Effects of Ficus pandurata Hance var. angustifolia Cheng Flavonoids on Intestinal Barrier and Cognitive Function by Regulating Intestinal Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Flavonoids from Ficus pandurata Hance var. angustifolia Cheng
2.3. Animal Experiment Design
2.4. Behavioral Assessment
2.5. Experimental Tissue Sampling
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Histological Examination
2.8. Detection of Occludin and ZO-1 Expression in Intestinal Tissue by Immunohistochemical Method
2.9. Immunofluorescence Detection Test
2.10. Analysis of Intestinal Flora
2.11. Metabolite Extraction and LC−MS Analysis
2.12. Statistical Analysis
3. Results
3.1. The Contents of FCF in the Extract
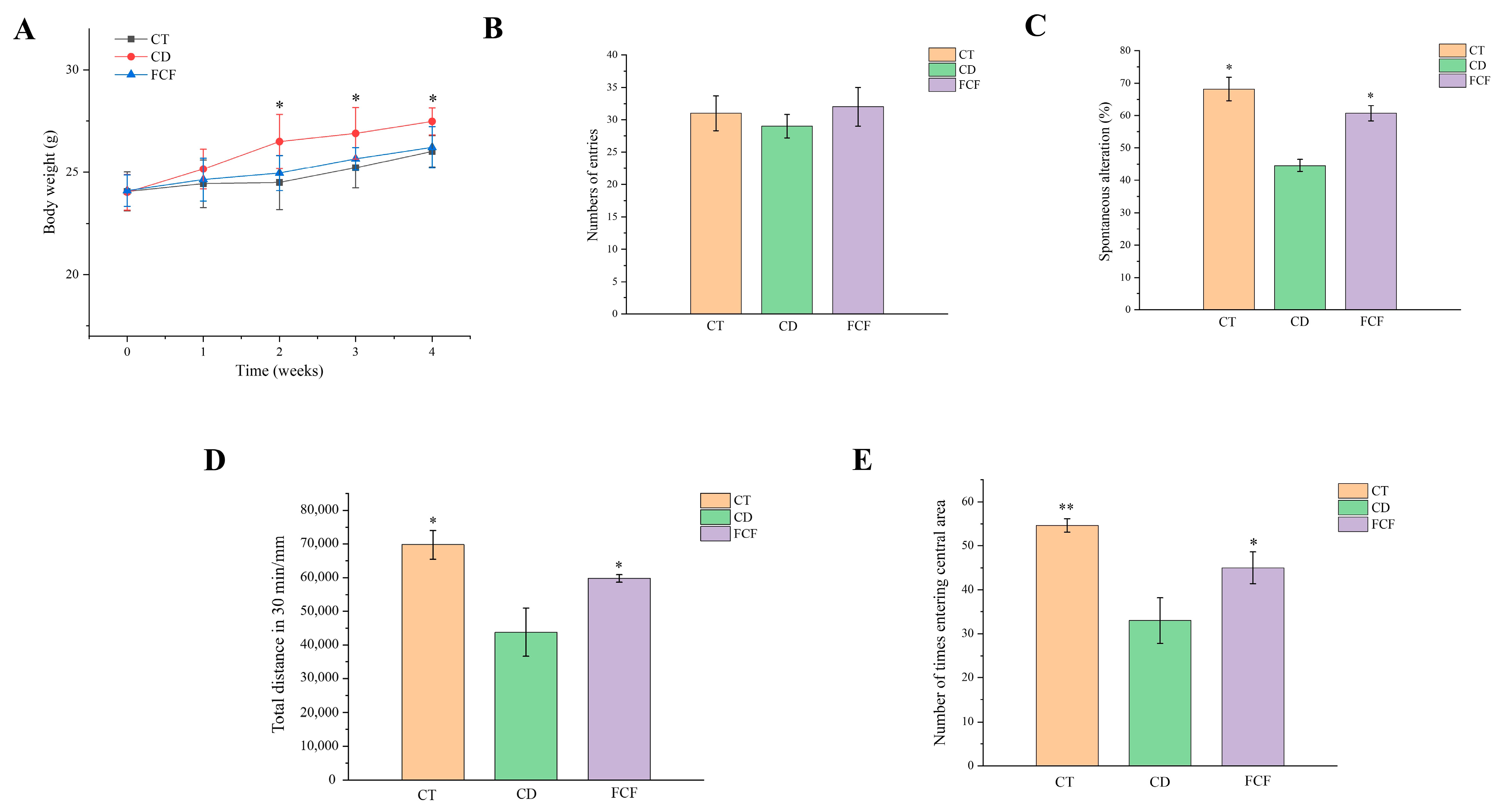
3.2. Effect of FCF on Body Weight and Caloric Intake in Mice with Circadian Rhythm Disorder
3.3. Effects of FCF on Cognitive Activity in Mice with Circadian Disturbance
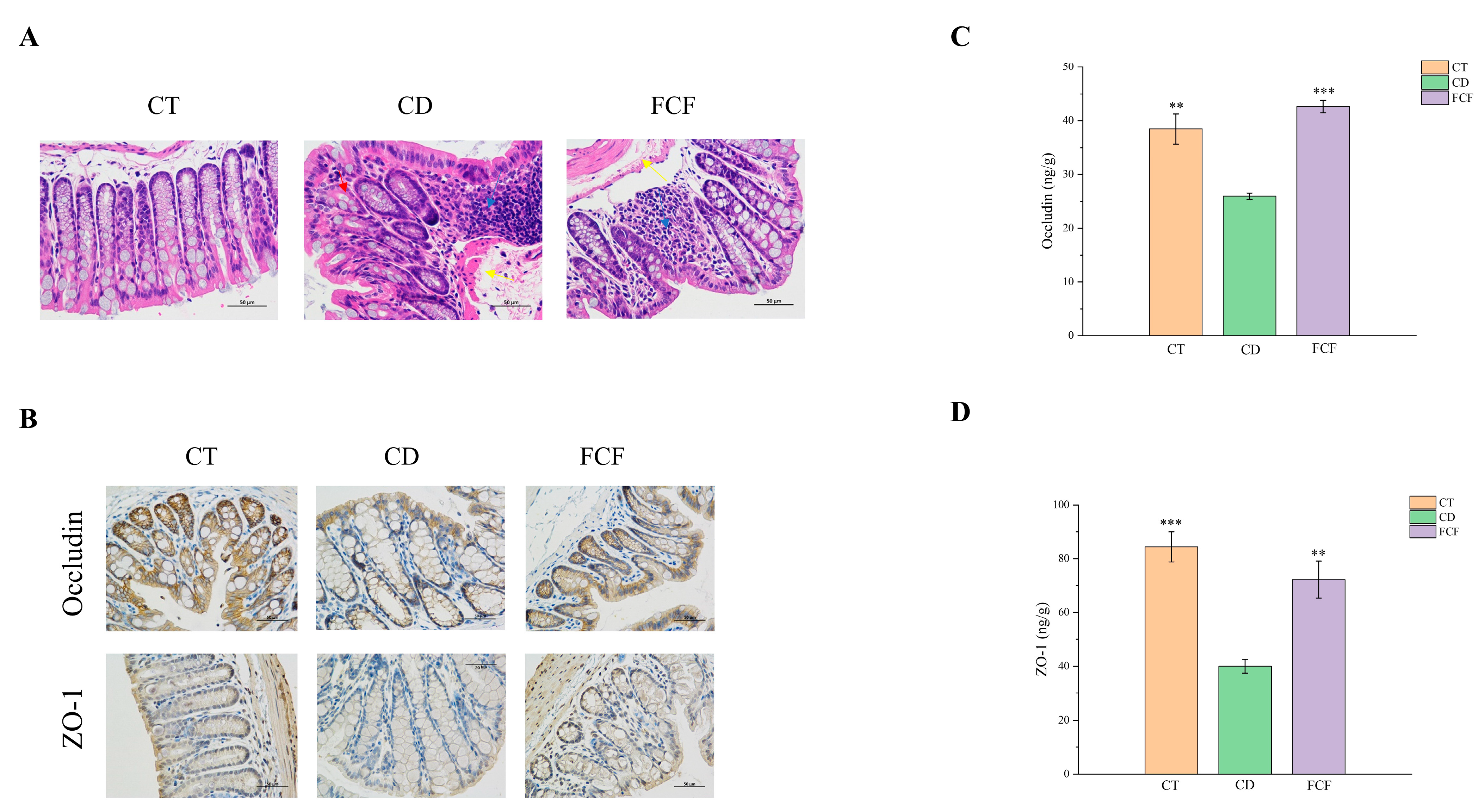
3.4. FCF Improved Intestinal Barrier Function in CD Mice
3.4.1. Intestinal Histopathological Changes in Each Group
3.4.2. The Expression of Occludin and ZO-1 in Intestinal Tissues of Mice in Each Group
3.5. Effects of FCF on the Diversity and Structure of Intestinal Flora in Mice with Circadian Rhythm Disturbance
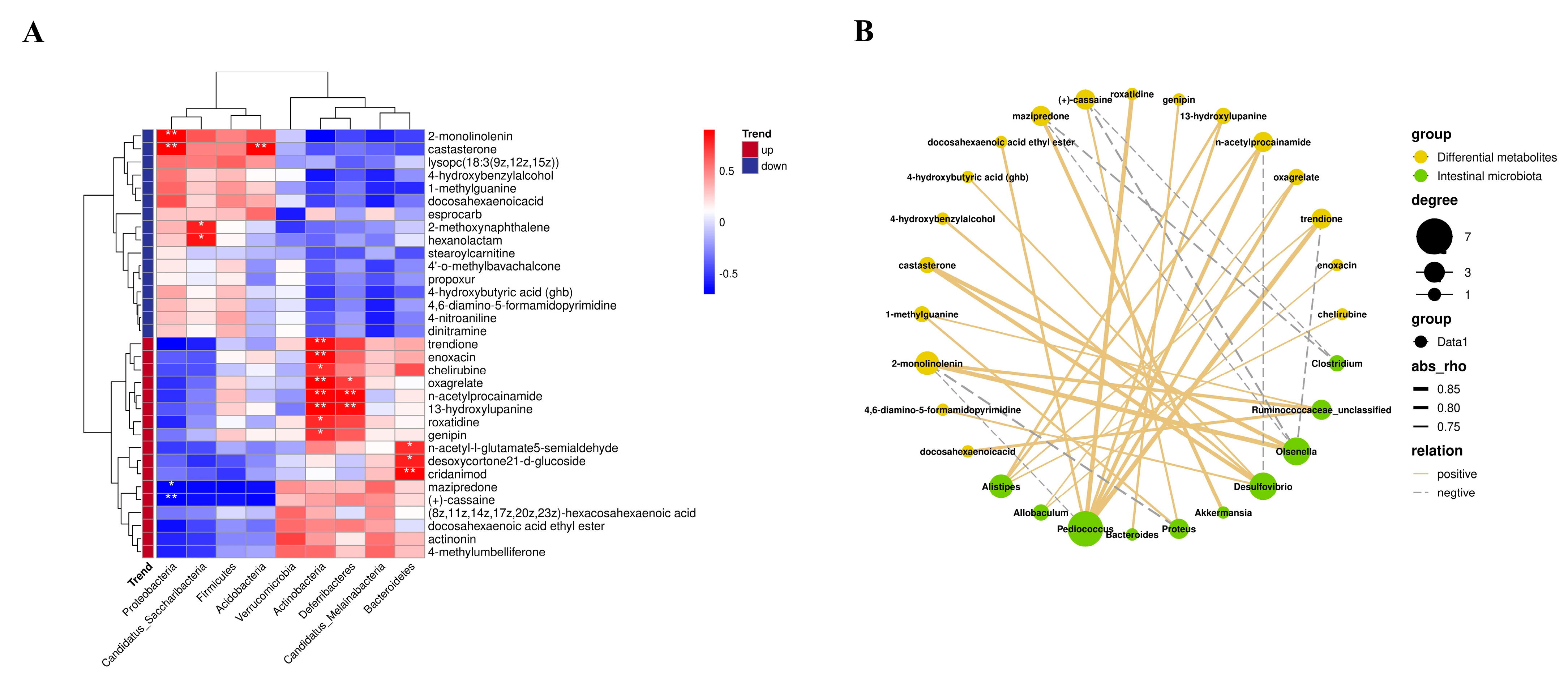
3.6. Influences of FCF on Intestinal Metabolites in Mice with Rhythm Disturbance
3.7. Correlation between Intestinal Microflora and Intestinal Metabolites
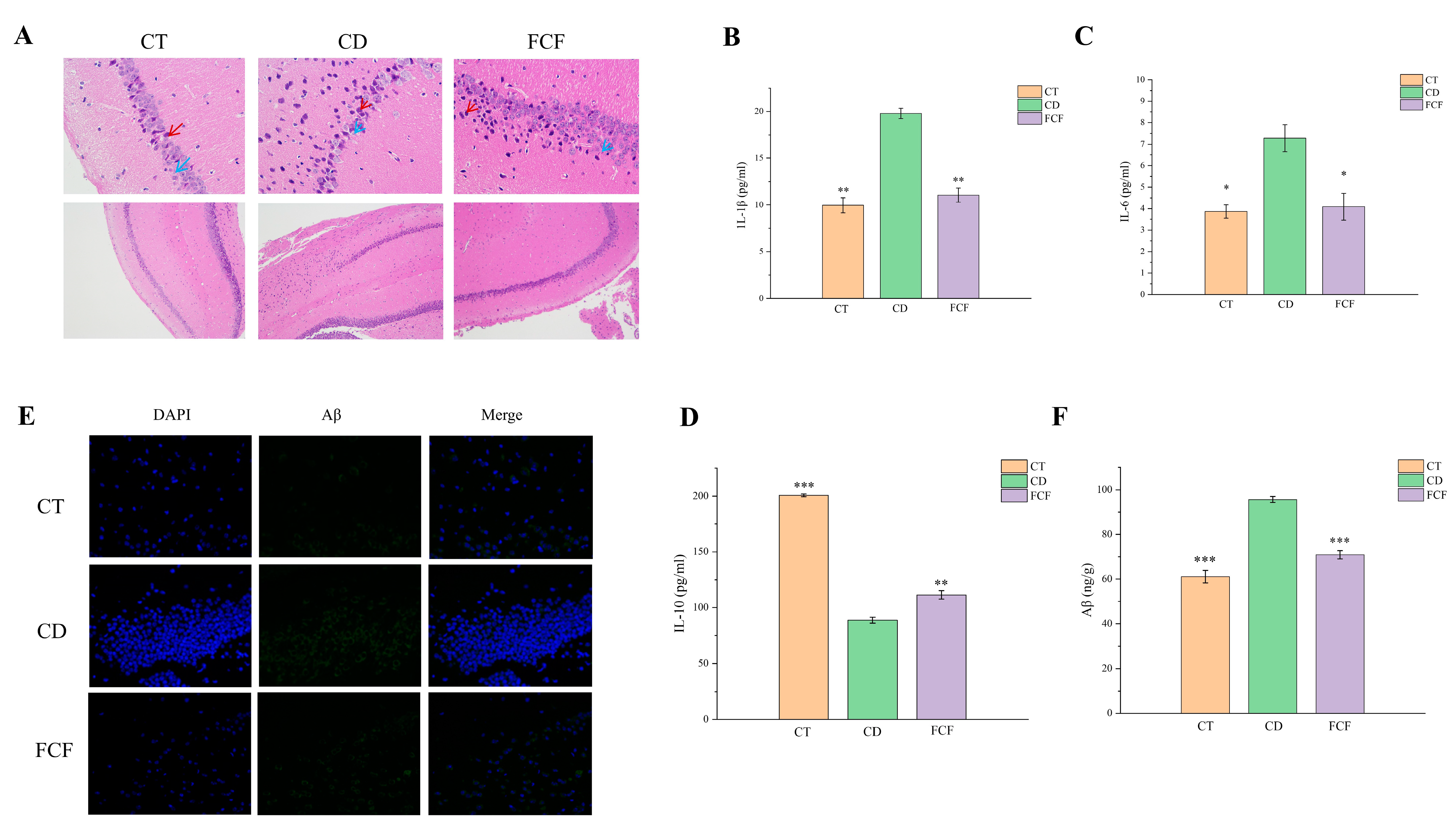
3.8. Physiological Mechanism of FCF Improving Cognitive Function in Mice with Circadian Disturbance
3.8.1. Morphological Comparison of Hippocampal Neurons in Each Group
3.8.2. Expression of Inflammatory Factors in the Hippocampus of Each Group
3.8.3. Expression of Aβ in the Hippocampus of Each Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Crnko, S.; Du Pré, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Yuan, X.; Eltzschig, H.K. Circadian rhythm as a therapeutic target. Nat. Rev. Drug Discov. 2021, 20, 287–307. [Google Scholar] [CrossRef]
