Implications of Organic Dairy Management on Herd Performance and Milk Fatty Acid Profiles and Interactions with Season
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Collection of Data and Milk Samples
2.2. Milk Analysis
2.3. Statistical Analysis
3. Results
3.1. Herd Composition and Feed Intake
3.1.1. Effect of Production System
| Production System | Production System × Month | ||||
|---|---|---|---|---|---|
| Conventional | Organic | SE | p-Value 2 | p-Value 2 | |
| n = 488 1 | n = 312 1 | ||||
| Milking herd size (number of cows) | 270 | 211 | 5.2 | 0.045 | <0.001 |
| Milking cows (% herd) | 86.3 | 84.9 | 0.50 | 0.638 | <0.001 |
| Estimated liveweight (kg) 3 | 656 | 637 | 2.4 | 0.111 | 0.181 |
| Breed composition (% herd) | |||||
| Holstein | 80.2 | 66.4 | 1.90 | 0.271 | 0.183 |
| British Friesian | 3.85 | 2.38 | 0.656 | 0.764 | 0.091 |
| NZ Friesian | 0.10 | 1.28 | 0.310 | 0.421 | 0.134 |
| Jersey | 0.35 | 0.81 | 0.110 | 0.476 | 0.313 |
| Scandinavian Red | 0.12 | 0.89 | 0.098 | 0.128 | 0.635 |
| Shorthorn | 0.01 | 0.76 | 0.035 | 0.001 | 0.018 |
| Ayrshire | 2.60 | 8.14 | 1.002 | 0.002 | 0.991 |
| Montbeliarde | 0.06 | 0.05 | 0.021 | 0.779 | 0.762 |
| Brown Swiss | 0.34 | 1.90 | 0.228 | 0.284 | 0.133 |
| Guernsey | 0.06 | 0.00 | 0.007 | 0.009 | 0.987 |
| Other breed or crossbreed | 12.3 | 17.4 | 1.68 | 0.534 | 0.356 |
| Diet composition (% DM offered unless otherwise stated) | |||||
| Offered feed (kg DM/day) 4 | 21.0 | 18.9 | 0.11 | <0.001 | 0.006 |
| Total forage | 61.0 | 74.2 | 0.46 | <0.001 | 0.089 |
| Total concentrate | 39.0 | 25.8 | 0.46 | <0.001 | 0.089 |
| Predicted grazing intake | 8.31 | 24.9 | 1.10 | <0.001 | <0.001 |
| Total silage intake | 50.2 | 47.7 | 0.97 | 0.720 | <0.001 |
