Development of Simultaneous Determination Method of Pesticide High Toxic Metabolite 3,4-Dichloroaniline and 3,5 Dichloroaniline in Chives Using HPLC-MS/MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Stock and Standard Solutions
2.3. Instruments and Analytical Conditions
2.4. Sample Preparation
2.5. Validation of the Method
2.6. Statistical Analysis
3. Results and Discussion
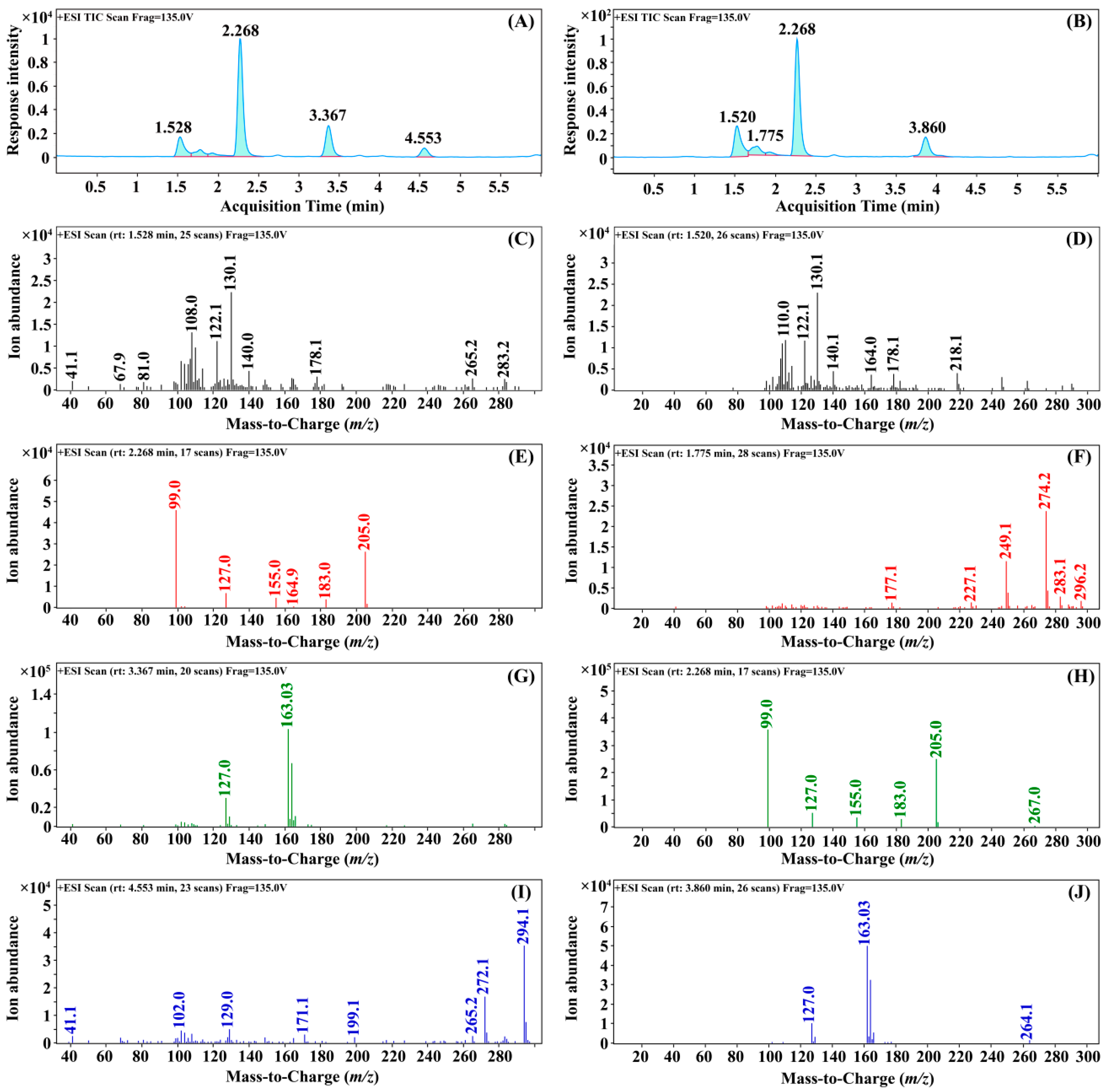
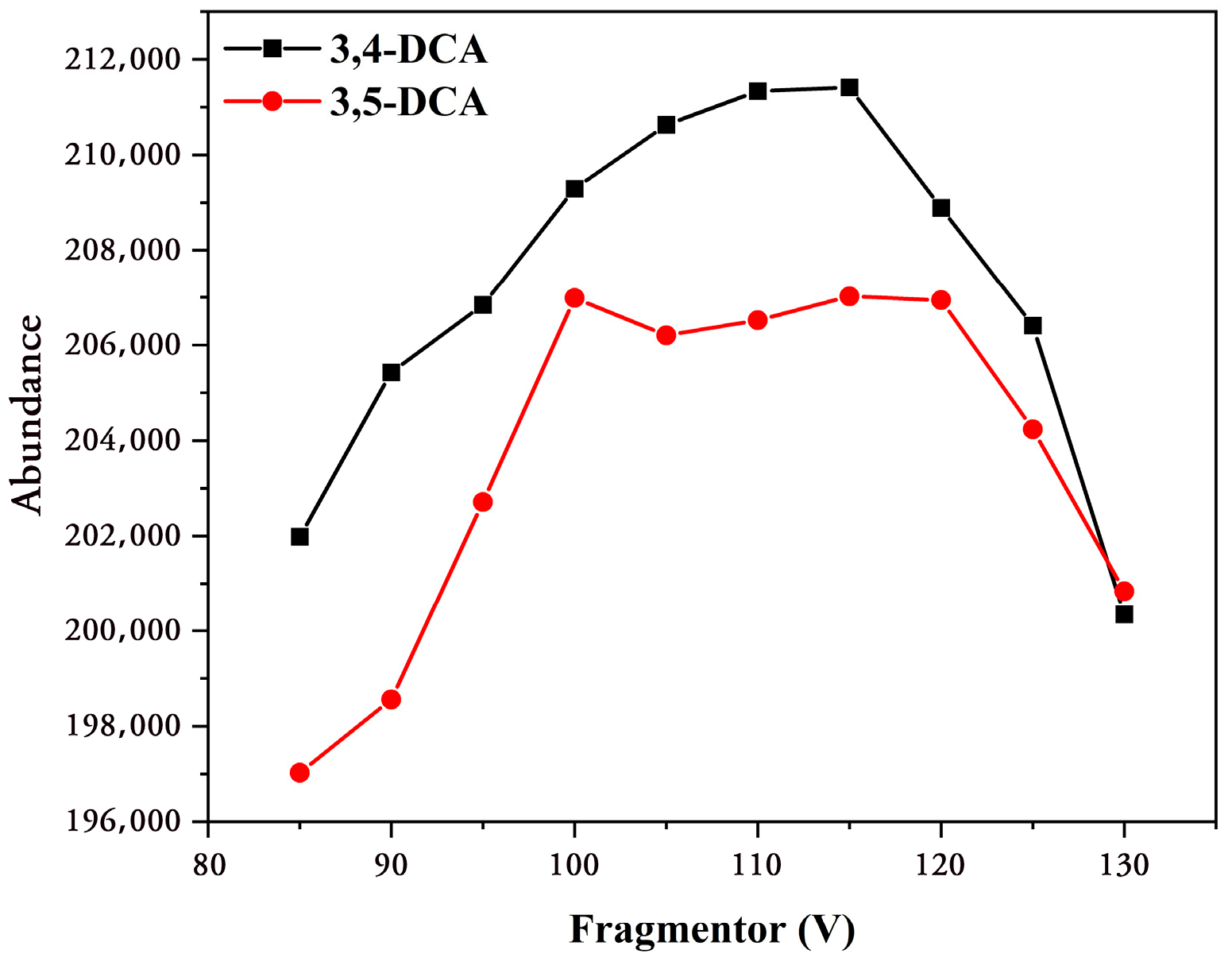
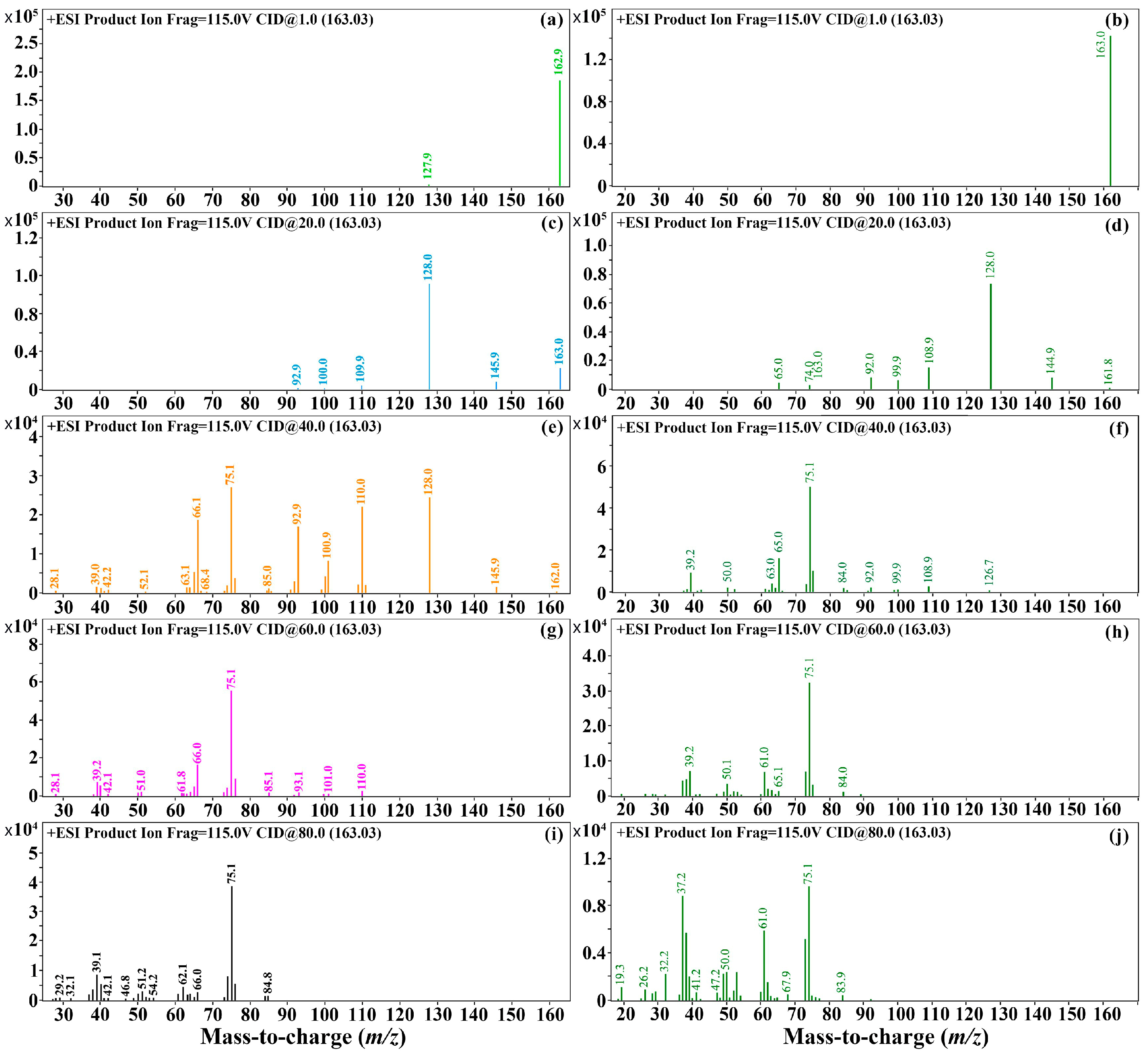
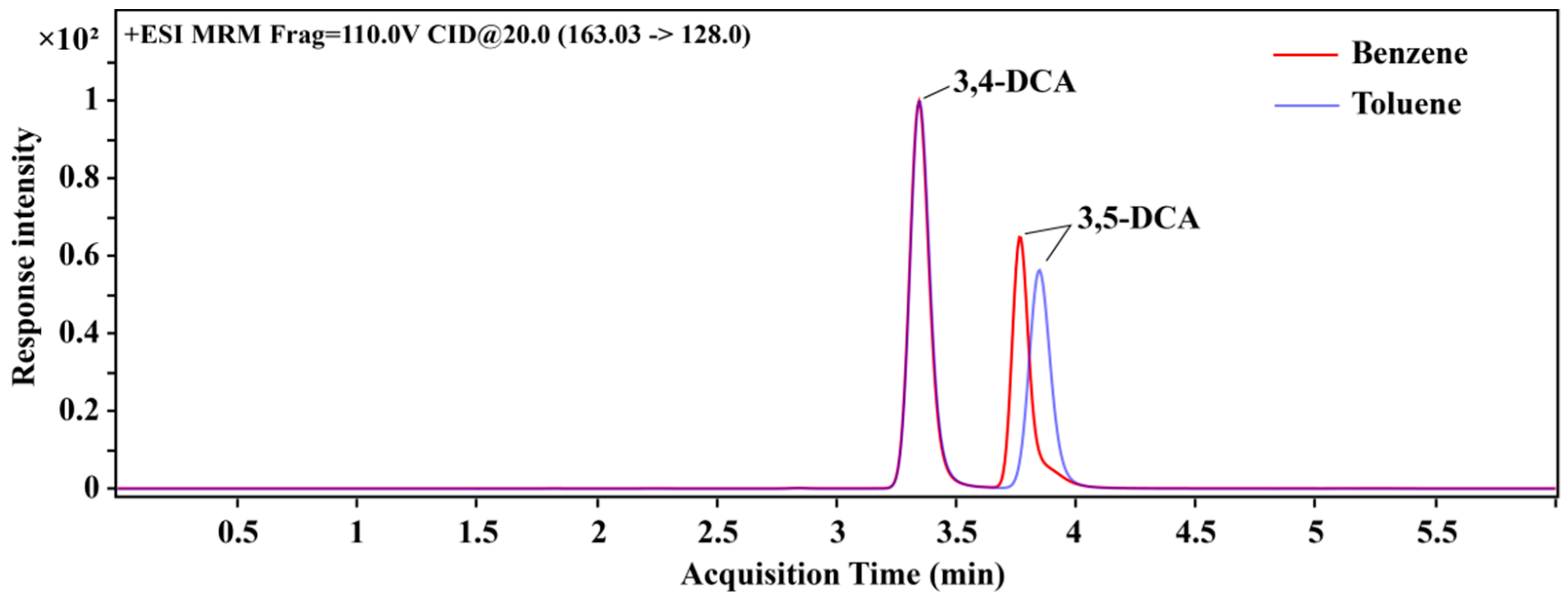
3.1. Optimization of Mass Spectrum Conditions
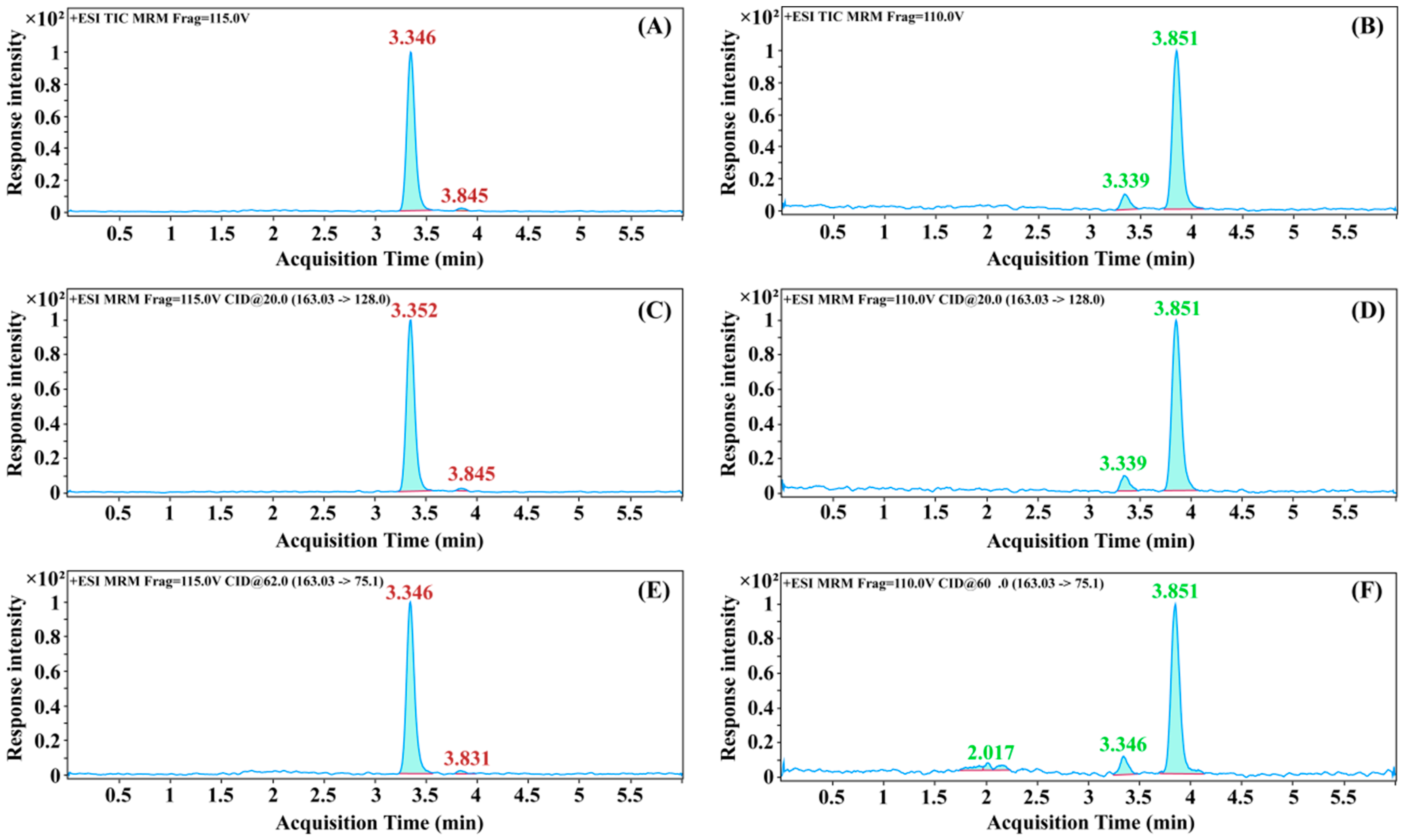
3.2. Optimization of Chromatographic Conditions
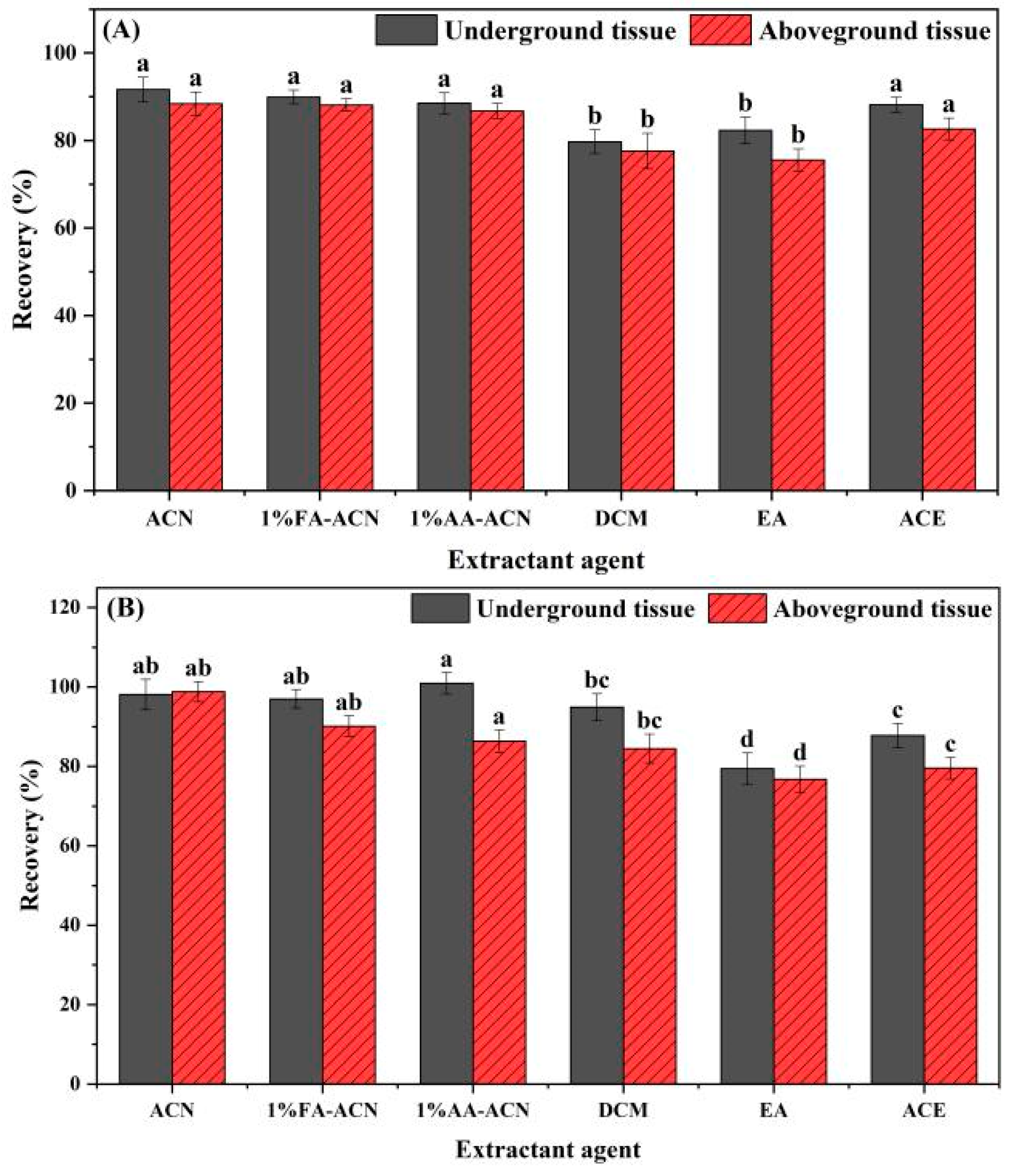
3.3. Optimization of Extractants in Chive Samples
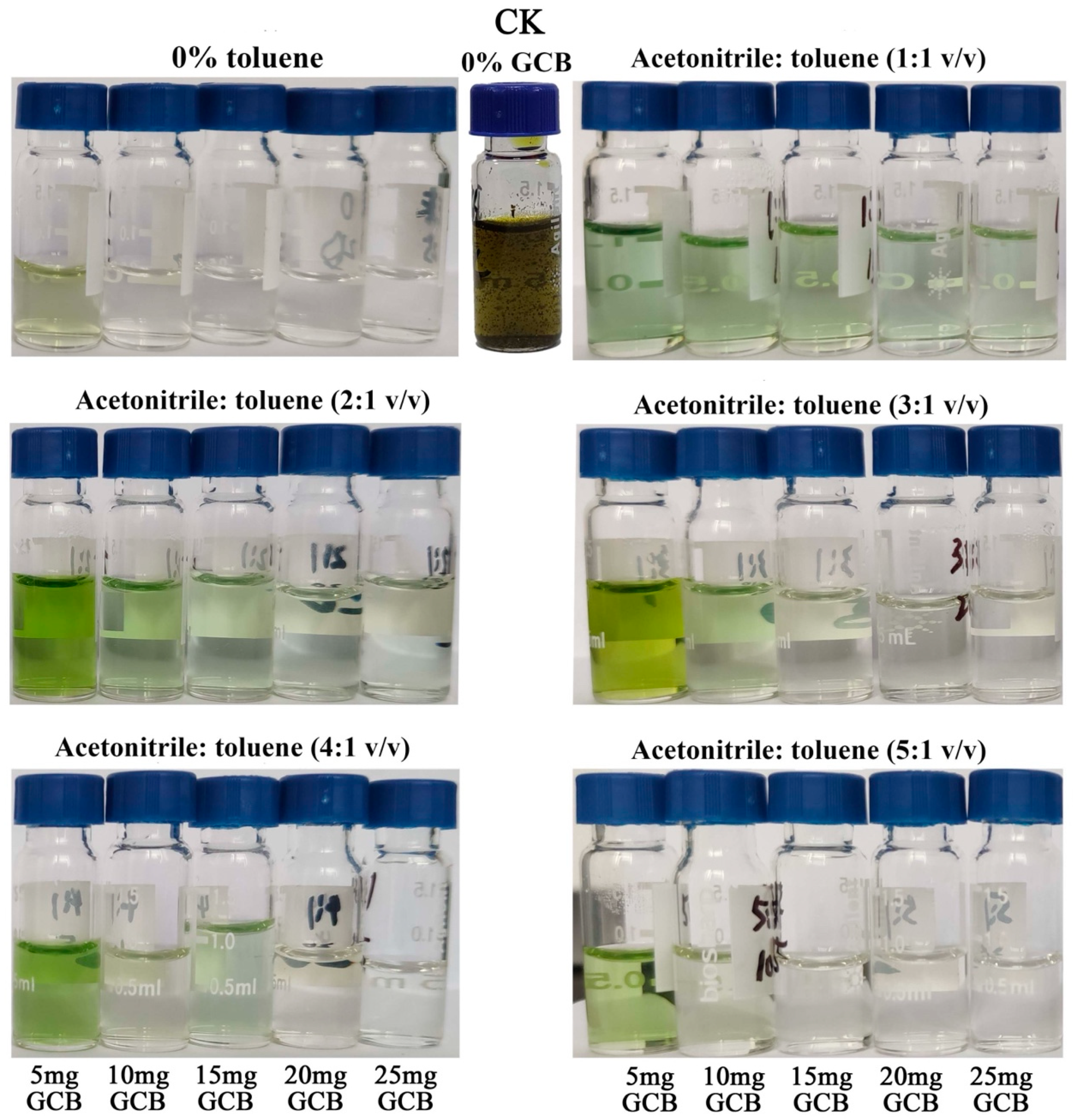
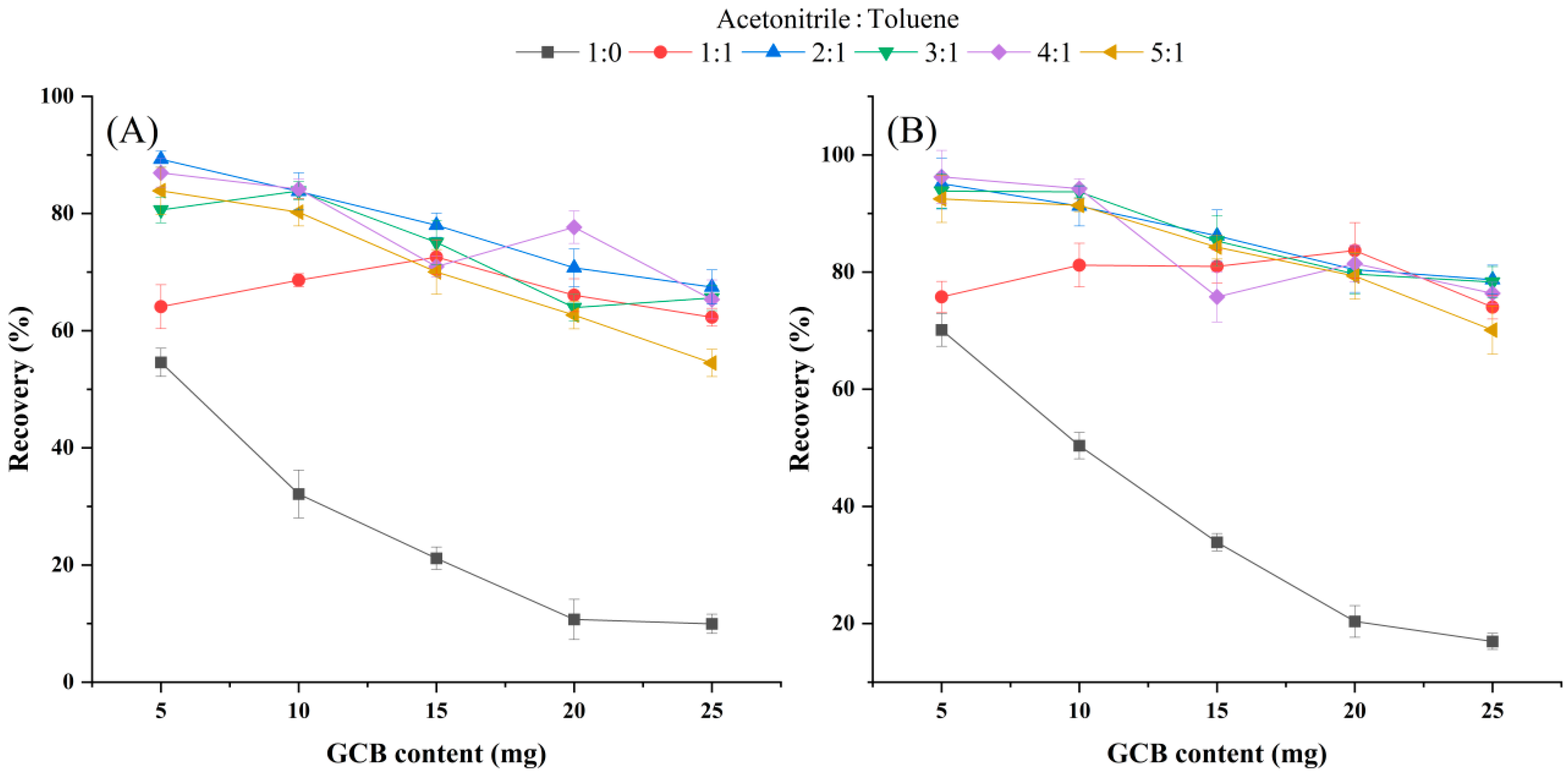
3.4. Evaluation of the Purification Effects on Chive Samples
3.5. Method Validation
3.5.1. Linearity, LOD, and LOQ
3.5.2. Accuracy and Precision
3.6. Application of Method for the Analysis of Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | ammonia water |
| ACE | acetone |
| ACN | acetonitrile |
| BEN | benzene |
| 3,4-DCA | 3,4-Dichloroaniline |
| 3,5-DCA | 3,5 Dichloroaniline |
| DCM | dichloromethane |
| EA | ethyl acetate |
| FA | formic acid |
| GCB | graphite carbon black |
| GC | gas chromatography |
| GC-MS | gas chromatography-mass spectrometry |
| HPLC | liquid chromatography |
| UPLC-MS/MS | ultra-high performance liquid chromatography-tandem mass spectrometry |
