Physical–Chemical and Sensory Quality of Oat Milk Produced Using Different Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
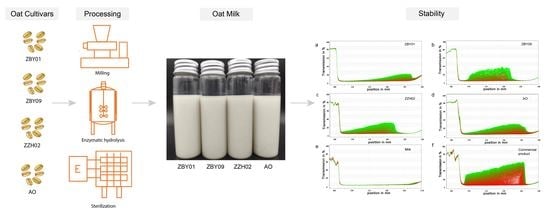
2.2. Preparation of Oat Milk
2.3. Nutritional Composition Analysis
2.4. Physical–Chemical Properties of Products
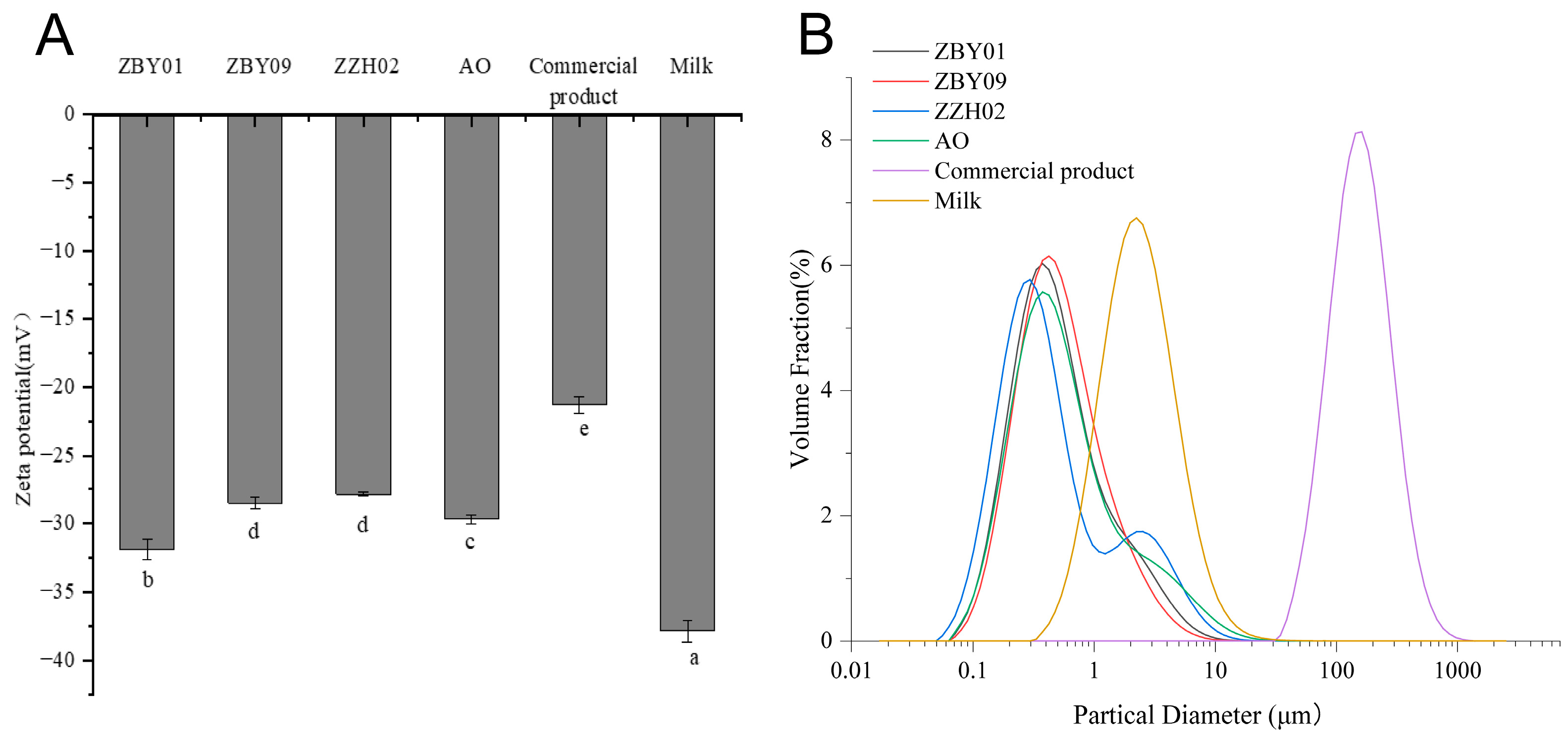
2.4.1. Particle Size Distribution
2.4.2. Zeta Potential
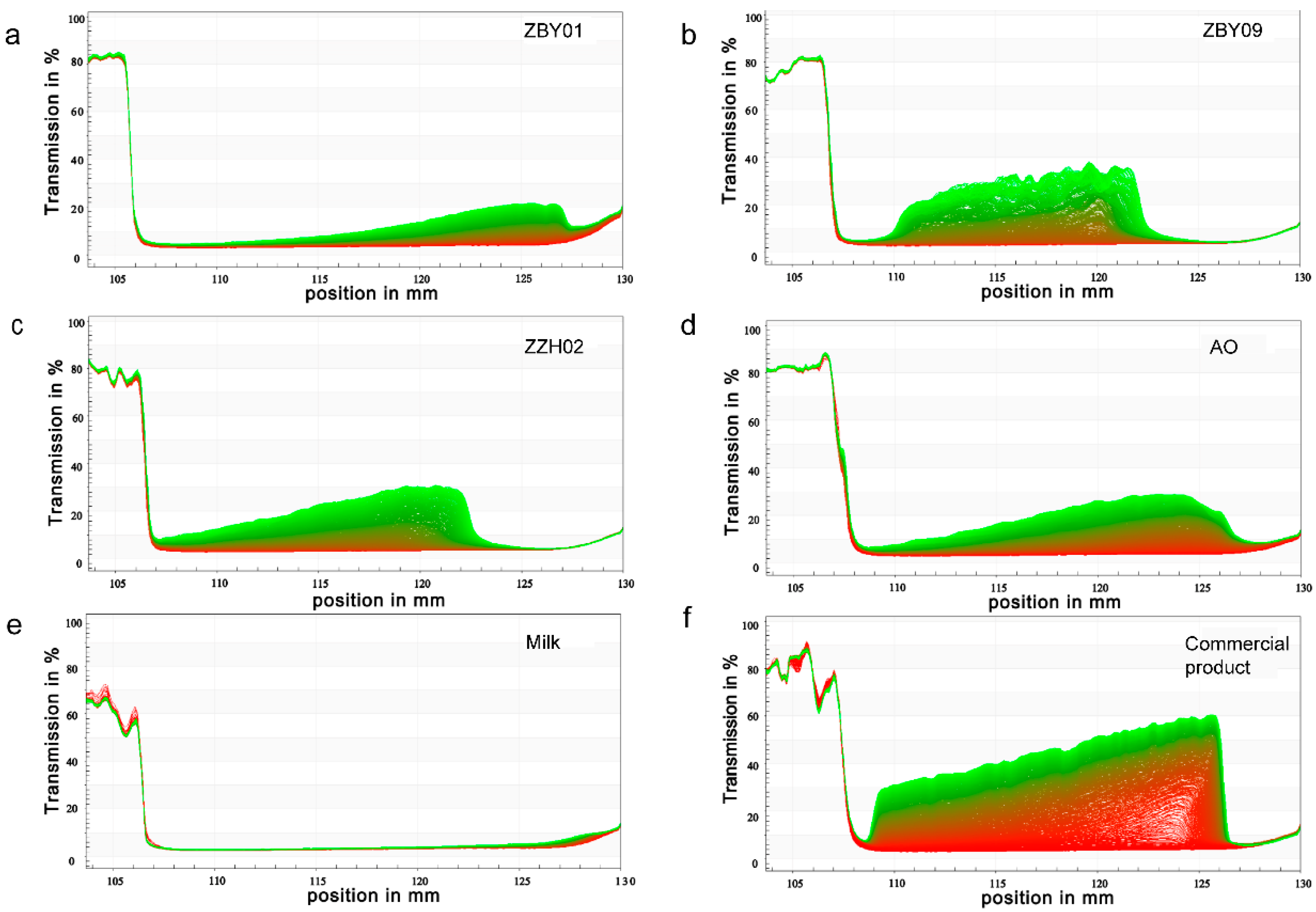
2.4.3. Physical Stability
2.4.4. Apparent Viscosity
2.5. Sensory Evaluation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Oat Cultivars
3.2. Physical Properties of Oat Milk
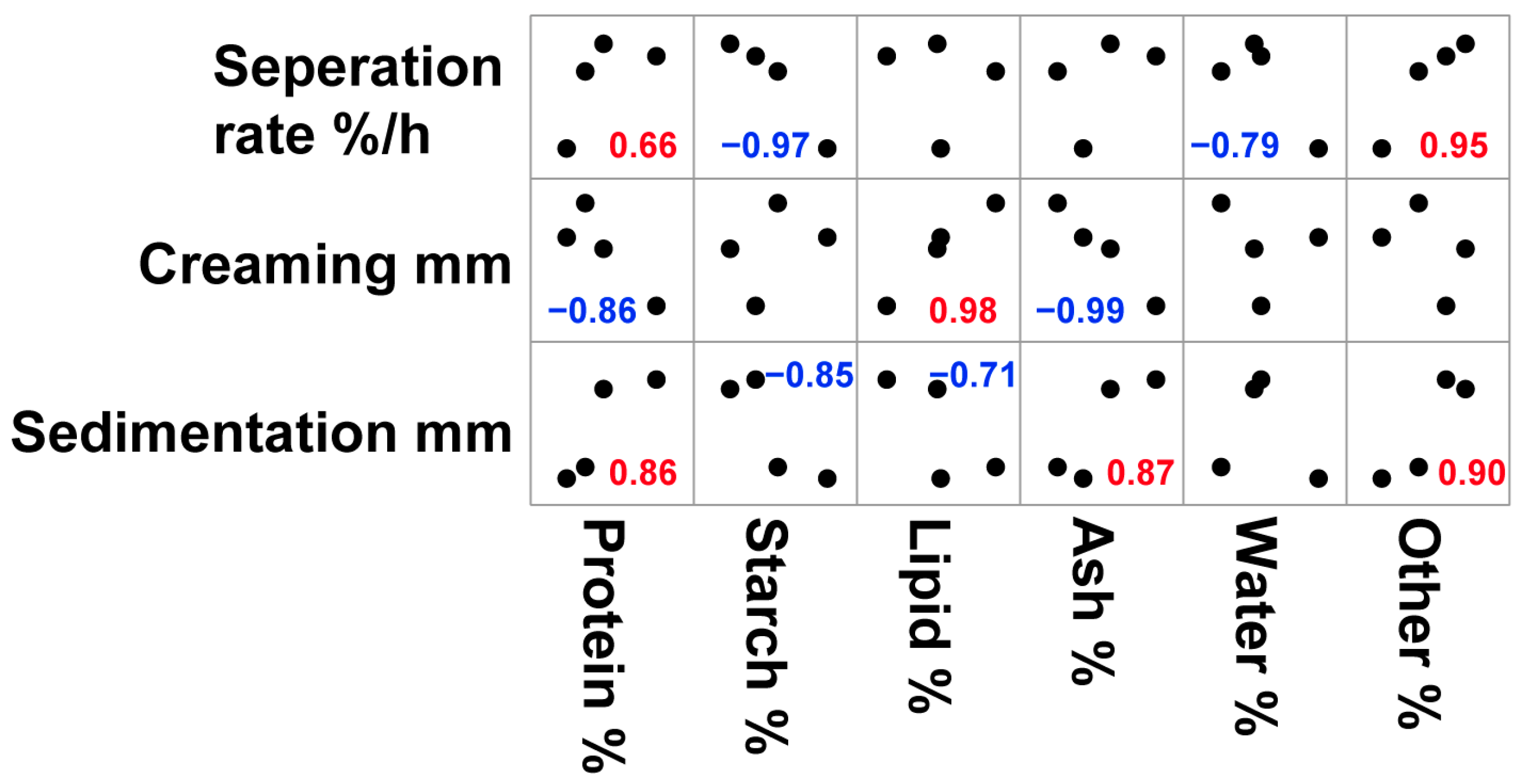
3.3. Correlation of Nutritional Composition of Cultivars and Physical Stability of Oat Milk
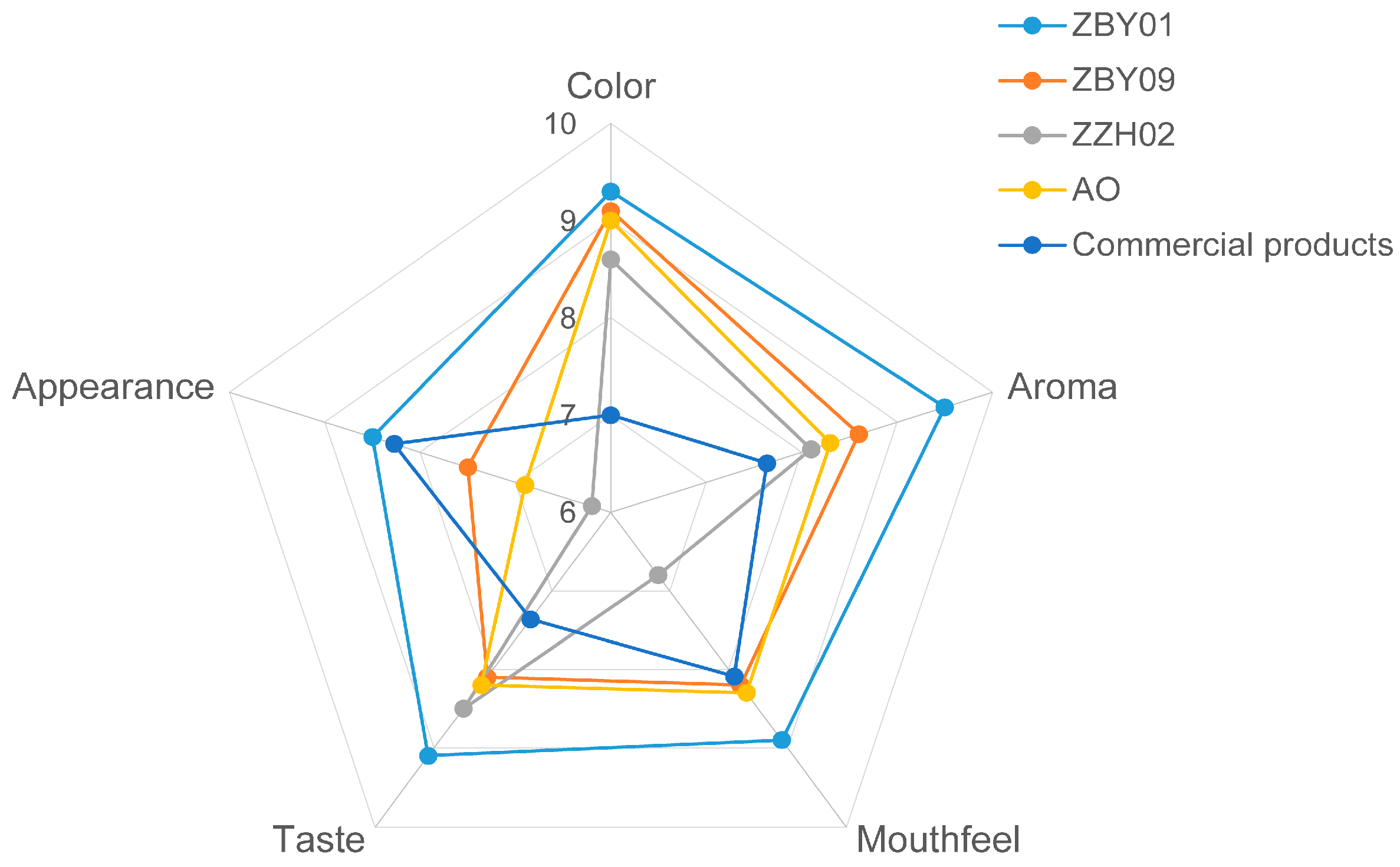
3.4. Sensory Evaluation of Oat Milk
3.5. Nutritional Composition of Oat Milk Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papageorgiou, M.; Skendi, A. 1-Introduction to cereal processing and by-products. In Sustainable Recovery and Reutilization of Cereal Processing By-Products; Galanakis, C.M., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 1–25. [Google Scholar]
- Hareland, G.A.; Manthey, F.A. OATS. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Fletcher, NC, USA, 2003; pp. 4213–4220. [Google Scholar]
- Price, R.K.; Welch, R.W. Cereal Grains. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Academic Press: Fletcher, NC, USA, 2013; pp. 307–316. [Google Scholar]
