Analysis of Microbial Diversity and Metabolites in Sauerkraut Products with and without Microorganism Addition
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Sauerkraut Samples
2.2. Physicochemical Analysis
2.3. Bioinformatics Analysis
2.4. Determination of Metabolite Groups
2.4.1. Determination of Nonvolatile Compounds
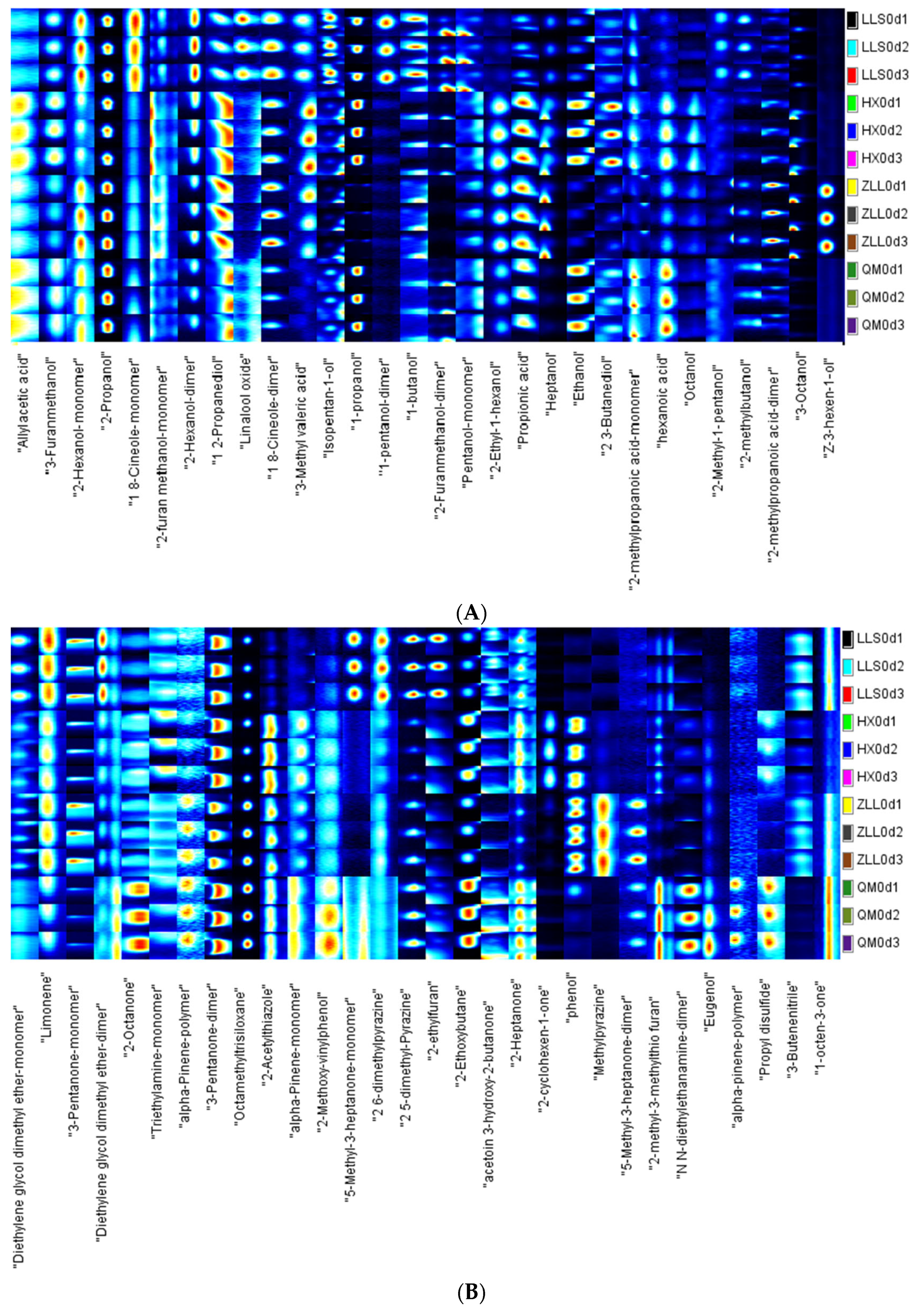
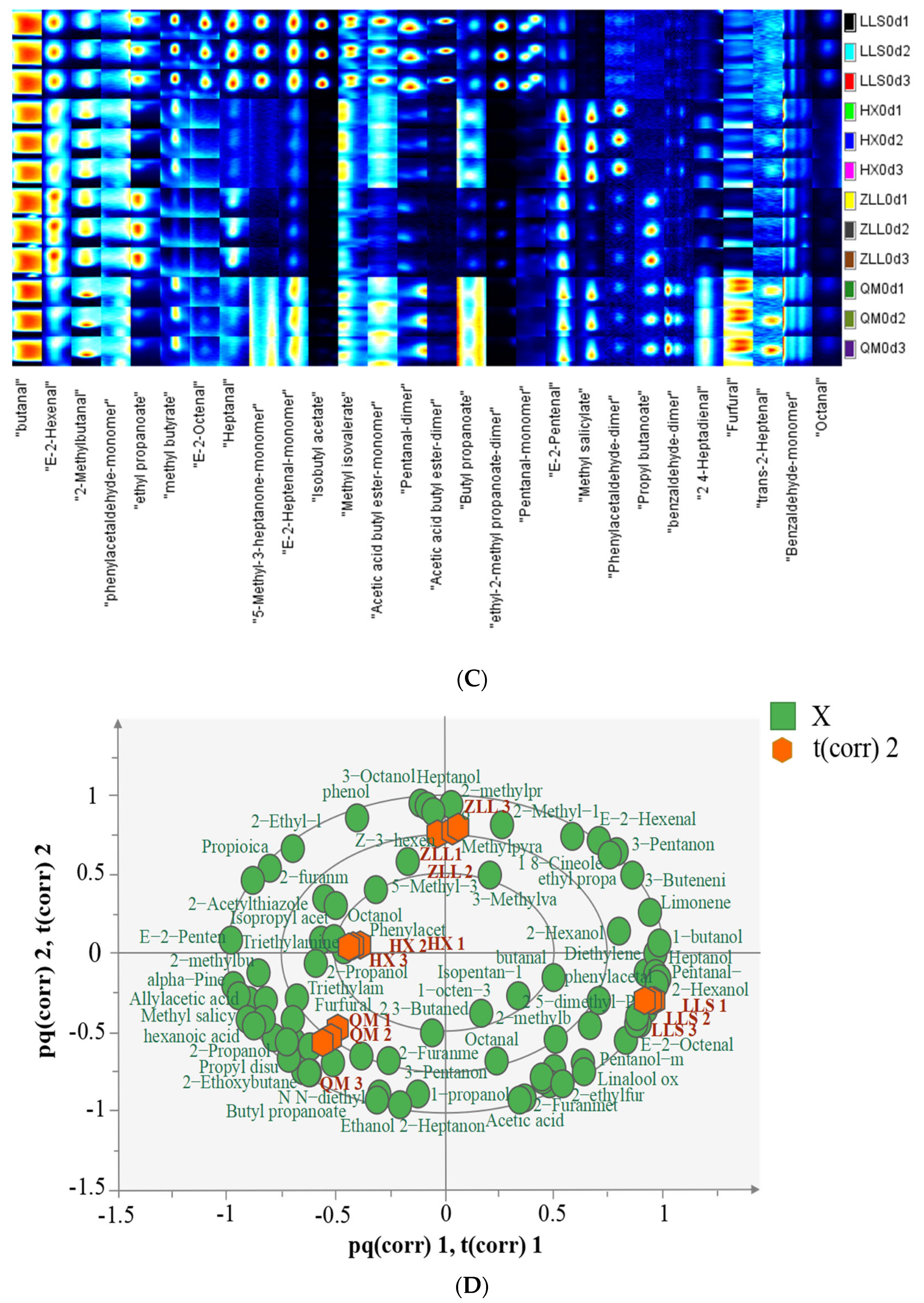
2.4.2. Determination of Volatile Compounds by GC-IMS
2.5. Data Analysis
3. Results and Analysis
3.1. Basic Physicochemical Properties
3.2. Microbiological Analysis
3.2.1. Microbial Statistical Analysis
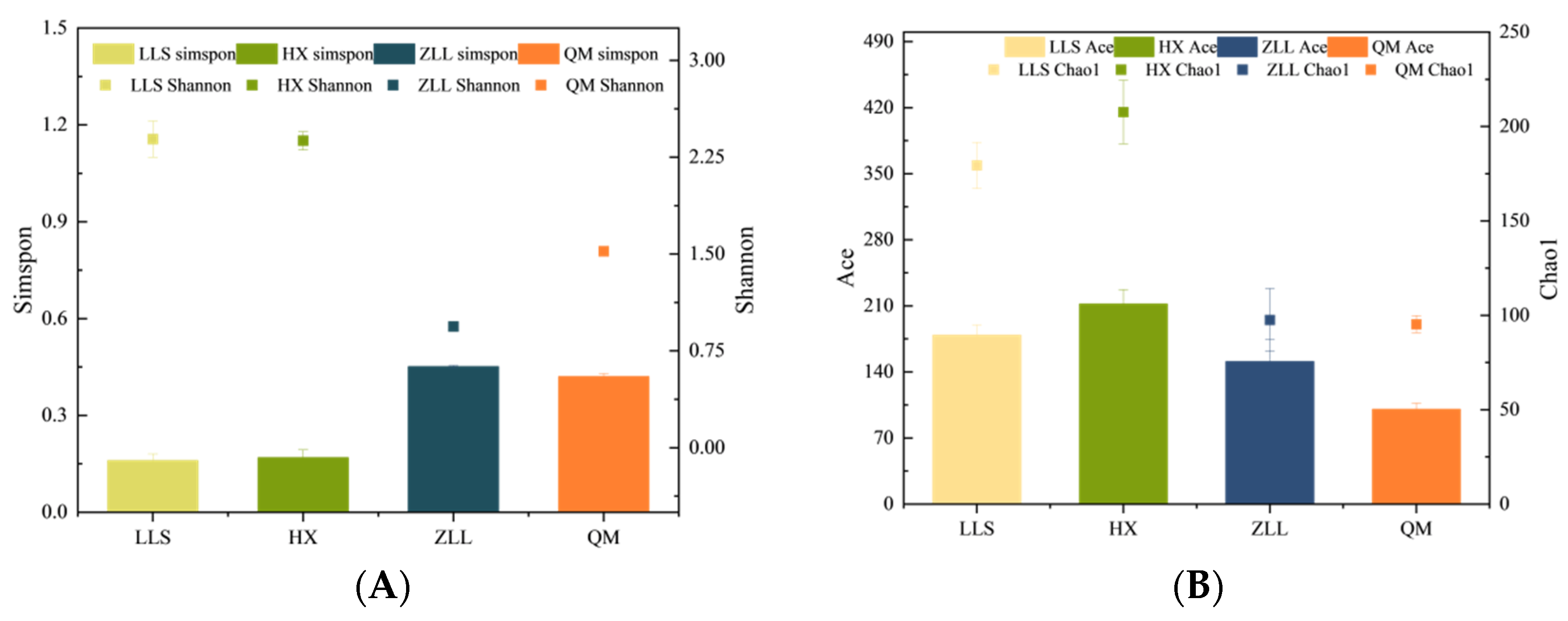
3.2.2. Alpha Diversity Analysis
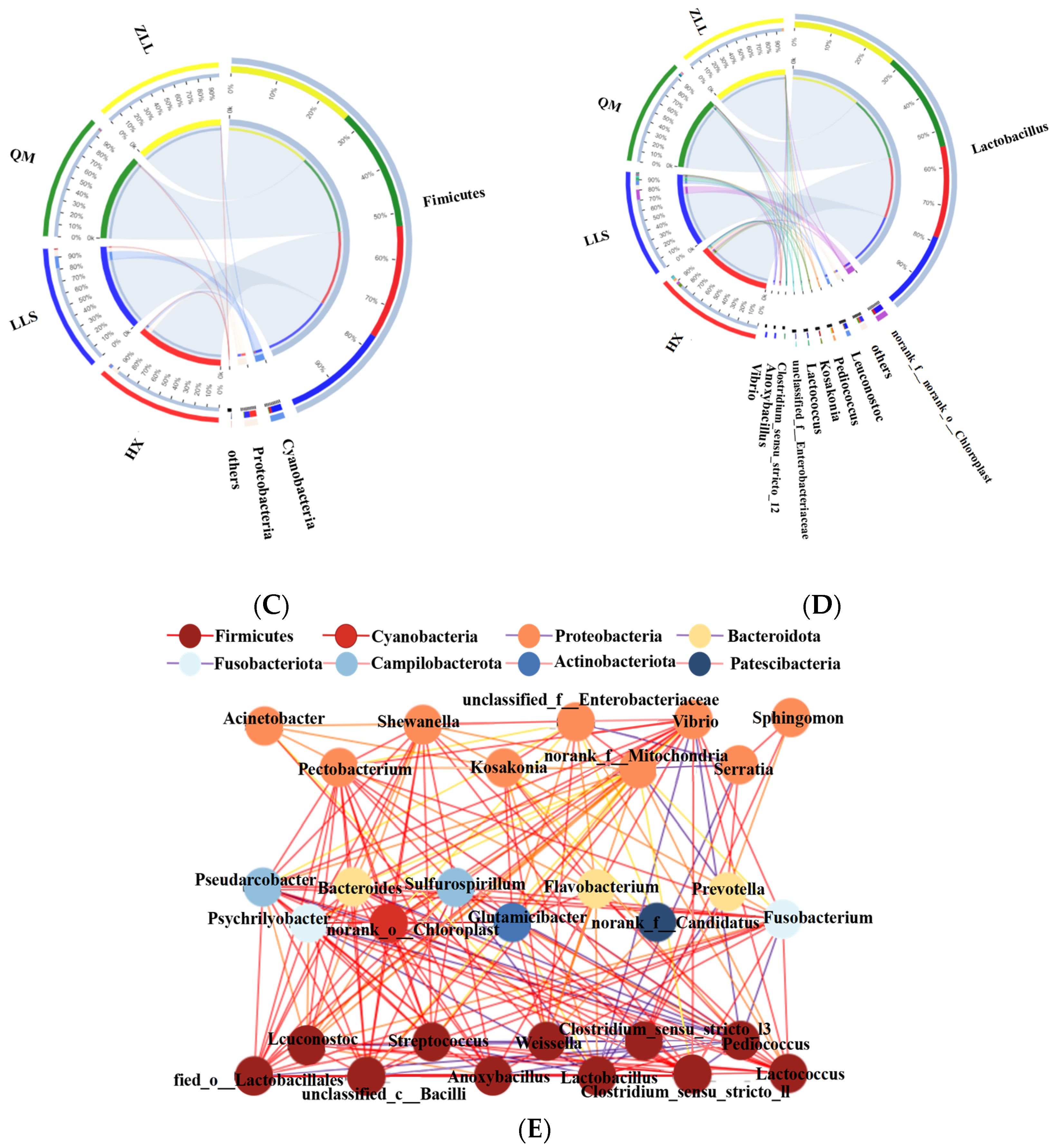
3.2.3. Structure of Bacterial Populations
3.2.4. Analysis of Microbial Beta Diversity
3.2.5. Network Characteristics of Sauerkraut Microorganisms
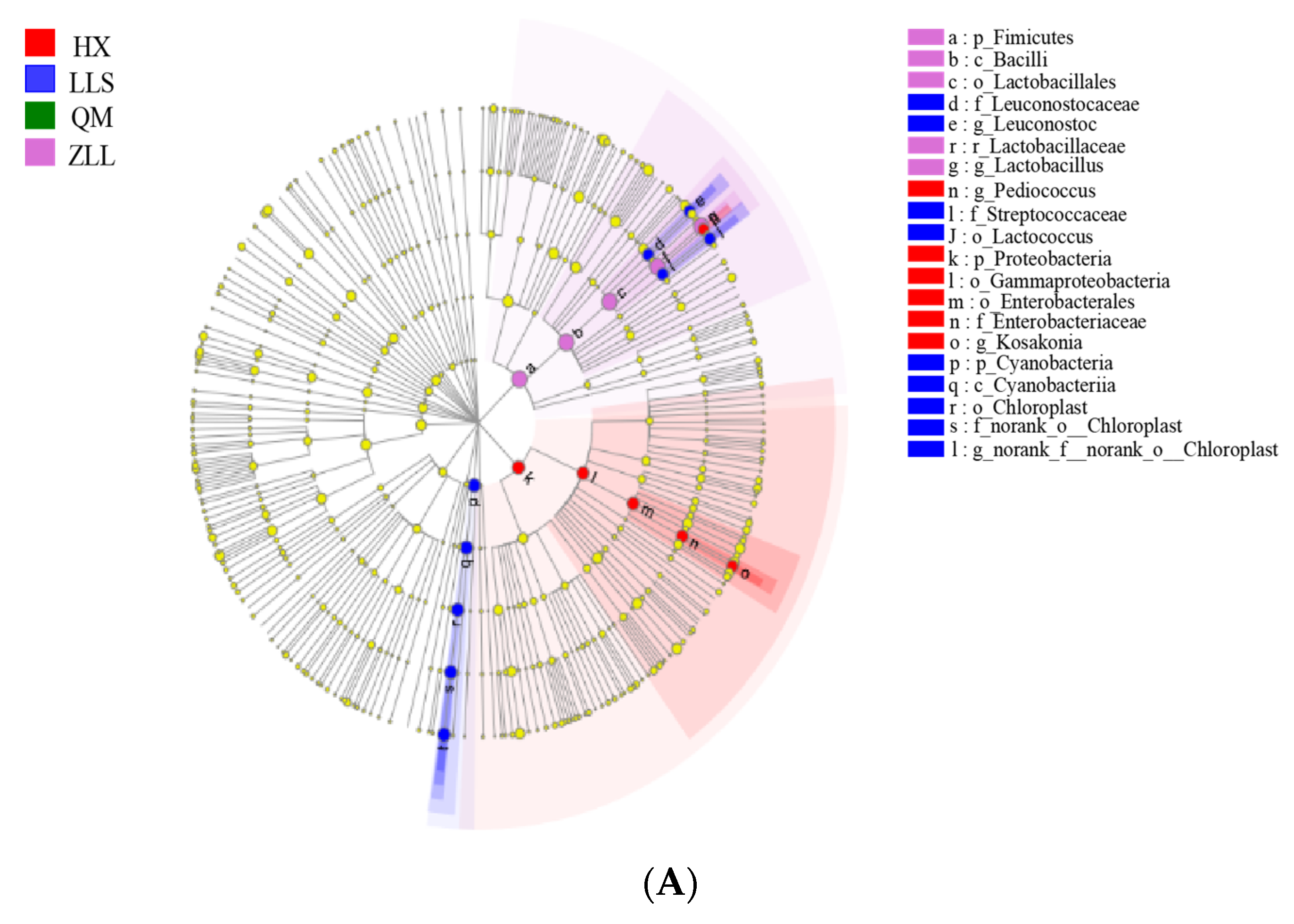
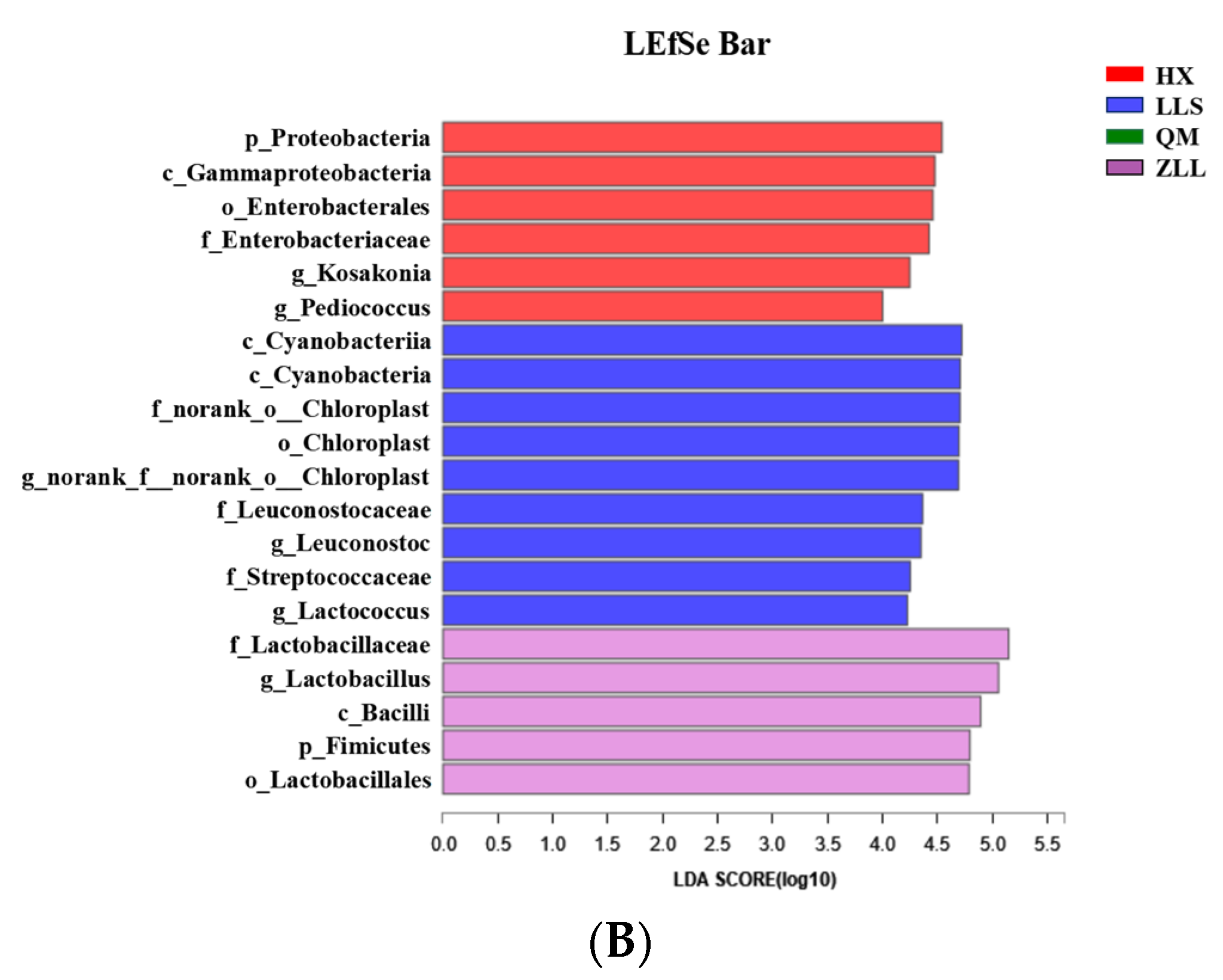
3.2.6. Discovery of Biomarkers in Sauerkraut
3.3. Analysis of Targeted Metabolomic Profiles
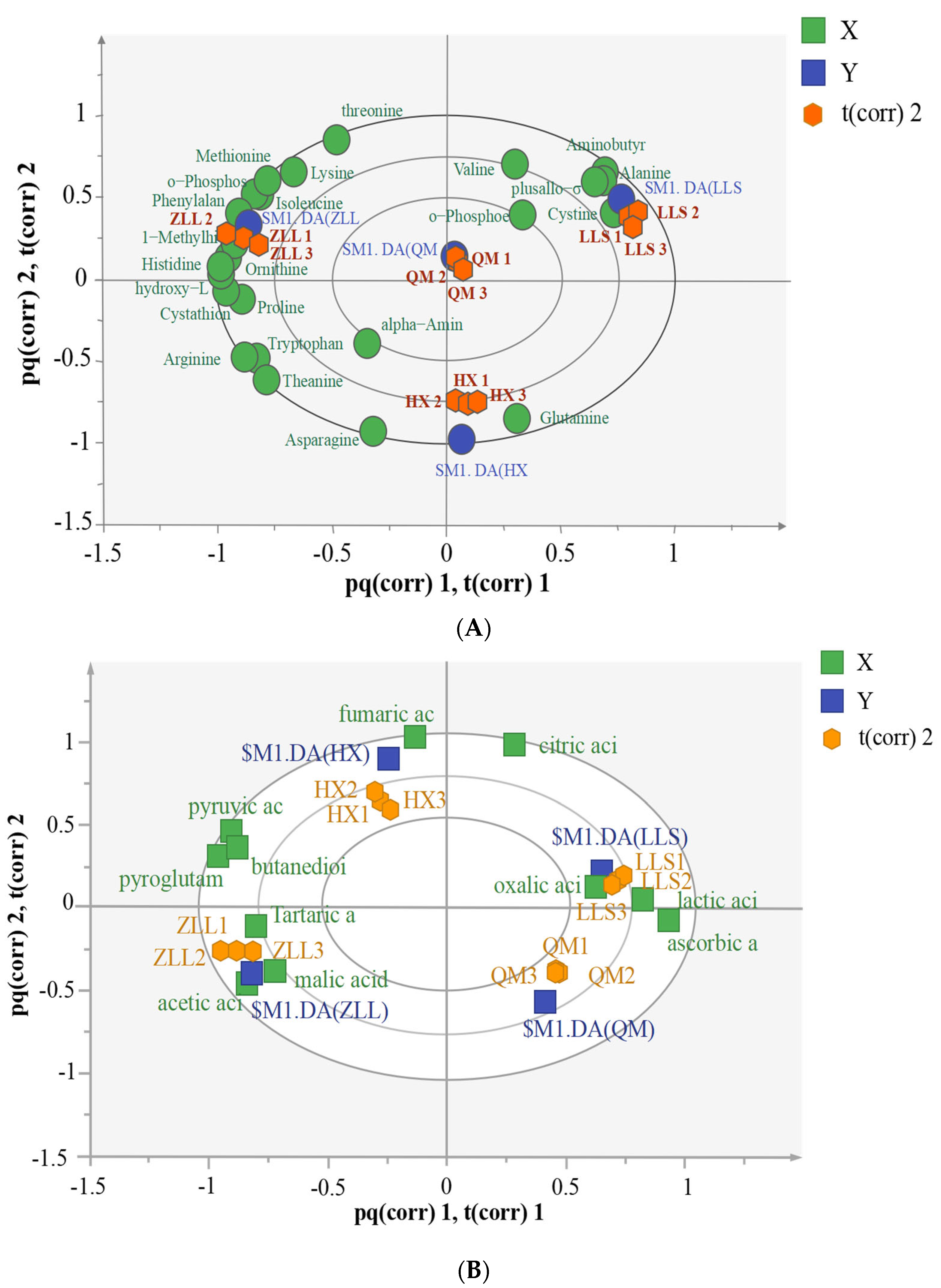
3.3.1. Detection and Analysis of Amino Acids in Sauerkraut
3.3.2. Detection and Analysis of Organic Acids in Sauerkraut
3.4. Analysis of Volatile Compounds
3.5. Associations between Bacterial Taxa and Metabolites
3.5.1. Analysis of Correlations among Microbial Taxa and Amino Acids
3.5.2. Analysis of the Correlations among Microbial Taxa and Organic Acids
3.5.3. Analysis of Correlations among Microbiota and Volatile Compounds