| HPLC-MS/MS | high-performance liquid chromatography-tandem mass spectrometry |
| LOD | limit of detection |
| LOQ | limit of quantitation) |
| ME | matrix effect |
| MEOH | methanol |
| MB | toluene |
| MRL | maximum residue limit |
| PSA | primary secondary amine |
Appendix A
References
- Sihtmäe, M.; Mortimer, M.; Kahru, A.; Blinova, I. Toxicity of Five Anilines to Crustaceans, Protozoa and Bacteria. J. Serb. Chem. Soc. 2010, 75, 1291–1302. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, X.; Wu, X.; Dong, F.; Liu, X.; Xu, J.; Wu, X.; Li, M.; Zheng, Y. Removal of dimethachlon from soils using immobilized cells and enzymes of a novel potential degrader Providencia stuartii JD. J. Hazard. Mater. 2019, 378, 120606. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.A.; van Dillewijn, P.; Nesme, J.; Sørensen, S.J.; Romero, E. Improvement of Pesticide Removal in Contaminated Media Using Aqueous Extracts from Contaminated Biopurification Systems. Sci. Total Environ. 2019, 691, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Ormad, M.P.; Miguel, N.; Claver, A.; Matesanz, J.M.; Ovelleiro, J.L. Pesticides Removal in the Process of Drinking Water Production. Chemosphere 2008, 71, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Kuckelkorn, J.; Nüßer, L.K.; Floehr, T.; Hennig, M.P.; Roß-Nickoll, M.; Schäffer, A.; Hollert, H. The Metabolite 3,4,3′,4′-Tetrachloroazobenzene (TCAB) Exerts a Higher Ecotoxicity than the Parent Compounds 3,4-Dichloroaniline (3,4-DCA) and Propanil. Sci. Total Environ. 2016, 551–552, 304–316. [Google Scholar] [CrossRef]
