1. Introduction
Morindae officinalis radix (MOR), the dried root of medicinal plant
Morinda officinalis How (Rubiaceae), has long been used in tonics and nutrient supplements (healthcare products, nourishing soups and drinks) in the southeast region of China for its action in nourishing the kidney, anti-osteoporosis, immune-enhancing effects, and for alleviating a wide spectrum of diseases. Various types of chemical constituents of MOR have been researched and reported, such as polysaccharides, mono- and oligosaccharides, iridoids, glycosides anthraquinones, volatile oils, organic acids, and other types of compounds [
1]. Based on that, MOR has been shown to have multiple biological activities, including anti-depression [
2,
3,
4,
5], anti-inflammation [
6,
7], anti-osteoporosis [
8,
9,
10], anti-fatigue [
11], anti-Alzheimer’s disease (AD) [
12,
13,
14], anti-oxidation [
11,
15,
16], immune-regulation [
17,
18], pro-fertility [
19,
20,
21], anti-radiation [
21,
22], regulation on gut microbiota [
15,
23,
24], etc. The content of saccharides in MOR was reported to be 49.79–58.25% [
12], and polysaccharides are the main active substances of MOR. Until now, studies on a variety of biological activities of the total polysaccharides or crude polysaccharides extracted from MOR, such as antioxidant [
15,
16,
25], pro-fertility [
26,
27], anti-fatigue [
11,
28], bone protection and anti-osteoporosis [
9,
29,
30,
31,
32], and anti-inflammatory [
33] have been reported, indicating that the polysaccharides have been supposed to play important roles in the pharmacological properties. In addition, more studies have revealed the structure and molecular mechanisms underlying the biological activities.
MOR has been approved since 2002 as a functional food for daily healthcare by the National Health Commission of China and has been used in health foods and Chinese prescriptions, such as
Baoshen Wan,
Tianjing Bushen Gao, and
Erxian Decoction [
1]. The development of health-functional foods and new food raw materials using natural products or extracts is actively advancing. Recently, the toxicological characteristics of fermented Morinda officinalis (FMO) have been assessed for supporting the safe use of FMO as a functional food and medicine [
34]. Most active extracts derived from herbal medicines are considered safe, but some of them have known toxicities [
35,
36]. Therefore, the scientific evaluation and basis for the safety of extracts are considered important and essential [
37,
38,
39]. The crude polysaccharides extracted from MOR have diverse pharmacological effects, which indicates great potential for applications in the development of new food raw material or functional food. However, the evaluation of potential toxicity of cMORP has not been reported so far. The evidence suggests that the biological activities are related to structural characteristics of polysaccharides (chemical structure, monosaccharide composition, molecular weight, etc.) affected by the selected methods of extraction [
40,
41,
42]. The extraction methods of polysaccharides are becoming increasingly diverse, including hot water extraction, alkali solution extraction, ultrasound-assisted extraction, microwave-assisted extraction, subcritical water extraction, and so on. It is hoped that the physical properties of polysaccharides will not change significantly under mild extraction conditions. The hot water procedure is usually the preferred method for extracting polysaccharides in industrial production, largely due to the process simplicity, facility, low-cost, and safety. A simple and effective extraction method of polysaccharides is the first step for development and application. Hot water extraction was chosen for the following experiments in view of simplicity and industrialization, and the extraction process should be optimized.
Therefore, in this study, the hot water extraction process of cMORP, including four variables (liquid/solid ratio, extraction temperature, number of extraction times, and extraction time), was investigated and optimized via a single-factor test and orthogonal experimental design. Then, ultraviolet–visible spectroscopy (UV-vis), Fourier-transform infrared spectroscopy (FT-IR), high-performance anion-exchange chromatography (HPAEC), matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF-MS), and scanning electron microscopy (SEM) were utilized for the preliminary characterization of cMORP. Additionally, a 14-day acute toxicity study in vivo with a single-dose and 30-day repeated tests at different doses were conducted to establish a preliminary safety evaluation. The aim of this research is to isolate water-soluble crude polysaccharides from Morindae officinalis radix and to evaluate the structural characteristics and safety. The results of this research are expected to provide a scientific basis and support for the practical application of cMORP as a new food raw material or functional food and in-depth study.
2. Materials and Methods
2.1. Reagents and Animals
MOR was obtained from Deqing County, Zhaoqing City, Guangdong Province, China. After drying and crushing, it was sieved through a 60-mesh sieve and placed in a dryer. Thirteen kinds of standard monosaccharides, including rhamnose (Rha), arabinose (Ara), glucose (Glc), galactose (Gal), fucose (Fuc), xylose (Xyl), mannose (Man), fructose (Fru), ribose (Rib), galacturonic acid (Gal-UA), glucuronic acid (Glc-UA), mannuronic acid (Man-UA), and guluronic acid (Gul-UA) were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). Other chemicals and reagents were of analytical grade.
The specific pathogen free (SPF) KM mice (half male and half female) were obtained from the Laboratory Animal Center of Sun Yat-Sen University (Guangzhou, China), and the license was SCXK (Y) 2021-0029. Adult healthy KM mice aged 6–8 weeks were used for the acute toxicity test (body weight, 25–30 g (males); 20–25 g (females)). Adult healthy KM mice aged 6–8 weeks were used for the repeated-dose 30-day oral toxicity study (body weight, 25–30 g (males); 25–30 g (females)). The selected female mice were non-pregnant and nulliparous. Male and female mice were housed separately and given sterilized food (Guangdong Medical Laboratory Animal Center, Guangdong, China) and purified water via a water bottle. After three days of acclimatization feeding, the mice were randomly assigned to different cages and labeled, and the temperature of the feeding environment was controlled at 22 ± 3 °C. The animal study was performed in accordance with the protocol approved by the Animal Ethical and Welfare Committee of Sun Yat-sen University (Approval No. SYSU-IACUC-2023-000015).