- Sontag-Strohm, T.; Lehtinen, P.; Kaukovirta-Norja, A. 8-Oat products and their current status in the celiac diet. In Gluten-Free Cereal Products and Beverages; Arendt, E.K., Dal Bello, F., Eds.; Academic Press: Fletcher, NC, USA, 2008; pp. 191–202. [Google Scholar]
- Yongfa, W.; Yongshan, Y.; Jiujin, X.; Ruofu, D.; Flatz, S.D.; Kühnau, W.; Flatz, G. Prevalence of primary adult lactose malabsorption in three populations of northern China. Hum. Genet. 1984, 67, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, M.; CUl, H.; B1AN, L.; Wang, Z. The prevalence of lactase deficiency and lactose intolerance in Chinese children of different ages. Chin. Med. J. 2000, 113, 1129–1132. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Peñas, E. Health benefits of oat: Current evidence and molecular mechanisms. Curr. Opin. Food Sci. 2017, 14, 26–31. [Google Scholar] [CrossRef]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Voss, G.B.; Coelho, M.C.; Pintado, M.E. Chapter 33-Food waste and by-product valorization as an integrated approach with zero waste: Future challenges. In Future Foods; Bhat, R., Ed.; Academic Press: Fletcher, CA, USA, 2022; pp. 569–596. [Google Scholar]
- Hu, X.-Z.; Zheng, J.-M.; Li, X.-P.; Xu, C.; Zhao, Q. Chemical composition and sensory characteristics of oat flakes: A comparative study of naked oat flakes from China and hulled oat flakes from western countries. J. Cereal Sci. 2014, 60, 297–301. [Google Scholar] [CrossRef]
- Zwer, P.K. Oats: Overview. In Encyclopedia of Food Grains, 2nd ed.; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Academic Press: Fletcher, NC, USA, 2016; pp. 173–183. [Google Scholar]
