Relationship between Dietary Polyphenols and Gut Microbiota: New Clues to Improve Cognitive Disorders, Mood Disorders and Circadian Rhythms
Abstract
1. Introduction
2. Mechanisms of Action of Gut Microbiota Intervention in the Central Nervous System
2.1. Gut Microbiota Modulates Mood through Neural Pathways
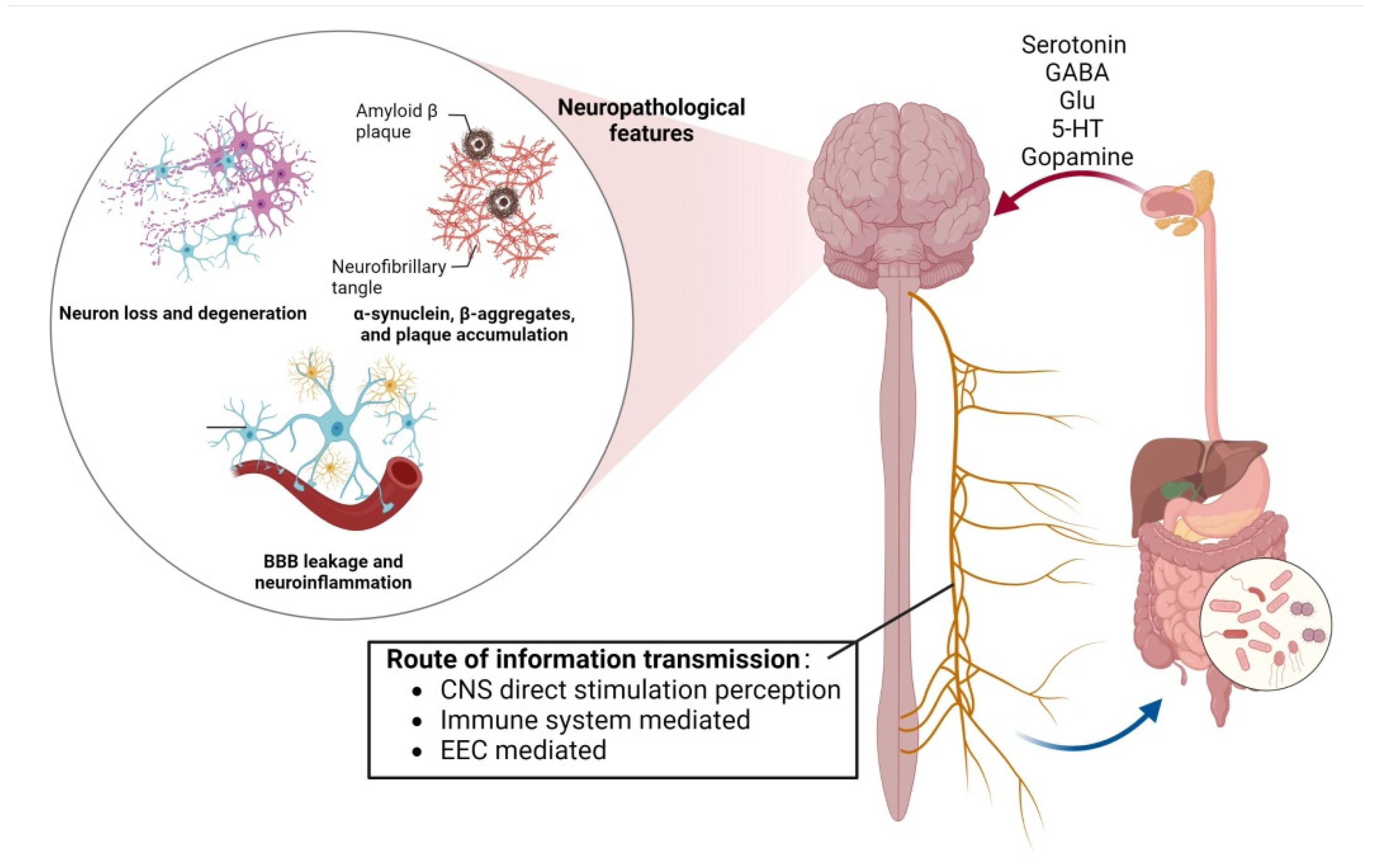
2.2. Mechanisms of Gut-Flora-Induced Cognitive Impairment
2.3. Intestinal Flora Interacts with Circadian Rhythms through the GBA Bidirectional Circulatory System
3. Biphasic Action of Dietary Polyphenols and Intestinal Flora to Intervene in Neurocentral Disorders
3.1. Metabolic Regulation of Dietary Polyphenols by Intestinal Microorganisms
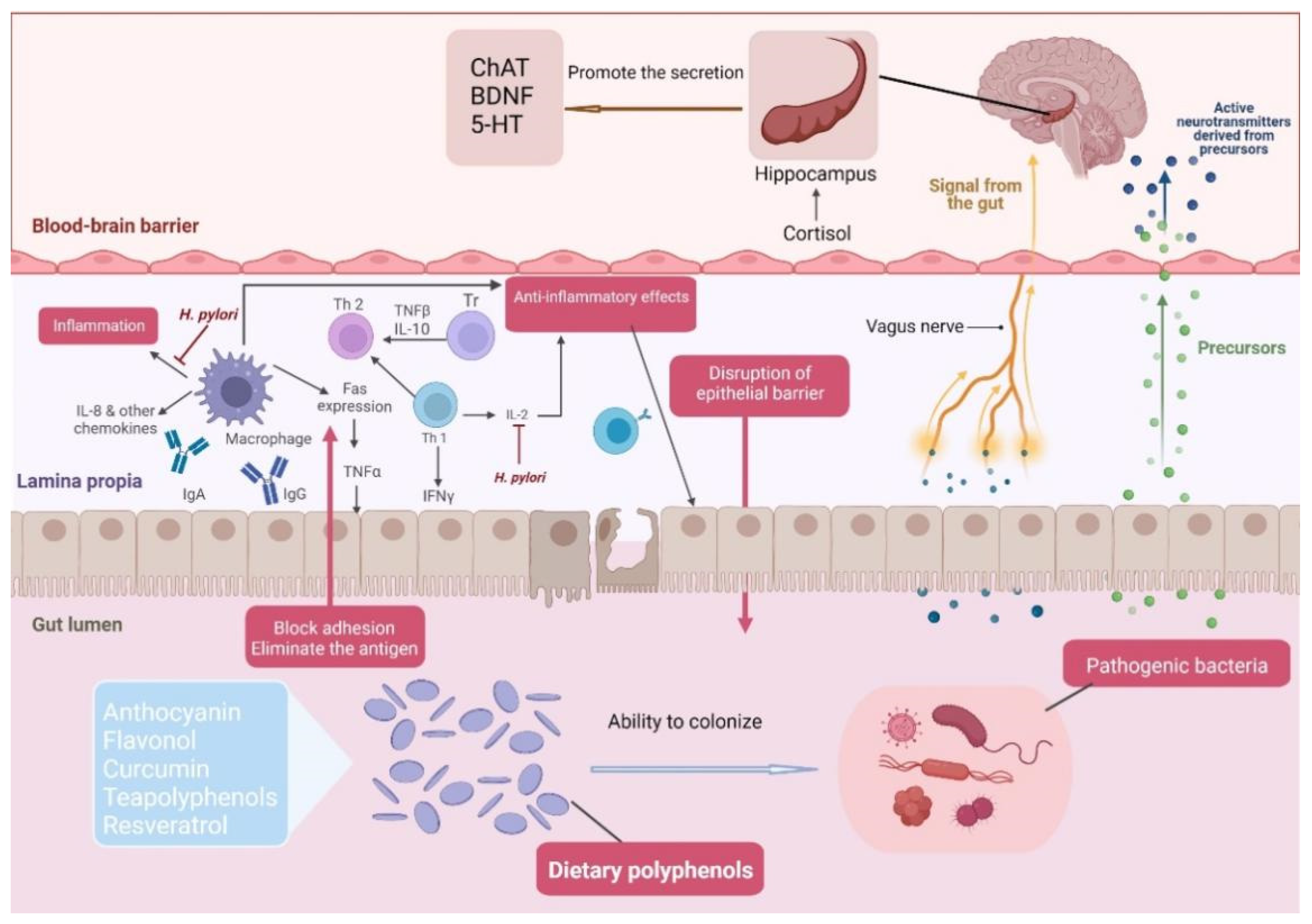
3.2. Regulation of Intestinal Flora by Dietary Polyphenols
3.3. Mechanisms of Dietary Polyphenol Intervention in the Central Nervous System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dinan, T.G.; Cryan, J.F. Gut Instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef]
- Houser, M.C.; Tansey, M.G. The Gut-Brain Axis: Is intestinal inflammation a silent driver of parkinson’s disease pathogenesis? Npj Park. Dis. 2017, 3, 3. [Google Scholar] [CrossRef]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of bifidobacterium and lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef]
- Salvetti, E.; O’Toole, P.W. When regulation challenges innovation: The case of the genus lactobacillus. Trends Food Sci. Technol. 2017, 66, 187–194. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef]
- Ojeda, P.; Bobe, A.; Dolan, K.; Leone, V.; Martinez, K. Nutritional modulation of gut microbiota—The impact on metabolic disease pathophysiology. J. Nutr. Biochem. 2016, 28, 191–200. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Jahromi, M.F.; Navidshad, B.; Liang, J.B. Effects of prebiotics on immune system and cytokine expression. Med. Microbiol. Immunol. 2017, 206, 1–9. [Google Scholar] [CrossRef]
- Kim, W.G.; Kim, H.I.; Kwon, E.K.; Han, M.J.; Kim, D.H. Lactobacillus plantarum lc27 and bifidobacterium longum lc67 mitigate alcoholic steatosis in mice by inhibiting lps-mediated nf-kappa b activation through restoration of the disturbed gut microbiota. Food Funct. 2018, 9, 4255–4265. [Google Scholar] [CrossRef]
- Pinto, C.J.G.; Ávila-Gálvez, M.Á.; Lian, Y.; Moura-Alves, P.; Nunes dos Santos, C. Targeting the aryl hydrocarbon receptor by gut phenolic metabolites: A strategy towards gut inflammation. Redox Biol. 2023, 61, 102622. [Google Scholar] [CrossRef] [PubMed]
- Tiozon, R.N., Jr.; Sartagoda, K.J.D.; Serrano, L.M.N.; Fernie, A.R.; Sreenivasulu, N. Metabolomics based inferences to unravel phenolic compound diversity in cereals and its implications for human gut health. Trends Food Sci. Technol. 2022, 127, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Frolinger, T.; Pasinetti, G. The gut microbiota composition affects polyphenol-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids (P20-038-19). Curr. Dev. Nutr. 2019, 3, 3130914. [Google Scholar] [CrossRef]
- Chen, H.D.; Jiang, M.Z.; Zhao, Y.Y.; Li, X.; Lan, H.; Yang, W.Q.; Lai, Y. Effects of breviscapine on cerebral ischemia-reperfusion injury and intestinal flora imbalance by regulating the tlr4/myd88/nf-kappab signaling pathway in rats. J. Ethnopharmacol. 2023, 300, 115691. [Google Scholar] [CrossRef] [PubMed]