| Grass silage | 23.3 | 32.5 | 0.84 | <0.001 | <0.001 |
| Grass:clover silage 5 | 0.86 | 3.19 | 0.329 | 0.018 | <0.001 |
| Maize silage | 24.9 | 0.97 | 0.427 | <0.001 | 0.056 |
| Cereal silage | 0.02 | 0.36 | 0.074 | 0.036 | 0.033 |
| Lucerne silage | 0.37 | 0.06 | 0.060 | 0.451 | 0.928 |
| Other mixed silage | 0.30 | 2.40 | 0.204 | <0.001 | 0.014 |
| Wholecrop | 0.45 | 8.23 | 0.316 | <0.001 | <0.001 |
| Hay and straw | 2.46 | 1.95 | 0.166 | 0.723 | 0.010 |
| Moist byproducts | 4.74 | 0.37 | 0.203 | <0.001 | 0.050 |
| Dry straights 6 | 10.5 | 0.91 | 0.412 | <0.001 | 0.922 |
| Cereals | 2.45 | 4.02 | 0.245 | 0.034 | 0.008 |
| Blends | 20.5 | 20.4 | 0.59 | 0.835 | 0.099 |
| Oil | 0.83 | 0.01 | 0.028 | <0.001 | 0.947 |
| Minerals (g/cow per day) | 134.0 | 71.5 | 4.98 | 0.013 | 0.144 |
| Vitamins (g/cow per day) | 10.8 | 0.00 | 1.373 | 0.091 | 0.041 |
3.1.2. Effect of Month
3.1.3. Effect of Production System × Month Interaction
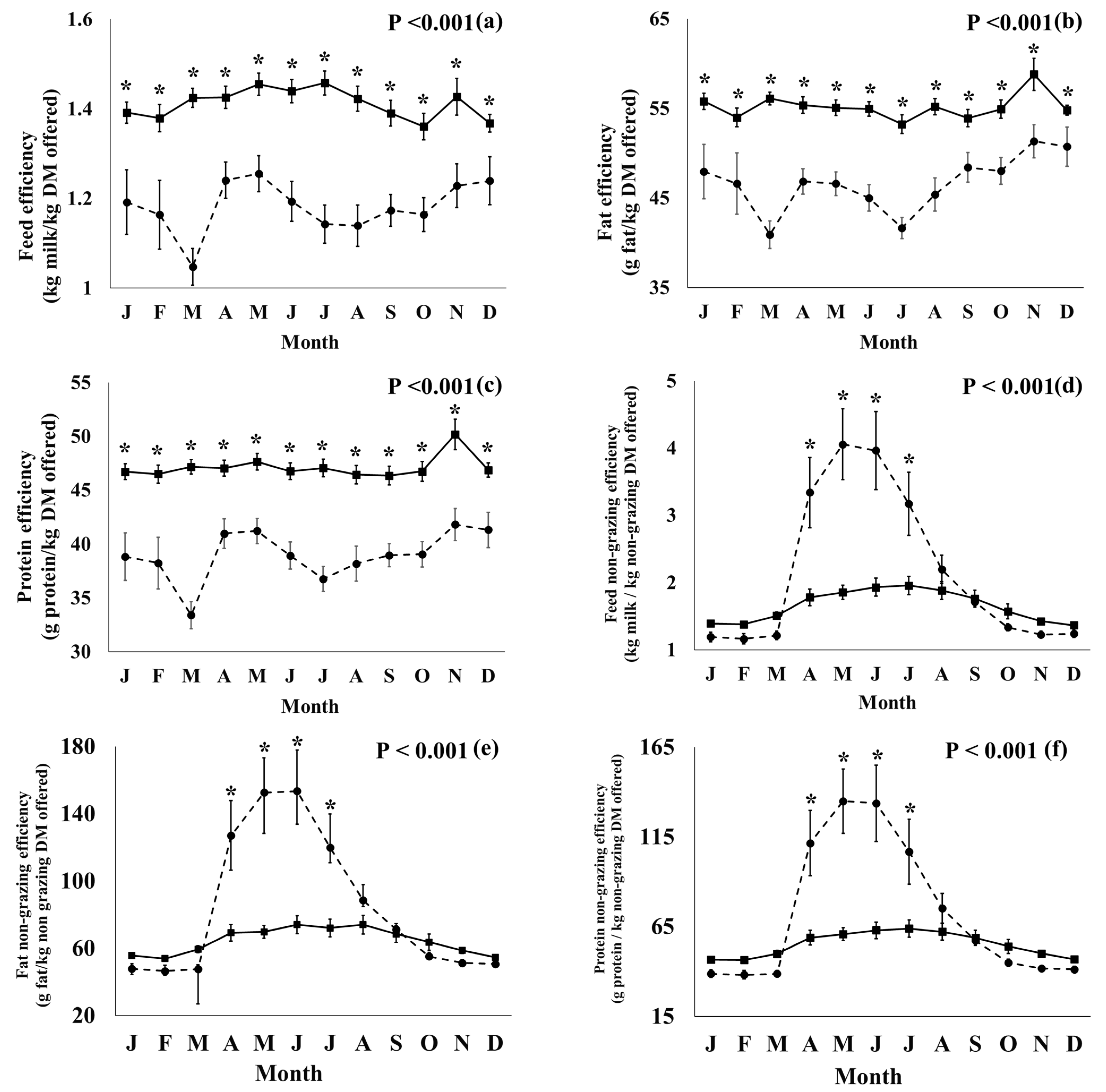
3.2. Productivity, Efficiency, and Health Parameters
3.2.1. Effect of Production System
| Production System | Production System × Month | ||||
|---|---|---|---|---|---|
| Conventional | Organic | SE | p-Value 2 | p-Value 2 | |
| n = 488 1 | n = 312 1 | ||||
| Productivity (kg/cow per day) | |||||
| Milk yield | 29.6 | 22.3 | 0.23 | <0.001 | <0.001 |
| ECMY | 30.3 | 22.8 | 0.22 | <0.001 | <0.001 |
| Milk fat yield | 1.15 | 0.88 | 0.008 | <0.001 | <0.001 |
| Milk protein yield | 0.99 | 0.74 | 0.007 | <0.001 | <0.001 |
| Basic composition (g/kg milk) | |||||
| Milk fat | 39.2 | 39.6 | 0.15 | 0.280 | 0.060 |
| Milk protein | 33.4 | 33.1 | 0.08 | 0.047 | <0.001 |
| Milk casein | 26.3 | 26.1 | 0.08 | 0.048 | <0.001 |
