Effect of Calcium Hydroxide on Physicochemical and In Vitro Digestibility Properties of Tartary Buckwheat Starch-Rutin Complex Prepared by Pre-Gelatinization and Co-Gelatinization Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Starch
2.3. Preparation of Pre-Gelatinized Complexes
2.4. Preparation of Co-Gelatinized Complexes
2.5. Solubility and Swelling Power (SP)
2.6. Scanning Electron Microscopy (SEM)
2.7. Gel Firmness (TPA)
2.8. Thermogravimetry Analysis (TGA)
2.9. X-ray Diffraction (XRD)
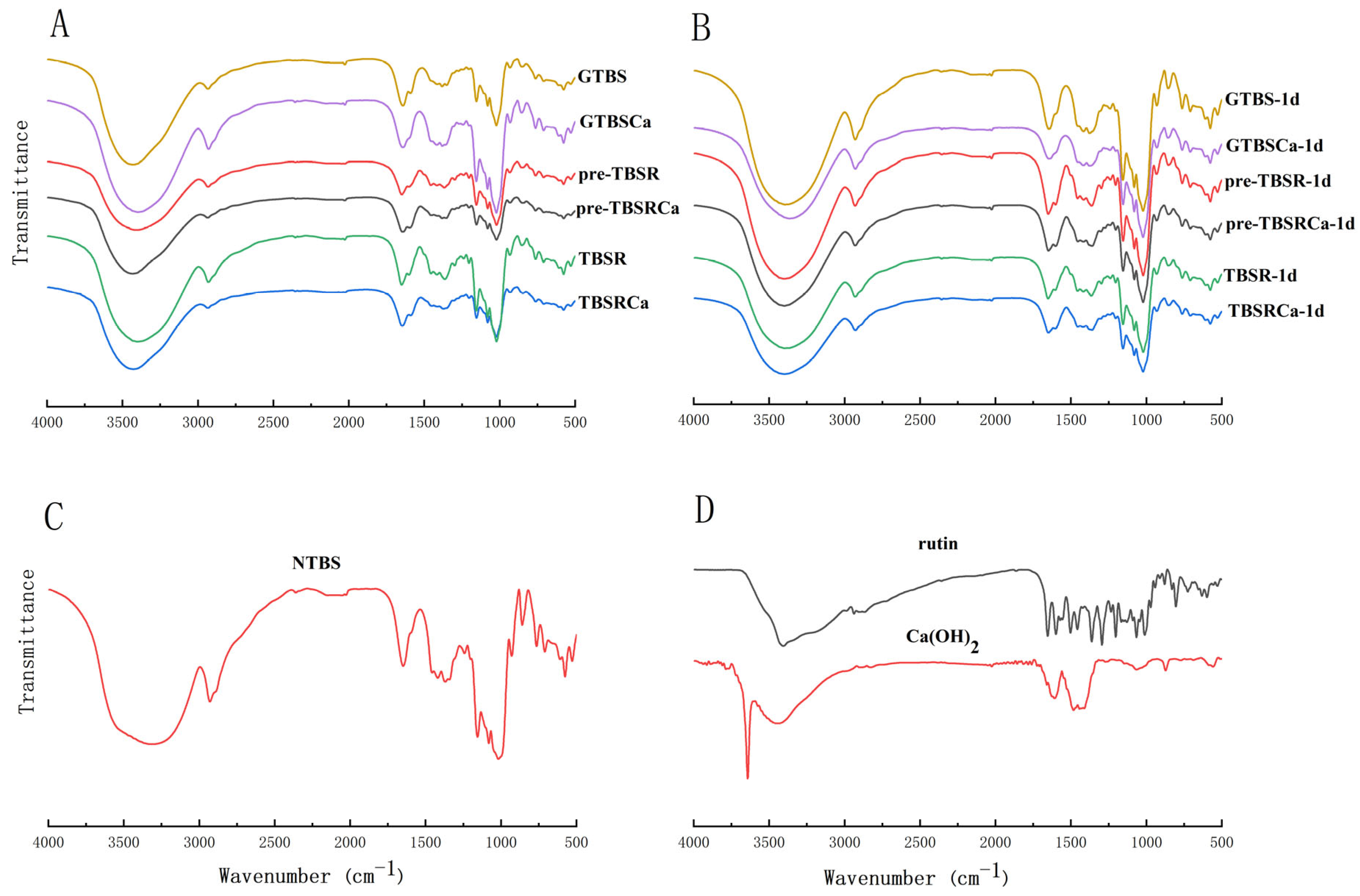
2.10. Fourier Transform Infrared Spectroscopy (FT-IR)
2.11. Differential Scanning Calorimetry (DSC)
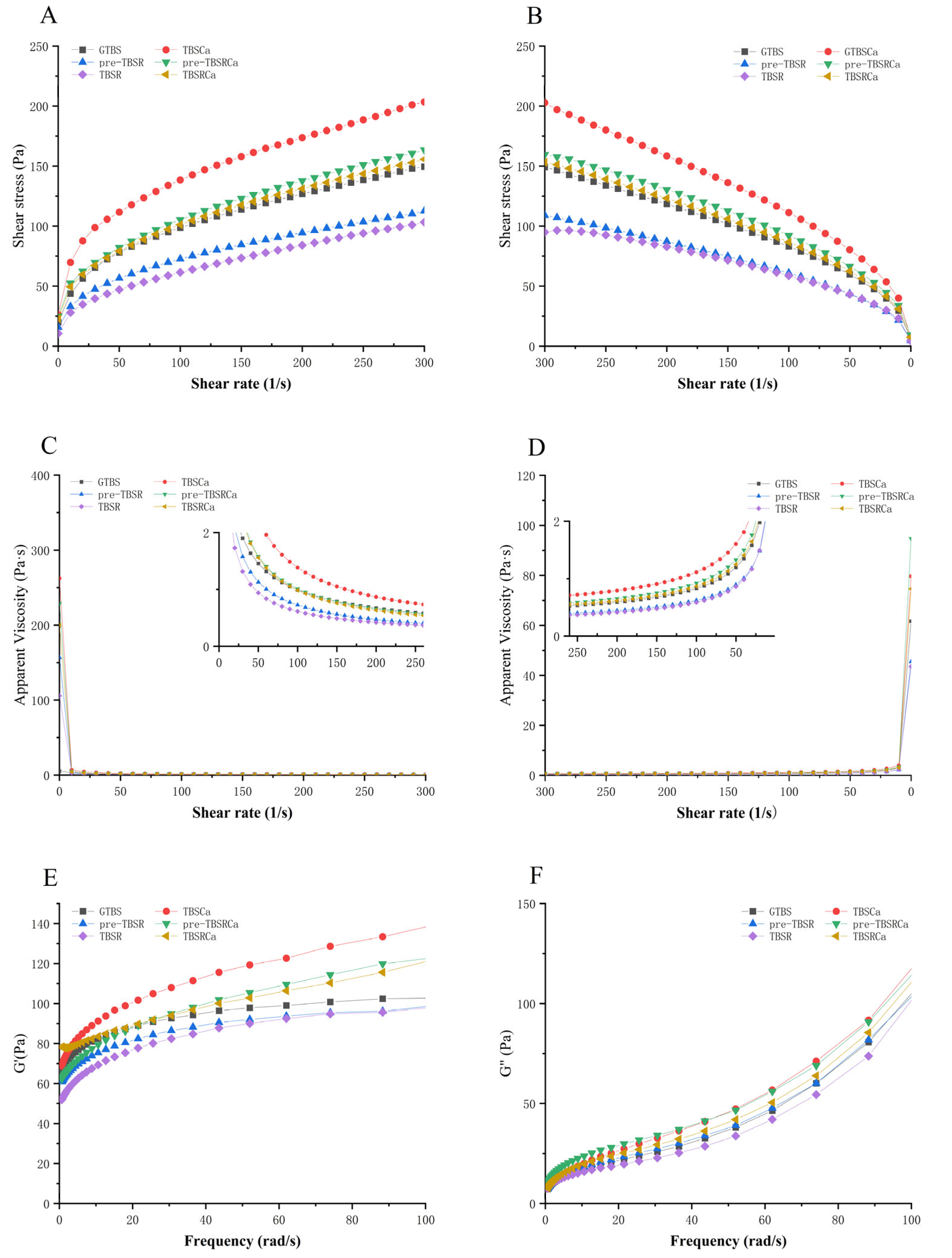
2.12. Rheological Properties
2.13. In Vitro Digestibility
2.14. Statistical Analysis
3. Results and Discussion
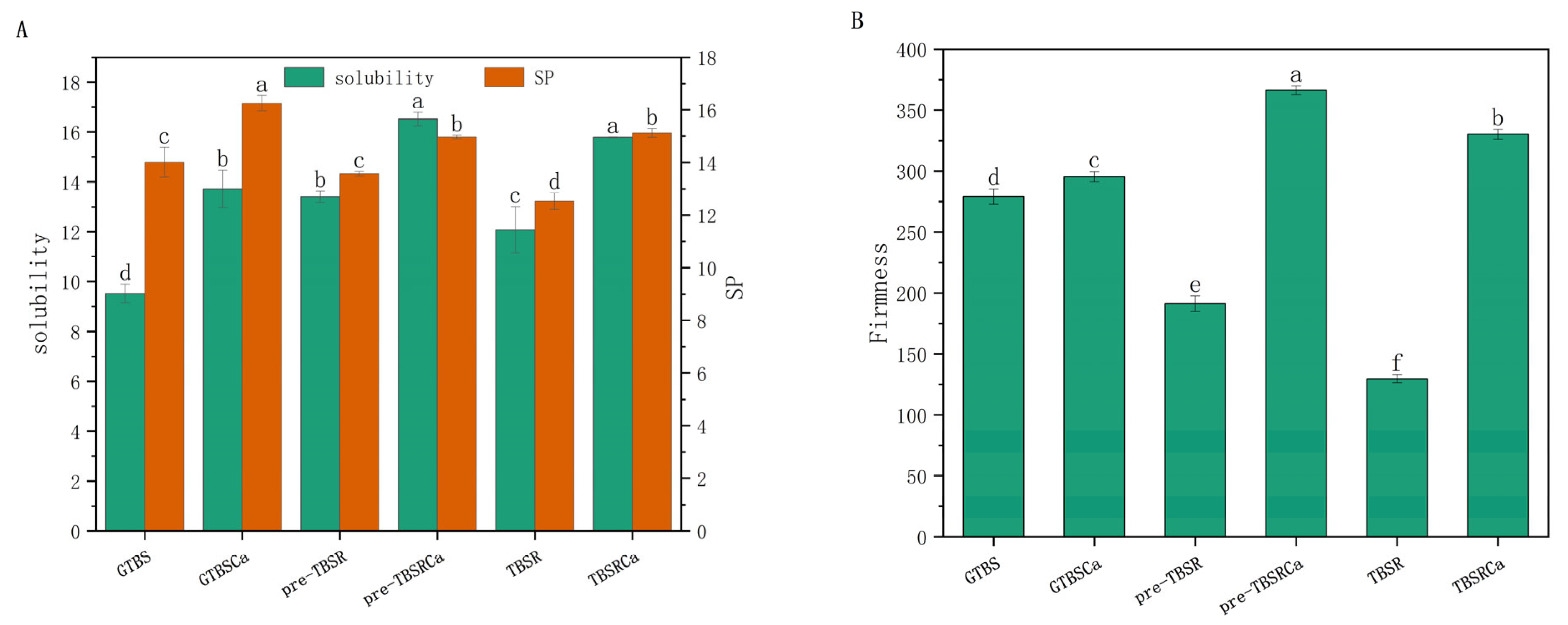
3.1. Solubility and Swelling Power (SP)
3.2. Gel Firmness
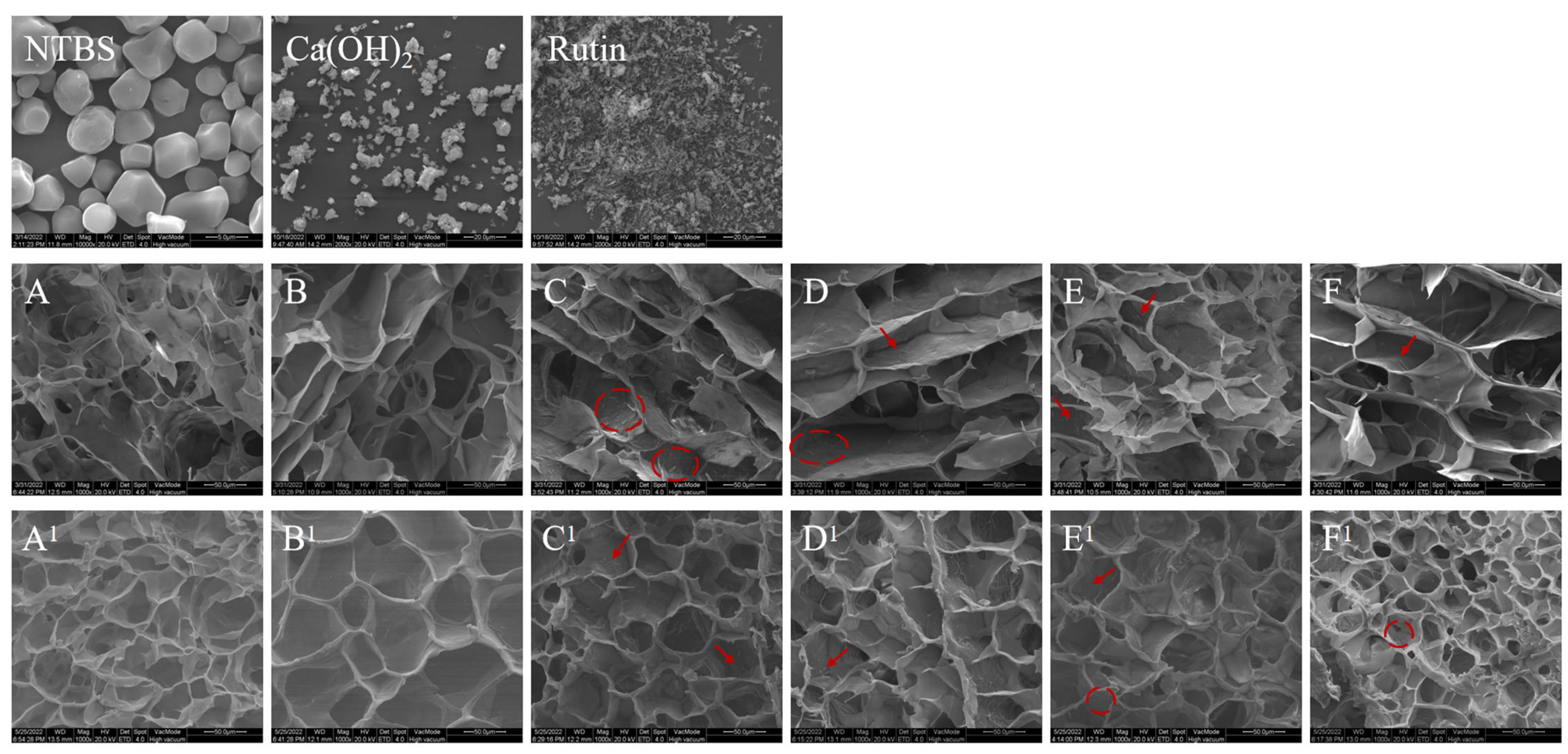
3.3. Morphological Characteristics
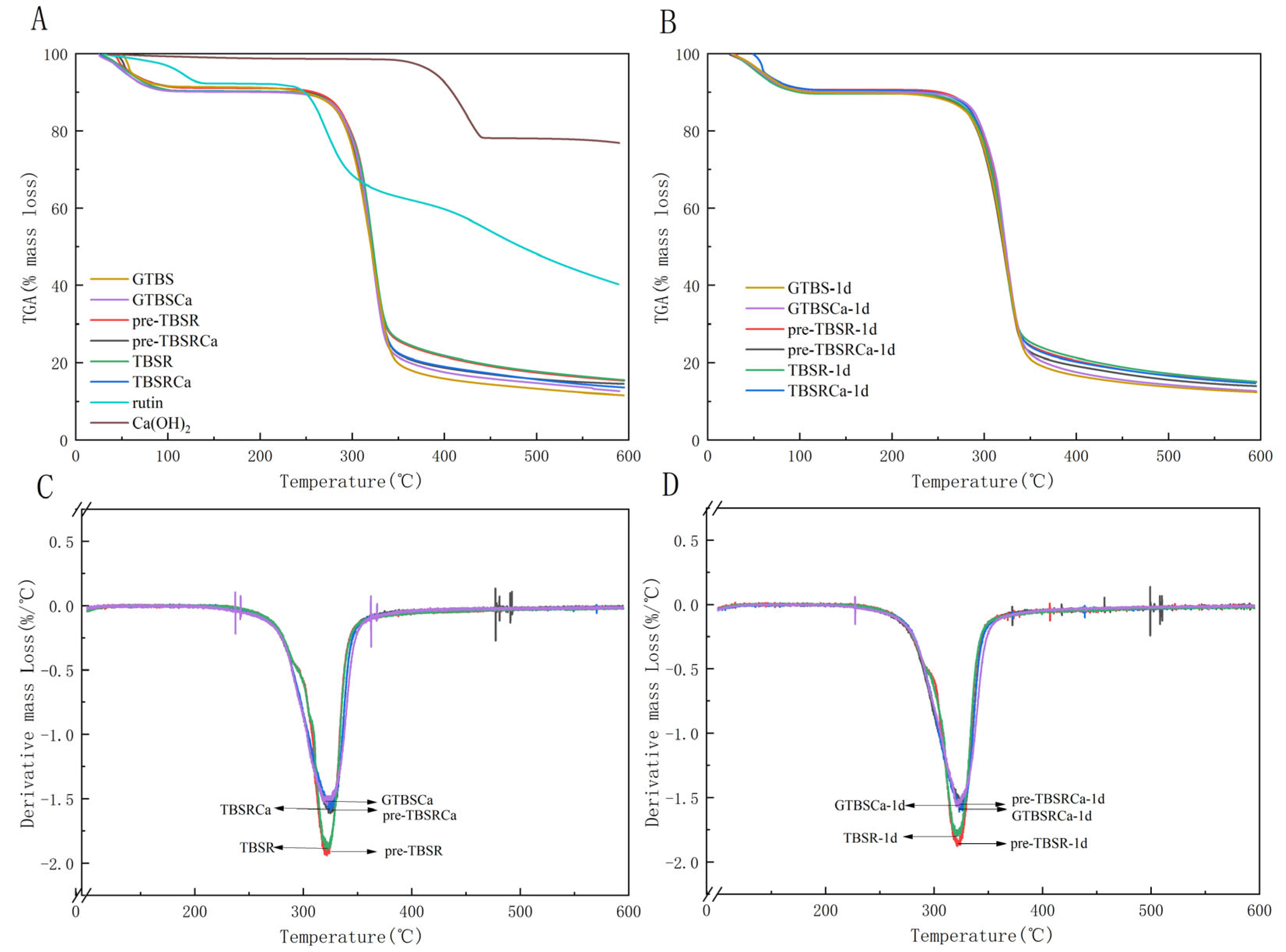
3.4. Thermogravimetry Analysis
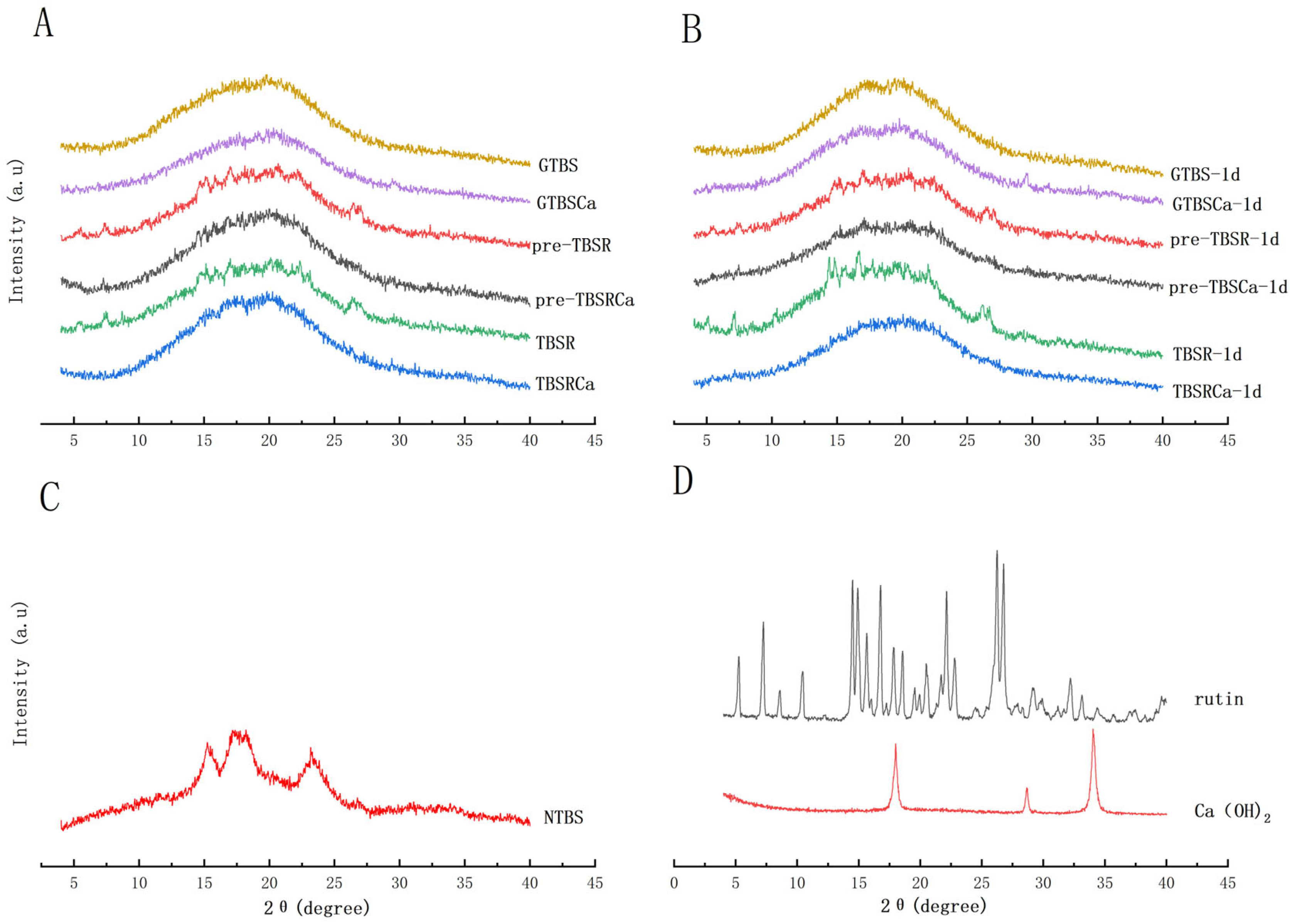
3.5. Crystalline Structure and Crystallinity
3.6. FT-IR Analysis
3.7. Differential Scanning Calorimetry
3.8. Rheological Properties
3.9. In Vitro Digestibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Li, Z.; Gao, Y.; Cui, S.W.; Wang, T.; Qiu, J. Diverse effects of rutin and quercetin on the pasting, rheological and structural properties of Tartary buckwheat starch. Food Chem. 2021, 335, 127556. [Google Scholar] [CrossRef]
- Fabjan, N.; Rode, J.; Košir, I.J.; Wang, Z.H.; Zhang, Z.; Kreft, I. Tartary buck-wheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercitrin. J. Agr. Food Chem. 2003, 51, 6452–6455. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015, 43, 129–143. [Google Scholar] [CrossRef]