- Luna, R.A.; Foster, J.A. Gut brain axis: Diet microbiota interactions and implications for modulation of anxiety and depression. Curr. Opin. Biotechnol. 2015, 32, 35–41. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, P.; Liu, Y.; Zhong, X.; Wang, H.; Guo, Y.; Xie, P. Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 647–655. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Gui, S.; Zeng, B.; Pu, J.; Zheng, P.; Zeng, L.; Luo, Y.; Wu, Y.; Zhou, C.; et al. Proteomics analysis of the gut-brain axis in a gut microbiota-dysbiosis model of depression. Transl. Psychiatry 2021, 11, 568. [Google Scholar] [CrossRef]
- Hori, T.; Matsuda, K.; Oishi, K. Probiotics: A dietary factor to modulate the gut microbiome, host immune system, and gut-brain interaction. Microorganisms 2020, 8, 1401. [Google Scholar] [CrossRef]
- Keita, A.V.; Soderholm, J.D. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol. Motil. 2010, 22, 718–733. [Google Scholar] [CrossRef]
- Sun, Y.; Ho, C.T.; Zhang, Y.; Hong, M.; Zhang, X. Plant polysaccharides utilized by gut microbiota: New players in ameliorating cognitive impairment. J. Tradit. Complement. Med. 2022, 13, 128–134. [Google Scholar] [CrossRef]
- Fetissov, S.O.; Dechelotte, P. The new link between gut-brain axis and neuropsychiatric disorders. Curr. Opin. Clin. Nutr. Metab. 2011, 14, 477–482. [Google Scholar] [CrossRef]
- Huo, R.; Zeng, B.; Zeng, L.; Cheng, K.; Li, B.; Luo, Y.; Wang, H.; Zhou, C.; Fang, L.; Li, W.; et al. Microbiota Modulate Anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front. Cell. Infect. Microbiol. 2017, 7, 489. [Google Scholar] [CrossRef] [PubMed]
- Heijtza, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Bjorkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.E.; Xu, B.; Lu, B.; Hempstead, B.L. New insights in the biology of bdnf synthesis and release: Implications in cns function. J. Neurosci. 2009, 29, 12764–12767. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Z.; Cheng, L.; Zhang, X.; Yang, H. The role of the intestinal microbiota in the pathogenesis of host depression and mechanism of tps relieving depression. Food Funct. 2021, 12, 7651–7663. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.; Farzi, A.; Zhu, W. Tryptophan metabolism: A link between the gut microbiota and brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, D.M.; Wohleb, E.S.; Duman, R.S. Emerging treatment mechanisms for depression: Focus on glutamate and synaptic plasticity. Drug Discov. Today 2016, 21, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain. Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Wang, X.; Wang, Z.; Zhang, J.; Jiang, R.; Wang, X.; Wang, K.; Liu, Z.; Xia, Z.; et al. Similar fecal microbiota signatures in patients with diarrhea-predominant irritable bowel syndrome and patients with depression. Clin. Gastroenterol. Hepatol. 2016, 14, 1602. [Google Scholar] [CrossRef] [PubMed]
- Lyte, M.; Li, W.; Opitz, N.; Gaykema, R.P.A.; Goehler, L.E. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia citrobacter rodentium. Physiol. Behav. 2006, 89, 350–357. [Google Scholar] [CrossRef]