- Rodrigues, A.D.; Dos Santos Montanholi, A.; Shimabukuro, A.A.; Yonekawa, M.K.A.; Cassemiro, N.S.; Silva, D.B.; Marchetti, C.R.; Weirich, C.E.; Beatriz, A.; Zanoelo, F.F.; et al. N-Acetylation of Toxic Aromatic Amines by Fungi: Strain Screening, Cytotoxicity and Genotoxicity Evaluation, and Application in Bioremediation of 3,4-Dichloroaniline. J. Hazard. Mater. 2023, 441, 129887. [Google Scholar] [CrossRef]
- Tasca, A.L.; Fletcher, A. State of the Art of the Environmental Behaviour and Removal Techniques of the Endocrine Disruptor 3,4-Dichloroaniline. J. Environ. Sci. Health Part A 2018, 53, 260–270. [Google Scholar] [CrossRef]
- Schiwy, S.; Herber, A.-K.; Hollert, H.; Brinkmann, M. New Insights into the Toxicokinetics of 3,4-Dichloroaniline in Early Life Stages of Zebrafish (Danio Rerio). Toxics 2020, 8, 16. [Google Scholar] [CrossRef]
- Boulahlib, S.; Boudina, A.; Si-Ahmed, K.; Bessekhouad, Y.; Trari, M. Development and Validation of a Fast and Simple HPLC Method for the Simultaneous Determination of Aniline and Its Degradation Products in Wastewater. Anal. Methods 2016, 8, 5949–5956. [Google Scholar] [CrossRef]
- Saleh, A.; Molaei, S.; Sheijooni Fumani, N.; Abedi, E. Antifouling Paint Booster Biocides (Irgarol 1051 and Diuron) in Marinas and Ports of Bushehr, Persian Gulf. Mar. Pollut. Bull. 2016, 105, 367–372. [Google Scholar] [CrossRef]