- Patra, T.; Rinnan, Å.; Olsen, K. The physical stability of plant-based drinks and the analysis methods thereof. Food Hydrocoll. 2021, 118, 106770. [Google Scholar] [CrossRef]
- Tong, L.-T.; Liu, L.-Y.; Zhong, K.; Wang, Y.; Guo, L.-N.; Zhou, S.-M. Effects of Cultivar on Phenolic Content and Antioxidant Activity of Naked Oat in China. J. Integr. Agric. 2014, 13, 1809–1816. [Google Scholar] [CrossRef]
- ISO. ISO 8968-1: 2014 (IDF 20-1: 2014) Milk and Milk Products: Determination of Nitrogen Content-Part 1: Kjeldahl Principle and Crude Protein Calculation; International Organization for Standardization: Geneva, Switzerland, 2014; pp. 1–18. [Google Scholar]
- ISO. 659: 2009, Oilseeds—Determination of Oil Content; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- International, A. AACC Methods 76-13.01; AACC International: St. Paul, MN, USA, 2000. [Google Scholar]
- Gibson, T.S.; Solah, V.A.; McCleary, B.V. A Procedure to Measure Amylose in Cereal Starches and Flours with Concanavalin A. J. Cereal Sci. 1997, 25, 111–119. [Google Scholar] [CrossRef]
- Delwiche, S. Official Method 923.03; Official Methods of Analysis of AOAC International: Rockville, MD, USA, 2003; Volume 2. [Google Scholar]
- AOAC. AOAC Official Method 925.10: Solids (Total) and Moisture in Flour; AOAC international: Rockville, MD, USA, 2010. [Google Scholar]
- AACC. International Method 32-23.01, β-Glucan Content of Barley and Oats—Rapid Enzymatic Procedure; American Association of Cereal Chemists: Saint Paul, MN, USA, 1999. [Google Scholar]
- Jeske, S.; Zannini, E.; Cronin, M.F.; Arendt, E.K. Impact of protease and amylase treatment on proteins and the product quality of a quinoa-based milk substitute. Food Funct. 2018, 9, 3500–3508. [Google Scholar] [CrossRef]