- Luo, A.; Xie, Z.; Wang, Y.; Wang, X.; Li, S.; Yan, J.; Zhan, G.; Zhou, Z.; Zhao, Y.; Li, S. Type 2 diabetes mellitus-associated cognitive dysfunction: Advances in potential mechanisms and therapies. Neurosci. Biobehav. Rev. 2022, 137, 104642. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Bermudez-Humaran, L.G.; Salinas, E.; Ortiz, G.G.; Ramirez-Jirano, L.J.; Alejandro Morales, J.; Bitzer-Quintero, O.K. From probiotics to psychobiotics: Live beneficial bacteria which act on the brain-gut axis. Nutrients 2019, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Julio-Pieper, M.; Hyland, N.P.; Bravo, J.A.; Dinan, T.G.; Cryan, J.F. A novel role for the metabotropic glutamate receptor-7: Modulation of faecal water content and colonic electrolyte transport in the mouse. Br. J. Pharmacol. 2010, 160, 367–375. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Q.; Zhang, X.; Wang, X.; Su, Y.; He, W.; Li, J.; Wan, H.; Jing, X. Electroacupuncture regulates inflammatory cytokines by activating the vagus nerve to enhance antitumor immunity in mice with breast tumors. Life Sci. 2022, 291, 119539. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, T.; Mallick, H.N.; Torii, K. Activation of the gut-brain axis by dietary glutamate and physiologic significance in energy homeostasis. Am. J. Clin. Nutr. 2009, 90, 832S–837S. [Google Scholar] [CrossRef]
- Lenz, K.M.; Nelson, L.H. Microglia and beyond: Innate immune cells as regulators of brain development and behavioral function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Edmonson, C.A.; Ziats, M.N.; Rennert, O.M. A non-inflammatory role for microglia in autism spectrum disorders. Front. Neurol. 2016, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Richwine, A.F.; Godbout, J.P.; Berg, B.M.; Chen, J.; Escobar, J.; Millard, D.K.; Johnson, R.W. Improved psychomotor performance in aged mice fed diet high in antioxidants is associated with reduced ex vivo brain interleukin-6 production. Brain. Behav. Immun. 2005, 19, 512–520. [Google Scholar] [CrossRef]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fak, F.; Jucker, M.; Lasser, T.; et al. Reduction of abeta amyloid pathology in appps1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 46856. [Google Scholar] [CrossRef]
- Wang, Q.; Jia, M.; Zhao, Y.; Hui, Y.; Pan, J.; Yu, H.; Yan, S.; Dai, X.; Liu, X.; Liu, Z. Supplementation of sesamin alleviates stress-induced behavioral and psychological disorders via reshaping the gut microbiota structure. J. Agric. Food Chem. 2019, 67, 12441–12451. [Google Scholar] [CrossRef]
- Mahmoudiandehkordi, S.; Arnold, M.; Nho, K.; Ahmad, S.; Ahmad, S.; Jia, W.; Xia, G.; Louie, G.; Kueider, A.; Moseley, M.A.; et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-an emerging role for gut microbiome. Alzheimers Dement. 2019, 15, 604. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.H.; Li, S.X.; He, Z.M.; Zhu, W.L.; Ji, Y.B.; Wang, Z.; Zhu, X.M.; Yuan, K.; Bao, Y.P.; et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol. Psychiatry 2021, 26, 6277–6292. [Google Scholar] [CrossRef] [PubMed]