| Milk whey protein | 7.12 | 7.05 | 0.014 | 0.153 | <0.001 |
| Milk lactose (g/kg milk) | 45.2 | 44.8 | 0.04 | 0.014 | <0.001 |
| Urea (g/kg milk) | 0.17 | 0.14 | 0.003 | <0.001 | <0.001 |
| Efficiency Parameters | |||||
| Feed efficiency (kg milk/kg DM offered) | 1.41 | 1.18 | 0.011 | <0.001 | <0.001 |
| Feed non-grazing efficiency (kg milk/kg non-grazing DM offered) | 1.65 | 2.15 | 0.061 | 0.004 | <0.001 |
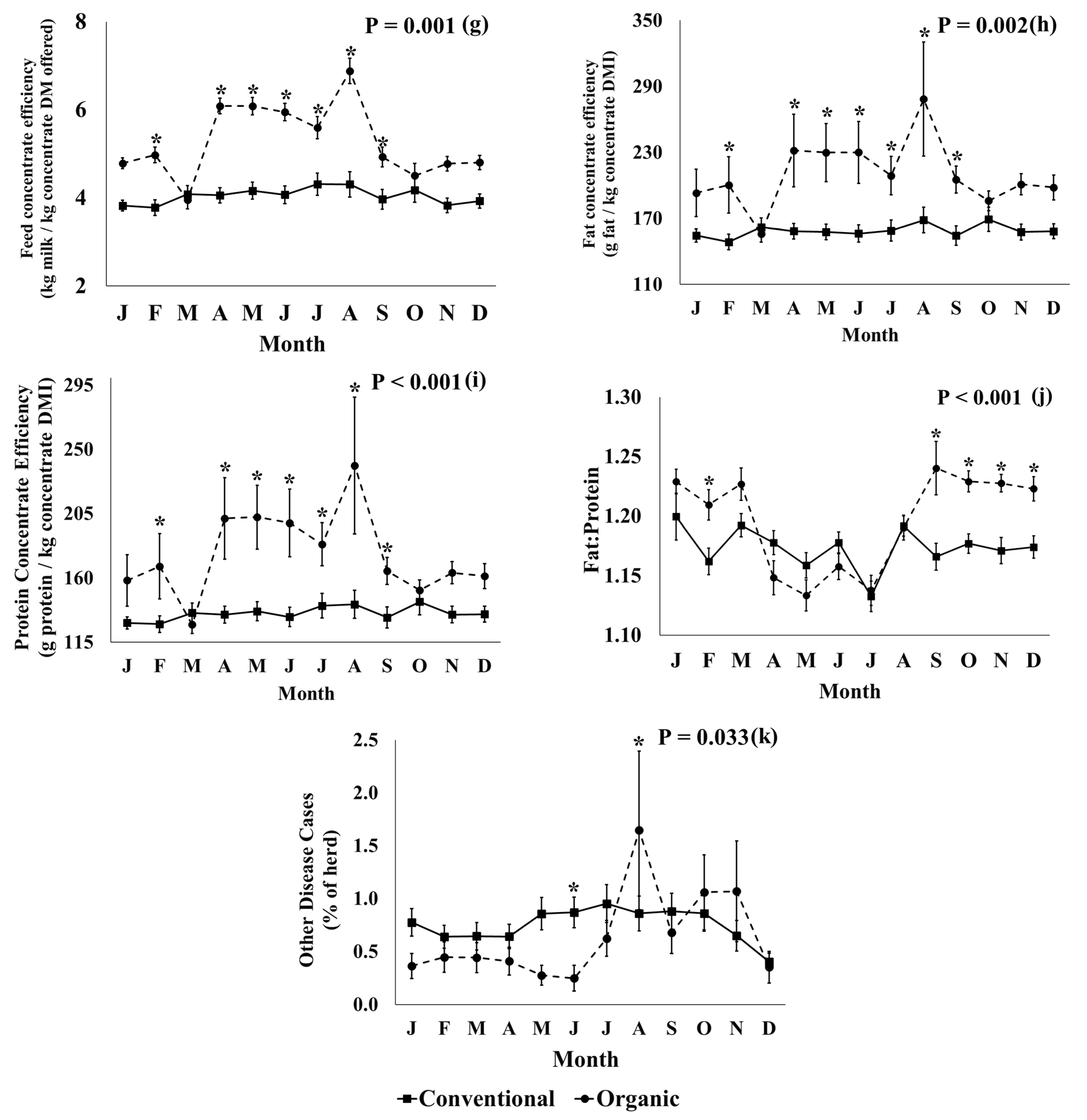
| Feed concentrate efficiency (kg milk/kg concentrate DM offered) | 4.05 | 5.28 | 0.103 | <0.001 | 0.001 |
| Fat efficiency (g fat/kg DM offered) | 55.2 | 46.6 | 0.410 | <0.001 | <0.001 |
| Fat non-grazing efficiency (g fat/kg non-grazing DM offered) | 64.7 | 84.8 | 2.42 | 0.004 | <0.001 |
| Fat concentrate efficiency (g fat/kg concentrate DM offered) | 159 | 210 | 4.3 | <0.001 | 0.002 |
| Protein efficiency (g protein/kg DM offered) | 47.1 | 39.0 | 0.333 | <0.001 | <0.001 |
| Protein non-grazing efficiency (g protein/kg non-grazing DM offered) | 55.3 | 72.3 | 2.13 | 0.007 | <0.001 |
| Protein concentrate efficiency (g protein/kg concentrate DM offered) | 135 | 177 | 3.7 | <0.001 | <0.001 |
| Health parameters and indicators | |||||
| Mastitis (% of herd) | 2.63 | 2.16 | 0.090 | 0.031 | 0.310 |
| Lameness (% of herd) | 2.05 | 2.65 | 0.122 | 0.279 | 0.396 |
| Other disease (% of herd) | 0.75 | 0.63 | 0.060 | 0.262 | 0.033 |
| Fat:protein | 1.17 | 1.20 | 0.004 | 0.002 | <0.001 |
| Milk SCC (×1000/mL milk) | 150 | 136 | 2.8 | 0.024 | 0.655 |
3.2.2. Effect of Month
3.2.3. Effect of Production System × Month Interaction
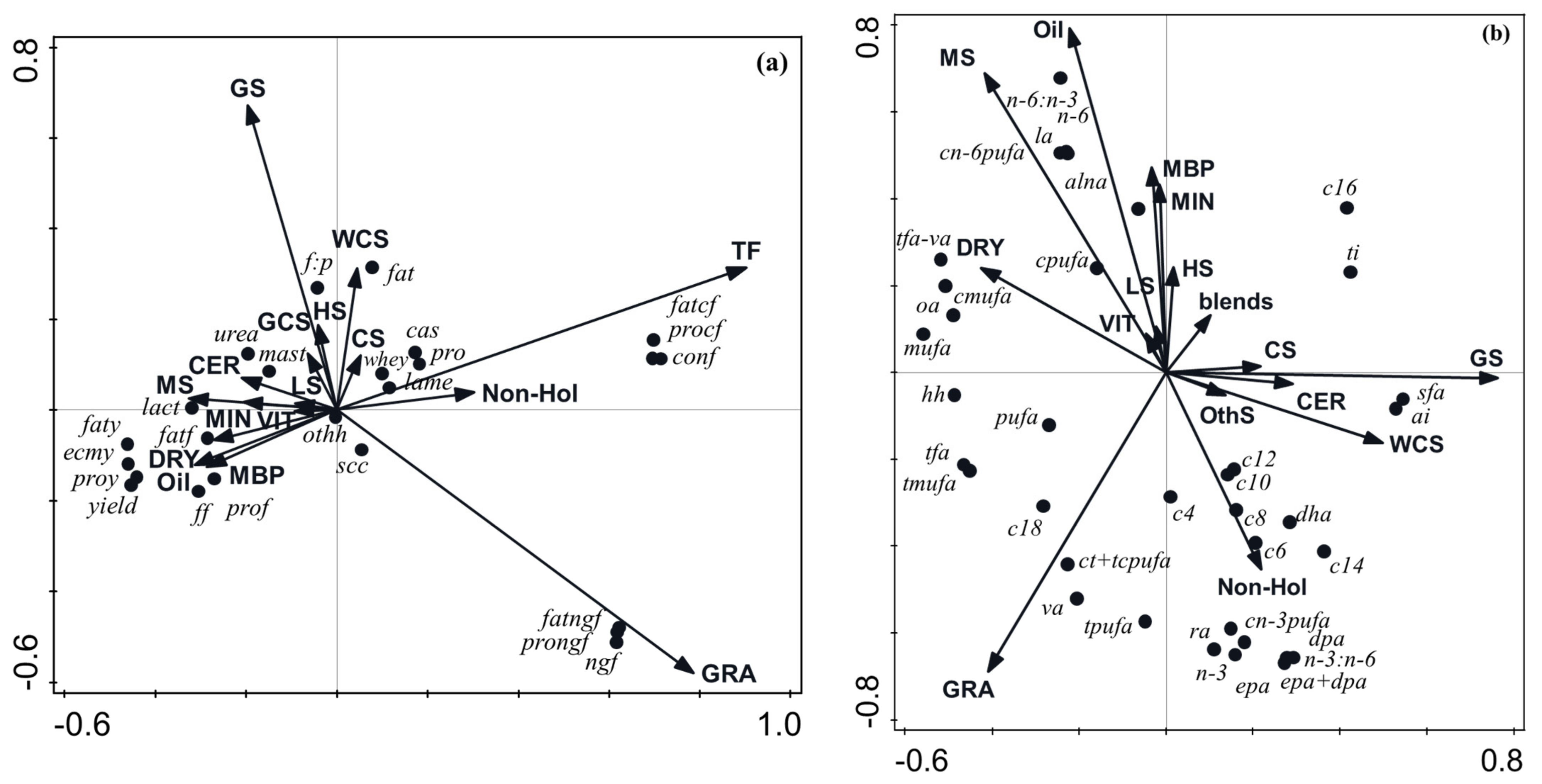
3.2.4. Multivariate Analysis of the Effect of Diet Composition on Milk Productivity, Efficiency, and Health Parameters