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Hu, W.; Jiang, A.; Xiu, Z.; Ji, Y.; Guan, Y.; Yang, X. Effect of salt concentration on quality of Chinese northeast sauerkraut fermented by Leuconostoc mesenteroides and Lactobacillus plantarum. Food Biosci. 2019, 30, 100421. [Google Scholar] [CrossRef]
- Park, S.-E.; Seo, S.-H.; Kim, E.-J.; Byun, S.; Na, C.-S.; Son, H.-S. Changes of microbial community and metabolite in kimchi inoculated with different microbial community starters. Food Chem. 2019, 274, 558–565. [Google Scholar] [CrossRef]
- Xiong, T.; Li, X.; Guan, Q.; Peng, F.; Xie, M. Starter culture fermentation of Chinese sauerkraut: Growth, acidification and metabolic analyses. Food Control 2014, 41, 122–127. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.H.; Jeon, C.O. Kimchi microflora: History, current status, and perspectives for industrial kimchi production. Appl. Microbiol. Biotechnol. 2014, 98, 2385–2393. [Google Scholar] [CrossRef]
- Pederson, C.S.; Albury, M.N. Variation among the heterofermentative lactic acid bacteria. J. Bacteriol. 1955, 70, 702–708. [Google Scholar] [CrossRef]
- Liang, H.; Yin, L.; Zhang, Y.; Chang, C.; Zhang, W. Dynamics and diversity of a microbial community during the fermentation of industrialized Qingcai paocai, a traditional Chinese fermented vegetable food, as assessed by Illumina MiSeq sequencing, DGGE and qPCR assay. Ann. Microbiol. 2018, 68, 111–122. [Google Scholar] [CrossRef]