- Ghaisas, S.; Maher, J.; Kanthasamy, A. Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol. Ther. 2016, 158, 52–62. [Google Scholar] [CrossRef]
- Zhao, K.; Yao, M.; Zhang, X.; Xu, F.; Shao, X.; Wei, Y.; Wang, H. Flavonoids and intestinal microbes interact to alleviate depression. J. Sci. Food Agric. 2022, 102, 1311–1318. [Google Scholar] [CrossRef]
- Li, R.; Xiao, J.; Cao, Y.; Huang, Q.; Ho, C.T.; Lu, M. Capsaicin attenuates oleic acid-induced lipid accumulation via the regulation of circadian clock genes in HepG2 cells. J. Agric. Food Chem. 2022, 70, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Young, J.F.; Sun, J. NADPH Oxidase-generated reactive oxygen species in mature follicles are essential for drosophila ovulation. Proc. Natl. Acad. Sci. USA 2018, 115, 7765–7770. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, H.M.A.; Lee, G.H.; Kim, H.R.; Chae, H.J. Endoplasmic reticulum stress and associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef]
- Vaccaro, A.; Dor, Y.K.; Nambara, K.; Pollina, E.A.; Lin, C.; Greenberg, M.E.; Rogulja, D. Sleep loss can cause death through accumulation of reactive oxygen species in the gut. Cell 2020, 181, 1307–1328.e15. [Google Scholar] [CrossRef]
- Kempf, A.; Song, S.M.; Talbot, C.B.; Miesenbock, G. A potassium channel beta-subunit couples mitochondrial electron transport to sleep. Nature 2019, 568, 230–234. [Google Scholar] [CrossRef]
- Bruening, F.; Noya, S.B.; Bange, T.; Koutsouli, S.; Rudolph, J.D.; Tyagarajan, S.; Cox, J.; Mann, M.; Brown, S.A.; Robles, M.S. Sleep-wake cycles drive daily dynamics of synaptic phosphorylation. Science 2019, 366, eaav3617. [Google Scholar] [CrossRef]
- Arola-Arnal, A.; Cruz-Carrión, Á.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Mulero, M.; Bravo, F.I.; Muguerza, B.; Arola, L.; Suárez, M. Chrononutrition and polyphenols: Roles and diseases. Nutrients 2019, 11, 2602. [Google Scholar] [CrossRef]
- Asher, G.; Sassone-Corsi, P. Time for food: The intimate interplay between nutrition, metabolism, and the circadian clock. Cell 2015, 161, 84–92. [Google Scholar] [CrossRef]
- Engin, A. Circadian Rhythms in Diet-Induced Obesity. In Obesity and Lipotoxicity; Engin, A.B., Engin, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 960, pp. 19–52. [Google Scholar]
- Welsh, D.; Logothetis, D.; Meister, M.; Reppert, S. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 1995, 14, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Zhao, B.; Tan, X.; Wang, L.; Liu, X. Role of food phytochemicals in the modulation of circadian clocks. J. Agric. Food Chem. 2019, 67, 8735–8739. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Voigt, R.M.; Forsyth, C.B.; Green, S.J.; Mutlu, E.; Engen, P.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. Circadian disorganization alters intestinal microbiota. PLoS ONE 2014, 9, e97500. [Google Scholar] [CrossRef]
- Deaver, J.A.; Eum, S.Y.; Toborek, M. Circadian disruption changes gut microbiome taxa and functional gene composition. Front. Microbiol. 2018, 9, 737. [Google Scholar] [CrossRef]
