From Medical Herb to Functional Food: Development of a Fermented Milk Containing Silybin and Protein from Milk Thistle
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
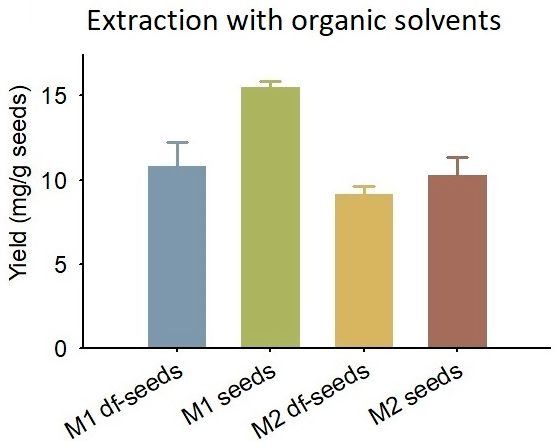
2.2. Extraction with Organic Solvents
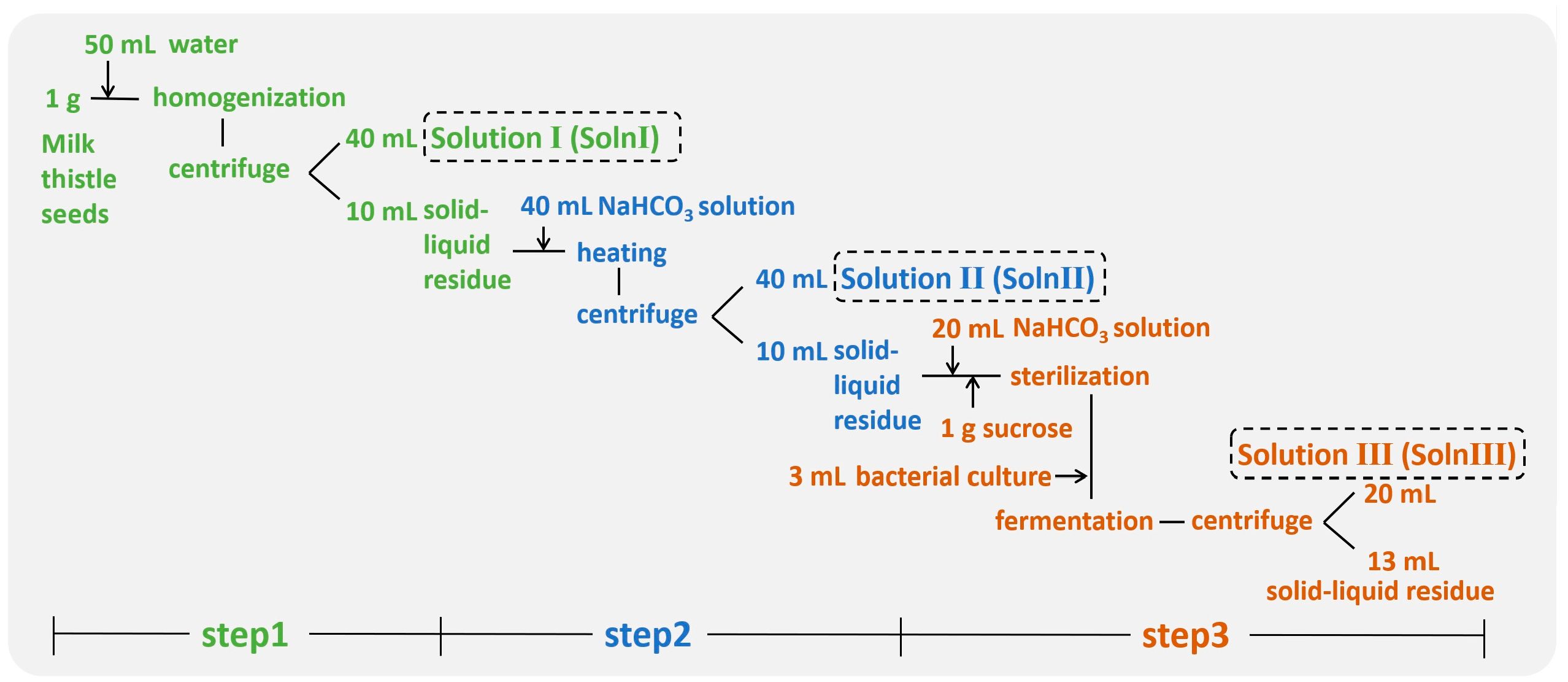
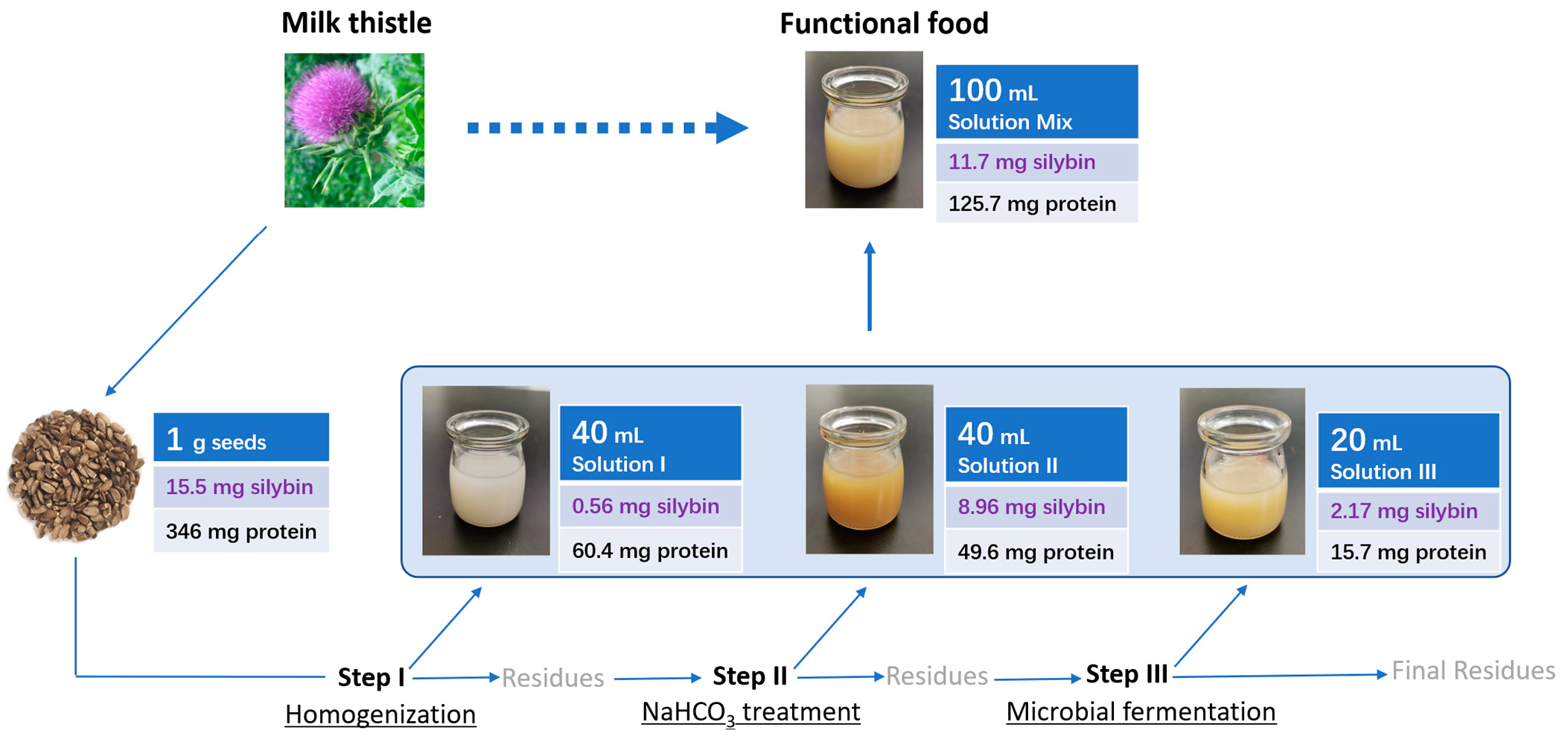
2.3. Design of the Three Step Procedure
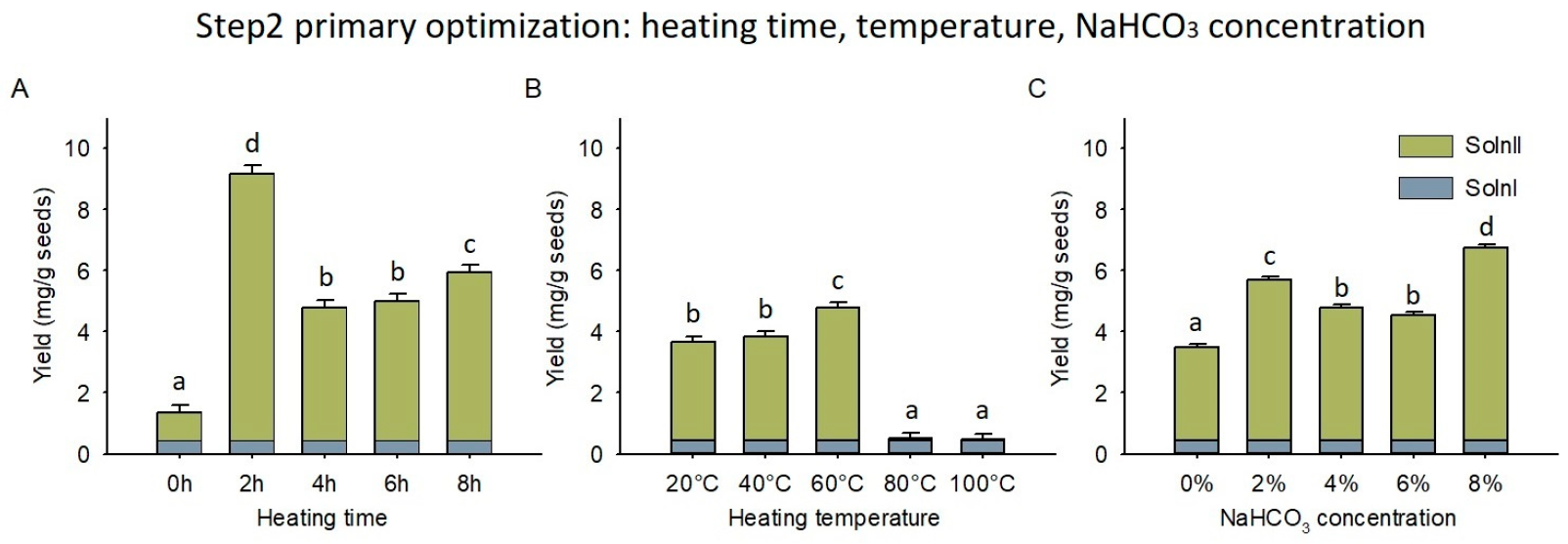
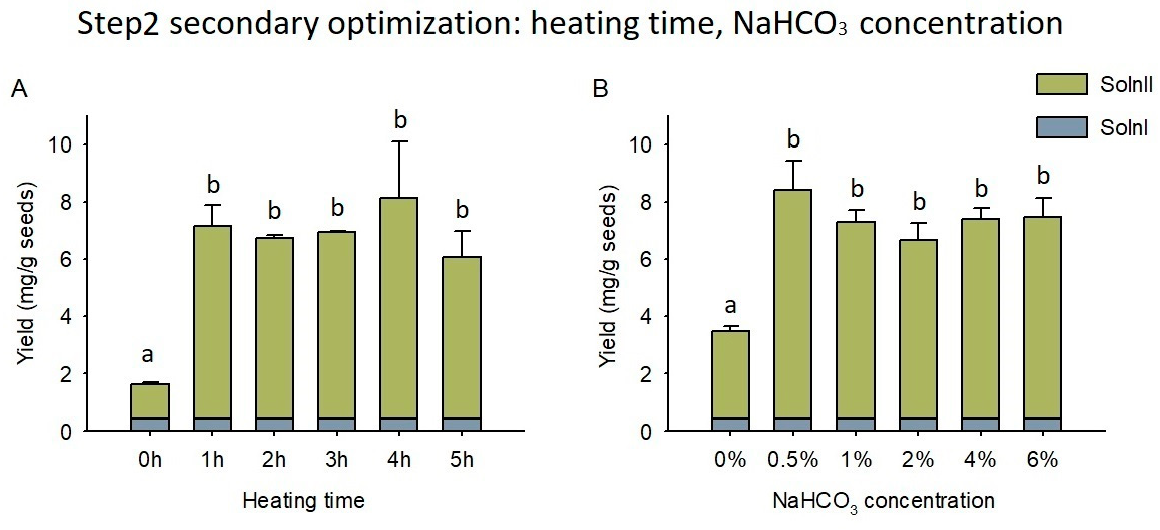
2.4. NaHCO3 Treatment and Optimization
2.5. Bacterial Fermentation
2.6. Protein Analysis
2.7. HPLC Analysis of Silybin Content
2.8. Statistical Analysis
3. Results and Discussion
3.1. HPLC Analysis
3.2. Extraction with Organic Solvents
3.3. NaHCO3 Thermal Treatment
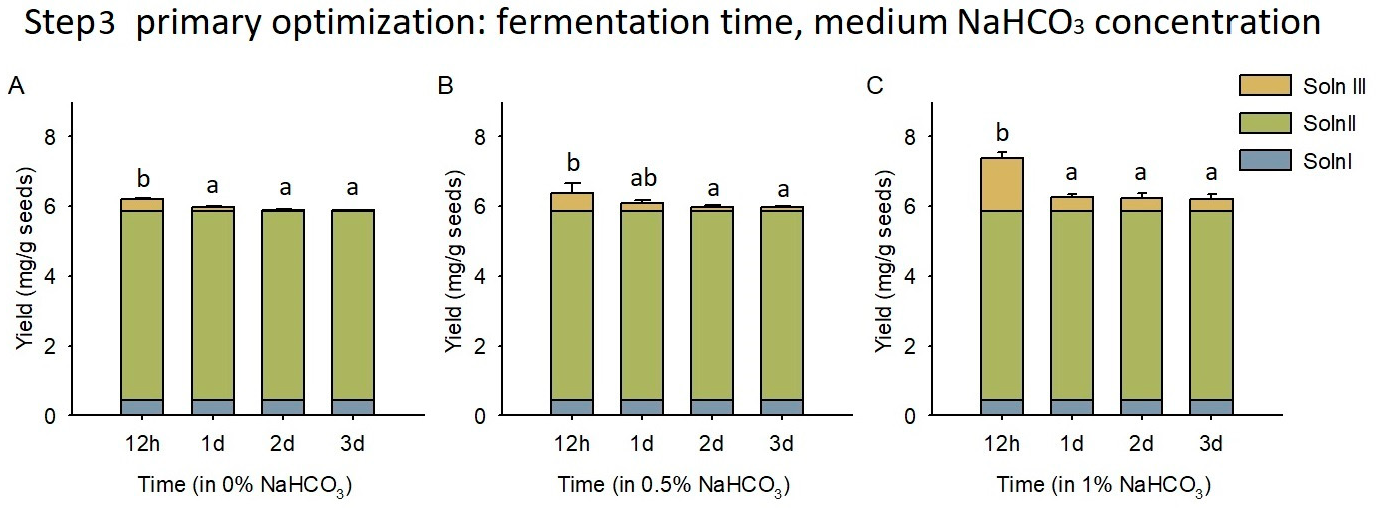
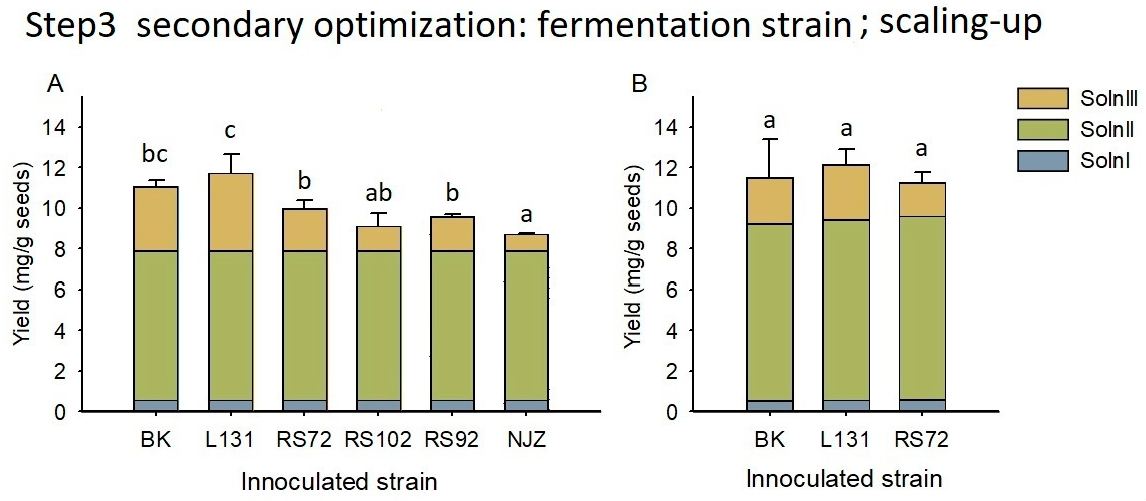
3.4. Microbial Fermentation
3.5. Protein Analysis
