Polyphenol-Dietary Fiber Conjugates from Fruits and Vegetables: Nature and Biological Fate in a Food and Nutrition Perspective
Abstract
1. Introduction
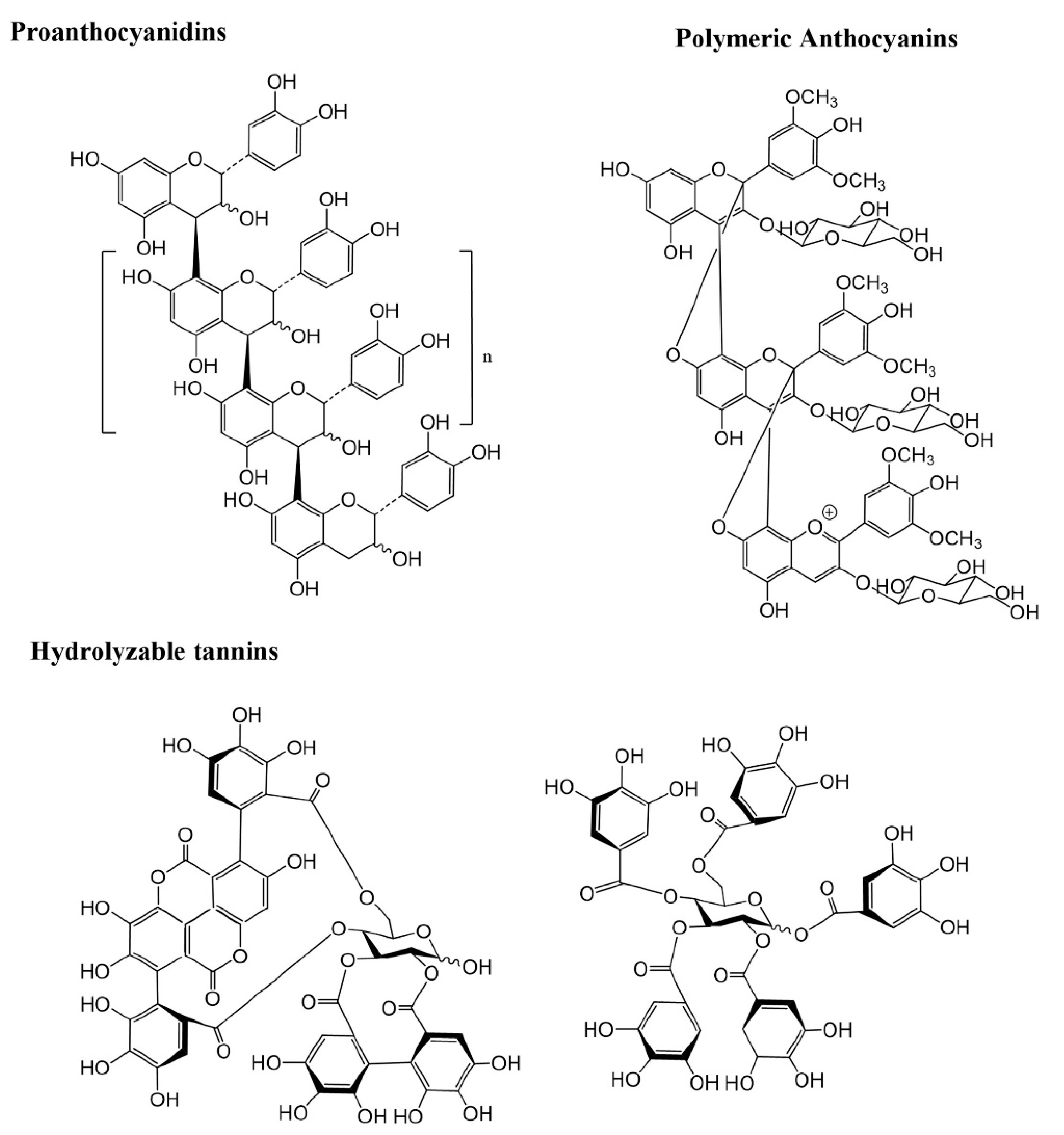
2. Polyphenols
3. Extractable and Non-Extractable Polyphenols
3.1. Types and Content of Non-Extractable Polyphenols in Foods
3.2. Release of Non-Extractable Polyphenols from Dietary Fibers
4. Biological Activity of Non-Extractable Polyphenol-Dietary Fibers
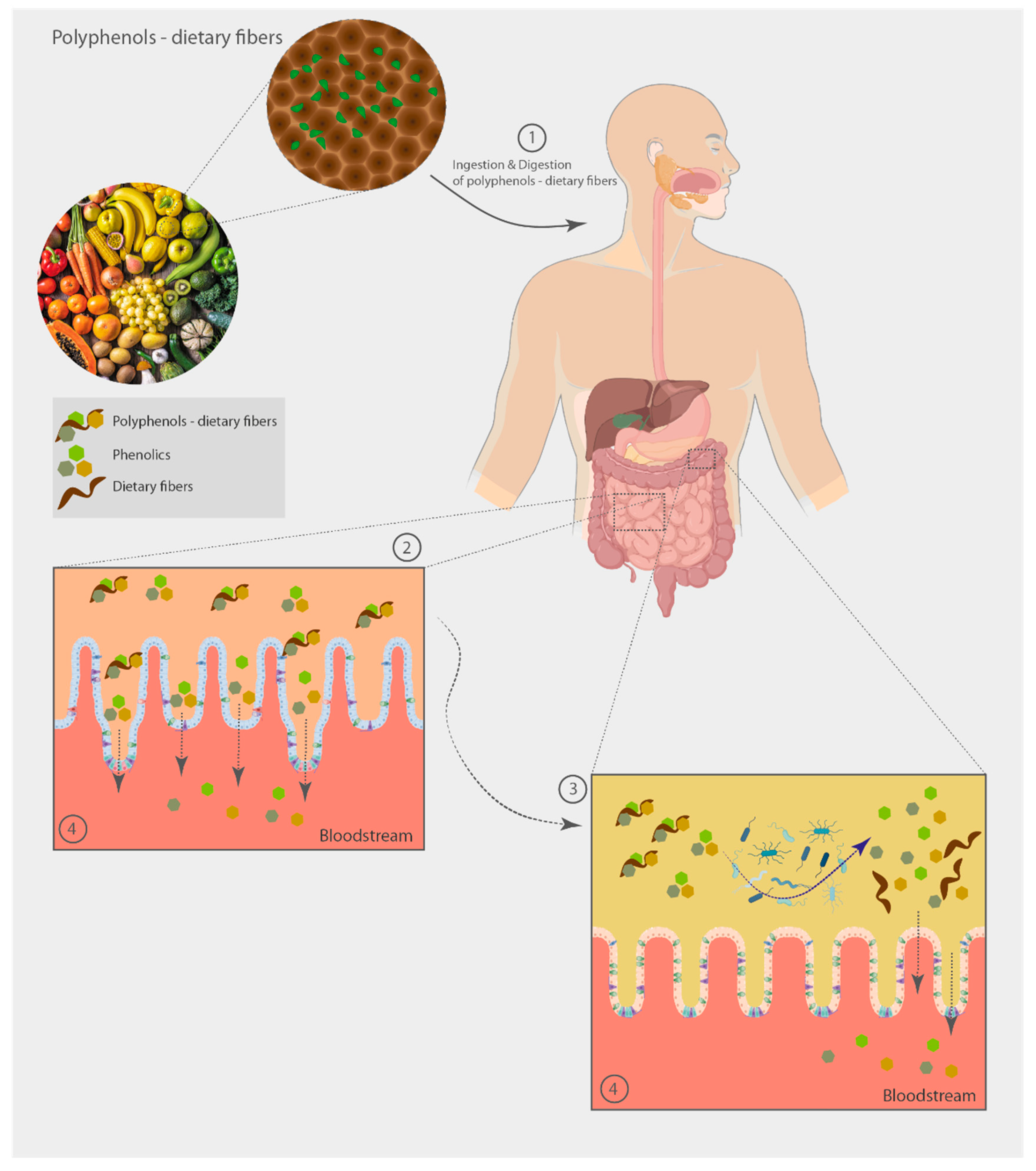
5. Non-Extractable Polyphenol-Dietary Fiber Fate through the Human Gut
6. Non-Extractable Polyphenol-Dietary Fibers: A Potential Functional Ingredient
7. Non-Extractable Polyphenol-Dietary Fibers: An Approach in Food Industry and Innovation Potential
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pérez-Jiménez, J.; Saura-Calixto, F. Macromolecular antioxidants or non-extractable polyphenols in fruit and vegetables: Intake in four European countries. Food Res. Int. 2015, 74, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Koch, W. Dietary Polyphenols-Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; Pereira, C.B.; Pérez-Gregorio, R.; Mateus, N.; Freitas, V. Recent advances on dietary polyphenol’s potential roles in Celiac Disease. Trends Food Sci. Technol. 2020, 107, 213–225. [Google Scholar] [CrossRef]
- Arranz, S.; Saura-Calixto, F.; Shaha, S.; Kroon, P.A. High contents of nonextractable polyphenols in fruits suggest that polyphenol contents of plant foods have been underestimated. J. Agric. Food Chem. 2009, 57, 7298–7303. [Google Scholar] [CrossRef]
- Li, Y.; Qin, C.; Dong, L.; Zhang, X.; Wu, Z.; Liu, L.; Yang, J.O.; Liu, L. Whole grain benefit: Synergistic effect of oat phenolic compounds and β-glucan on hyperlipidemia via gut microbiota in high-fat-diet mice. Food Funct. 2022, 13, 12686–12696. [Google Scholar] [CrossRef]
- Dong, L.; Qin, C.; Li, Y.; Wu, Z.; Liu, L. Oat phenolic compounds regulate metabolic syndrome in high fat diet-fed mice via gut microbiota. Food Biosci. 2022, 50, 101946. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Concept and health-related properties of nonextractable polyphenols: The missing dietary polyphenols. J. Agric. Food Chem. 2012, 60, 11195–11200. [Google Scholar] [CrossRef]
- Serpen, A.; Capuano, E.; Fogliano, V.; Gökmen, V. A New Procedure To Measure the Antioxidant Activity of Insoluble Food Components. J. Agric. Food Chem. 2007, 55, 7676–7681. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Motilva, M.J. Metabolic and Microbial Modulation of the Large Intestine Ecosystem by Non-Absorbed Diet Phenolic Compounds: A Review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef]
- Quatrin, A.; Pauletto, R.; Maurer, L.H.; Minuzzi, N.; Nichelle, S.M.; Carvalho, J.F.C.; Maróstica, M.R.; Rodrigues, E.; Bochi, V.C.; Emanuelli, T. Characterization and quantification of tannins, flavonols, anthocyanins and matrix-bound polyphenols from jaboticaba fruit peel: A comparison between Myrciaria trunciflora and M. jaboticaba. J. Food Compos. Anal. 2019, 78, 59–74. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Non-extractable polyphenols, a major dietary antioxidant: Occurrence, metabolic fate and health effects. Nutr. Res. Rev. 2013, 26, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Saeed Ur, R.; Bilal, M.; Huang, D.-f. Role of flavonoids in plant interactions with the environment and against human pathogens—A review. J. Integr. Agric. 2019, 18, 211–230. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- Soto-Vaca, A.; Gutierrez, A.; Losso, J.N.; Xu, Z.; Finley, J.W. Evolution of Phenolic Compounds from Color and Flavor Problems to Health Benefits. J. Agric. Food Chem. 2012, 60, 6658–6677. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Pérez-Gregorio, R.; Soares, S.; Mateus, N.; de Freitas, V. Bioactive Peptides and Dietary Polyphenols: Two Sides of the Same Coin. Molecules 2020, 25, 3443. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, L.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Heimler, D.; Romani, A.; Ieri, F. Plant polyphenol content, soil fertilization and agricultural management: A review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Hellström, J.K.; Törrönen, A.R.; Mattila, P.H. Proanthocyanidins in Common Food Products of Plant Origin. J. Agric. Food Chem. 2009, 57, 7899–7906. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, S.; Ge, S.; Lin, S. Review of Distribution, Extraction Methods, and Health Benefits of Bound Phenolics in Food Plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Li, H.; Deng, Z.; Tsao, R. A review on insoluble-bound phenolics in plant-based food matrix and their contribution to human health with future perspectives. Trends Food Sci. Technol. 2020, 105, 347–362. [Google Scholar] [CrossRef]
- Beckman, C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Torres, J.L. Analysis of nonextractable phenolic compounds in foods: The current state of the art. J. Agric. Food Chem. 2011, 59, 12713–12724. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J.D. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic Compounds and Their Role in Disease Resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Jansen, M.A.; van den Noort, R.E.; Tan, M.Y.; Prinsen, E.; Lagrimini, L.M.; Thorneley, R.N. Phenol-oxidizing peroxidases contribute to the protection of plants from ultraviolet radiation stress. Plant Physiol 2001, 126, 1012–1023. [Google Scholar] [CrossRef]