- Gao, J.; Kreft, I.; Chao, G.; Wang, Y.; Liu, X.; Wang, L.; Wang, P.; Gao, X.; Feng, B. Tartary buckwheat (Fagopyrum tataricum Gaertn.) starch, a side product in functional food production, as a potential source of retrograded starch. Food Chem. 2016, 190, 552–558. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Li, S.; Gao, W. Preparation, physicochemical characterization and in vitro digestibility on solid complex of maize starches with quercetin. LWT-Food Sci. Technol. 2011, 44, 787–792. [Google Scholar] [CrossRef]
- He, C.; Zhang, Z.; Liu, H.; Gao, J.; Li, Y.; Wang, M. Effect of rutin and quercetin on the physicochemical properties of Tartary buckwheat starch. Starch-Stärke 2018, 70, 1700038. [Google Scholar] [CrossRef]
- Deng, N.; Deng, Z.; Tang, C.; Liu, C.; Luo, S.; Chen, T.; Hu, X. Formation, structure and properties of the starch-polyphenol inclusion complex: A review. Trends Food Sci. Technol. 2021, 112, 667–675. [Google Scholar] [CrossRef]
- Xu, T.; Li, X.; Ji, S.; Zhong, Y.; Simal-Gandara, J.; Capanoglu, E.; Xiao, J.; Lu, B. Starch modification with phenolics: Methods, physicochemical property alteration, and mechanisms of glycaemic control. Trends Food Sci. Technol. 2021, 111, 12–26. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Cheng, Z.; Fan, X.; Ma, Z.; Hu, X.; Wu, G.; Xing, Y. Modified Tartary buckwheat (Fagopyrum tataricum Gaertn.) starch by gaseous ozone: Structural, physicochemical and in vitro digestible properties. Food Hydrocoll. 2022, 125, 107365. [Google Scholar] [CrossRef]
