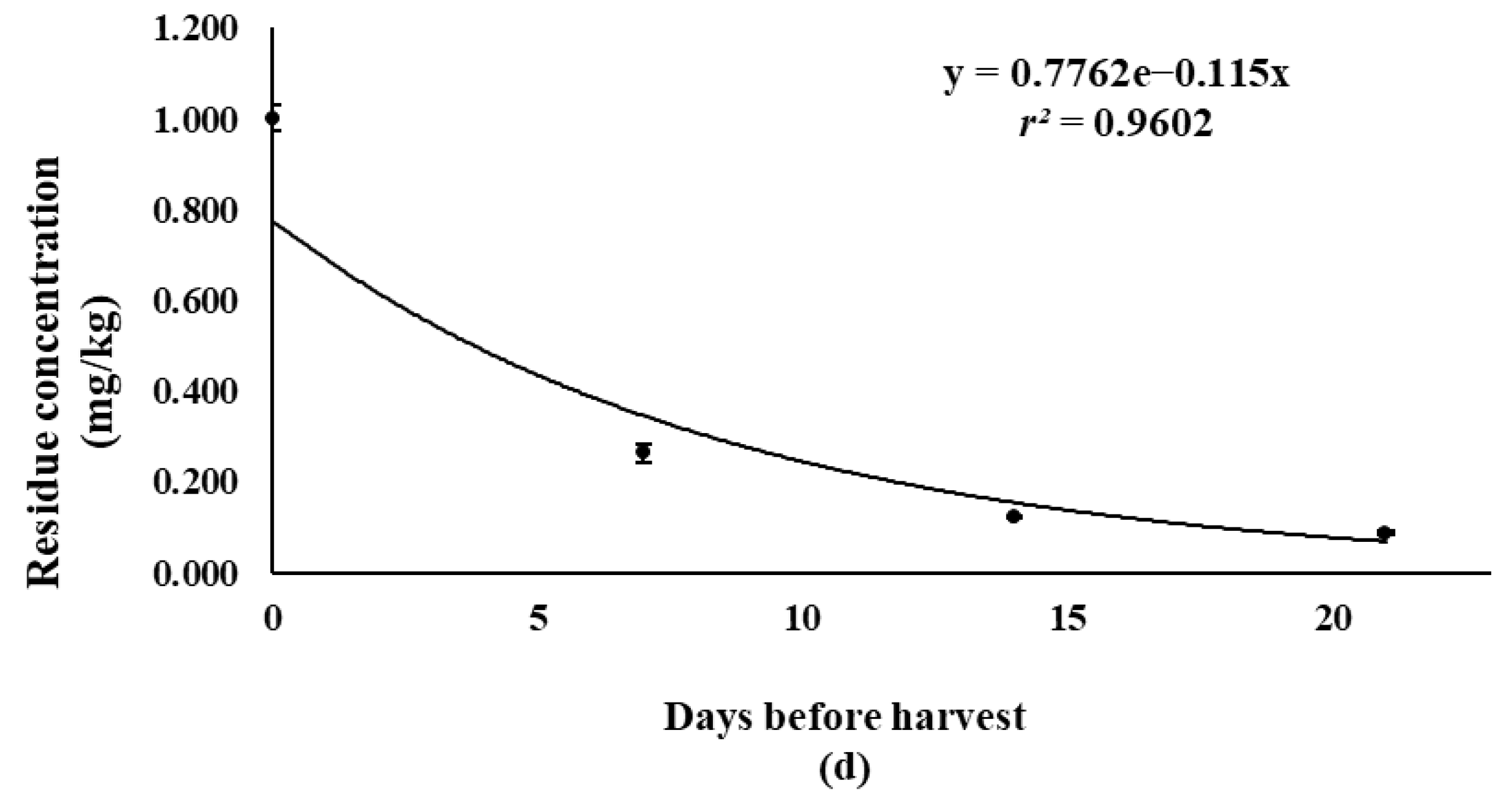
| Pesticide | Treatment Group | Pesticide Spraying Days Before Harvest | Concentration (mg/kg) | Average ± SD 1 (mg/kg) | ||
|---|---|---|---|---|---|---|
| Rep. 1 | Rep. 2 | Rep. 3 | ||||
| Bifenthrin | T1 | 7-0 | 0.971 | 1.025 | 1.010 | 1.002 ± 0.028 |
| T2 | 14-7 | 0.279 | 0.270 | 0.241 | 0.263 ± 0.020 | |
| T3 | 21-14 | 0.122 | 0.126 | 0.124 | 0.124 ± 0.002 | |
| T4 | 30-21 | 0.085 | 0.091 | 0.087 | 0.087 ± 0.003 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Lee, Y.-H.; Jeong, M.-J.; Gwon, D.-Y.; Lee, J.-H.; Shin, Y.; Choi, H. LC-MS/MS Method Minimizing Matrix Effect for the Analysis of Bifenthrin and Butachlor in Chinese Chives and Its Application for Residual Study. Foods 2023, 12, 1683. https://doi.org/10.3390/foods12081683
Kim S-H, Lee Y-H, Jeong M-J, Gwon D-Y, Lee J-H, Shin Y, Choi H. LC-MS/MS Method Minimizing Matrix Effect for the Analysis of Bifenthrin and Butachlor in Chinese Chives and Its Application for Residual Study. Foods. 2023; 12(8):1683. https://doi.org/10.3390/foods12081683
Chicago/Turabian StyleKim, So-Hee, Yoon-Hee Lee, Mun-Ju Jeong, Da-Yeong Gwon, Ji-Ho Lee, Yongho Shin, and Hoon Choi. 2023. "LC-MS/MS Method Minimizing Matrix Effect for the Analysis of Bifenthrin and Butachlor in Chinese Chives and Its Application for Residual Study" Foods 12, no. 8: 1683. https://doi.org/10.3390/foods12081683
APA StyleKim, S.-H., Lee, Y.-H., Jeong, M.-J., Gwon, D.-Y., Lee, J.-H., Shin, Y., & Choi, H. (2023). LC-MS/MS Method Minimizing Matrix Effect for the Analysis of Bifenthrin and Butachlor in Chinese Chives and Its Application for Residual Study. Foods, 12(8), 1683. https://doi.org/10.3390/foods12081683