- Rombouts, F.M.; Thibault, J.-F. Feruloylated pectic substances from sugar-beet pulp. Carbohydr. Res. 1986, 154, 177–187. [Google Scholar] [CrossRef]
- Fry, S.C. Feruloylated pectins from the primary cell wall: Their structure and possible functions. Planta 1983, 157, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Tuyet Lam, T.B.; Iiyama, K.; Stone, B.A. Cinnamic acid bridges between cell wall polymers in wheat and phalaris internodes. Phytochemistry 1992, 31, 1179–1183. [Google Scholar] [CrossRef]
- Ishii, T. Isolation and characterization of a diferuloyl arabinoxylan hexasaccharide from bamboo shoot cell-walls. Carbohydr. Res. 1991, 219, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Grabber, J.H.; Hatfield, R.D.; Ralph, J.; Zoń, J.; Amrhein, N. Ferulate cross-linking in cell walls isolated from maize cell suspensions. Phytochemistry 1995, 40, 1077–1082. [Google Scholar] [CrossRef]
- Eran Nagar, E.; Okun, Z.; Shpigelman, A. Digestive fate of polyphenols: Updated view of the influence of chemical structure and the presence of cell wall material. Curr. Opin. Food Sci. 2020, 31, 38–46. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Le Bourvellec, C.; Renard, C.M.G.C.; Nunes, F.M.; Bastos, R.; Coelho, E.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Revisiting the chemistry of apple pomace polyphenols. Food Chem. 2019, 294, 9–18. [Google Scholar] [CrossRef]
- Ferreira, D.; Guyot, S.; Marnet, N.; Delgadillo, I.; Renard, C.M.; Coimbra, M.A. Composition of phenolic compounds in a Portuguese pear (Pyrus communis L. var. S. Bartolomeu) and changes after sun-drying. J. Agric. Food Chem. 2002, 50, 4537–4544. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Interactions between apple (Malus x domestica Borkh.) polyphenols and cell walls modulate the extractability of polysaccharides. Carbohydr. Polym. 2009, 75, 251–261. [Google Scholar] [CrossRef]
- Liu, X.; Le Bourvellec, C.; Renard, C.M.G.C. Interactions between cell wall polysaccharides and polyphenols: Effect of molecular internal structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Dietary Fiber as a Carrier of Dietary Antioxidants: An Essential Physiological Function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.M.G.C.; Watrelot, A.A.; Le Bourvellec, C. Interactions between polyphenols and polysaccharides: Mechanisms and consequences in food processing and digestion. Trends Food Sci. Technol. 2017, 60, 43–51. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chu, Y.F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Saura-Calixto, F. Fruit peels as sources of non-extractable polyphenols or macromolecular antioxidants: Analysis and nutritional implications. Food Res. Int. 2018, 111, 148–152. [Google Scholar] [CrossRef]
- Chu, Y.F.; Sun, J.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef] [PubMed]
- Peričin, D.; Krimer, V.; Trivić, S.; Radulović, L. The distribution of phenolic acids in pumpkin’s hull-less seed, skin, oil cake meal, dehulled kernel and hull. Food Chem. 2009, 113, 450–456. [Google Scholar] [CrossRef]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. The distribution of phenolic acids in rice. Food Chem. 2004, 87, 401–406. [Google Scholar] [CrossRef]
- White, B.L.; Howard, L.R.; Prior, R.L. Release of Bound Procyanidins from Cranberry Pomace by Alkaline Hydrolysis. J. Agric. Food Chem. 2010, 58, 7572–7579. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiang, J.; Zheng, B.; Sun, J.; Luo, D.; Li, P.; Fan, J. Diversity of phenolics including hydroxycinnamic acid amide derivatives, phenolic acids contribute to antioxidant properties of proso millet. LWT 2022, 154, 112611. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Silva, A.M.S.; Evtuguin, D.V.; Nunes, F.M.; Wessel, D.F.; Cardoso, S.M.; Coimbra, M.A. The hydrophobic polysaccharides of apple pomace. Carbohydr. Polym. 2019, 223, 115132. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Tomás-Barberán, F.A.; Espín, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tsao, R.; Yang, R.; Liu, C.; Zhu, H.; Young, J.C. Polyphenolic Profiles and Antioxidant Activities of Heartnut (Juglans ailanthifolia Var. cordiformis) and Persian Walnut (Juglans regia L.). J. Agric. Food Chem. 2006, 54, 8033–8040. [Google Scholar] [CrossRef]
- John, J.A.; Shahidi, F. Phenolic compounds and antioxidant activity of Brazil nut (Bertholletia excelsa). J. Funct. Foods 2010, 2, 196–209. [Google Scholar] [CrossRef]
- Kapasakalidis, P.G.; Rastall, R.A.; Gordon, M.H. Effect of a cellulase treatment on extraction of antioxidant phenols from black currant (Ribes nigrum L.) pomace. J. Agric. Food Chem. 2009, 57, 4342–4351. [Google Scholar] [CrossRef]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, M.J. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Díaz-Rubio, M.E.; Saura-Calixto, F. Dietary Fiber Complex in Beer. J. Am. Soc. Brew. Chem. 2009, 67, 38–43. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Arranz, S.; Serrano, J.; Goñi, I. Dietary fiber content and associated antioxidant compounds in Roselle flower (Hibiscus sabdariffa L.) beverage. J. Agric. Food Chem. 2007, 55, 7886–7890. [Google Scholar] [CrossRef]