- Kori, A.H.; Mahesar, S.A.; Sherazi, S.T.H.; Khatri, U.A.; Laghari, Z.H.; Panhwar, T. Effect of process parameters on emulsion stability and droplet size of pomegranate oil-in-water. Grasas Y Aceites 2021, 72, e410. [Google Scholar] [CrossRef]
- Biel, W.; Jacyno, E.; Kawęcka, M. Chemical composition of hulled, dehulled and naked oat grains. S. Afr. J. Anim. Sci. 2014, 44, 189. [Google Scholar] [CrossRef]
- Moisio, T.; Forssell, P.; Partanen, R.; Damerau, A.; Hill, S.E. Reorganisation of starch, proteins and lipids in extrusion of oats. J. Cereal Sci. 2015, 64, 48–55. [Google Scholar] [CrossRef]
- Kumar, L.; Sehrawat, R.; Kong, Y. Oat proteins: A perspective on functional properties. Lwt 2021, 152, 112307. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. 3-Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Nimesh, S., Chandra, R., Gupta, N., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 43–58. [Google Scholar]
- Tangsuphoom, N.; Coupland, J.N. Effect of pH and ionic strength on the physicochemical properties of coconut milk emulsions. J. Food Sci. 2008, 73, E274–E280. [Google Scholar] [CrossRef]
- Bernat, N.; Cháfer, M.; Rodríguez-García, J.; Chiralt, A.; González-Martínez, C. Effect of high pressure homogenisation and heat treatment on physical properties and stability of almond and hazelnut milks. LWT-Food Sci. Technol. 2015, 62 Pt 2, 488–496. [Google Scholar] [CrossRef]
- Durand, A.; Franks, G.V.; Hosken, R.W. Particle sizes and stability of UHT bovine, cereal and grain milks. Food Hydrocoll. 2003, 17, 671–678. [Google Scholar] [CrossRef]