- Han, L.; Lu, Z.; Hao, X.; Cheng, Y.; Li, L. Impact Of Calcium Hydroxide on the Textural Properties Of Buckwheat Noodles. J. Texture Stud. 2012, 43, 227–234. [Google Scholar] [CrossRef]
- Palacios-Fonseca, A.J.; Castro-Rosas, J.; Gómez-Aldapa, C.A.; Tovar-Benítez, T.; Millán-Malo, B.M.; del Real, A.; Rodríguez-García, M.E. Effect of the alkaline and acid treatments on the physicochemical properties of corn starch. CyTA J. Food 2013, 11 (Suppl. S1), 67–74. [Google Scholar] [CrossRef]
- Cornejo-Villegas, M.d.l.Á.; Rincón-Londoño, N.; Del Real-López, A.; Rodríguez-García, M.E. The effect of Ca2+ ions on the pasting, morphological, structural, vibrational, and mechanical properties of corn starch–water system. J. Cereal Sci. 2018, 79, 174–182. [Google Scholar] [CrossRef]
- De la Hera, E.; Gomez, M.; Rosell, C.M. Particle size distribution of rice flour affecting the starch enzymatic hydrolysis and hydration properties. Carbohydr. Polym. 2013, 98, 421–427. [Google Scholar] [CrossRef]
- Gao, S.; Liu, H.; Sun, L.; Cao, J.; Yang, J.; Lu, M.; Wang, M. Rheological, thermal and in vitro digestibility properties on complex of plasma modified Tartary buckwheat starches with quercetin. Food Hydrocoll. 2021, 110, 106209. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.-S.; Chen, H.-H.; Li, Q.-Q.; Wang, Q. The gelatinization and retrogradation properties of wheat starch with the addition of stearic acid and sodium alginate. Food Hydrocolloid 2018, 81, 77–86. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.; Cummings, J. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar]
- Han, X.; Zhang, M.; Zhang, R.; Huang, L.; Jia, X.; Huang, F.; Liu, L. Physicochemical interactions between rice starch and different polyphenols and structural characterization of their complexes. LWT 2020, 125, 109227. [Google Scholar] [CrossRef]