- Chen, G.-L.; Zhang, X.; Chen, S.-G.; Han, M.-D.; Gao, Y.-Q. Antioxidant activities and contents of free, esterified and insoluble-bound phenolics in 14 subtropical fruit leaves collected from the south of China. J. Funct. Foods 2017, 30, 290–302. [Google Scholar] [CrossRef]
- Drossard, C.; Fröhling, B.; Dietrich, H.; Kersting, M. Anthocyanin analysis in banana fruit-a mistake. Am. J. Clin. Nutr. 2011, 93, 865–866. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Kay, C.; Rimm, E.B. Reply to C Drossard et al. Am. J. Clin. Nutr. 2011, 93, 866–867. [Google Scholar] [CrossRef]
- Campos, F.; Peixoto, A.F.; Fernandes, P.A.R.; Coimbra, M.A.; Mateus, N.; de Freitas, V.; Fernandes, I.; Fernandes, A. The Antidiabetic Effect of Grape Pomace Polysaccharide-Polyphenol Complexes. Nutrients 2021, 13, 4495. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.; Ribeiro, M.; Cruz, A.C.; Domingues, M.R.; Coimbra, M.A.; Bunzel, M.; Nunes, F.M. Nature of phenolic compounds in coffee melanoidins. J. Agric. Food Chem. 2014, 62, 7843–7853. [Google Scholar] [CrossRef]
- Ding, Y.; Morozova, K.; Scampicchio, M.; Ferrentino, G. Non-Extractable Polyphenols from Food By-Products: Current Knowledge on Recovery, Characterisation, and Potential Applications. Processes 2020, 8, 925. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Jokić, S.; Cvjetko, M.; Božić, Đ.; Fabek, S.; Toth, N.; Vorkapić-Furač, J.; Redovniković, I.R. Optimisation of microwave-assisted extraction of phenolic compounds from broccoli and its antioxidant activity. Int. J. Food Sci. Technol. 2012, 47, 2613–2619. [Google Scholar] [CrossRef]
- Herrera, M.C.; Luque de Castro, M.D. Ultrasound-assisted extraction for the analysis of phenolic compounds in strawberries. Anal. Bioanal. Chem. 2004, 379, 1106–1112. [Google Scholar] [CrossRef]
- Buranov, A.U.; Mazza, G. Extraction and purification of ferulic acid from flax shives, wheat and corn bran by alkaline hydrolysis and pressurised solvents. Food Chem. 2009, 115, 1542–1548. [Google Scholar] [CrossRef]
- Đurović, S.; Nikolić, B.; Luković, N.; Jovanović, J.; Stefanović, A.; Šekuljica, N.; Mijin, D.; Knežević-Jugović, Z. The impact of high-power ultrasound and microwave on the phenolic acid profile and antioxidant activity of the extract from yellow soybean seeds. Ind. Crops Prod. 2018, 122, 223–231. [Google Scholar] [CrossRef]
- Chen, X.; Sun, K.; Zhuang, K.; Ding, W. Comparison and Optimization of Different Extraction Methods of Bound Phenolics from Jizi439 Black Wheat Bran. Foods 2022, 11, 1478. [Google Scholar] [CrossRef] [PubMed]
- Wanyo, P.; Meeso, N.; Siriamornpun, S. Effects of different treatments on the antioxidant properties and phenolic compounds of rice bran and rice husk. Food Chem. 2014, 157, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Lohani, U.C.; Muthukumarappan, K. Application of the pulsed electric field to release bound phenolics in sorghum flour and apple pomace. Innov. Food Sci. Emerg. Technol. 2016, 35, 29–35. [Google Scholar] [CrossRef]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.Z.; Hwang, I.W.; Chung, S.K. Enhancing polyphenol extraction from unripe apples by carbohydrate-hydrolyzing enzymes. J. Zhejiang Univ. Sci. B 2009, 10, 912–919. [Google Scholar] [CrossRef]
- Landbo, A.K.; Meyer, A.S. Enzyme-assisted extraction of antioxidative phenols from black currant juice press residues (Ribes nigrum). J. Agric. Food Chem. 2001, 49, 3169–3177. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Saura-Calixto, F. Proanthocyanidin content in foods is largely underestimated in the literature data: An approach to quantification of the missing proanthocyanidins. Food Res. Int. 2009, 42, 1381–1388. [Google Scholar] [CrossRef]
- Mencin, M.; Jamnik, P.; Mikulič Petkovšek, M.; Veberič, R.; Terpinc, P. Enzymatic treatments of raw, germinated and fermented spelt (Triticum spelta L.) seeds improve the accessibility and antioxidant activity of their phenolics. LWT 2022, 169, 114046. [Google Scholar] [CrossRef]
- Abdel-Aty, A.M.; Barakat, A.Z.; Bassuiny, R.I.; Mohamed, S.A. Improved production of antioxidant-phenolic compounds and certain fungal phenolic-associated enzymes under solid-state fermentation of chia seeds with Trichoderma reesei: Response surface methodology-based optimization. J. Food Meas. Charact. 2022, 16, 3488–3500. [Google Scholar] [CrossRef]
- Abdel-Aty, A.M.; Bassuiny, R.I.; Barakat, A.Z.; Mohamed, S.A. Upgrading the phenolic content, antioxidant and antimicrobial activities of garden cress seeds using solid-state fermentation by Trichoderma reesei. J. Appl. Microbiol. 2019, 127, 1454–1467. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Adebiyi, J.A.; Kayitesi, E. Co-influence of fermentation time and temperature on physicochemical properties, bioactive components and microstructure of ting (a Southern African food) from whole grain sorghum. Food Biosci. 2018, 25, 118–127. [Google Scholar] [CrossRef]
- Ti, H.; Zhang, R.; Zhang, M.; Li, Q.; Wei, Z.; Zhang, Y.; Tang, X.; Deng, Y.; Liu, L.; Ma, Y. Dynamic changes in the free and bound phenolic compounds and antioxidant activity of brown rice at different germination stages. Food Chem. 2014, 161, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.D.; Shahidi, F. Critical Evaluation of Changes in the Ratio of Insoluble Bound to Soluble Phenolics on Antioxidant Activity of Lentils during Germination. J. Agric. Food Chem. 2015, 63, 379–381. [Google Scholar] [CrossRef]