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-based Milks: A Review of the Science Underpinning Their Design, Fabrication, and Performance. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2047–2067. [Google Scholar] [CrossRef]
- Jeske, S.; Zannini, E.; Arendt, E.K. Evaluation of Physicochemical and Glycaemic Properties of Commercial Plant-Based Milk Substitutes. Plant Foods Hum. Nutr. 2017, 72, 26–33. [Google Scholar] [CrossRef]
- Farooq, Z.; Boye, J.I. 11-Novel food and industrial applications of pulse flours and fractions. In Pulse Foods; Tiwari, B.K., Gowen, A., McKenna, B., Eds.; Academic Press: Fletcher, NC, USA, 2011; pp. 283–323. [Google Scholar]
- Runyon, J.R.; Sunilkumar, B.A.; Nilsson, L.; Rascon, A.; Bergenståhl, B. The effect of heat treatment on the soluble protein content of oats. J. Cereal Sci. 2015, 65, 119–124. [Google Scholar] [CrossRef]
- Uthumporn, U.; Zaidul, I.S.M.; Karim, A.A. Hydrolysis of granular starch at sub-gelatinization temperature using a mixture of amylolytic enzymes. Food Bioprod. Process. 2010, 88, 47–54. [Google Scholar] [CrossRef]
- Deswal, A.; Deora, N.S.; Mishra, H.N. Optimization of Enzymatic Production Process of Oat Milk Using Response Surface Methodology. Food Bioprocess Technol. 2014, 7, 610–618. [Google Scholar] [CrossRef]
- Yao, Y.; He, W.; Cai, X.; Bekhit, A.E.-D.A.; Xu, B. Sensory, physicochemical and rheological properties of plant-based milk alternatives made from soybean, peanut, adlay, adzuki bean, oat and buckwheat. Int. J. Food Sci. Technol. 2022, 57, 4868–4878. [Google Scholar] [CrossRef]