2.2. Optimal Design of Hot Water Extraction Process for cMORP
2.2.1. Hot Water Extraction Process
The cMORP was extracted by a hot water procedure. The dried powder was added to water at a ratio of 1:20 (
w/
v) and extracted twice at 80 °C for 2 h each time, and the residue was filtered. The polysaccharide extract solution was obtained, and the content of polysaccharides was determined by the phenol-sulfuric acid method [
43]. The calculation formula of yield of polysaccharides was as follows:
where
C,
V,
D, and
M are the concentration of polysaccharides (g/mL), volume of the solution (mL), dilution factor, and the mass of the sample (g), respectively.
2.2.2. Single-Factor Experiment
The medium value of the optimal range for each factor was determined by single-factor experiments. The values of other three factors remained fixed when one factor changed. The yield of polysaccharide was affected by multiple factors, including extraction temperature, extraction time, liquid/solid ratio, and extraction times [
44]. The four factors, including liquid/solid ratios of 10, 15, 20, 25, and 30 mL/g; extraction temperatures of 60, 70, 80, 90, and 100 °C; numbers of extraction times of 1, 2, 3, 4, and 5; and extraction times of 0.5, 1, 2, 3, and 4 h, were set as the experimental conditions and investigated.
2.2.3. Orthogonal Experimental Design
Based on a preliminary study of extraction factors (liquid/solid ratio, extraction temperature, number of extraction times, and extraction time) in the single-factor trials, the orthogonal table L
9(3
4) was selected to optimize the parameters, reflecting the orthogonality, representativeness, and comprehensive comparability. The assessment indicator was the yield of polysaccharides, and then the optimal extraction process conditions were determined.
Table 1 shows the test factors and levels of the experiments.
2.2.4. Obtention of cMORP
The extraction process was carried out according to the optimized method. The extract was concentrated to one-third of the volume by a rotary evaporator, decolorized twice with 1% activated carbon (w/v), and filtered. The supernatants were recovered and concentrated to a quarter of volume, and the polysaccharides extract solution was obtained. cMORP was obtained by the alcohol precipitation method. Subsequently, the concentrated extract was mixed with 95% ethanol to an ethanol concentration of 80%, stirred, and kept overnight. The precipitate was collected by centrifugation (15 min at 5000 rpm). The precipitate was dried under vacuum and stored in a light-proof desiccator.
2.3. Characterization of cMORP
2.3.1. Determination of Chemical Composition
The polysaccharide content was determined by the phenol-sulfuric acid method with glucose as the standard [
45]. In short, 1 mL polysaccharide solution or glucose solution at a concentration of 50 μg/mL was mixed with 1 mL 5% phenol solution and 5 mL sulfuric acid and then reacted at 100 °C for 15 min. After cooling to room temperature, the absorbance at 490 nm was measured. The uronic acid content was determined by the vitriol-carbazole method with D-glucuronic acid as the standard [
46]. Briefly, 1 mL polysaccharide solution or D-glucuronic acid solution was mixed with 6 mL sodium tetraborate-sulfuric acid solution in an ice-water bath and then reacted at 100 °C for 10 min. After cooling to room temperature, it was mixed with 100 μL of m-hydroxybiphenyl solution with the absorbance measured at 524 nm. The protein content of cMORP was determined using Coomassie brilliant blue G-250 with bovine serum albumin (BSA) as the standard [
47]. In short, 1 mL polysaccharide solution or BSA solution was mixed with 1 mL Coomassie brilliant blue G-250 solution and then reacted at room temperature for 30 min with absorbance measured at 590 nm.
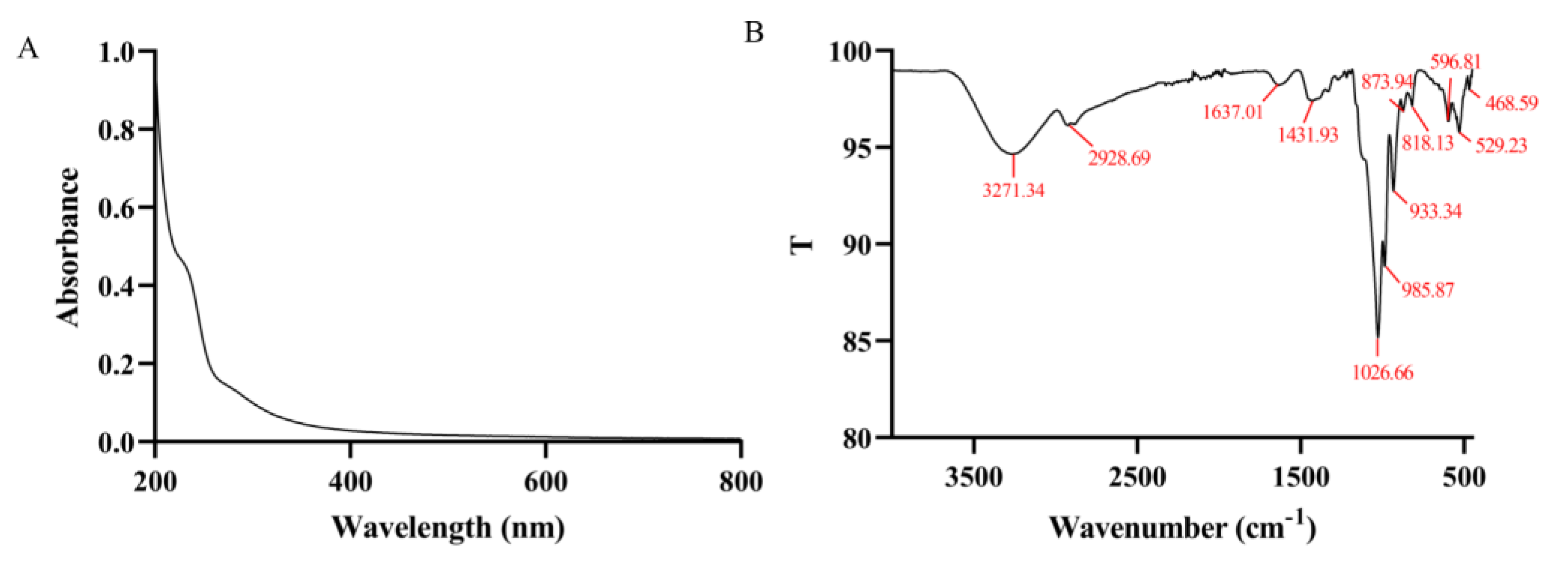
2.3.2. UV-Vis and FT-IR
For the analysis, 1 mg/mL aqueous solution of cMORP was prepared and scanned from 200 to 800 nm at room temperature with a UV-2600 ultraviolet spectrophotometer (Shimadzu, Japan), and the UV-vis spectrum was obtained. cMORP functional groups were determined by FT-IR. Then 10 mg of the sample was pressed into the disk. The IR spectrum was recorded at room temperature (25 ± 0.5 °C) with a spectral resolution of 2 cm
−1 under dry air in the range of 4000–450 cm
−1 on an FT-IR spectrophotometer (Frontier, PerkinElmer, Waltham, MA, USA) [
48].