- Bordenave, N.; Hamaker, B.R.; Ferruzzi, M.G. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014, 5, 18–34. [Google Scholar] [CrossRef]
- Hedayati, S.; Shahidi, F.; Majzoobi, M.; Koocheki, A.; Farahnaky, A. Structural, rheological, pasting and textural properties of granular cold water swelling maize starch: Effect of NaCl and CaCl(2). Carbohydr. Polym. 2020, 242, 116406. [Google Scholar] [CrossRef]
- Aleixandre, A.; Benavent-Gil, Y.; Moreira, R.; Rosell, C. In vitro digestibility of gels from different starches: Relationship between kinetic parameters and microstructure. Food Hydrocoll. 2021, 120, 106909. [Google Scholar] [CrossRef]
- Zhu, F.; Cai, Y.-Z.; Sun, M.; Corke, H. Effect of phytochemical extracts on the pasting, thermal, and gelling properties of wheat starch. Food Chem. 2009, 112, 919–923. [Google Scholar] [CrossRef]
- Wang, M.; Shen, Q.; Hu, L.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Physicochemical properties, structure and in vitro digestibility on complex of starch with lotus (Nelumbo nucifera Gaertn.) leaf flavonoids. Food Hydrocoll. 2018, 81, 191–199. [Google Scholar] [CrossRef]
- Nascimento, T.A.; Calado, V.; Carvalho, C.W.P. Development and characterization of flexible film based on starch and passion fruit mesocarp flour with nanoparticles. Food Res. Int. 2012, 49, 588–595. [Google Scholar] [CrossRef]
- Chanjarujit, W.; Hongsprabhas, P.; Chaiseri, S. Physicochemical properties and flavor retention ability of alkaline calcium hydroxide-mungbean starch films. Carbohydr. Polym. 2018, 198, 473–480. [Google Scholar] [CrossRef]