- Lee, J.-J.; Choi, Y.-J.; Lee, M.J.; Park, S.J.; Oh, S.J.; Yun, Y.-R.; Min, S.G.; Seo, H.-Y.; Park, S.-H.; Lee, M.-A. Effects of combining two lactic acid bacteria as a starter culture on model kimchi fermentation. Food Res. Int. 2020, 136, 109591. [Google Scholar] [CrossRef]
- Yang, J.; Ji, Y.; Park, H.; Lee, J.; Park, S.; Yeo, S.; Shin, H.; Holzapfel, W.H. Selection of functional lactic acid bacteria as starter cultures for the fermentation of Korean leek (Allium tuberosum Rottler ex Sprengel.). Int. J. Food Microbiol. 2014, 191, 164–171. [Google Scholar] [CrossRef]
- Liu, A.; Li, X.; Pu, B.; Ao, X.; Zhou, K.; He, L.; Chen, S.; Liu, S. Use of psychrotolerant lactic acid bacteria (Lactobacillus spp. and Leuconostoc spp.) Isolated from Chinese Traditional Paocai for the Quality Improvement of Paocai Products. J. Agric. Food Chem. 2017, 65, 2580–2587. [Google Scholar] [CrossRef]
- Minervini, F.; Missaoui, J.; Celano, G.; Calasso, M.; Achour, L.; Saidane, D.; Gobbetti, M.; De Angelis, M. Use of autochthonous Lactobacilli to increase the safety of Zgougou. Microorganisms 2019, 8, 29. [Google Scholar] [CrossRef]
- Johanningsmeier, S.D.; Fleming, H.P.; Thompson, R.; Mcfeeters, R.F. Chemical and sensory properties of sauerkraut produced with Leuconostoc mesenteroides starter cultures of differing malolactic phenotypes. J. Food Sci. 2005, 70, S343–S349. [Google Scholar] [CrossRef]