- Palau-Casellas, A.; Hutchinson, T.H. Acute Toxicity of Chlorinated Organic Chemicals to the Embryos and Larvae of the Marine WormPlatynereis Dumerilii (Polychaeta: Nereidae). Environ. Toxicol. Water Qual. 1998, 13, 149–155. [Google Scholar] [CrossRef]
- Sosak-Świderska, B.; Tyrawska, D.; Maślikowska, B. Microalgal Ecotoxicity Test with 3,4-Dichloroaniline. Chemosphere 1998, 37, 2975–2982. [Google Scholar] [CrossRef]
- Ramos, E.U.; Vaal, M.A.; Hermens, J.L.M. Interspecies Sensitivity in the Aquatic Toxicity of Aromatic Amines. Environ. Toxicol. Phar. 2002, 11, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Liu, T.; Hu, X.; Wang, G. Developmental Toxicity of 3,4-Dichloroaniline on Rare Minnow (Gobiocypris Rarus) Embryos and Larvae. Chemosphere 2013, 90, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, M.D.; Janssen, C.R.; Andreu, E.; Persoone, G. Ecotoxicological Studies with the Freshwater Rotifer Brachionus Calyciflorus II. An Assessment of the Chronic Toxicity of Lindane and 3,4-Dichloroaniline Using Life Tables. Hydrobiologia 1993, 255–256, 33–40. [Google Scholar] [CrossRef]
- Lampert, I.T.W. The Juvenile Growth Rate of Daphnia: A Short-Term Alternative to Measuring the Per Capita Rate of Increase in Ecotoxicology? Arch. Environ. Contam. Toxicol. 2002, 42, 193–198. [Google Scholar] [CrossRef]
- Vanni, A.; Gamberini, R.; Calabria, A.; Pellegrino, V. Determination of Presence of Fungicides by Their Common Metabolite, 3,5-DCA, in Compost. Chemosphere 2000, 41, 453–458. [Google Scholar] [CrossRef]