3.6. Summary of the Three Step Procedure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Post-White, J.; Ladas, E.J.; Kelly, K.M. Advances in the use of milk thistle (Silybum marianum). Integr. Cancer Ther. 2007, 6, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Valková, V.; Ďúranová, H.; Bilčíková, J.; Habán, M. Milk Thistle (Silybum Marianum): A Valuable Medicinal Plant with Several Therapeutic Purposes. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 836–843. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef]
- Gillessen, A.; Schmidt, H.H.J. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef]
- Hackett, E.S.; Twedt, D.C.; Gustafson, D.L. Milk Thistle and Its Derivative Compounds: A Review of Opportunities for Treatment of Liver Disease. J. Vet. Intern. Med. 2013, 27, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Luangchosiri, C.; Thakkinstian, A.; Chitphuk, S.; Stitchantrakul, W.; Petraksa, S.; Sobhonslidsuk, A. A double-blinded randomized controlled trial of silymarin for the prevention of antituberculosis drug-induced liver injury. BMC Complement. Altern. Med. 2015, 15, 334. [Google Scholar] [CrossRef]
- Morishima, C.; Shuhart, M.C.; Wang, C.C.; Paschal, D.M.; Apodaca, M.C.; Liu, Y.; Sloan, D.D.; Graf, T.; Oberlies, N.H.; Lee, D.Y.; et al. Silymarin Inhibits In Vitro T-Cell Proliferation and Cytokine Production in Hepatitis C Virus Infection. Gastroenterology 2010, 138, 671–681. [Google Scholar] [CrossRef]
- Chambers, C.S.; Holečková, V.; Petrásková, L.; Biedermann, D.; Valentová, K.; Buchta, M.; Křen, V. The silymarin composition… and why does it matter??? Food Res. Int. 2017, 100, 339–353. [Google Scholar] [CrossRef]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)-Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.X.; Tan, C.H.; Chen, J.; Zhang, Y.W.; Xia, G.H. Milk