- Chen, A.-J.; Luo, W.; Peng, Y.-T.; Niu, K.-L.; Liu, X.-Y.; Shen, G.-H.; Zhang, Z.-Q.; Wan, H.; Luo, Q.-Y.; Li, S.-S. Quality and microbial flora changes of radish paocai during multiple fermentation rounds. Food Control 2019, 106, 106733. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Ji, Y.; Guan, Y.; Feng, K. Comparison of northeast sauerkraut fermentation between single lactic acid bacteria strains and traditional fermentation. Food Res. Int. 2020, 137, 109553. [Google Scholar] [CrossRef]
- Hu, W.; Yang, X.; Ji, Y.; Guan, Y. Effect of starter cultures mixed with different autochthonous lactic acid bacteria on microbial, metabolome and sensory properties of Chinese northeast sauerkraut. Food Res. Int. 2021, 148, 110605. [Google Scholar] [CrossRef] [PubMed]
- Pino, A.; Vaccalluzzo, A.; Solieri, L.; Romeo, F.V.; Todaro, A.; Caggia, C.; Arroyo-López, F.N.; Bautista-Gallego, J.; Randazzo, C.L. Effect of sequential inoculum of beta-glucosidase positive and probiotic strains on brine fermentation to obtain low salt Sicilian table olives. Front. Microbiol. 2019, 10, 174. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An open source software for exploring and manipulating networks. In Proceedings of the International AAAI Conference on Web and Social Media, San Jose, CA, USA, 17–20 May 2009. [Google Scholar]
- Vick-Majors, T.J.; Priscu, J.C.; Amaral-Zettler, L.A. Modular community structure suggests metabolic plasticity during the transition to polar night in ice-covered Antarctic lakes. ISME J. 2014, 8, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Yang, H.J.; Kim, M.J.; Han, E.-S.; Kim, H.-J.; Kwon, D.Y. Metabolomic analysis of meju during fermentation by ultra performance liquid chromatography-quadrupole-time of flight mass spectrometry (UPLC-Q-TOF MS). Food Chem. 2011, 127, 1056–1064. [Google Scholar] [CrossRef]