3.3. Milk Fatty Acid Profile
3.3.1. Effect of Production System
| Production System | Production System × Month | ||||
|---|---|---|---|---|---|
| Conventional n = 485 1 | Organic n = 309 1 | SE | p-Value 2 | p-Value 2 | |
| Individual FA (g/kg total FA) | |||||
| SFA | |||||
| C4:0 | 26.2 | 26.8 | 0.07 | <0.001 | <0.001 |
| C6:0 | 18.4 | 19.2 | 0.06 | <0.001 | <0.001 |
| C8:0 | 11.6 | 12.0 | 0.05 | 0.029 | <0.001 |
| C10:0 | 27.1 | 27.9 | 0.17 | 0.292 | <0.001 |
| C12:0 | 34.0 | 34.5 | 0.24 | 0.968 | <0.001 |
| C14:0 | 106 | 113 | 0.4 | <0.001 | 0.122 |
| C16:0 | 314 | 309 | 0.1 | 0.905 | <0.001 |
| C18:0 | 99.3 | 102 | 0.6 | 0.080 | <0.001 |
| MUFA | |||||
| VA (C18:1 t11) | 11.9 | 16.8 | 0.28 | <0.001 | <0.001 |
| OA (C18:1 c9) | 198 | 185 | 0.89 | <0.001 | 0.015 |
| PUFA | |||||
| LA (18:2 c9c12) | 19.1 | 16.0 | 0.21 | <0.001 | 0.365 |
| RA | 5.94 | 8.00 | 0.123 | <0.001 | <0.001 |
| ALNA (18:3 c9c12c15) | 4.53 | 6.90 | 0.063 | <0.001 | 0.013 |
| EPA (20:5 c5c8c11c14c17) | 0.44 | 0.66 | 0.006 | <0.001 | <0.001 |
| DPA (C22:5 c7c10c13c16c19) | 0.74 | 1.05 | 0.007 | <0.001 | 0.002 |
| DHA (C22:6 c4c7c10c13c16c19) | 0.05 | 0.07 | 0.001 | <0.001 | 0.002 |
| FA groups (g/kg total FA) | |||||
| SFA 3 | 678 | 692 | 1.4 | <0.001 | <0.001 |
| MUFA 4 | 279 | 263 | 1.2 | <0.001 | <0.001 |
| cis MUFA 6 | 242 | 226 | 0.9 | <0.001 | 0.018 |
| trans MUFA 7 | 36.7 | 37.0 | 0.41 | 0.405 | <0.001 |
| PUFA 5 | 43.3 | 45.7 | 0.30 | 0.014 | <0.001 |
| cis PUFA 8 | 27.8 | 27.6 | 0.22 | 0.132 | 0.036 |
| trans PUFA 9 | 0.24 | 0.41 | 0.009 | <0.001 | <0.001 |
| cis,trans + trans,cis PUFA 10 | 15.3 | 17.7 | 0.19 | <0.001 | <0.001 |
| n-3 11 | 8.30 | 12.5 | 0.19 | <0.001 | <0.001 |
| n-6 12 | 22.7 | 19.1 | 0.234 | <0.001 | 0.207 |