thistle extraction and purification process based on shell kernel separation. Chin. Tradit. Pat. Med. 2016, 38, 686–689. [Google Scholar]
- Zhang, L.F.; Lin, X.; Feng, X.H.; Gao, D.X. Optimization of the process of ultrasound-assisted organic solvent reflux extraction of silymarin from milk thistle seeds. Chin. Tradit. Pat. Med. 2019, 41, 185–188. [Google Scholar]
- Wang, X. Studies on Constitutes of Milk Thistle Seeds by Microwave-Assisted Extraction; Northeast Agricultural University: Harbin, China, 2009. [Google Scholar]
- Ren, R.B.; Zhao, Y.Y.; Xu, B.H.; Wu, J.L.; Chen, J.; Liang, C.Y.; Lv, H.; Li, W.L. Studies on silymarin extraction and silybin purification. Lishizhen Med. Mater. Med. Res. 2012, 23, 655–656. [Google Scholar]
- Zhu, S.Y.; Dong, Y.; Xiao, X.; Zhang, S.S.; Qin, Y.Y. Study on Protein and Amino Acid Composition of Milk Thistle Meal and Functional Properties. J. Chin. Cereals Oils Assoc. 2011, 26, 71–74+83. [Google Scholar]
- Zhu, S.Y.; Dong, Y.; Chen, X.D.; Zhou, Y. Physico-Chemical Properties of Silibum Marianum Protein Component. J. Chin. Cereals Oils Assoc. 2013, 28, 15–20. [Google Scholar]
- Harrabi, S.; Romdhane, H.; Daassa, M.; Fellah, H. Fatty Acid and Triacylglycerol Compositions of Milk Thistle Seeds Growing Wild in Tunisia (Silybum Marianum L.). Acta Aliment. 2015, 44, 304–310. [Google Scholar] [CrossRef]
- Li, S.K.; Wu, X.X.; Wu, X.Y. Study on the production process of silymarin extracted by ultrasound. All Health 2014, 8, 18. [Google Scholar]
- GB2760-2014-25; National Food Safety Standards. Standards for the Use of Food Additives. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2014; p. 220.