- Moss, R.; Barker, S.; Falkeisen, A.; Gorman, M.; Knowles, S.; McSweeney, M.B. An investigation into consumer perception and attitudes towards plant-based alternatives to milk. Food Res. Int. 2022, 159, 111648. [Google Scholar] [CrossRef] [PubMed]
- Pointke, M.; Albrecht, E.H.; Geburt, K.; Gerken, M.; Traulsen, I.; Pawelzik, E. A Comparative Analysis of Plant-Based Milk Alternatives Part 1: Composition, Sensory, and Nutritional Value. Sustainability 2022, 14, 7996. [Google Scholar] [CrossRef]
| Index | Evaluation Standards | Score |
|---|---|---|
| Color | Uniform color, milky white | 8–10 |
| (10 points) | Slightly dull, nearly milky white | 6–7 |
| Dull and tends to be gray | 4–5 | |
| Dull and not acceptable | 1–3 | |
| Aroma | Oat aroma and strong | 8–10 |
| (10 points) | Oat aroma and not strong | 6–7 |
| Little oat aroma, clear fishy smell | 4–5 | |
| No oat aroma, fishy smell and not acceptable | 1–3 | |
| Mouthfeel | Smoothy and no graininess | 8–10 |
| (10 points) | Less smoothy, slightly graininess | 6–7 |
| Not smoothy, more graininess | 4–5 | |
| Rough taste and serious graininess | 1–3 | |
| Taste | Moderate sweetness, pleasant taste | 8–10 |
| (10 points) | Moderate sweetness, slightly bitter or oily | 6–7 |
| Plain taste, bitter or oily | 4–5 | |
| Obvious bitter or oily taste, not acceptable | 1–3 | |
| Appearance | Uniform system, no visible separation | 8–10 |
| (10 points) | Relatively uneven system, slight wall hanging or separation | 6–7 |
| Non-uniform system, certain wall hanging phenomenon | 4–5 | |