- Hood, S.; Amir, S. The aging clock: Circadian rhythms and later life. J. Clin. Investig. 2017, 127, 437–446. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of Diurnal Variation of Gut Microbes and High-Fat Feeding on Host Circadian Clock Function and Metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Kalsbeek, A.; Cheeseman, J.F. Potential Role for the Gut Microbiota in Modulating Host Circadian Rhythms and Metabolic Health. Microorganisms 2019, 7, 41. [Google Scholar] [CrossRef]
- Brancaccio, M.; Patton, A.P.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Astrocytes Control Circadian Timekeeping in the Suprachiasmatic Nucleus via Glutamatergic Signaling. Neuron 2017, 93, 1420–1435.e5. [Google Scholar] [CrossRef]
- Logan, R.W.; McClung, C.A. Rhythms of life: Circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci. 2019, 20, 49–65. [Google Scholar] [CrossRef]
- Pearson, J.A.; Voisey, A.C.; Boest-Bjerg, K.; Wong, F.S.; Wen, L. Circadian Rhythm Modulation of Microbes During Health and Infection. Front. Microbiol. 2021, 12, 721004. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Z.; Cheng, P.; Qian, C.; Xu, F.; Yang, Y.; Wang, A.; Chen, W.; Sun, Z.; Lu, Y. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics 2020, 10, 10665–10679. [Google Scholar] [CrossRef]
- Ren, B.; Wang, L.; Mulati, A.; Liu, Y.; Liu, Z.; Liu, X. Methionine Restriction Improves Gut Barrier Function by Reshaping Diurnal Rhythms of Inflammation-Related Microbes in Aged Mice. Front. Nutr. 2021, 8, 746592. [Google Scholar] [CrossRef] [PubMed]
- Rosshart, S.P.; Vassallo, B.G.; Angeletti, D.; Hutchinson, D.S.; Morgan, A.P.; Takeda, K.; Hickman, H.D.; McCulloch, J.A.; Badger, J.H.; Ajami, N.J.; et al. Wild Mouse Gut Microbiota Promotes Host Fitness and Improves Disease Resistance. Cell 2017, 171, 1015–1028.e13. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, X.; Liang, S.; Li, W.; Wu, X.; Wang, L.; Jin, F. Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Benef. Microbes 2015, 6, 707–717. [Google Scholar] [CrossRef]