- Roskhrua, P.; Tran, T.; Chaiwanichsiri, S.; Kupongsak, S.; Pradipasena, P. Physicochemical properties of thermal alkaline treated pigeonpea (Cajanus cajan L.) flour. Food Sci. Biotechnol. 2014, 23, 381–388. [Google Scholar] [CrossRef]
- Wang, S.; Copeland, L. Effect of alkali treatment on structure and function of pea starch granules. Food Chem. 2012, 135, 1635–1642. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I. Research advances on structural characterization of resistant starch and its structure-physiological function relationship: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1059–1083. [Google Scholar] [CrossRef]
- Ren, N.; Ma, Z.; Xu, J.; Hu, X. Insights into the supramolecular structure and techno-functional properties of starch isolated from oat rice kernels subjected to different processing treatments. Food Chem. 2020, 317, 126464. [Google Scholar] [CrossRef]
- Lopez-Rubio, A.; Flanagan, B.M.; Shrestha, A.K.; Gidley, M.J.; Gilbert, E.P. Molecular rearrangement of starch during in vitro digestion: Toward a better un-derstanding of enzyme resistant starch formation in processed starches. Biomacromolecules 2008, 9, 1951–1958. [Google Scholar] [CrossRef]
- Chai, Y.; Wang, M.; Zhang, G. Interaction between amylose and tea polyphenols modulates the postprandial glycemic response to high-amylose maize starch. J. Agric. Food Chem. 2013, 61, 8608–8615. [Google Scholar] [CrossRef]
- Contreras-Jimenez, B.; Vazquez-Contreras, G.; de Los Angeles Cornejo-Villegas, M.; Del Real-Lopez, A.; Rodriguez-Garcia, M.E. Structural, morphological, chemical, vibrational, pasting, rheological, and thermal characterization of isolated jicama (Pachyrhizus spp.) starch and jicama starch added with Ca(OH)2. Food Chem. 2019, 283, 83–91. [Google Scholar] [CrossRef]