| Unstable system, obvious separation | 1–3 |
| Chemical Composition, % | Cultivar | |||
|---|---|---|---|---|
| ZBY01 | ZBY09 | ZZH02 | AO | |
| Protein | 11.85 ± 0.20 b | 12.98 ± 0.54 ab | 14.60 ± 0.68 a | 12.42 ± 0.68 ab |
| Starch | 66.63 ± 0.86 a | 59.82 ± 0.17 c | 61.61 ± 1.89 bc | 63.16 ± 0.52 b |
| Amylose | 37.09 ± 0.42 a | 36.34 ± 0.82 a | 38.73 ± 1.35 a | 36.41 ± 1.62 a |
| Lipid | 6.59 ± 0.31 b | 6.44 ± 0.39 b | 4.23 ± 0.12 c | 9.00 ± 0.44 a |
| Ash | 1.57 ± 0.05 bc | 1.76 ± 0.06 b | 2.08 ± 0.06 a | 1.39 ± 0.01 c |
| Water | 10.41 ± 0.20 a | 8.72 ± 0.02 b | 8.90 ± 0.08 b | 7.85 ± 0.10 c |
| Other | 2.95 ± 0.81 d | 10.28 ± 0.62 a | 8.58 ± 0.14 b | 6.18 ± 0.89 c |
| Sample | Separation Rate (%/h) | Sediment (mm) | Creaming (mm) | Instability Index |
|---|---|---|---|---|
| ZBY01 | 1.06 ± 0.05 d | 2.55 ± 0.05 d | 1.65 ± 0.15 b | 0.092 ± 0.003 c |
| ZBY09 | 2.16 ± 0.02 b | 4.90 ± 0.20 b | 1.60 ± 0.20 b | 0.185 ± 0.001 b |
| ZZH02 | 2.03 ± 0.02 b | 5.15 ± 0.35 a | 1.35 ± 0.25 c | 0.177 ± 0.001 b |
| AO | 1.87 ± 0.05 c | 2.85 ± 0.05 d | 1.80 ± 0.00 b | 0.152 ± 0.010 b |
| UHT bovine milk | 0.16 ± 0.01 e | 0.60 ± 0.20 e | 1.05 ± 0.15 c | 0.012 ± 0.001 d |
| Commercial product | 5.74 ± 0.12 a | 3.75 ± 0.05 c | 2.05 ± 0.35 a | 0.384 ± 0.004 a |
| Sample | Apparent Viscosity | ||
|---|---|---|---|
| Consistency Index k (mPa sn) | Flow Behavior Index n | R2 | |
| ZBY01 | 31.35 ± 0.50 a | 0.492 ± 0.020 a | 0.999 |
| ZBY09 | 30.30 ± 10.47 a | 0.513 ± 0.089 a | 0.999 |
| ZZH02 | 32.2 ± 2.82 a | 0.463 ± 0.026 a | 0.999 |
| AO | 27.35 ± 2.33 a | 0.452 ± 0.006 a | 0.999 |