- Khapre, R.V.; Kondratova, A.A.; Susova, O.; Kondratov, R.V. Circadian clock protein BMAL1 regulates cellular senescence in vivo. Cell Cycle 2011, 10, 4162–4169. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalova, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota diumal rhythmicity programs host transcriptome oscillations. Cell 2016, 167, 1495–1510.e12. [Google Scholar] [CrossRef] [PubMed]
- Summa, K.C.; Voigt, R.M.; Forsyth, C.B.; Shaikh, M.; Cavanaugh, K.; Tang, Y.; Vitaterna, M.H.; Song, S.; Turek, F.W.; Keshavarzian, A. Disruption of the circadian clock in mice increases intestinal permeability and promotes alcohol-induced hepatic pathology and inflammation. PLoS ONE 2013, 8, e67102. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Xing, X.; Wang, S. Health benefits of dietary polyphenols: Insight into interindividual variability in absorption and metabolism. Curr. Opin. Food Sci. 2022, 48, 100941. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef] [PubMed]
- Molinari, R.; Merendino, N.; Costantini, L. Polyphenols as modulators of pre-established gut microbiota dysbiosis: State-of-the-art. BioFactors 2022, 48, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Scapagnini, G. Interactions between dietary polyphenols and aging gut microbiota: A review. BioFactors 2022, 48, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Piwowarski, J.P.; Stanisławska, I.; Granica, S. Dietary polyphenol and microbiota interactions in the context of prostate health. Ann. N. Y. Acad. Sci. 2022, 1508, 54–77. [Google Scholar] [CrossRef]
- Couteau, D.; McCartney, A.L.; Gibson, G.R.; Williamson, G.; Faulds, C.B. Isolation and characterization of human colonic bacteria able to hydrolyse chlorogenic acid. J. Appl. Microbiol. 2001, 90, 873–881. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Li, H.; Deng, Z.; Tsao, R. A review on insoluble-bound phenolics in plant-based food matrix and their contribution to human health with future perspectives. Trends Food Sci. Technol. 2020, 105, 347–362. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.Y.; Fang, Z.; Ng, K. Dietary fiber-based colon-targeted delivery systems for polyphenols. Trends Food Sci. Technol. 2020, 100, 333–348. [Google Scholar] [CrossRef]
- Elghali, S.; Mustafa, S.; Amid, M.; Abd Manap, M.Y.; Ismail, A.; Abas, F. Bioconversion of daidzein to equol by bifidobacterium breve 15700 and bifidobacterium longum BB536. J. Funct. Foods 2012, 4, 736–745. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, Y.; Lee, P.K.; Wong, H.C. Effect of oral supplementation of gordonibacter urolithinfaciens on gut microbiota and bioavailability of ellagic acid and urolithins in mice. Curr. Dev. Nutr. 2022, 6, 6009344. [Google Scholar] [CrossRef]
- Rasmussen, S.E.; Frederiksen, H.; Krogholm, K.S.; Poulsen, L. Dietary proanthocyanidins: Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res. 2005, 49, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Nef, S. Soy, Phytoestrogens and metabolism: A review. Mol. Cell. Endocrinol. 2009, 304, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Mendis, M.; Leclerc, E.; Simsek, S. Arabinoxylans, Gut microbiota and immunity. Carbohydr. Polym. 2016, 139, 159–166. [Google Scholar] [CrossRef]
- Fernandes, J.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.S.; Comelli, E.M. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr. Diabetes 2014, 4, e121. [Google Scholar] [CrossRef]