- Rodríguez, M.E.; Yánez-Limón, M.; Alvarado-Gil, J.J.; Vargas, H.; Sánchez-Sinencio, F.; Figueroa, J.D.C.; Miranda, L.C.M. Influence of the structural changes during alkaline cooking on the thermal, rheological, and dielectric properties of corn tortillas. Cereal Chem. 1996, 73, 593–600. [Google Scholar]
- Dangi, N.; Yadav, B.S.; Yadav, R.B. Pasting, rheological, thermal and gel textural properties of pearl millet starch as modified by guar gum and its acid hydrolysate. Int. J. Biol. Macromol. 2019, 139, 387–396. [Google Scholar] [CrossRef]
- Santiago-Ramos, D.; Figueroa-Cardenas, J.D.; Veles-Medina, J.J. Viscoelastic behaviour of masa from corn flours obtained by nixtamalization with different calcium sources. Food Chem. 2018, 248, 21–28. [Google Scholar] [CrossRef]
- Liu, S.; Lin, L.; Shen, M.; Wang, W.; Xiao, Y.; Xie, J. Effect of Mesona chinensis polysaccharide on the pasting, thermal and rheological properties of wheat starch. Int. J. Biol. Macromol. 2018, 118, 945–951. [Google Scholar] [CrossRef]
- Tang, M.; Hong, Y.; Gu, Z.; Zhang, Y.; Cai, X. The effect of xanthan on short and long-term retrogradation of rice starch. Starch-Stärke 2013, 65, 702–708. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, S.; Shen, M.; Jiang, L.; Ren, Y.; Luo, Y.; Wen, H.; Xie, J. Physicochemical, rheological and thermal properties of Mesona chinensis polysaccharides obtained by sodium carbonate assisted and cellulase assisted extraction. Int. J. Biol. Macromol. 2019, 126, 30–36. [Google Scholar] [CrossRef]