- Wu, R.; Yu, M.; Liu, X.; Meng, L.; Wang, Q.; Xue, Y.; Wu, J.; Yue, X. Changes in flavour and microbial diversity during natural fermentation of suan-cai, a traditional food made in Northeast China. Int. J. Food Microbiol. 2015, 211, 23–31. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, X.; Hadiatullah, H.; Zhang, J.; Zhao, G. Determination of microbial diversities and aroma characteristics of Beitang shrimp paste. Food Chem. 2021, 344, 128695. [Google Scholar] [CrossRef]
- Li, Q.; Kang, J.; Ma, Z.; Li, X.; Liu, L.; Hu, X. Microbial succession and metabolite changes during traditional serofluid dish fermentation. LWT 2017, 84, 771–779. [Google Scholar] [CrossRef]
- Trail, A.; Fleming, H.; Young, C.; Mcfeeters, R. Chemical and sensory characterization of commercial sauerkraut 1. J. Food Qual. 1996, 19, 15–30. [Google Scholar] [CrossRef]
- Martorana, A.; Alfonzo, A.; Gaglio, R.; Settanni, L.; Corona, O.; La Croce, F.; Vagnoli, P.; Caruso, T.; Moschetti, G.; Francesca, N. Evaluation of different conditions to enhance the performances of Lactobacillus pentosus OM13 during industrial production of Spanish-style table olives. Food Microbiol. 2017, 61, 150–158. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičová, J. Optimisation of method of fermentation of cabbage juice. Czech J. Food Sci. 2004, 22, 39. [Google Scholar] [CrossRef]