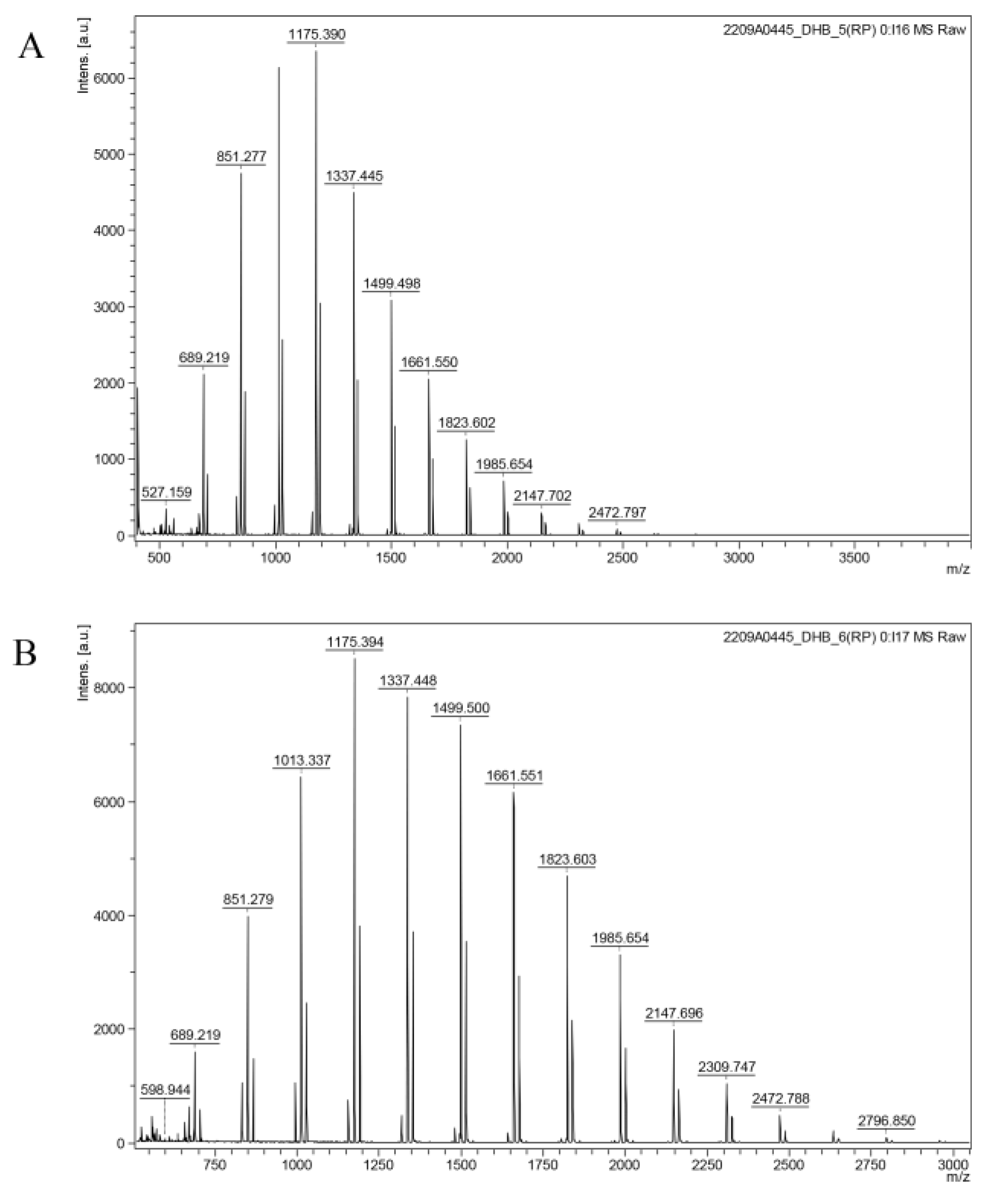
2.3.3. Determination of Molecular Weight (Mw)
MALDI-TOF-MS is sensitive to the detection of low molecular weight substances and can determine the molecular mass distribution of crude polysaccharides. The sample were diluted with distilled water to a concentration of 1 mg/mL and filtered. The signals were recorded in the range of 400–4000 m/z.