- Hullmann, M.; Albrecht, C.; van Berlo, D.; Gerlofs-Nijland, M.E.; Wahle, T.; Boots, A.W.; Krutmann, J.; Cassee, F.R.; Bayer, T.A.; Schins, R.P.F. Diesel engine exhaust accelerates plaque formation in a mouse model of Alzheimer’s disease. Part. Fibre Toxicol. 2017, 14, 35. [Google Scholar] [CrossRef]
- Gowd, V.; Jia, Z.; Chen, W. Anthocyanins as promising molecules and dietary bioactive components against diabetes—A review of recent advances. Trends Food Sci. Technol. 2017, 68, 1–13. [Google Scholar] [CrossRef]
- Nie, H.; Chen, H.; Li, G.; Su, K.; Song, M.; Duan, Z.; Li, X.; Cao, X.; Huang, J.; Huang, S.; et al. Comparison of flavonoids and phenylpropanoids compounds in Chinese water chestnut processed with different methods. Food Chem. 2021, 335, 127662. [Google Scholar] [CrossRef]
- Muñoz, Y.; Garrido, A.; Valladares, L. Equol is more active than soy isoflavone itself to compete for binding to thromboxane A2 receptor in human platelets. Thromb. Res. 2009, 123, 740–744. [Google Scholar] [CrossRef]
- Cheng, N.; Bell, L.; Lamport, D.J.; Williams, C.M. Dietary Flavonoids and Human Cognition: A Meta-Analysis. Mol. Nutr. Food Res. 2022, 66, e2100976. [Google Scholar] [CrossRef]
- Ramadan, M.; Ahmad, A.; Nafady, A.; Mansour, A. Chemical composition of the stem bark and leaves of Ficus pandurate Hance. Nat. Prod. Res. 2009, 23, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Chen, C.; Feng, H.; Li, G.; Peng, W.; Liu, X.; Yang, J.; Hu, X. Protection of Ficus pandurata Hance against acute alcohol-induced liver damage in mice via suppressing oxidative stress, inflammation, and apoptosis. J. Ethnopharmacol. 2021, 275, 114140. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Zhao, B.; Tan, X.; Wang, L.; Liu, X. Role of Food Phytochemicals in the Modulation of Circadian Clocks. J. Agric. Food Chem. 2019, 67, 8735–8739. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Kadyan, P.; Gahlaut, A.; Dabur, R. Nontargeted Identification of the Phenolic and Other Compounds of Saraca asoca by High Performance Liquid Chromatography-Positive Electrospray Ionization and Quadrupole Time-of-Flight Mass Spectrometry. ISRN Pharm. 2013, 2013, 293935. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Z.; Weng, P. Antioxidant and hepatoprotective effect of (-)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3″Me) from Chinese oolong tea. J. Agric. Food Chem. 2014, 62, 10046–10054. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zhang, X.; Miao, Y.; Cao, J.; Wu, Z.; Weng, P. The modulatory effect of (-)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3″Me) on intestinal microbiota of high fat diet-induced obesity mice model. Food Res. Int. 2017, 92, 9–16. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. J. Chromatogr. A 2020, 1635, 461770. [Google Scholar] [CrossRef] [PubMed]
- Sahar, S.; Sassone-Corsi, P. Regulation of metabolism: The circadian clock dictates the time. Trends Endocrinol. Metab. 2012, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Ovais, M.; Ullah, I.; Ahmed, J.; Shahid, M. Flavonoids as Prospective Neuroprotectants and Their Therapeutic Propensity in Aging Associated Neurological Disorders. Front. Aging Neurosci. 2019, 11, 155. [Google Scholar] [CrossRef]
- Voigt, R.M.; Forsyth, C.B.; Green, S.; Mutlu, E.; Engen, P.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. Circadian Disorganization Alters Intestinal Microbiota. PLoS ONE 2014, 9, e97500. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Han, H.; Ma, J.; Liu, G.; Wu, X.; Huang, X.; Fang, R.; Baba, K.; Bin, P.; et al. Administration of Exogenous Melatonin Improves the Diurnal Rhythms of the Gut Microbiota in Mice Fed a High-Fat Diet. Msystems 2020, 5, e00002-20. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Tamana, S.K.; Tun, H.M.; Konya, T.; Chari, R.S.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Moraes, T.J.; Turvey, S.E.; Subbarao, P.; et al. Bacteroides-dominant gut microbiome of late infancy is associated with enhanced neurodevelopment. Gut Microbes 2021, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tooley, K. Effects of the Human Gut Microbiota on Cognitive Performance, Brain Structure and Function: A Narrative Review. Nutrients 2020, 12, 3009. [Google Scholar] [CrossRef]
- Chen, C.; You, L.-J.; Huang, Q.; Fu, X.; Zhang, B.; Liu, R.-H.; Li, C. Modulation of gut microbiota by mulberry fruit polysaccharide treatment of obese diabetic db/db mice. Food Funct. 2018, 9, 3732–3742. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.; Zhang, S.; Duan, Z.; Ding, Y.; Chen, T.; Wang, R.; Feng, Z.; Shi, G.; Zhou, J.; Chen, Y. A critical review on the relationship of herbal medicine, Akkermansia muciniphila, and human health. Biomed. Pharmacother. 2020, 128, 110352. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cui, Y.; Xu, B.; Wang, Y.; Lv, F.; Li, Z.; Li, H.; Chen, X.; Peng, X.; Chen, Y.; et al. Main active components of Jiawei Gegen Qinlian decoction protects against ulcerative colitis under different dietary environments in a gut microbiota-dependent manner. Pharmacol. Res. 2021, 170, 105694. [Google Scholar] [CrossRef]
- Higarza, S.G.; Arboleya, S.; Arias, J.L.; Gueimonde, M.; Arias, N. Akkermansia muciniphila and environmental enrichment reverse cognitive impairment associated with high-fat high-cholesterol consumption in rats. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Wang, X.; Feng, J.; Su, X.; Liang, J.; Li, H.; Zhang, J. Impact of chronic exposure to trichlorfon on intestinal barrier, oxidative stress, inflammatory response and intestinal microbiome in common carp (Cyprinus carpio L.). Environ. Pollut. 2020, 259, 113846. [Google Scholar] [CrossRef]