- Miyahira, R.F.; Lopes, J.O.; Antunes, A.E.C. The Use of Sprouts to Improve the Nutritional Value of Food Products: A Brief Review. Plant Foods Hum. Nutr. 2021, 76, 143–152. [Google Scholar] [CrossRef]
- Xiang, Z.; Deng, J.; Yang, K.; Zhu, Y.; Xia, C.; Chen, J.; Liu, T. Effect of processing on the release of phenolic compounds and antioxidant activity during in vitro digestion of hulless barley. Arab. J. Chem. 2021, 14, 103447. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Y.; Dong, L.; Jia, X.; Liu, L.; Huang, F.N.; Chi, J.; Xiao, J.; Zhang, M.; Zhang, R. Extrusion and fungal fermentation change the profile and antioxidant activity of free and bound phenolics in rice bran together with the phenolic bioaccessibility. LWT 2019, 115, 108461. [Google Scholar] [CrossRef]
- Ee, K.Y.; Agboola, S.; Rehman, A.; Zhao, J. Characterisation of phenolic components present in raw and roasted wattle (Acacia victoriae Bentham) seeds. Food Chem. 2011, 129, 816–821. [Google Scholar] [CrossRef]
- Ragaee, S.; Seetharaman, K.; Abdel-Aal, E.-S.M. The Impact of Milling and Thermal Processing on Phenolic Compounds in Cereal Grains. Crit. Rev. Food Sci. Nutr. 2014, 54, 837–849. [Google Scholar] [CrossRef]
- Cardoso, L.d.M.; Pinheiro, S.S.; de Carvalho, C.W.P.; Queiroz, V.A.V.; de Menezes, C.B.; Moreira, A.V.B.; de Barros, F.A.R.; Awika, J.M.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Phenolic compounds profile in sorghum processed by extrusion cooking and dry heat in a conventional oven. J. Cereal Sci. 2015, 65, 220–226. [Google Scholar] [CrossRef]
- Burgos, G.; Amoros, W.; Muñoa, L.; Sosa, P.; Cayhualla, E.; Sanchez, C.; Díaz, C.; Bonierbale, M. Total phenolic, total anthocyanin and phenolic acid concentrations and antioxidant activity of purple-fleshed potatoes as affected by boiling. J. Food Compos. Anal. 2013, 30, 6–12. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Yeo, J.D.; Shahidi, F. Effect of hydrothermal processing on changes of insoluble-bound phenolics of lentils. J. Funct. Foods 2017, 38, 716–722. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; López-Pacheco, F.; Alvarez, M.M.; Serna-Saldívar, S.O. In vivo anti-inflammatory effects of isorhamnetin glycosides isolated from Opuntia ficus-indica (L.) Mill cladodes. Ind. Crops Prod. 2015, 76, 803–808. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, B.; Li, X.; Chen, P.X.; Zhang, H.; Liu, R.H.; Tsao, R. Bound Phenolics of Quinoa Seeds Released by Acid, Alkaline, and Enzymatic Treatments and Their Antioxidant and α-Glucosidase and Pancreatic Lipase Inhibitory Effects. J. Agric. Food Chem. 2016, 64, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Ricardo, M.; Moreno-García, B.E.; Gutiérrez-Uribe, J.A.; Aráiz-Hernández, D.; Alvarez, M.M.; Serna-Saldivar, S.O. Induction of Apoptosis in Colon Cancer Cells Treated with Isorhamnetin Glycosides from Opuntia Ficus-indica Pads. Plant Foods Hum. Nutr. 2014, 69, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, X.; Hu, X.; Li, T.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.). Food Chem. 2017, 217, 773–781. [Google Scholar] [CrossRef]
- Eitsuka, T.; Tatewaki, N.; Nishida, H.; Kurata, T.; Nakagawa, K.; Miyazawa, T. Synergistic inhibition of cancer cell proliferation with a combination of δ-tocotrienol and ferulic acid. Biochem. Biophys. Res. Commun. 2014, 453, 606–611. [Google Scholar] [CrossRef]
- Liao, W.; Chen, L.; Ma, X.; Jiao, R.; Li, X.; Wang, Y.-M. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur. J. Med. Chem. 2016, 114, 24–32. [Google Scholar] [CrossRef]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid regulates hepatic GLUT2 gene expression in high fat and fructose-induced type-2 diabetic adult male rat. Eur. J. Pharmacol. 2015, 761, 391–397. [Google Scholar] [CrossRef]
- Papillo, V.A.; Vitaglione, P.; Graziani, G.; Gokmen, V.; Fogliano, V. Release of antioxidant capacity from five plant foods during a multistep enzymatic digestion protocol. J. Agric. Food Chem. 2014, 62, 4119–4126. [Google Scholar] [CrossRef]
- Cui, J.; Lian, Y.; Zhao, C.; Du, H.; Han, Y.; Gao, W.; Xiao, H.; Zheng, J. Dietary Fibers from Fruits and Vegetables and Their Health Benefits via Modulation of Gut Microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1514–1532. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.D.T.; Williams, B.A.; Netzel, G.; Mikkelsen, D.; D’Arcy, B.R.; Gidley, M.J. Independent fermentation and metabolism of dietary polyphenols associated with a plant cell wall model. Food Funct. 2020, 11, 2218–2230. [Google Scholar] [CrossRef] [PubMed]
- Tarko, T.; Duda-Chodak, A. Influence of Food Matrix on the Bioaccessibility of Fruit Polyphenolic Compounds. J. Agric. Food Chem. 2020, 68, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Bagano Vilas Boas, P.; Lepercq, P.; Comtet-Marre, S.; Auffret, P.; Ruiz, P.; Bott, R.; Renard, C.M.G.C.; Dufour, C.; Chatel, J.-M.; et al. Procyanidin—Cell Wall Interactions within Apple Matrices Decrease the Metabolization of Procyanidins by the Human Gut Microbiota and the Anti-Inflammatory Effect of the Resulting Microbial Metabolome In Vitro. Nutrients 2019, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef]