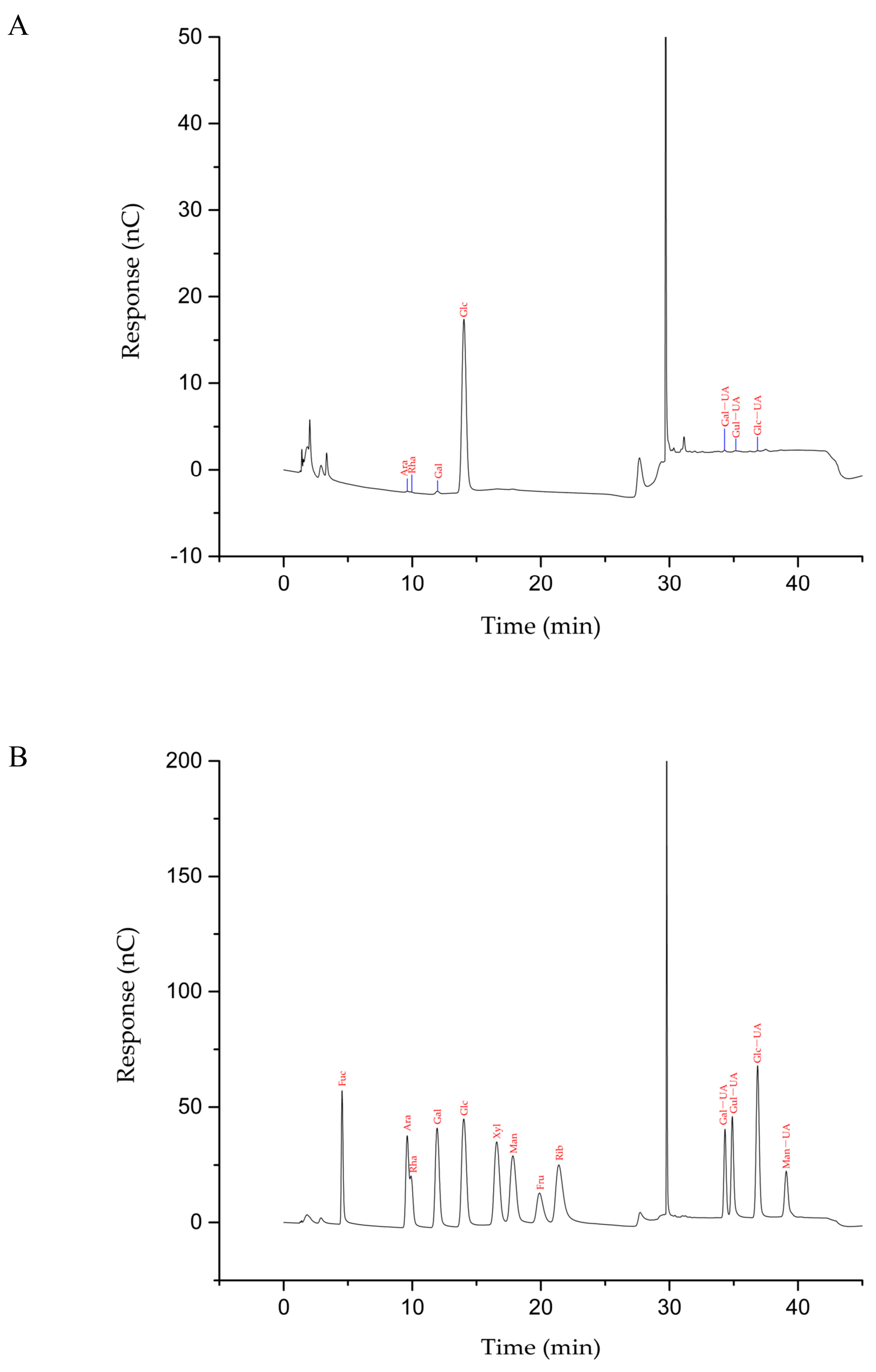
2.3.4. Monosaccharide Identification
The monosaccharide composition was determined by HPAEC. The polysaccharides were hydrolyzed in a sealed chromatographic bottle with 2 M trifluoroacetic acid (TFA) for 2 h at 121 °C and redissolved with water and analyzed by HPAEC following the removal of TFA. The retention times and standard curves of monosaccharide standards, including Rha, Ara, Glc, Gal, Fuc, Xyl, Man, Fru, Rib, Gal-UA, Glc-UA, Man-UA, and Gul-UA, were determined. By comparing the chromatographic peak and retention time of the sample with that of the standards, the composition and content of the monosaccharide in the sample can be determined.
Chromatographic conditions were as follows: Dionex™ CarboPac™ PA-20 column (3.0 mm × 150 mm, 10 µm); Dionex™ ICS-5000 system with pulsed amperometric detector (PAD); flow rate: 0.5 mL/min; column temperature: 30 °C; injection volume: 5 µL; mobile phase: ddH2O (A); 0.1 M NaOH (B); 0.1 M NaOH, 0.2 M NaAc (C); gradient elution (0–26 min, 95–85% A, 5–5% B, 0–10% C; 26–42 min, 85% A, 5% B, 10% C; 42–42.1 min, 85–60% A, 5–0% B, 10–40% C; 42.1–52 min, 60–60% A, 0–40% B, 40–0% C).