- Endo, A.; Maeno, S.; Tanizawa, Y.; Kneifel, W.; Arita, M.; Dicks, L.; Salminen, S. Fructophilic lactic acid bacteria, a unique group of fructose-fermenting microbes. Appl. Environ. Microbiol. 2018, 84, e01290-18. [Google Scholar] [CrossRef]
- Tereshonok, V.; Markarova, M.Y.; Posokina, N.; Bondareva, L.; Nadezhkin, S. Influence of varietal characteristics the cabbage in the quality of the products used for pickling after a long storage period. Ovoŝi Rossii 2019, 23, 91–95. [Google Scholar] [CrossRef]
- Pérez-Díaz, I.; Dickey, A.; Fitria, R.; Ravishankar, N.; Hayes, J.; Campbell, K.; Arritt, F. Modulation of the bacterial population in commercial cucumber fermentations by brining salt type. J. Appl. Microbiol. 2020, 128, 1678–1693. [Google Scholar] [CrossRef]
- Peng, Q.; Jiang, S.; Chen, J.; Ma, C.; Huo, D.; Shao, Y.; Zhang, J. Unique microbial diversity and metabolic pathway features of fermented vegetables from Hainan, China. Front. Microbiol. 2018, 9, 399. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, N.; Lu, X.; Zheng, Z.; Zhang, M.; Qiao, X. Characterization of microbial community structure and metabolic potential using Illumina MiSeq platform during the black garlic processing. Food Res. Int. 2018, 106, 428–438. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, F.; Zhang, C.; Yang, L.; Fan, G.; Xu, Y.; Sun, B.; Li, X. Dynamic microbial succession of Shanxi aged vinegar and its correlation with flavor metabolites during different stages of acetic acid fermentation. Sci. Rep. 2018, 8, 8612. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, Q.; Chi, N.; Zhang, Q. Diversity of bacterial community of Chinese Northeast sauerkraut fermentation at the prophase and anaphase. Chin. Brew. 2022, 41, 42–46. [Google Scholar]
- Sun, W. Bacterial Diversity Research of Luzhou Flavor Liquor and Suancai based on Illumina Miseq Sequencing. Tianjin Univ. 2017, 1, 45–66. [Google Scholar]
- Zhou, Q.; Zang, S.; Zhao, Z.; Li, X. Dynamic changes of bacterial communities and nitrite character during northeastern Chinese sauerkraut fermentation. Food Sci. Biotechnol. 2018, 27, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Abubakr, M.A.; Al-Adiwish, W.M. Isolation and identification of lactic acid bacteria from different fruits with proteolytic activity. Int. J. Microbiol. Biotechnol. 2017, 2, 58–64. [Google Scholar]
- Satora, P.; Skotniczny, M.; Strnad, S.; Piechowicz, W. Chemical composition and sensory quality of sauerkraut produced from different cabbage varieties. LWT 2021, 136, 110325. [Google Scholar] [CrossRef]
- Smid, E.; Kleerebezem, M. Production of aroma compounds in lactic fermentations. Annu. Rev. Food Sci. Technol. 2014, 5, 313–326. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Zhao, D.; Du, R.; Wang, Y.; Wang, Q.; Na, J.; Guo, S.; Ge, J. Comparative Evaluation of Metabolite Composition and Quality of Lactobacillus paracasei HD1.7 Fermented and Commercial Pickled Chinese Cabbage. Food Sci. 2017, 38, 6–11. [Google Scholar]