| cis n-3 PUFA 13 | 5.91 | 8.97 | 0.073 | <0.001 | 0.015 |
| cis n-6 PUFA 14 | 21.8 | 18.4 | 0.23 | <0.001 | 0.216 |
| n-3:n-6 ratio | 0.40 | 0.68 | 0.106 | <0.001 | <0.001 |
| n-6:n-3 ratio | 2.91 | 1.62 | 0.384 | <0.001 | <0.001 |
| EPA+DHA | 0.48 | 0.74 | 0.007 | <0.001 | <0.001 |
| trans FA 15 | 38.5 | 38.6 | 0.44 | 0.526 | <0.001 |
| trans FA (exc. VA) | 26.7 | 22.0 | 0.23 | <0.001 | 0.028 |
| Human health related indices | |||||
| AI 16 | 2.43 | 2.63 | 0.020 | <0.001 | <0.001 |
| TI 17 | 2.92 | 2.88 | 0.019 | 0.529 | <0.001 |
| HH 18 | 0.54 | 0.51 | 0.004 | 0.004 | 0.011 |
| Δ9-desaturase activity indicators | |||||
| Δ9I 19 | 0.28 | 0.27 | 0.001 | <0.001 | 0.007 |
| C14:1:C14:0 | 0.02 | 0.02 | 0.000 | <0.001 | <0.001 |
| C16:1:C16:0 | 0.03 | 0.03 | 0.000 | <0.001 | 0.009 |
| OA:C18:0 | 2.01 | 1.81 | 0.008 | <0.001 | 0.006 |
| RA:VA | 0.17 | 0.16 | 0.006 | <0.001 | <0.001 |
3.3.2. Effect of Month
3.3.3. Effect of Production System × Month Interaction
3.3.4. Multivariate Analysis of the Effect of Diet Composition on Milk Fatty Acid Profile
4. Discussion
4.1. Effect of Production System on Productivity and Efficiency
4.2. Effect of Production System on Animal Health
4.3. Effect of Production System on Milk Fatty Acid Profile
4.4. Nutritional Implications for Organic Milk Consumers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Association, S. Soil Association Standards Farming and Growing. Available online: https://www.soilassociation.org/media/15931/farming-and-growing-standards.pdf (accessed on 17 July 2022).
- Association, S. Organic Market Report. Available online: https://www.soilassociation.org/certification/organic-market-report/ (accessed on 9 December 2022).