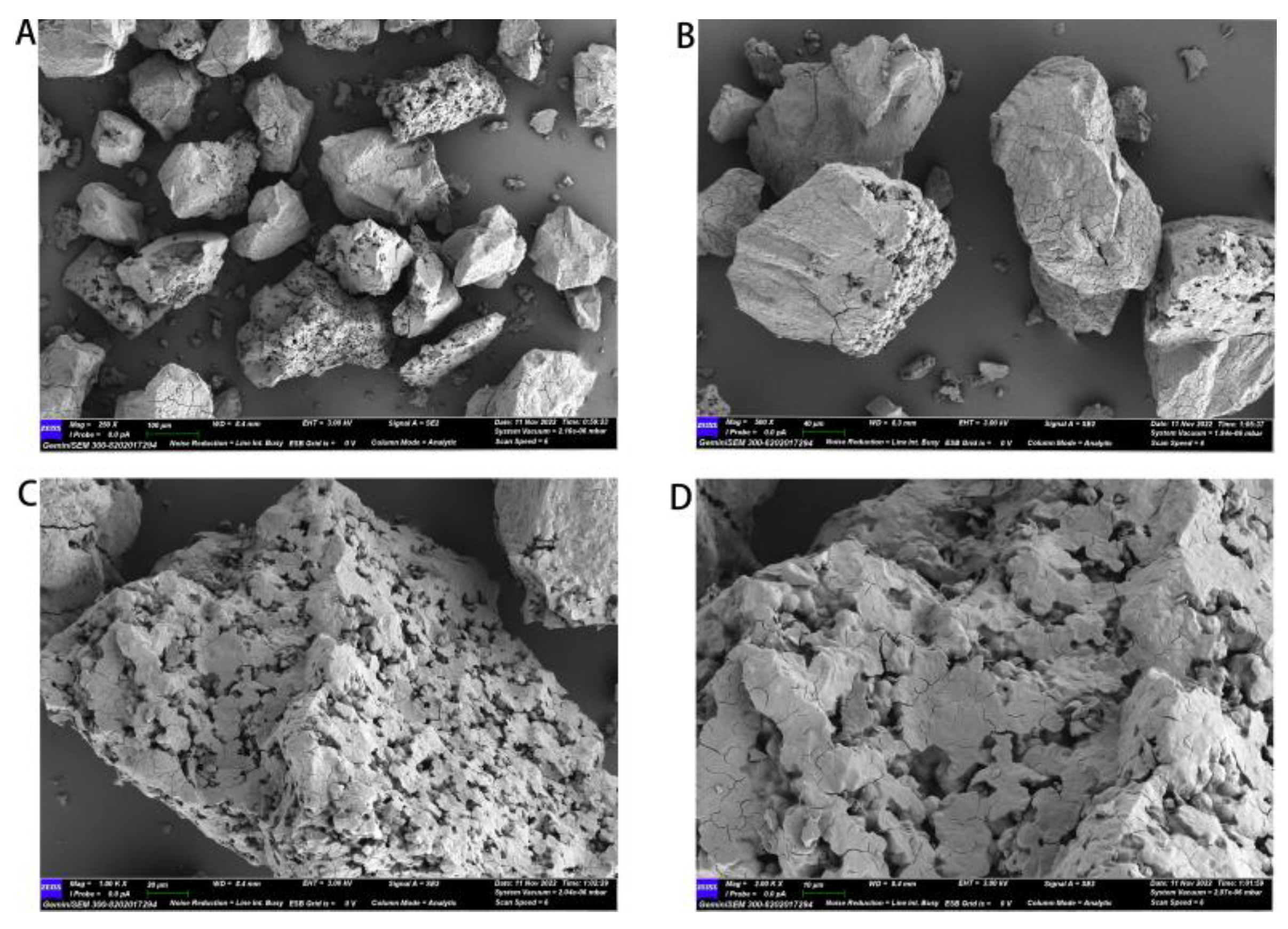
2.3.5. SEM Analysis
The dried sample was sieved through a 100-mesh sieve and sputter-coated with a gold layer. The surface structural characteristics of cMORP were examined via a scanning electron microscope (Merlin Compact, Zeiss, Germany), and then the images were recorded at different magnifications (250–2000 times) at an acceleration voltage of 3 kV.
2.4. Safety Study
2.4.1. Single-Dose Acute Oral Toxicity Study
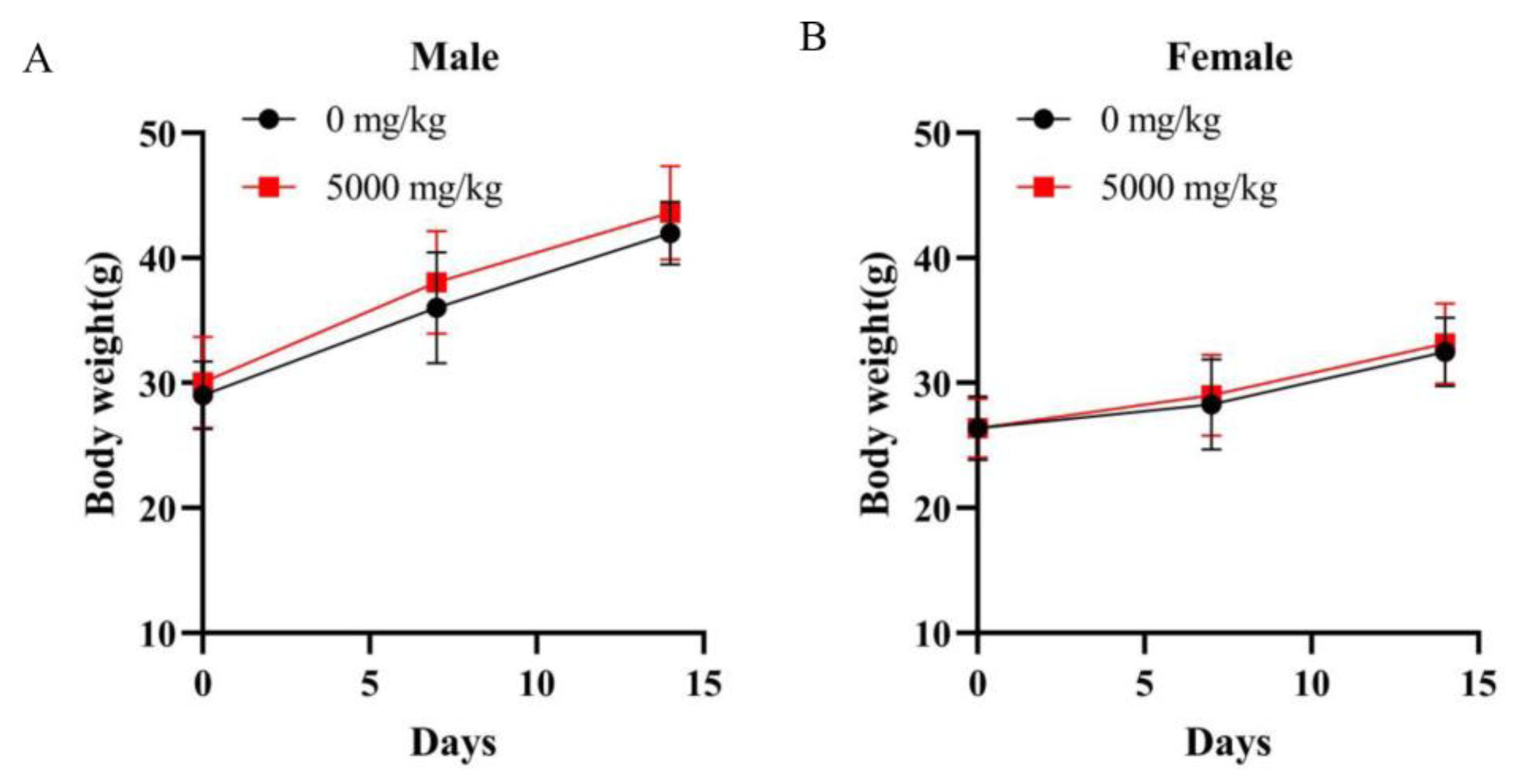
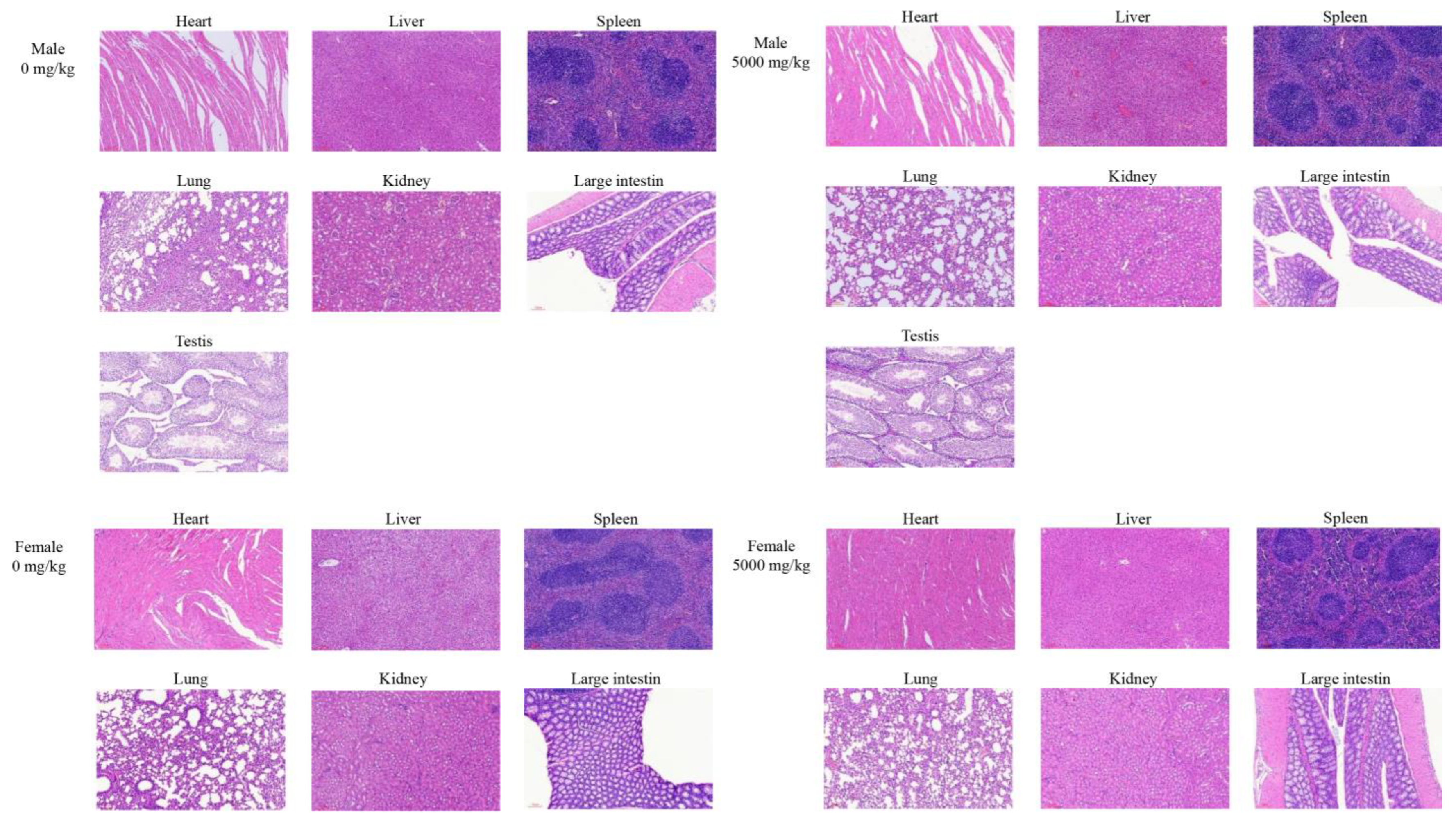
The experiments were performed according to Technical Guidelines for Acute Toxicity Research of Traditional Chinese Medicine and Natural Medicine. For acute oral toxicity, healthy adult mammals of half female sex were generally used. A total of 40 mice with half male and half female was randomly divided into the control group (0 mg/kg) and test group (5000 mg/kg). The samples were diluted with distilled water. The negative control group was administered with distilled water. After a 3-day acclimatization period, body weights were measured before administration (10/sex/group). After a 12 h fast, a single oral dose of cMORP or distilled water was administered separately by an oral gavage needle (40 mL/kg). Food was provided 4 h after the administration. Furthermore, signs of toxicity and behavioral changes were observed carefully within 4 h after administration, including water consumption, appearance, behavior, secretions, excreta, etc. The signs of clinical symptoms and mortality of experimental animals were observed for 14 days thereafter. Additionally, the body weights were measured on days 0, 7, and 14. The day of administration was designated as day 0. On the 14th day after administration, all mice were fasted for 16 h and then euthanized. The major organs, including the heart, liver, spleen, lung, kidneys, and testes, were visually examined following animal autopsies and collected for pathological section observation.