- Terry, P.; Giovannucci, E.; Michels, K.B.; Bergkvist, L.; Hansen, H.; Holmberg, L.; Wolk, A. Fruit, Vegetables, Dietary Fiber, and Risk of Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2001, 93, 525–533. [Google Scholar] [CrossRef]
- Swann, O.G.; Kilpatrick, M.; Breslin, M.; Oddy, W.H. Dietary fiber and its associations with depression and inflammation. Nutr. Rev. 2020, 78, 394–411. [Google Scholar] [CrossRef]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef]
- Gökmen, V.; Serpen, A.; Fogliano, V. Direct measurement of the total antioxidant capacity of foods: The ‘QUENCHER’ approach. Trends Food Sci. Technol. 2009, 20, 278–288. [Google Scholar] [CrossRef]
- Tufan, A.N.; Celik, S.E.; Ozyürek, M.; Güçlü, K.; Apak, R. Direct measurement of total antioxidant capacity of cereals: QUENCHER-CUPRAC method. Talanta 2013, 108, 136–142. [Google Scholar] [CrossRef]
- Cömert, E.D.; Gökmen, V. Antioxidants Bound to an Insoluble Food Matrix: Their Analysis, Regeneration Behavior, and Physiological Importance. Compr. Rev. Food Sci. Food Saf. 2017, 16, 382–399. [Google Scholar] [CrossRef]
- Abderrahim, F.; Huanatico, E.; Segura, R.; Arribas, S.; Gonzalez, M.C.; Condezo-Hoyos, L. Physical features, phenolic compounds, betalains and total antioxidant capacity of coloured quinoa seeds (Chenopodium quinoa Willd.) from Peruvian Altiplano. Food Chem. 2015, 183, 83–90. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Condezo-Hoyos, L.; Abderrahim, F.; Arriba, S.M.; Carmen González, M. A novel, micro, rapid and direct assay to assess total antioxidant capacity of solid foods. Talanta 2015, 138, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Del Pino-García, R.; García-Lomillo, J.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. Adaptation and Validation of QUick, Easy, New, CHEap, and Reproducible (QUENCHER) Antioxidant Capacity Assays in Model Products Obtained from Residual Wine Pomace. J. Agric. Food Chem. 2015, 63, 6922–6931. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Çelik, E.E.; Gökmen, V.; Fogliano, V. Soluble Antioxidant Compounds Regenerate the Antioxidants Bound to Insoluble Parts of Foods. J. Agric. Food Chem. 2013, 61, 10329–10334. [Google Scholar] [CrossRef]
- Çelik, E.E.; Gökmen, V. Investigation of the interaction between soluble antioxidants in green tea and insoluble dietary fiber bound antioxidants. Food Res. Int. 2014, 63, 266–270. [Google Scholar] [CrossRef]
- Kosmala, M.; Zduńczyk, Z.; Karlińska, E.; Juśkiewicz, J. The effects of strawberry, black currant, and chokeberry extracts in a grain dietary fiber matrix on intestinal fermentation in rats. Food Res. Int. 2014, 64, 752–761. [Google Scholar] [CrossRef]
- Gorjanović, S.; Micić, D.; Pastor, F.; Tosti, T.; Kalušević, A.; Ristić, S.; Zlatanović, S. Evaluation of Apple Pomace Flour Obtained Industrially by Dehydration as a Source of Biomolecules with Antioxidant, Antidiabetic and Antiobesity Effects. Antioxidants 2020, 9, 413. [Google Scholar] [CrossRef]
- Urquiaga, I.; D’Acuña, S.; Pérez, D.; Dicenta, S.; Echeverría, G.; Rigotti, A.; Leighton, F. Wine grape pomace flour improves blood pressure, fasting glucose and protein damage in humans: A randomized controlled trial. Biol. Res. 2015, 48, 49. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; Troncoso, D.; Mackenna, M.J.; Urzúa, C.; Pérez, D.; Dicenta, S.; De la Cerda, P.M.; Amigo, L.; Carreño, J.C.; Echeverría, G.; et al. The Consumption of Beef Burgers Prepared with Wine Grape Pomace Flour Improves Fasting Glucose, Plasma Antioxidant Levels, and Oxidative Damage Markers in Humans: A Controlled Trial. Nutrients 2018, 10, 1388. [Google Scholar] [CrossRef] [PubMed]
- Rivera, K.; Salas-Pérez, F.; Echeverría, G.; Urquiaga, I.; Dicenta, S.; Pérez, D.; de la Cerda, P.; González, L.; Andia, M.E.; Uribe, S.; et al. Red Wine Grape Pomace Attenuates Atherosclerosis and Myocardial Damage and Increases Survival in Association with Improved Plasma Antioxidant Activity in a Murine Model of Lethal Ischemic Heart Disease. Nutrients 2019, 11, 2135. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Egashira, Y.; Sanada, H. Digestion and absorption of ferulic acid sugar esters in rat gastrointestinal tract. J. Agric. Food Chem. 2003, 51, 5534–5539. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, M.F.; Kroon, P.A.; Williamson, G.; Garcia-Conesa, M.-T. Esterase Activity Able To Hydrolyze Dietary Antioxidant Hydroxycinnamates Is Distributed along the Intestine of Mammals. J. Agric. Food Chem. 2001, 49, 5679–5684. [Google Scholar] [CrossRef]
- Kroon, P.A.; Faulds, C.B.; Ryden, P.; Robertson, J.A.; Williamson, G. Release of Covalently Bound Ferulic Acid from Fiber in the Human Colon. J. Agric. Food Chem. 1997, 45, 661–667. [Google Scholar] [CrossRef]
- Andreasen, M.F.; Kroon, P.A.; Williamson, G.; Garcia-Conesa, M.T. Intestinal release and uptake of phenolic antioxidant diferulic acids. Free Radic. Biol. Med. 2001, 31, 304–314. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Pérez-Jiménez, J.; Touriño, S.; Serrano, J.; Fuguet, E.; Torres, J.L.; Goñi, I. Proanthocyanidin metabolites associated with dietary fibre from in vitro colonic fermentation and proanthocyanidin metabolites in human plasma. Mol. Nutr. Food Res. 2010, 54, 939–946. [Google Scholar] [CrossRef]