- Chumsri, P.; Panpipat, W.; Cheong, L.Z.; Nisoa, M.; Chaijan, M. Comparative Evaluation of Hydrothermally Produced Rice Starch-Phenolic Complexes: Contributions of Phenolic Type, Plasma-Activated Water, and Ultrasonication. Foods 2022, 11, 3826. [Google Scholar] [CrossRef]
- Li, X.; Peng, Y.; Liu, H.; Xu, Y.; Wang, X.; Zhang, C.; Ma, X. Comparative studies on the interaction of nine flavonoids with trypsin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 238, 118440. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Wang, T.; Li, Z.; Gao, Y.; Cui, S.W.; Qiu, J. Comparison of quercetin and rutin inhibitory influence on Tartary buckwheat starch digestion in vitro and their differences in binding sites with the digestive enzyme. Food Chem. 2022, 367, 130762. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhang, L.; Li, K.; He, J.; Xin, H.; Wang, Q. Effect of gelatinization processing on the antioxidant, digestion, and physicochemical properties of wheat starch enhanced with tannic acid. LWT 2020, 125, 109228. [Google Scholar] [CrossRef]

| RC (%) | RC (%)-1d | DO | DO-1d | |
|---|---|---|---|---|
| GTBS | 7.41 ± 0.05 a | 10.04 ± 0.03 a | 1.17 ± 0.006 a | 1.27 ± 0.008 a |
| GTBSCa | 7.10 ± 0.11 b | 8.54 ± 0.1 b | 1.17 ± 0.005 a | 1.23 ± 0.006 b |
| pre-TBSR | 5.98 ± 0.04 c | 7.01 ± 0.03 c | 1.10 ± 0.005 b | 1.18 ± 0.004 c |
| pre-TBSRCa | 5.60 ± 0.03 d | 6.81 ± 0.03 d | 1.05 ± 0.002 e | 1.14 ± 0.004 d |
| TBSR | 5.41 ± 0.05 e | 6.81 ± 0.13 d | 1.08 ± 0.001 c | 1.13 ± 0.004 e |
| TBSRCa | 5.35 ± 0.05 e | 6.64 ± 0.07 e | 1.07 ± 0.002 d | 1.08 ± 0.002 f |
| TO (°C) | TP (°C) | TC (°C) | △H (J/g) | △Hr (J/g) | |
|---|---|---|---|---|---|
| GTBS | 60.69 ± 0.07 d | 67.17 ± 0.02 d | 74.57 ± 0.3 b | 9.44 ± 0.34 a | 2.21 ± 0.09 a |
| GTBSCa | 65.56 ± 0.05 a | 71.11 ± 0.1 a | 77.34 ± 0.09 a | 7.09 ± 0.12 b | 1.92 ± 0.07 b |