2.4.2. Repeated Dose 30-Day Oral Toxicity Study
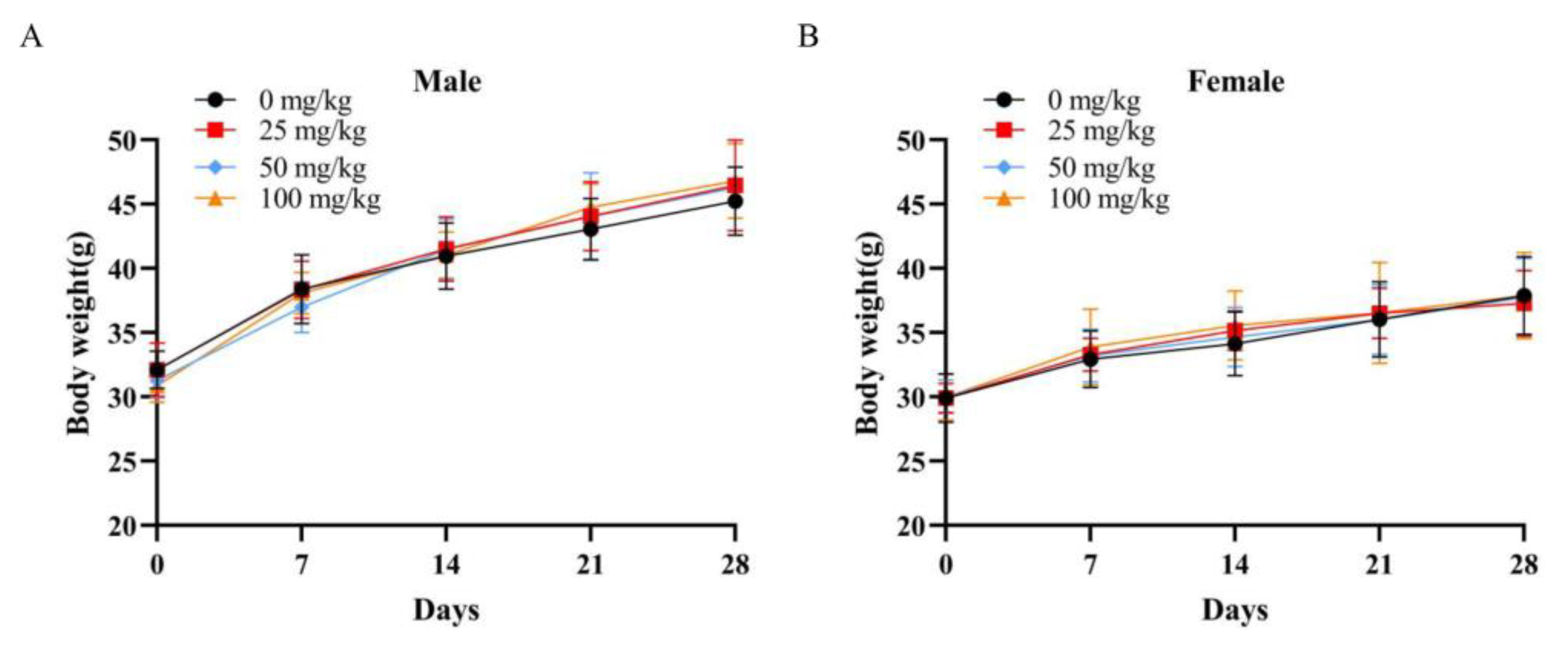
After a 3-day acclimatization feeding, 80 mice, half male and half female, were divided randomly into four groups, namely, a low-dose group, a medium-dose group, and a high-dose group (0, 25, 50, and 100 mg/kg, respectively), as well as a negative control group. The samples were diluted with distilled water. The negative control group was administered with distilled water. An oral dose of cMORP or distilled water was administered separately by an oral gavage needle (40 mL/kg) once a day. The signs of clinical symptoms and mortality of experimental animals were observed twice a day for 30 days thereafter. The weights of each animal were recorded on days 0, 7, 14 and 28. The day of administration was designated as day 0.