- Ellis, K.A.; Innocent, G.; Grove-White, D.; Cripps, P.; McLean, W.G.; Howard, C.V.; Mihm, M. Comparing the fatty acid composition of organic and conventional milk. J. Dairy Sci. 2006, 89, 1938–1950. [Google Scholar] [CrossRef] [PubMed]
- Butler, G.; Nielsen, J.H.; Slots, T.; Seal, C.; Eyre, M.D.; Sanderson, R.; Leifert, C. Fatty acid and fat-soluble antioxidant concentrations in milk from high- and low-input conventional and organic systems: Seasonal variation. J. Sci. Food Agric. 2008, 88, 1431–1441. [Google Scholar] [CrossRef]
- Stergiadis, S.; Leifert, C.; Seal, C.J.; Eyre, M.D.; Larsen, M.K.; Slots, T.; Nielsen, J.H.; Butler, G. A 2-year study on milk quality from three pasture-based dairy systems of contrasting production intensities in Wales. J. Agric. Sci. 2015, 153, 708–731. [Google Scholar] [CrossRef]
- Rodríguez-Bermúdez, R.; Fouz, R.; Miranda, M.; Orjales, I.; Minervino, A.H.H.; López-Alonso, M. Organic or conventional dairy farming in northern Spain: Impacts on cow reproductive performance. Reprod. Domest. Anim. 2019, 54, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Croissant, A.E.; Washburn, S.P.; Dean, L.L.; Drake, M.A. Chemical Properties and Consumer Perception of Fluid Milk from Conventional and Pasture-Based Production Systems. J. Dairy Sci. 2007, 90, 4942–4953. [Google Scholar] [CrossRef]
- Davis, H.; Stergiadis, S.; Chatzidimitriou, E.; Sanderson, R.; Leifert, C.; Butler, G. Meeting Breeding Potential in Organic and Low-Input Dairy Farming. Front. Vet. Sci. 2020, 7, 544149. [Google Scholar] [CrossRef]
- Stergiadis, S.; Leifert, C.; Seal, C.J.; Eyre, M.D.; Nielsen, J.H.; Larsen, M.K.; Slots, T.; Steinshamn, H.; Butler, G. Effect of Feeding Intensity and Milking System on Nutritionally Relevant Milk Components in Dairy Farming Systems in the North East of England. J. Agric. Food Chem. 2012, 60, 7270–7281. [Google Scholar] [CrossRef]
- SACN. Saturated Fats and Health. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/814995/SACN_report_on_saturated_fat_and_health.pdf (accessed on 12 June 2022).
- Gaudaré, U.; Pellerin, S.; Benoit, M.; Durand, G.; Dumont, B.; Barbieri, P.; Nesme, T. Comparing productivity and feed-use efficiency between organic and conventional livestock animals. Environ. Res. Lett. 2021, 16, 024012. [Google Scholar] [CrossRef]
- Brito, A.F.; Silva, L.H.P. Symposium review: Comparisons of feed and milk nitrogen efficiency and carbon emissions in organic versus conventional dairy production systems. J. Dairy Sci. 2020, 103, 5726–5739. [Google Scholar] [CrossRef]
- AHDB. Is All-Year-Round Calving Really the Best Option. Available online: https://ahdb.org.uk/news/is-all-year-round-calving-really-the-best-option (accessed on 21 March 2022).
- Qin, N.; Faludi, G.; Beauclercq, S.; Pitt, J.; Desnica, N.; Pétursdóttir, Á.; Newton, E.E.; Angelidis, A.; Givens, I.; Juniper, D.; et al. Macromineral and trace element concentrations and their seasonal variation in milk from organic and conventional dairy herds. Food Chem. 2021, 359, 129865. [Google Scholar] [CrossRef]
- Chilliard, Y.; Martin, C.; Rouel, J.; Doreau, M. Milk fatty acids in dairy cows fed whole crude linseed, extruded linseed, or linseed oil, and their relationship with methane output1. J. Dairy Sci. 2009, 92, 5199–5211. [Google Scholar] [CrossRef] [PubMed]
- Ulberth, F.; Gabernig, R.G.; Schrammel, F. Flame-ionization detector response to methyl, ethyl, propyl, and butyl esters of fatty acids. J. Amer. Oil Chem. Soc. 1999, 76, 263–266. [Google Scholar] [CrossRef]
- Mierlita, D. Effects of diets containing hemp seeds or hemp cake on fatty acid composition and oxidative stability of sheep milk. S. Afr. J. Anim. 2018, 48, 504–515. [Google Scholar] [CrossRef]
- Stergiadis, S.; Berlitz, C.B.; Hunt, B.; Garg, S.; Ian Givens, D.; Kliem, K.E. An update to the fatty acid profiles of bovine retail milk in the United Kingdom: Implications for nutrition in different age and gender groups. Food Chem. 2019, 276, 218–230. [Google Scholar] [CrossRef]
- VSN International. Genstat for Windows, 18th ed.; VSN International Ltd.: Hempstead, UK, 2020. [Google Scholar]
- Canoco5, Software for Multivariate Data Exploration, Testing and Summarization; Microcomputor Power: Ithaca, NY, USA, 2012.