- Carrillo, C.; Buvé, C.; Panozzo, A.; Grauwet, T.; Hendrickx, M. Role of Structural Barriers in the in Vitro Bioaccessibility of Anthocyanins in Comparison with Carotenoids. Food Chem. 2017, 227, 271–279. [Google Scholar] [CrossRef]
- Rifai, A.; Souissi, Y.; Genty, C.; Clavaguera, C.; Bourcier, S.; Jaber, F.; Bouchonnet, S. Ultraviolet Degradation of Procymidone—Structural Characterization by Gas Chromatography Coupled with Mass Spectrometry and Potential Toxicity of Photoproducts Using in Silico Tests: UV Degradation of Procymidone. Rapid Commun. Mass Spectrom. 2013, 27, 1505–1516. [Google Scholar] [CrossRef]
- Perkins, A.N.; Inayat-Hussain, S.H.; Deziel, N.C.; Johnson, C.H.; Ferguson, S.S.; Garcia-Milian, R.; Thompson, D.C.; Vasiliou, V. Evaluation of Potential Carcinogenicity of Organic Chemicals in Synthetic Turf Crumb Rubber. Environ. Res. 2019, 169, 163–172. [Google Scholar] [CrossRef]
- Valentovic, M.A.; Lo, H.-H.; Brown, P.I.; Rankin, G.O. 3,5-Dichloroaniline Toxicity in Fischer 344 Rats Pretreated with Inhibitors and Inducers of Cytochrome P450. Toxicol. Lett. 1995, 78, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Sun, X.; Li, L.; Li, D.; Wang, M.; Shi, H. Toxicity Effects of Procymidone, Iprodione and Their Metabolite of 3,5-Dichloroaniline to Zebrafish. Chemosphere 2021, 272, 129577. [Google Scholar] [CrossRef] [PubMed]