- Yi, W.; Fischer, J.; Krewer, G.; Akoh, C.C. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J. Agric. Food Chem. 2005, 53, 7320–7329. [Google Scholar] [CrossRef]
- Touriño, S.; Fuguet, E.; Vinardell, M.P.; Cascante, M.; Torres, J.L. Phenolic Metabolites of Grape Antioxidant Dietary Fiber in Rat Urine. J. Agric. Food Chem. 2009, 57, 11418–11426. [Google Scholar] [CrossRef]
- Han, F.; Yang, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends Food Sci. Technol. 2019, 83, 211–224. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Van Rymenant, E.; Abrankó, L.; Tumova, S.; Grootaert, C.; Van Camp, J.; Williamson, G.; Kerimi, A. Chronic exposure to short-chain fatty acids modulates transport and metabolism of microbiome-derived phenolics in human intestinal cells. J. Nutr. Biochem. 2017, 39, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Rondini, L.; Peyrat-Maillard, M.N.; Marsset-Baglieri, A.; Fromentin, G.; Durand, P.; Tomé, D.; Prost, M.; Berset, C. Bound ferulic acid from bran is more bioavailable than the free compound in rat. J. Agric. Food Chem. 2004, 52, 4338–4343. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Espín, J.C.; Tomás-Barberán, F.A. Non-extractable polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical-scavenging effects. Trends Food Sci. Technol. 2017, 69, 281–288. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, L.; Liu, L.; Wu, Z.; Pan, D.; Liu, L. Recent Advances of Stimuli-Responsive Polysaccharide Hydrogels in Delivery Systems: A Review. J. Agric. Food Chem. 2022, 70, 6300–6316. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Matić, P. Non-covalent dietary fiber—Polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Veiga, M.; Costa, E.M.; Silva, S.; Pintado, M. Impact of plant extracts upon human health: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 873–886. [Google Scholar] [CrossRef]
- Holscher, H.D. Diet Affects the Gastrointestinal Microbiota and Health. J. Acad. Nutr. Diet. 2020, 120, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Yong, H.; Kan, J.; Jin, C. Recent advances in flavonoid-grafted polysaccharides: Synthesis, structural characterization, bioactivities and potential applications. Int. J. Biol. Macromol. 2018, 116, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, W.; Chen, Z.; Chen, H. Physicochemical characterization of the oolong tea polysaccharides with high molecular weight and their synergistic effects in combination with polyphenols on hepatocellular carcinoma. Biomed. Pharm. 2017, 90, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Quirós-Sauceda, A.E.; Palafox-Carlos, H.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Bello-Perez, L.A.; Alvarez-Parrilla, E.; de la Rosa, L.A.; González-Córdova, A.F.; González-Aguilar, G.A. Dietary fiber and phenolic compounds as functional ingredients: Interaction and possible effect after ingestion. Food Funct. 2014, 5, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.F.; Ooi, K.L. Antioxidant and α-Glucosidase Inhibitory Activities of 40 Tropical Juices from Malaysia and Identification of Phenolics from the Bioactive Fruit Juices of Barringtonia racemosa and Phyllanthus acidus. J. Agric. Food Chem. 2014, 62, 9576–9585. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.F.R.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic Compounds and Antimicrobial Activity of Olive (Olea europaea L. Cv. Cobrançosa) Leaves. Molecules 2007, 12, 1153–1162. [Google Scholar]
- Gowd, V.; Xie, L.; Zheng, X.; Chen, W. Dietary fibers as emerging nutritional factors against diabetes: Focus on the involvement of gut microbiota. Crit. Rev. Biotechnol. 2019, 39, 524–540. [Google Scholar] [CrossRef]
- Ahmad, M.; Wani, T.A.; Wani, S.M.; Masoodi, F.A.; Gani, A. Incorporation of carrot pomace powder in wheat flour: Effect on flour, dough and cookie characteristics. J. Food Sci. Technol. 2016, 53, 3715–3724. [Google Scholar] [CrossRef]
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M.J. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 57, 59–81. [Google Scholar] [CrossRef]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Madane, P.; Das, A.K.; Pateiro, M.; Nanda, P.K.; Bandyopadhyay, S.; Jagtap, P.; Barba, F.J.; Shewalkar, A.; Maity, B.; Lorenzo, J.M. Drumstick (Moringa oleifera) Flower as an Antioxidant Dietary Fibre in Chicken Meat Nuggets. Foods 2019, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Dobson, C.C.; Mottawea, W.; Rodrigue, A.; Buzati Pereira, B.L.; Hammami, R.; Power, K.A.; Bordenave, N. Chapter Three—Impact of molecular interactions with phenolic compounds on food polysaccharides functionality. In Advances in Food and Nutrition Research; Ferreira, I.C.F.R., Barros, L., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 90, pp. 135–181. [Google Scholar]
- Jin, W.; Xiang, L.; Peng, D.; Liu, G.-S.; He, J.; Cheng, S.; Li, B.; Huang, Q. Study on the coupling progress of thermo-induced anthocyanins degradation and polysaccharides gelation. Food Hydrocoll. 2020, 105, 105822. [Google Scholar] [CrossRef]
- Tudorache, M.; Bordenave, N. Phenolic compounds mediate aggregation of water-soluble polysaccharides and change their rheological properties: Effect of different phenolic compounds. Food Hydrocoll. 2019, 97, 105193. [Google Scholar] [CrossRef]