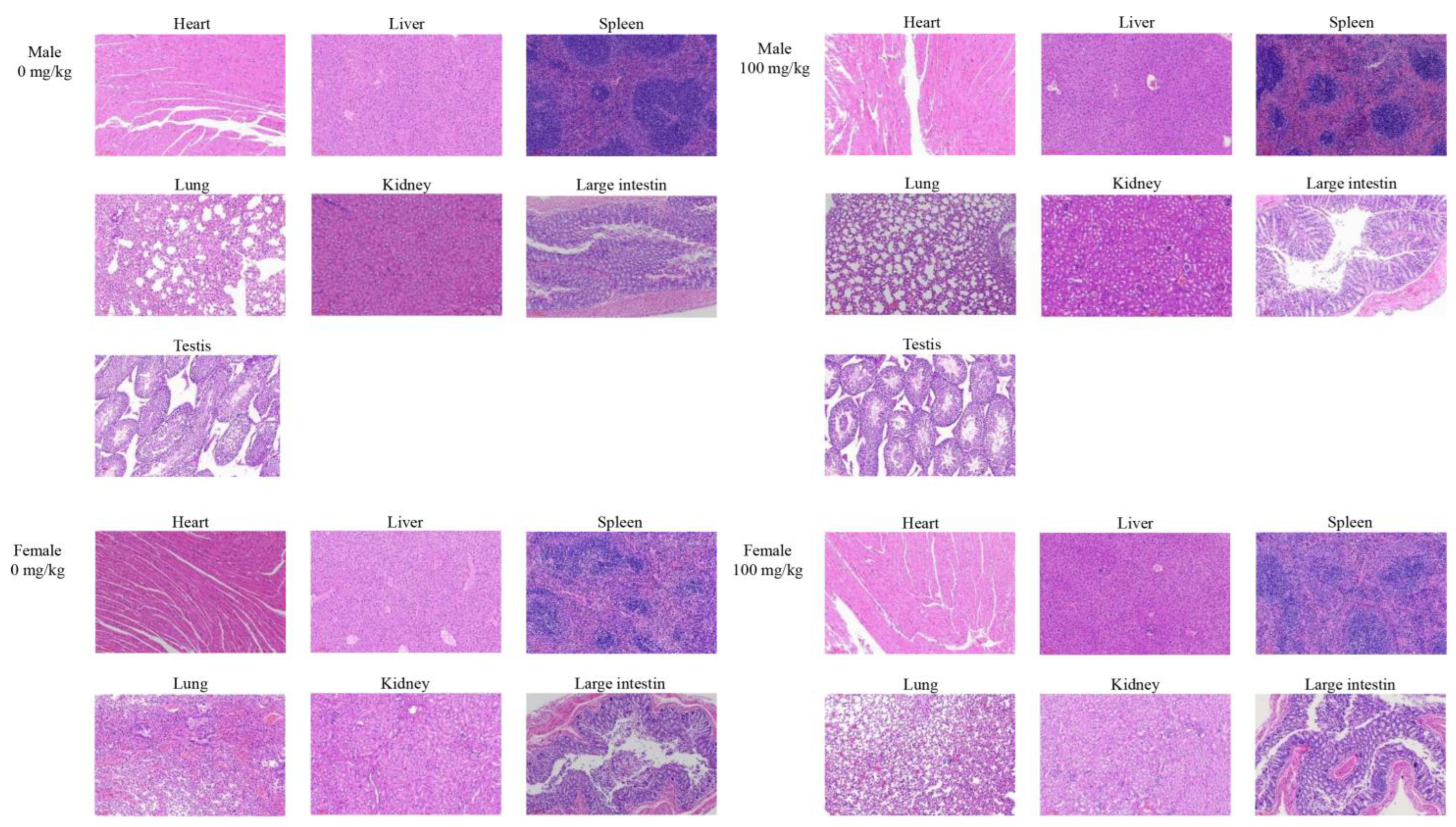
After 30 days, animals were fasted for 16 h and then euthanized. Blood samples were collected for hematological examination and serological analysis. The major organs, including the heart, liver, spleen, lung, kidneys, and testes, were visually examined following animal autopsies, weighed, calculated as the relative weight, and collected for histological examination.
2.5. Statistical Analyses
The experimental data are represented as the mean ± standard deviation (SD). Where appropriate, one-way analysis of variance (ANOVA) with LSD’s post test was used to assess the differences between multiple groups. Statistical analysis was conducted using SPSS version 22. In this study, the significance level was set at p < 0.05. The data were processed and obtained by the software GraphPad Prism 5.
4. Conclusions
In this study, the hot water extraction of crude polysaccharides from Morindae officinalis radix was optimized by the single-factor test and the orthogonal design method. The optimal extraction process involved an extraction temperature of 80 °C, an extraction time of 2 h, a liquid/solid ratio of 15 mL/g, and an extraction time number of 1, and it had an extraction yield of 8.37 ± 2.44%. The preliminary characterization of cMORP demonstrated that it had obvious and characteristic peaks of polysaccharides in the FT-IR spectrum, and the relative molecular masses were mainly distributed in the range of 689.22–2796.85 Da in the MALDI-TOF-MS spectrum. The analysis of monosaccharide composition showed that cMORP primarily comprised glucose (92.15%), guluronic acid (2.28%), galacturonic acid (1.98%), galactose (1.51%), rhamnose (0.88%), arabinose (0.81%), and glucuronic acid (0.40%). Moreover, a single-dose acute oral toxicity test and a repeated-dose 30-day oral toxicity study were performed to evaluate the feasibility of cMORP as a new food raw material. The preliminary study of safety suggested that an LD50 of cMORP was estimated to be greater than 5000 mg/kg BW, and oral administration of cMORP at a concentration of 0, 25, 50, and 100 mg/kg presented no abnormal symptoms and adverse toxicological effects, as evidenced by the absence of treatment-related changes in gross appearance, body weight, behavior, histological examination, and hematological and serological parameters of male and female KM mice. Therefore, it was concluded that cMORP can be initially considered non-toxic, and this study can provide valuable information for future development and application.