| Commercial products | 6.75 ± 0.50 b | 0.843 ± 0.001 b | 0.990 |
| Protein, % | Lipid, % | Carbohydrate, mg/mL | β-Glucan, mg/mL | Total Polyphenols, mg GAE/100 g DW | |
|---|---|---|---|---|---|
| ZBY01 | 0.95 ± 0.01 a | 1.88 ± 0.01 b | 60.35 ± 0.30 d | 9.84 ± 0.44 a | 70.96 ± 0.15 a |
| ZBY09 | 1.05 ± 0.05 a | 1.87 ± 0.02 b | 63.22 ± 0.45 c | 9.12 ± 0.68 b | 60.03 ± 0.92 c |
| ZZH02 | 1.11 ± 0.01 a | 1.51 ± 0.03 c | 71.86 ± 0.71 a | 8.77 ± 0.40 c | 62.96 ± 0.77 b |
| AO | 1.09 ± 0.12 a | 2.01 ± 0.04 a | 73.47 ± 0.40 a | 8.09 ± 0.04 d | 60.79 ± 1.39 c |
| Commercial product | 1.01 ± 0.02 a | 1.52 ± 0.02 c | 66.15 ± 0.28 b | 4.69 ± 0.12 e | 47.40 ± 1.9 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Jia, Q.; Cui, L.; Dai, Y.; Li, R.; Tang, J.; Lu, J. Physical–Chemical and Sensory Quality of Oat Milk Produced Using Different Cultivars. Foods 2023, 12, 1165. https://doi.org/10.3390/foods12061165
Zhou S, Jia Q, Cui L, Dai Y, Li R, Tang J, Lu J. Physical–Chemical and Sensory Quality of Oat Milk Produced Using Different Cultivars. Foods. 2023; 12(6):1165. https://doi.org/10.3390/foods12061165
Chicago/Turabian StyleZhou, Sumei, Qiuju Jia, Lulu Cui, Ying Dai, Ruoning Li, Jian Tang, and Jing Lu. 2023. "Physical–Chemical and Sensory Quality of Oat Milk Produced Using Different Cultivars" Foods 12, no. 6: 1165. https://doi.org/10.3390/foods12061165
APA StyleZhou, S., Jia, Q., Cui, L., Dai, Y., Li, R., Tang, J., & Lu, J. (2023). Physical–Chemical and Sensory Quality of Oat Milk Produced Using Different Cultivars. Foods, 12(6), 1165. https://doi.org/10.3390/foods12061165