- Vasileiadis, S.; Puglisi, E.; Papadopoulou, E.S.; Pertile, G.; Suciu, N.; Pappolla, R.A.; Tourna, M.; Karas, P.A.; Papadimitriou, F.; Kasiotakis, A.; et al. Blame It on the Metabolite: 3,5-Dichloroaniline Rather than the Parent Compound Is Responsible for the Decreasing Diversity and Function of Soil Microorganisms. Appl. Environ. Microbiol. 2018, 84, e01536-18. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, T.; Mou, L.; Ou, G.; Hu, D.; Zhang, Y. Identification and Quantification of Dimethachlon Degradation Products in Soils and Their Effects on Soil Enzyme Activities. J. Agric. Food Chem. 2023, 71, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Wittke, K.; Hajimiragha, H.; Dunemann, L.; Begerow, J. Determination of Dichloroanilines in Human Urine by GC–MS, GC–MS–MS, and GC–ECD as Markers of Low-Level Pesticide Exposure. J. Chromatogr. B 2001, 755, 215–228. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Q.; Li, J.; Zhang, W.; Wu, X. Rapid Elimination of Dicarboximide Fungicides and Their Metabolite 3,5-Dichloroaniline from Soils by Immobilized Bacterial Consortia. Environ. Technol. Innov. 2023, 30, 103120. [Google Scholar] [CrossRef]
- Quirantes, M.; Nogales, R.; Romero, E. Sorption Potential of Different Biomass Fly Ashes for the Removal of Diuron and 3,4-Dichloroaniline from Water. J. Hazard. Mater. 2017, 331, 300–308. [Google Scholar] [CrossRef]
- Tsochatzis, E.; Karayannakidis, P.; Kalogiannis, S. Determination of Selected Dichloroanilines and Phthalates in Lyophilised Mussels Samples with Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry after QuEChERS Clean-Up. Food Addit. Contam. Part A 2019, 36, 1253–1260. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, Y.; Jiang, Y.; Yang, B. Structure Identification of the Oligosaccharides by UPLC-MS/MS. Food Hydrocoll. 2023, 139, 108558. [Google Scholar] [CrossRef]