| Sample | L. paracasei | L. plantarum | L. acidophilus |
|---|---|---|---|
| LLS | + | — | — |
| HX | — | + | — |
| QM | — | + | + |
| ZLL | — | — | — |
| Physical and Chemical Properties | LLS | HX | QM | ZLL |
|---|---|---|---|---|
| pH | 3.67 | 3.59 | 3.62 | 3.80 |
| Total acid (g/kg) | 10.98 | 14.06 | 10.44 | 9.00 |
| Reduced sugar (g/100 g) | 3.254 | 1.009 | 0.781 | 3.030 |
| Salts (g/100 g) | 1.40 | 1.87 | 0.94 | 1.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, X.; Li, F.; Shi, H.; He, M.; Ge, J.; Ling, H.; Cheng, K. Analysis of Microbial Diversity and Metabolites in Sauerkraut Products with and without Microorganism Addition. Foods 2023, 12, 1164. https://doi.org/10.3390/foods12061164
Liu Y, Chen X, Li F, Shi H, He M, Ge J, Ling H, Cheng K. Analysis of Microbial Diversity and Metabolites in Sauerkraut Products with and without Microorganism Addition. Foods. 2023; 12(6):1164. https://doi.org/10.3390/foods12061164
Chicago/Turabian StyleLiu, Yueyi, Xiaochun Chen, Fuxiang Li, Huiling Shi, Mingyi He, Jingping Ge, Hongzhi Ling, and Keke Cheng. 2023. "Analysis of Microbial Diversity and Metabolites in Sauerkraut Products with and without Microorganism Addition" Foods 12, no. 6: 1164. https://doi.org/10.3390/foods12061164
APA StyleLiu, Y., Chen, X., Li, F., Shi, H., He, M., Ge, J., Ling, H., & Cheng, K. (2023). Analysis of Microbial Diversity and Metabolites in Sauerkraut Products with and without Microorganism Addition. Foods, 12(6), 1164. https://doi.org/10.3390/foods12061164