- Tudorache, M.; McDonald, J.-L.; Bordenave, N. Gallic acid reduces the viscosity and water binding capacity of soluble dietary fibers. Food Funct. 2020, 11, 5866–5874. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yao, F.; Zhang, M.; Khalifa, I.; Li, K.; Li, C. Effect of persimmon tannin on the physicochemical properties of maize starch with different amylose/amylopectin ratios. Int. J. Biol. Macromol. 2019, 132, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F. Antioxidant Dietary Fiber Product: A New Concept and a Potential Food Ingredient. J. Agric. Food Chem. 1998, 46, 4303–4306. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Madane, P.; Biswas, S.; Das, A.K.; Zhang, W.; Lorenzo, J.M. A comprehensive review on antioxidant dietary fibre enriched meat-based functional foods. Trends Food Sci. Technol. 2020, 99, 323–336. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Costa, C.M.; Bonifácio-Lopes, T.; Silva, S.; Veiga, M.; Monforte, A.; Nunes, J.; Vicente, A.A.; Pintado, M. Prebiotic effects of olive pomace powders in the gut: In vitro evaluation of the inhibition of adhesion of pathogens, prebiotic and antioxidant effects. Food Hydrocoll. 2021, 112, 106312. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.; Coelho, M.; Veiga, M.; Costa, E.M.; Silva, S.; Nunes, J.; Vicente, A.A.; Pintado, M. Are olive pomace powders a safe source of bioactives and nutrients? J. Sci. Food Agric. 2021, 101, 1963–1978. [Google Scholar] [CrossRef]
- Yapo, B.M.; Besson, V.; Koubala, B.B.; Koffi, K.L. Adding Value to Cacao Pod Husks as a Potential Antioxidant-Dietary Fiber Source. Am. J. Food Nutr. 2013, 1, 38–46. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Rincón, M.; Pulido, R.; Saura-Calixto, F. Guava fruit (Psidium guajava L.) as a new source of antioxidant dietary fiber. J. Agric. Food Chem. 2001, 49, 5489–5493. [Google Scholar] [CrossRef] [PubMed]
- Ajila, C.M.; Aalami, M.; Leelavathi, K.; Rao, U.J.S.P. Mango peel powder: A potential source of antioxidant and dietary fiber in macaroni preparations. Innov. Food Sci. Emerg. Technol. 2010, 11, 219–224. [Google Scholar] [CrossRef]
- Chantaro, P.; Devahastin, S.; Chiewchan, N. Production of antioxidant high dietary fiber powder from carrot peels. LWT-Food Sci. Technol. 2008, 41, 1987–1994. [Google Scholar] [CrossRef]
- Vergara-Valencia, N.; Granados-Pérez, E.; Agama-Acevedo, E.; Tovar, J.; Ruales, J.; Bello-Pérez, L.A. Fibre concentrate from mango fruit: Characterization, associated antioxidant capacity and application as a bakery product ingredient. LWT-Food Sci. Technol. 2007, 40, 722–729. [Google Scholar] [CrossRef]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Noor, S.A.A.; Siti, N.M.; Mahmad, N.J. Chemical Composition, Antioxidant Activity and Functional Properties of Mango (Mangifera indica L. var Perlis Sunshine) Peel Flour (MPF). Appl. Mech. Mater. 2015, 754–755, 1065–1070. [Google Scholar] [CrossRef]
- Malav, O.P.; Sharma, B.D.; Kumar, R.R.; Talukder, S.; Ahmed, S.R.; A, I. Antioxidant potential and quality characteristics of functional mutton patties incorporated with cabbage powder. Nutr. Food Sci. 2015, 45, 542–563. [Google Scholar] [CrossRef]
- Rufino, M.d.S.M.; Pérez-Jiménez, J.; Arranz, S.; Alves, R.E.; de Brito, E.S.; Oliveira, M.S.P.; Saura-Calixto, F. Açaí (Euterpe oleraceae) ‘BRS Pará’: A tropical fruit source of antioxidant dietary fiber and high antioxidant capacity oil. Food Res. Int. 2011, 44, 2100–2106. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Jiménez-Escrig, A.; Saura-Calixto, F.; Borderías, A.J. Effect of grape antioxidant dietary fibre on the prevention of lipid oxidation in minced fish: Evaluation by different methodologies. Food Chem. 2007, 101, 372–378. [Google Scholar] [CrossRef]
- Sudha, M.L.; Baskaran, V.; Leelavathi, K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- Tagliani, C.; Perez, C.; Curutchet, A.; Arcia, P.; Cozzano, S. Blueberry pomace, valorization of an industry by-product source of fibre with antioxidant capacity. Food Sci. Technol. 2019, 39, 644–651. [Google Scholar] [CrossRef]
- Salama, M.F.; Abozed, S.S.; Abozeid, W.M. Potentiality of Local Wastes as a Source of Natural Antioxidant Dietary Fibers on Dry Pasta. J. Biol. Sci. 2019, 19, 92–100. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Recovery of Phenolic Antioxidants and Functional Compounds from Coffee Industry By-Products. Food Bioprocess Technol. 2012, 5, 897–903. [Google Scholar] [CrossRef]
- Llobera, A.; Cañellas, J. Dietary fibre content and antioxidant activity of Manto Negro red grape (Vitis vinifera): Pomace and stem. Food Chem. 2007, 101, 659–666. [Google Scholar] [CrossRef]
- Garau, M.C.; Simal, S.; Rosselló, C.; Femenia, A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. Food Chem. 2007, 104, 1014–1024. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Sendra-Nadal, E.; Navarro, C.; Sayas, E.; Viuda-Martos, M.; Alvarez, J.A.P. Storage stability of a high dietary fibre powder from orange by-products. Int. J. Food Sci. Technol. 2009, 44, 748–756. [Google Scholar] [CrossRef]
- Agama-Acevedo, E.; Sañudo-Barajas, J.A.; Vélez De La Rocha, R.; González-Aguilar, G.A.; Bello-Peréz, L.A. Potential of plantain peels flour (Musa paradisiaca L.) as a source of dietary fiber and antioxidant compound. CyTA-J. Food 2016, 14, 117–123. [Google Scholar] [CrossRef]