- Pang, X.X.; Ansari, A.R.; Yang, W.J.; Niu, X.Y.; Dong, L.; Li, H.Z.; Xu, F.L.; Zhang, Z.W.; Xiao, K.; Hui, S. Visfatin Regulates Inflammatory Mediators in Mouse Intestinal Mucosa Through Toll-Like Receptors Signaling Under Lipopolysaccharide Stress. Arch. Immunol. Ther. Exp. 2021, 69, 11. [Google Scholar] [CrossRef]
- Malev, A.L.; Zakharova, A.N.; Kaliberdenko, V.B.; Fominykh, T.A.; Kulanthaivel, S.; Balasundaram, K. Structural and Morphological Changes in the Liver Due to Intestinal Endotoxins. Rev. Recent Clin. Trials 2020, 15, 205–213. [Google Scholar] [CrossRef]
- Yuan, J.W.; Che, S.Y.; Zhang, L.; Ruan, Z. Reparative effects of ethanol-induced intestinal barrier injury by flavonoid luteolin via MAPK/NF-κB/MLCK and Nrf2 signaling pathways. J. Agric. Food Chem. 2021, 69, 4101–4110. [Google Scholar] [CrossRef]
- Peng, J.-H.; Cui, T.; Huang, F.; Chen, L.; Zhao, Y.; Xu, L.; Xu, L.-L.; Feng, Q.; Hu, Y.-Y. Puerarin Ameliorates Experimental Alcoholic Liver Injury by Inhibition of Endotoxin Gut Leakage, Kupffer Cell Activation, and Endotoxin Receptors Expression. Experiment 2013, 344, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, C.; O’shea, A.; Kraft, J.N.; Albizu, A.; Evangelista, N.D.; Hausman, H.K.; Boutzoukas, E.M.; Van Etten, E.J.; Bharadwaj, P.K.; Song, H.; et al. Contributions of Hippocampal Volume to Cognition in Healthy Older Adults. Front. Aging Neurosci. 2020, 12, 593833. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Parashar, A.; Udayabanu, M. Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiol. Behav. 2017, 171, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-H.; Bi, W.; Qi, R.-B.; Wang, H.-D.; Wang, Z.-G.; Zeng, Q.; Zhao, Y.-R.; Lu, D.-X. Luteolin reduces primary hippocampal neurons death induced by neuroinflammation. Neurol. Res. 2011, 33, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kelley, K.W.; Johnson, R.W. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc. Natl. Acad. Sci. USA 2008, 105, 7534–7539. [Google Scholar] [CrossRef]