- Uyanga, V.A.; Amevor, F.K.; Liu, M.; Cui, Z.; Zhao, X.; Lin, H. Potential implications of citrulline and quercetin on gut functioning of monogastric animals and humans: A comprehensive review. Nutrients 2021, 13, 3782. [Google Scholar] [CrossRef]
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Hijova, E.; Bertkova, I.; Stofilova, J.F. Dietary fibre as prebiotics in nutrition. Cent. Eur. J. Public Health 2019, 27, 251–255. [Google Scholar] [CrossRef]
- Cheng, L.; Kong, L.; Xia, C.; Zeng, X.; Wu, Z.; Guo, Y.; Pan, D. Sources, Processing-related transformation, and gut axisregulation of conventional and potential prebiotics. J. Agric. Food Chem. 2022, 70, 4509–4521. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Shitut, S.; Preussger, D.; Yousif, G.; Waschina, S.; Kost, C. Ecology and evolution of metabolic cross-feeding interactions in bacteria. Nat. Prod. Rep. 2018, 35, 455–488. [Google Scholar] [CrossRef]
- Guo, T.; Ho, C.T.; Zhang, X.; Cao, J.; Wang, H.; Shao, X.; Pan, D.; Wu, Z. Oolong tea polyphenols ameliorate circadian rhythm of intestinal microbiome and liver clock genes in mouse model. J. Agric. Food Chem. 2019, 67, 11969–11976. [Google Scholar] [CrossRef] [PubMed]
- Din, A.U.; Mazhar, M.; Waseem, M.; Ahmad, W.; Bibi, A.; Hassan, A.; Ali, N.; Gang, W.; Qian, G.; Ullah, R.; et al. SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed. Pharmacother. 2021, 133, 110947. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef]
- Mukherjee, R.; Yun, J.W. Lactobionic Acid reduces body weight gain in diet-induced obese rats by targeted inhibition of Galectin-1. Biochem. Biophys. Res. Commun. 2015, 463, 1311–1316. [Google Scholar] [CrossRef]
- Canfora, E.E.; van der Beek, C.M.; Hermes, G.D.A.; Goossens, G.H.; Jocken, J.W.E.; Holst, J.J.; van Eijk, H.M.; Venema, K.; Smidt, H.; Zoetendal, E.G.; et al. Supplementation of diet with galacto-oligosaccharides increases bifidobacteria, but not insulin sensitivity, in obese prediabetic individuals. Gastroenterology 2017, 153, 87–97.e3. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- Burokas, A.; Moloney, R.D.; Dinan, T.G.; Cryan, J.F. Microbiota regulation of the mammalian gut-brain axis. Adv. Appl. Microbiol. 2015, 91, 1–62. [Google Scholar]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Westfall, S.; Pasinetti, G. Design of a novel synbiotic formulation to optimize gut-derived phenolic acid mediated gut-brain-axis signals for the treatment of stress-induced depression and anxiety (OR23-03-19). Curr. Dev. Nutr. 2019, 3, 3130871. [Google Scholar] [CrossRef]
- That, L.F.M.L.N.; Xu, B.; Pandohee, J. Could foodomics hold the key to unlocking the role of prebiotics in gut microbiota and immunity? Curr. Opin. Food Sci. 2022, 48, 100920. [Google Scholar] [CrossRef]
- Musilova, S.; Modrackova, N.; Hermanova, P.; Hudcovic, T.; Svejstil, R.; Rada, V.; Tejnecky, V.; Bunesova, V. Assessment of the synbiotic properites of human milk oligosaccharides and bifidobacterium longum subsp infantis in vitro and in humanised mice. Benef. Microbes 2017, 8, 281–289. [Google Scholar] [CrossRef]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.S. The impact of ellagitannins and their metabolites through gut microbiome on the gut health and brain wellness within the gut-brain axis. Foods 2023, 12, 270. [Google Scholar] [CrossRef]