- Stergiadis, S.; Bieber, A.; Chatzidimitriou, E.; Franceschin, E.; Isensee, A.; Rempelos, L.; Baranski, M.; Maurer, V.; Cozzi, G.; Bapst, B.; et al. Impact of US Brown Swiss genetics on milk quality from low-input herds in Switzerland: Interactions with season. Food Chem. 2018, 251, 93–102. [Google Scholar] [CrossRef]
- Średnicka-Tober, D.; Barański, M.; Seal, C.; Sanderson, R.; Benbrook, C.; Steinshamn, H.; Gromadzka-Ostrowska, J.; Rembiałkowska, E.; Skwarło-Sońta, K.; Eyre, M.; et al. Composition differences between organic and conventional meat: A systematic literature review and meta-analysis. Br. J. Nutr. 2016, 115, 994–1011. [Google Scholar] [CrossRef]
- Kay, J.K.; Mackle, T.R.; Auldist, M.J.; Thomson, N.A.; Bauman, D.E. Endogenous Synthesis of cis-9, trans-11 Conjugated Linoleic Acid in Dairy Cows Fed Fresh Pasture. J. Dairy Sci. 2004, 87, 369–378. [Google Scholar] [CrossRef]
- Adler, S.A.; Jensen, S.K.; Govasmark, E.; Steinshamn, H. Effect of short-term versus long-term grassland management and seasonal variation in organic and conventional dairy farming on the composition of bulk tank milk. J. Dairy Sci. 2013, 96, 5793–5810. [Google Scholar] [CrossRef]
- White, S.L.; Bertrand, J.A.; Wade, M.R.; Washburn, S.P.; Green, J.T.; Jenkins, T.C. Comparison of Fatty Acid Content of Milk from Jersey and Holstein Cows Consuming Pasture or a Total Mixed Ration. J. Dairy Sci. 2001, 84, 2295–2301. [Google Scholar] [CrossRef]
- Schwendel, B.H.; Wester, T.J.; Morel, P.C.H.; Tavendale, M.H.; Deadman, C.; Shadbolt, N.M.; Otter, D.E. Invited review: Organic and conventionally produced milk—An evaluation of factors influencing milk composition. J. Dairy Sci. 2015, 98, 721–746. [Google Scholar] [CrossRef]
- Lorenz, H.; Reinsch, T.; Hess, S.; Taube, F. Is low-input dairy farming more climate friendly? A meta-analysis of the carbon footprints of different production systems. J. Clean. Prod. 2019, 211, 161–170. [Google Scholar] [CrossRef]
- Ellis, K.A.; Innocent, G.T.; Mihm, M.; Cripps, P.; McLean, W.G.; Howard, C.V.; Grove-White, D. Dairy cow cleanliness and milk quality on organic and conventional farms in the UK. J. Dairy Res. 2007, 74, 302–310. [Google Scholar] [CrossRef]
- Ward, W.R.; Hughes, J.W.; Faull, W.B.; Cripps, P.J.; Sutherland, J.P.; Sutherst, J.E. Observational study of temperature, moisture, pH and bacteria in straw bedding, and faecal consistency, cleanliness and mastitis in cows in four dairy herds. Vet. Rec. 2002, 151, 199–206. [Google Scholar] [CrossRef]
- Prendiville, R.; Pierce, K.M.; Buckley, F. A comparison between Holstein-Friesian and Jersey dairy cows and their F1 cross with regard to milk yield, somatic cell score, mastitis, and milking characteristics under grazing conditions. J. Dairy Sci. 2010, 93, 2741–2750. [Google Scholar] [CrossRef]
- Friggens, N.C.; Ridder, C.; Løvendahl, P. On the Use of Milk Composition Measures to Predict the Energy Balance of Dairy Cows. J. Dairy Sci. 2007, 90, 5453–5467. [Google Scholar] [CrossRef]
- Cabezas-Garcia, E.H.; Gordon, A.W.; Mulligan, F.J.; Ferris, C.P. Revisiting the Relationships between Fat-to-Protein Ratio in Milk and Energy Balance in Dairy Cows of Different Parities, and at Different Stages of Lactation. Animals 2021, 11, 3256. [Google Scholar] [CrossRef]
- Poljak, F.; Mijić, P.; Lončarić, Z.; Steiner, Z.; Gantner, V. The analysis of variability of indicators associated with prevalence of subclinical ketosis/acidosis in dairy cattle. Agric. Conspec. Sci. 2021, 86, 259–263. [Google Scholar]
- Zhang, R.; Liu, J.; Jiang, L.; Mao, S. Effect of high-concentrate diets on microbial composition, function, and the VFAs formation process in the rumen of dairy cows. Anim. Feed Sci. Technol. 2020, 269, 114619. [Google Scholar] [CrossRef]
- Schroeder, G.F.; Delahoy, J.E.; Vidaurreta, I.; Bargo, F.; Gagliostro, G.A.; Muller, L.D. Milk Fatty Acid Composition of Cows Fed a Total Mixed Ration or Pasture Plus Concentrates Replacing Corn with Fat. J. Dairy Sci. 2003, 86, 3237–3248. [Google Scholar] [CrossRef]
- Ormston, S.; Davis, H.; Butler, G.; Chatzidimitriou, E.; Gordon, A.W.; Theodoridou, K.; Huws, S.; Yan, T.; Leifert, C.; Stergiadis, S. Performance and milk quality parameters of Jersey crossbreds in low-input dairy systems. Sci. Rep. 2022, 12, 7550. [Google Scholar] [CrossRef]