- Heckmann, B.L.; Teubner, B.J.W.; Tummers, B.; Boada-Romero, E.; Harris, L.; Yang, M.; Guy, C.S.; Zakharenko, S.S.; Green, D.R. LC3-associated endocytosis facilitates beta-amyloid clearance and mitigates neurodegeneration in murine Alzheimer’s Disease. Cell 2019, 178, 536–551.e14. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, A.K.Y.; Ip, N.Y. Synaptic dysfunction in Alzheimer’s disease: Mechanisms and therapeutic strategies. Pharmacol. Ther. 2019, 195, 186–198. [Google Scholar] [CrossRef]
- Li, S.M.; Selkoe, D.J. A mechanistic hypothesis for the impairment of synaptic plasticity by soluble A beta oligomers from Alzheimer’s brain. J. Neurochem. 2020, 154, 583–597. [Google Scholar] [CrossRef]
- Musiek, E.S.; Holtzman, D.M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 2016, 354, 1004–1008. [Google Scholar] [CrossRef]
- Sorriento, D.; Santulli, G.; Del Giudice, C.; Anastasio, A.; Trimarco, B.; Iaccarino, G. Endothelial Cells Are Able to Synthesize and Release Catecholamines Both In Vitro and In Vivo. Hypertension 2012, 60, 129–136. [Google Scholar] [CrossRef]
- Johnson, C.; Gravelle, S. Catecholamine compounds as inhibitors of amyloid beta-42 aggregation in Alzheimer’s disease. Abstr. Pap. Am. Chem. Soc. 2018, 255. [Google Scholar]
- Lv, C.; Wang, L.; Liu, X.; Yan, S.; Yan, S.S.; Wang, Y.; Zhang, W. Multi-faced neuroprotective effects of geniposide depending on the RAGE-mediated signaling in an Alzheimer mouse model. Neuropharmacology 2015, 89, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kitson, A.P.; Metherel, A.H.; Chen, C.T.; Domenichiello, A.F.; Trépanier, M.-O.; Berger, A.; Bazinet, R.P. Effect of dietary docosahexaenoic acid (DHA) in phospholipids or triglycerides on brain DHA uptake and accretion. J. Nutr. Biochem. 2016, 33, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Shock, D.D.; Beard, W.A.; Greenberg, M.M.; Freudenthal, B.D.; Wilson, S.H. A guardian residue hinders insertion of a Fapy center dot dGTP analog by modulating the open-closed DNA polymerase transition. Nucleic Acids Res. 2019, 47, 3197–3207. [Google Scholar] [CrossRef] [PubMed]