- Męczykowska, H.; Stepnowski, P.; Caban, M. Impact of Humic Acids, Temperature and Stirring on Passive Extraction of Pharmaceuticals from Water by Trihexyl (Tetradecyl) Phosphonium Dicyanamide. Microchem. J. 2019, 144, 500–505. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, H.; Cao, X.; Du, P.; Shao, H.; Jin, F.; Jin, M.; Wang, J. Determination of Hymexazol in 26 Foods of Plant Origin by Modified QuEChERS Method and Liquid Chromatography Tandem-Mass Spectrometry. Food Chem. 2017, 228, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Łozowicka, B.; Mojsak, P.; Kaczyński, P.; Konecki, R.; Borusiewicz, A. The Fate of Spirotetramat and Dissipation Metabolites in Apiaceae and Brassicaceae Leaf-Root and Soil System under Greenhouse Conditions Estimated by Modified QuEChERS/LC–MS/MS. Sci. Total Environ. 2017, 603–604, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Guedes, J.A.C.; de Oliveira Silva, R.; Lima, C.G.; Milhome, M.A.L.; do Nascimento, R.F. Matrix Effect in Guava Multiresidue Analysis by QuEChERS Method and Gas Chromatography Coupled to Quadrupole Mass Spectrometry. Food Chem. 2016, 199, 380–386. [Google Scholar] [CrossRef]
- SANTE Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed; SANTE/12682/2019, Implemented by 01.01.2020; European Commission Directorate General for Health and Food Safety Brussels: Brussels, Belgium, 2020.