- Jin, N.; Zhang, S.; Sun, S.; Wu, M.; Yang, X.; Xu, J.; Ma, K.; Guan, S.; Xu, W. An Organic Solvent-Free Method for the Extraction of Ellagic Acid Compounds from Raspberry Wine Pomace with Assistance of Sodium Bicarbonate. Molecules 2022, 27, 2145. [Google Scholar] [CrossRef]
- Fawzy, M.A.; El-Naeb, E.H.; Hifney, A.F.; Adam, M.S.; Gomaa, M. Growth behavior, phenol removal and lipid productivity of microalgae in mixotrophic and heterotrophic conditions under synergistic effect of phenol and bicarbonate for biodiesel production. J. Appl. Phycol. 2022, 34, 2981–2994. [Google Scholar] [CrossRef]
- Zhao, C.J.; Schieber, A.; Ganzle, M.G. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations—A review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef]
- Jia, L.N. Extraction Study of the Active Ingredients of Milk Thistle; Liaoning Normal University: Dalian, China, 2005. [Google Scholar]
- Jin, N.; Liu, Y.; Zhang, S.; Sun, S.; Wu, M.; Dong, X.; Tong, H.; Xu, J.; Zhou, H.; Guan, S.; et al. C/N-Dependent Element Bioconversion Efficiency and Antimicrobial Protein Expression in Food Waste Treatment by Black Soldier Fly Larvae. Int. J. Mol. Sci. 2022, 23, 5036. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, P.; Ma, Y.; Jin, N.; Sun, S.; Dong, X.; Li, X.; Xu, J.; Zhou, X.; Xu, W. Transformation of food waste to source of antimicrobial proteins by black soldier fly larvae for defense against marine Vibrio parahaemolyticus. Sci. Total Environ. 2022, 826, 154163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.S.; Zhang, N.Z.; Liu, M.; Deng, F.M.; Wu, M.H. Purification and preparation of silybin and isosilybinby solid phase extraction. Chin. J. Chromatogr. 2017, 35, 70–74. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 20 November 2022).
- Liu, J.X.; Qin, W.; Peng, X.F.; Yuan, D. Simultaneous analysis of seven active components in silymarin from fruits, peels, nutlets and commercial extracts of Silybum marianum by HPLC. J. Shenyang Pharm. Univ. 2016, 33, 56–62. [Google Scholar]
- Ruan, H.S.; Jia, G.Y.; Wu, Z.J.; Zheng, X.L. Optimization of Ultrasonic-assisted Aqueous Two-phase System Extraction Silymarin from Silybum marianum L. Gaertn. J. Heilongjiang Bayi Agric. Univ. 2017, 29, 59–63+146. [Google Scholar]
- Liu, F.; Zhai, X.M.; Zhao, T.; Li, Z.Y.; Liu, Y.X.; Guo, D.L. Optimization of β-Cyclodextrin Assisted Extraction of Silybin by Response Surface Methodology. Food Ind. 2021, 42, 130–135. [Google Scholar]
- Li, Y.C.; Wang, J.X.; Ren, S.Y.; Wang, Y.F.; Niu, Z.P. Study on the stability of four silymarin monomers. China Food Addit. 2021, 32, 23–28. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wu, M.; Ren, M.; Bao, H.; Wang, Q.; Wang, N.; Sun, S.; Xu, J.; Yang, X.; Zhao, X.; et al. From Medical Herb to Functional Food: Development of a Fermented Milk Containing Silybin and Protein from Milk Thistle. Foods 2023, 12, 1308. https://doi.org/10.3390/foods12061308
Liu Y, Wu M, Ren M, Bao H, Wang Q, Wang N, Sun S, Xu J, Yang X, Zhao X, et al. From Medical Herb to Functional Food: Development of a Fermented Milk Containing Silybin and Protein from Milk Thistle. Foods. 2023; 12(6):1308. https://doi.org/10.3390/foods12061308
Chicago/Turabian StyleLiu, Yanxia, Minghuo Wu, Miaomiao Ren, Haijun Bao, Qing’an Wang, Nan Wang, Shibo Sun, Jianqiang Xu, Xiaojing Yang, Xu Zhao, and et al. 2023. "From Medical Herb to Functional Food: Development of a Fermented Milk Containing Silybin and Protein from Milk Thistle" Foods 12, no. 6: 1308. https://doi.org/10.3390/foods12061308
APA StyleLiu, Y., Wu, M., Ren, M., Bao, H., Wang, Q., Wang, N., Sun, S., Xu, J., Yang, X., Zhao, X., Bao, Y., He, G., & Xu, W. (2023). From Medical Herb to Functional Food: Development of a Fermented Milk Containing Silybin and Protein from Milk Thistle. Foods, 12(6), 1308. https://doi.org/10.3390/foods12061308