- Velagapudi, R.; Lepiarz, I.; El-Bakoush, A.; Katola, F.O.; Bhatia, H.; Fiebich, B.L.; Olajide, O.A. Induction of autophagy and activation of SIRT-1 deacetylation mechanisms mediate neuroprotection by the pomegranate metabolite urolithin A in BV2 microglia and differentiated 3D human neural progenitor cells. Mol. Nutr. Food Res. 2019, 63, e1801237. [Google Scholar] [CrossRef] [PubMed]
- Toney, A.M.; Albusharif, M.; Works, D.; Polenz, L.; Schlange, S.; Chaidez, V.; Ramer-Tait, A.E.; Chung, S. Differential effects of whole red raspberry polyphenols and their gut metabolite urolithin a on neuroinflammation in BV-2 microglia. Int. J. Environ. Res. Public. Health 2020, 18, 68. [Google Scholar] [CrossRef]
- Xu, J.; Yuan, C.; Wang, G.; Luo, J.; Ma, H.; Xu, L.; Mu, Y.; Li, Y.; Seeram, N.P.; Huang, X.; et al. Urolithins attenuate LPS-induced neuroinflammation in BV2Microglia via MAPK, Akt, and NF-ΚB signaling pathways. J. Agric. Food Chem. 2018, 66, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Park, J.S.; Lee, E.J.; Ahn, J.H.; Kim, H.S. Anti-inflammatory and antioxidant mechanisms of urolithin B in activated microglia. phytomedicine int. J. Phytother. Phytopharm. 2019, 55, 50–57. [Google Scholar]
- Xu, Q.; Shen, M.; Han, Y.; Diao, H. Effects of ellagic acid supplementation on jejunal morphology, digestive enzyme activities, antioxidant capacity, and microbiota in mice. Front. Microbiol. 2021, 12, 793576. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, M.; Mo, J.; Lan, G.; Liang, J. Dietary supplementation ellagic acid on the growth, intestinal immune response, microbiota, and inflammation in weaned piglets. Front. Vet. Sci. 2022, 9, 980271. [Google Scholar] [CrossRef]
- Kshirsagar, S.; Alvir, R.V.; Pradeepkiran, J.A.; Hindle, A.; Vijayan, M.; Ramasubramaniam, B.; Kumar, S.; Reddy, A.P.; Reddy, P.H. A combination therapy of urolithin A+EGCG has stronger protective effects than single drug urolithin a in a humanized amyloid beta knockin mice for late-onset Alzheimer’s disease. Cells 2022, 11, 2660. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, L.; Liu, T.; Wang, J.; Wen, A.; Ding, Y. Ellagic acid protects mice against sleep deprivation-induced memory impairment and anxiety by inhibiting TLR4 and activating Nrf2. Aging 2020, 12, 10457–10472. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, M.; Chen, W.; Liu, Z. The neuroprotective effects of intermittent fasting on brain aging and neurodegenerative diseases via regulating mitochondrial function. Free Radic. Biol. Med. 2022, 182, 206–218. [Google Scholar] [CrossRef]