- Guidelines on Performance Criteria for Methods of Analysis for the Determination of Pesticide Residues in Food and Feed. Available online: http://www.fao.org/fao-who-codexalimentarius/thematic-areas/pesticides/en/ (accessed on 24 March 2023).
- Zhang, Z.; Feng, M.; Zhu, K.; Han, L.; Sapozhnikova, Y.; Lehotay, S.J. Multiresidue Analysis of Pesticides in Straw Roughage by Liquid Chromatography–Tandem Mass Spectrometry. J. Agric. Food Chem. 2016, 64, 6091–6099. [Google Scholar] [CrossRef] [PubMed]

| Retention Time/min | Flow Rate/(mL/min) | Mobile Phase A/% | Mobile Phase B/% |
|---|---|---|---|
| 0 | 0.5 | 30 | 70 |
| 1 | 0.5 | 30 | 70 |
| 3 | 0.5 | 10 | 90 |
| 5 | 0.5 | 30 | 70 |
| 6 | 0.5 | 30 | 70 |
| Type of Elution | Compound | Peak Area 1 | Peak Area 2 | Peak Area 3 | Mean Peak Area | Response Improvement Rate (%) |
|---|---|---|---|---|---|---|
| Isocratic elution | 3,4-DCA | 605.95 | 666.08 | 585.39 | 619.14 | — |
| 3,5-DCA | 298.19 | 286.52 | 307.86 | 297.52 | — | |
| Gradient elute | 3,4-DCA | 742.75 | 744.61 | 779.67 | 755.68 | 22.05 |
| 3,5-DCA | 458.88 | 465.01 | 461.95 | 461.95 | 55.26 |
| Compound | Matrix | Linear Equation | R2 | LOD (μg/kg) | LOQ (μg/kg) | ME (%) |
|---|---|---|---|---|---|---|
| 3,4-DCA | Aboveground tissue | 0.9972 | 0.6 | 2.0 | −9.0 | |
| Underground tissue | 0.9999 | −2.6 | ||||
| Solvent | 0.9999 * | — | — | — | ||
| 3,5-DCA | Aboveground tissue | 0.9969 | 1.0 | 3.0 | −4.4 | |
| Underground tissue | 0.9999 | 2.3 | ||||
| Solvent | 0.9999 * | — | — | — |
| Compound | Matrix | Addition Level (mg/kg) | Average Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 3,4-DCA | Aboveground tissue | 0.001 | 75.3 | 8.5 |
| 0.010 | 85.4 | 4.3 | ||
| 0.100 | 80.1 | 2.6 | ||
| 1.000 | 84.4 | 2.1 | ||
| Underground tissue | 0.001 | 78.5 | 6.7 | |
| 0.010 | 77.3 | 3.8 | ||
| 0.100 | 81.2 | 3.2 | ||
| 1.000 | 86.0 | 2.7 | ||
| 3,5-DCA | Aboveground tissue | 0.001 | 78.2 | 9.4 |
| 0.010 | 98.1 | 7.5 | ||
| 0.100 | 87.1 | 5.1 | ||
| 1.000 | 94.3 | 2.1 | ||
| Underground tissue | 0.001 | 79.6 | 10.6 | |
| 0.010 | 82.0 | 11.9 | ||
| 0.100 | 87.2 | 2.9 | ||
| 1.000 | 93.4 | 1.4 |
| Compound | Parent Compounds | Vegetable | Average (mg/kg) | Median (mg/kg) | Range (mg/kg) |
|---|---|---|---|---|---|
| 3,4-DCA | Diuron | Chive | 0.076 | 0.082 | 0.01–0.176 |
| Carrot | 0.016 | 0.024 | 0.002–0.053 | ||
| Onions | — | — | — | ||
| Leek | — | — | — | ||
| Parsley | — | — | — | ||
| Propanil | Spinach | — | — | — | |
| Tomato | — | — | — | ||
| Cucumber | — | — | — | ||
| Potato | — | — | — | ||
| Pepper | — | — | — | ||
| 3,5-DCA | Iprodione | Chive | 0.083 | 0.067 | 0.01–0.2 |
| Leek | 0.273 | 0.312 | 0.02–1.07 | ||
| Tomato | 0.084 | 0.067 | 0.01–0.154 | ||
| Onion | — | — | — | ||
| Cucumber | — | — | — | ||
| Procymidone | Potato | — | — | — | |
| Pepper | — | — | — | ||
| Parsley | — | — | — | ||
| Spinach | — | — | — | ||
| Carrot | — | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Yao, X.; Zhang, W.; Wu, X. Development of Simultaneous Determination Method of Pesticide High Toxic Metabolite 3,4-Dichloroaniline and 3,5 Dichloroaniline in Chives Using HPLC-MS/MS. Foods 2023, 12, 2875. https://doi.org/10.3390/foods12152875
Dong Y, Yao X, Zhang W, Wu X. Development of Simultaneous Determination Method of Pesticide High Toxic Metabolite 3,4-Dichloroaniline and 3,5 Dichloroaniline in Chives Using HPLC-MS/MS. Foods. 2023; 12(15):2875. https://doi.org/10.3390/foods12152875
Chicago/Turabian StyleDong, Yibo, Xiaolong Yao, Wanping Zhang, and Xiaomao Wu. 2023. "Development of Simultaneous Determination Method of Pesticide High Toxic Metabolite 3,4-Dichloroaniline and 3,5 Dichloroaniline in Chives Using HPLC-MS/MS" Foods 12, no. 15: 2875. https://doi.org/10.3390/foods12152875
APA StyleDong, Y., Yao, X., Zhang, W., & Wu, X. (2023). Development of Simultaneous Determination Method of Pesticide High Toxic Metabolite 3,4-Dichloroaniline and 3,5 Dichloroaniline in Chives Using HPLC-MS/MS. Foods, 12(15), 2875. https://doi.org/10.3390/foods12152875