| pre-TBSR | 60.43 ± 0.08 e | 66.12 ± 0.09 e | 72.40 ± 0.25 c | 7.03 ± 0.08 bc | 1.57 ± 0.03 c |
| pre-TBSRCa | 63.78 ± 0.04 b | 68.50 ± 0.17 b | 74.61 ± 0.13 b | 5.89 ± 0.09 d | 1.27 ± 0.04 e |
| TBSR | 60.21 ± 0.05f | 66.11 ± 0.26e | 72.16 ± 0.27c | 6.61 ± 0.23c | 1.41 ± 0.04d |
| TBSRCa | 63.56 ± 0.06 c | 67.98 ± 0.04 c | 74.60 ± 0.14 b | 5.24 ± 0.31 e | 1.12 ± 0.01 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Li, X.; Cai, Q.; Ma, Z.; Ren, T.; Hu, X. Effect of Calcium Hydroxide on Physicochemical and In Vitro Digestibility Properties of Tartary Buckwheat Starch-Rutin Complex Prepared by Pre-Gelatinization and Co-Gelatinization Methods. Foods 2023, 12, 951. https://doi.org/10.3390/foods12050951
Ding X, Li X, Cai Q, Ma Z, Ren T, Hu X. Effect of Calcium Hydroxide on Physicochemical and In Vitro Digestibility Properties of Tartary Buckwheat Starch-Rutin Complex Prepared by Pre-Gelatinization and Co-Gelatinization Methods. Foods. 2023; 12(5):951. https://doi.org/10.3390/foods12050951
Chicago/Turabian StyleDing, Xinxin, Xiaoping Li, Qiling Cai, Zhen Ma, Tian Ren, and Xinzhong Hu. 2023. "Effect of Calcium Hydroxide on Physicochemical and In Vitro Digestibility Properties of Tartary Buckwheat Starch-Rutin Complex Prepared by Pre-Gelatinization and Co-Gelatinization Methods" Foods 12, no. 5: 951. https://doi.org/10.3390/foods12050951
APA StyleDing, X., Li, X., Cai, Q., Ma, Z., Ren, T., & Hu, X. (2023). Effect of Calcium Hydroxide on Physicochemical and In Vitro Digestibility Properties of Tartary Buckwheat Starch-Rutin Complex Prepared by Pre-Gelatinization and Co-Gelatinization Methods. Foods, 12(5), 951. https://doi.org/10.3390/foods12050951