- Gao, Y.; Li, B.; Liu, H.; Tian, Y.; Gu, C.; Du, X.; Bu, R.; Gao, J.; Liu, Y.; Li, G. Cistanche deserticola polysaccharides alleviate cognitive decline in aging model mice by restoring the gut microbiota-brain axis. Aging 2021, 13, 15320–15335. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Q.; Jia, M.; Fu, S.; Pan, J.; Chu, C.; Liu, X.; Liu, X.; Liu, Z. (+)-Sesamin attenuates chronic unpredictable mild stress-induced depressive-like behaviors and memory deficits via suppression of neuroinflammation. J. Nutr. Biochem. 2019, 64, 61–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Xie, Q.; Wang, Y.; Yu, J.; Ma, X. A comprehensive review of the principles, key factors, application, and assessment of thawing technologies for muscle foods. Compr. Rev. Food Sci. Food Saf. 2023, 22, 107–134. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin inhibits lps-induced neuroinflammation by promoting microglial M2 polarization via TREM2/ TLR4/ NF-ΚB pathways in BV2 cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Xiong, L.; Zhang, L.; Sun, D.; Liu, H. Green tea polyphenols inhibit cognitive impairment induced by chronic cerebral hypoperfusion via modulating oxidative stress. J. Nutr. Biochem. 2010, 21, 741–748. [Google Scholar] [PubMed]
- Zaplatic, E.; Bule, M.; Shah, S.Z.A.; Uddin, M.d.S.; Niaz, K. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 2019, 224, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.; Chen, W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci. Technol. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, Y.; Zhang, X.; Zheng, X.; Cao, J.; Wu, Z.; Qin, W.; Cheng, K. A Metagenomic analysis of the modulatory effect of cyclocarya paliurus flavonoids on the intestinal microbiome in a high-fat diet-induced obesity mouse model. J. Sci. Food Agric. 2019, 99, 3967–3975. [Google Scholar] [CrossRef]
- Stoupi, S.; Williamson, G.; Drynan, J.W.; Barron, D.; Clifford, M.N. A comparison of the in vitro biotransformation of (-)-epicatechin and procyanidin B2 by human faecal microbiota. Mol. Nutr. Food Res. 2010, 54, 747–759. [Google Scholar] [CrossRef]
- Pekovic-Vaughan, V.; Gibbs, J.; Yoshitane, H.; Yang, N.; Pathiranage, D.; Guo, B.; Sagami, A.; Taguchi, K.; Bechtold, D.; Loudon, A.; et al. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014, 28, 548–560. [Google Scholar] [CrossRef]
- Miro-Blanch, J.; Yanes, O. Epigenetic regulation at the interplay between gut microbiota and host metabolism. Front. Genet. 2019, 10, 638. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Cheng, L.; Liu, Y.; Zhan, S.; Wu, Z.; Zhang, X. Relationship between Dietary Polyphenols and Gut Microbiota: New Clues to Improve Cognitive Disorders, Mood Disorders and Circadian Rhythms. Foods 2023, 12, 1309. https://doi.org/10.3390/foods12061309
Liu S, Cheng L, Liu Y, Zhan S, Wu Z, Zhang X. Relationship between Dietary Polyphenols and Gut Microbiota: New Clues to Improve Cognitive Disorders, Mood Disorders and Circadian Rhythms. Foods. 2023; 12(6):1309. https://doi.org/10.3390/foods12061309
Chicago/Turabian StyleLiu, Siyu, Lu Cheng, Yanan Liu, Shengnan Zhan, Zufang Wu, and Xin Zhang. 2023. "Relationship between Dietary Polyphenols and Gut Microbiota: New Clues to Improve Cognitive Disorders, Mood Disorders and Circadian Rhythms" Foods 12, no. 6: 1309. https://doi.org/10.3390/foods12061309
APA StyleLiu, S., Cheng, L., Liu, Y., Zhan, S., Wu, Z., & Zhang, X. (2023). Relationship between Dietary Polyphenols and Gut Microbiota: New Clues to Improve Cognitive Disorders, Mood Disorders and Circadian Rhythms. Foods, 12(6), 1309. https://doi.org/10.3390/foods12061309