| Compound | Content (mg/g) |
|---|---|
| rutin | 169.77 ± 13.27 |
| vitexin | 143.52 ± 14.81 |
| apigenin | 119.98 ± 11.51 |
| epicatechin | 38.69 ± 1.63 |
| luteolin | 23.41 ± 0.85 |
| Sample | Chao1 | Shannon | Simpson |
|---|---|---|---|
| CT-0 | 846.03 ± 32.95 a | 5.38 ± 0.54 b | 0.96 ± 0.03 c |
| CT-4 | 879.61 ± 34.65 c | 8.04 ± 0.51 d | 0.99 ± 0.02 d |
| CD-0 | 841.08 ± 43.05 a | 5.26 ± 0.54 b | 0.94 ± 0.07 c |
| CD-4 | 846.06 ± 31.15 a | 4.34 ± 0.51 a | 0.85 ± 0.02 a |
| FCF-0 | 840.26 ± 44.15 a | 5.28 ± 0.28 b | 0.91 ± 0.04 b |
| FCF-4 | 868.56 ± 30.19 b | 7.12 ± 0.59 c | 0.92 ± 0.02 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Pan, J.; Liu, Y.; Zhang, X.; Cheng, K. Effects of Ficus pandurata Hance var. angustifolia Cheng Flavonoids on Intestinal Barrier and Cognitive Function by Regulating Intestinal Microbiota. Foods 2023, 12, 1682. https://doi.org/10.3390/foods12081682
Zhang Y, Pan J, Liu Y, Zhang X, Cheng K. Effects of Ficus pandurata Hance var. angustifolia Cheng Flavonoids on Intestinal Barrier and Cognitive Function by Regulating Intestinal Microbiota. Foods. 2023; 12(8):1682. https://doi.org/10.3390/foods12081682
Chicago/Turabian StyleZhang, Yuting, Junjie Pan, Yanan Liu, Xin Zhang, and Kejun Cheng. 2023. "Effects of Ficus pandurata Hance var. angustifolia Cheng Flavonoids on Intestinal Barrier and Cognitive Function by Regulating Intestinal Microbiota" Foods 12, no. 8: 1682. https://doi.org/10.3390/foods12081682
APA StyleZhang, Y., Pan, J., Liu, Y., Zhang, X., & Cheng, K. (2023). Effects of Ficus pandurata Hance var. angustifolia Cheng Flavonoids on Intestinal Barrier and Cognitive Function by Regulating Intestinal Microbiota. Foods, 12(8), 1682. https://doi.org/10.3390/foods12081682