- Stergiadis, S.; Bieber, A.; Franceschin, E.; Isensee, A.; Eyre, M.D.; Maurer, V.; Chatzidimitriou, E.; Cozzi, G.; Bapst, B.; Stewart, G.; et al. Impact of US Brown Swiss genetics on milk quality from low-input herds in Switzerland: Interactions with grazing intake and pasture type. Food Chem. 2015, 175, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.V.; Malau-Aduli, B.S.; Cavalieri, J.; Malau-Aduli, A.E.O.; Nichols, P.D. Enhancing omega-3 long-chain polyunsaturated fatty acid content of dairy-derived foods for human consumption. Nutrients 2019, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Ferlay, A.; Mansbridge, R.M.; Doreau, M. Ruminant milk fat plasticity: Nutritional control of saturated, polyunsaturated, trans and conjugated fatty acids. Ann. Zootech. 2000, 49, 181–205. [Google Scholar] [CrossRef]
- Givens, D.I. Milk in the diet: Good or bad for vascular disease? Proc. Nutr. Soc. 2012, 71, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Nantapo, C.T.W.; Muchenje, V.; Hugo, A. Atherogenicity index and health-related fatty acids in different stages of lactation from Friesian, Jersey and Friesian×Jersey cross cow milk under a pasture-based dairy system. Food Chem. 2014, 146, 127–133. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Evans, R.T.; Scollan, N.D.; Moorby, J.M.; Merry, R.J.; Wilkins, R.J. Comparison of Grass and Legume Silages for Milk Production. 2. In Vivo and In Sacco Evaluations of Rumen Function. J. Dairy Sci. 2003, 86, 2612–2621. [Google Scholar] [CrossRef]
- Stergiadis, S.; Leifert, C.; Seal, C.J.; Eyre, M.D.; Steinshamn, H.; Butler, G. Improving the fatty acid profile of winter milk from housed cows with contrasting feeding regimes by oilseed supplementation. Food Chem. 2014, 164, 293–300. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, Y.; Xu, Q.; Zheng, N.; Zhao, S.; Liu, K.; Qu, X.; Yu, J.; Wang, J. DHA content in milk and biohydrogenation pathway in rumen: A review. PeerJ 2020, 8, e10230. [Google Scholar] [CrossRef]
- Givens, I.D.; Gibbs, R.A. Current intakes of EPA and DHA in European populations and the potential of animal-derived foods to increase them: Symposium on ‘How can the n-3 content of the diet be improved? Proc. Nutr. Soc. 2008, 67, 273–280. [Google Scholar] [CrossRef]
- Givens, D.I. Milk and meat in our diet: Good or bad for health? Animal 2010, 4, 1941–1952. [Google Scholar] [CrossRef]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef]
- England, P.H. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/943114/NDNS_UK_Y9-11_report.pdf (accessed on 19 June 2022).
- Jahreis, G.; Dawczynski, C. Chapter 4—Trans and conjugated fatty acids in dairy products: Cause for concern? In Milk and Dairy Foods; Givens, D.I., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 87–120. [Google Scholar]
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef]
- Verneque, B.J.F.; Machado, A.M.; de Abreu Silva, L.; Lopes, A.C.S.; Duarte, C.K. Ruminant and industrial trans-fatty acids consumption and cardiometabolic risk markers: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2050–2060. [Google Scholar] [CrossRef]
- Givens, D.I. Manipulation of lipids in animal-derived foods: Can it contribute to public health nutrition? Eur. J. Lipid Sci. Technol. 2015, 117, 1306–1316. [Google Scholar] [CrossRef]
- Lavie Carl, J.; Milani Richard, V.; Mehra Mandeep, R.; Ventura Hector, O. Omega-3 Polyunsaturated Fatty Acids and Cardiovascular Diseases. J. Am. Coll. Cardiol. 2009, 54, 585–594. [Google Scholar] [CrossRef]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br. J. Nutr. 2002, 88, 355–363. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ormston, S.; Qin, N.; Faludi, G.; Pitt, J.; Gordon, A.W.; Theodoridou, K.; Yan, T.; Huws, S.A.; Stergiadis, S. Implications of Organic Dairy Management on Herd Performance and Milk Fatty Acid Profiles and Interactions with Season. Foods 2023, 12, 1589. https://doi.org/10.3390/foods12081589
Ormston S, Qin N, Faludi G, Pitt J, Gordon AW, Theodoridou K, Yan T, Huws SA, Stergiadis S. Implications of Organic Dairy Management on Herd Performance and Milk Fatty Acid Profiles and Interactions with Season. Foods. 2023; 12(8):1589. https://doi.org/10.3390/foods12081589
Chicago/Turabian StyleOrmston, Sabrina, Nanbing Qin, Gergely Faludi, Joe Pitt, Alan W. Gordon, Katerina Theodoridou, Tianhai Yan, Sharon A. Huws, and Sokratis Stergiadis. 2023. "Implications of Organic Dairy Management on Herd Performance and Milk Fatty Acid Profiles and Interactions with Season" Foods 12, no. 8: 1589. https://doi.org/10.3390/foods12081589
APA StyleOrmston, S., Qin, N., Faludi, G., Pitt, J., Gordon, A. W., Theodoridou, K., Yan, T., Huws, S. A., & Stergiadis, S. (2023). Implications of Organic Dairy Management on Herd Performance and Milk Fatty Acid Profiles and Interactions with Season. Foods, 12(8), 1589. https://doi.org/10.3390/foods12081589