- Polka, D.; Podsędek, A.; Koziołkiewicz, M. Comparison of Chemical Composition and Antioxidant Capacity of Fruit, Flower and Bark of Viburnum opulus. Plant Foods Hum. Nutr. 2019, 74, 436–442. [Google Scholar] [CrossRef]
- Zafra-Rojas, Q.; Cruz-Cansino, N.; Delgadillo-Ramírez, A.; Alanís-García, E.; Añorve-Morga, J.; Quintero-Lira, A.; Castañeda-Ovando, A.; Ramírez-Moreno, E. Organic Acids, Antioxidants, and Dietary Fiber of Mexican Blackberry (Rubus fruticosus) Residues cv. Tupy. J. Food Qual. 2018, 2018, 5950761. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chem. 2012, 135, 1520–1526. [Google Scholar] [CrossRef]
- Meena, L.; Neog, R.; Yashini, M.; Sunil, C.K. Pineapple pomace powder (freeze-dried): Effect on the texture and rheological properties of set-type yogurt. Food Chem. Adv. 2022, 1, 100101. [Google Scholar] [CrossRef]
- Nieto Calvache, J.; Cueto, M.; Farroni, A.; de Escalada Pla, M.; Gerschenson, L.N. Antioxidant characterization of new dietary fiber concentrates from papaya pulp and peel (Carica papaya L.). J. Funct. Foods 2016, 27, 319–328. [Google Scholar] [CrossRef]
- Ajila, C.M.; Leelavathi, K.; Prasada Rao, U.J.S. Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. J. Cereal Sci. 2008, 48, 319–326. [Google Scholar] [CrossRef]
- Santos, D.; Lopes da Silva, J.A.; Pintado, M. Fruit and vegetable by-products’ flours as ingredients: A review on production process, health benefits and technological functionalities. LWT 2022, 154, 112707. [Google Scholar] [CrossRef]
- Araújo-Rodrigues, H.; Santos, D.; Campos, D.A.; Ratinho, M.; M. Rodrigues, I.; E. Pintado, M. Development of Frozen Pulps and Powders from Carrot and Tomato by-Products: Impact of Processing and Storage Time on Bioactive and Biological Properties. Horticulturae 2021, 7, 185. [Google Scholar] [CrossRef]
- Campos, D.A.; Coscueta, E.R.; Valetti, N.W.; Pastrana-Castro, L.M.; Teixeira, J.A.; Picó, G.A.; Pintado, M.M. Optimization of bromelain isolation from pineapple byproducts by polysaccharide complex formation. Food Hydrocoll. 2019, 87, 792–804. [Google Scholar] [CrossRef]
- Liu, J.; Bai, R.; Liu, Y.; Zhang, X.; Kan, J.; Jin, C. Isolation, structural characterization and bioactivities of naturally occurring polysaccharide-polyphenolic conjugates from medicinal plants—A review. Int. J. Biol. Macromol. 2018, 107, 2242–2250. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Pawlaczyk-Graja, I. Polyphenolic-polysaccharide conjugates from flowers and fruits of single-seeded hawthorn (Crataegus monogyna Jacq.): Chemical profiles and mechanisms of anticoagulant activity. Int. J. Biol. Macromol. 2018, 116, 869–879. [Google Scholar] [CrossRef]
- Szejk, M.; Poplawski, T.; Czubatka-Bienkowska, A.; Olejnik, A.K.; Pawlaczyk-Graja, I.; Gancarz, R.; Zbikowska, H.M. A comparative study on the radioprotective potential of the polyphenolic glycoconjugates from medicinal plants of Rosaceae and Asteraceae families versus their aglycones. J. Photochem. Photobiol. B Biol. 2017, 171, 50–57. [Google Scholar] [CrossRef]
- Saluk-Juszczak, J.; Pawlaczyk, I.; Olas, B.; Kołodziejczyk, J.; Ponczek, M.; Nowak, P.; Tsirigotis-Wołoszczak, M.; Wachowicz, B.; Gancarz, R. The effect of polyphenolic-polysaccharide conjugates from selected medicinal plants of Asteraceae family on the peroxynitrite-induced changes in blood platelet proteins. Int. J. Biol. Macromol. 2010, 47, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk-Czepas, J.; Bijak, M.; Saluk, J.; Ponczek, M.B.; Zbikowska, H.M.; Nowak, P.; Tsirigotis-Maniecka, M.; Pawlaczyk, I. Radical scavenging and antioxidant effects of Matricaria chamomilla polyphenolic-polysaccharide conjugates. Int. J. Biol. Macromol. 2015, 72, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Saluk, J.; Tsirigotis-Maniecka, M.; Komorowska, H.; Wachowicz, B.; Zaczyńska, E.; Czarny, A.; Czechowski, F.; Nowak, P.; Pawlaczyk, I. The influence of conjugates isolated from Matricaria chamomilla L. on platelets activity and cytotoxicity. Int. J. Biol. Macromol. 2013, 61, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Šutovská, M.; Capek, P.; Fraňová, S.; Pawlaczyk, I.; Gancarz, R. Antitussive and bronchodilatory effects of Lythrum salicaria polysaccharide-polyphenolic conjugate. Int. J. Biol. Macromol. 2012, 51, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.A.; Godfrey, D.; Fukumoto, L. The chemical composition, antioxidant activity and α-glucosidase inhibitory activity of water-extractable polysaccharide conjugates from northern Manitoba lingonberry. Cogent Food Agric. 2015, 1, 1109781. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, L.; Jia, X.; Liu, L.; Chi, J.; Huang, F.N.; Ma, Q.; Zhang, M.; Zhang, R. Bound Phenolics Ensure the Antihyperglycemic Effect of Rice Bran Dietary Fiber in db/db Mice via Activating the Insulin Signaling Pathway in Skeletal Muscle and Altering Gut Microbiota. J. Agric. Food Chem. 2020, 68, 4387–4398. [Google Scholar] [CrossRef]
- Chen, G.-L.; Xie, M.; Wan, P.; Chen, D.; Dai, Z.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Fuzhuan Brick Tea Polysaccharides Attenuate Metabolic Syndrome in High-Fat Diet Induced Mice in Association with Modulation in the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 2783–2795. [Google Scholar] [CrossRef]
- Ho, T.C.; Kiddane, A.T.; Sivagnanam, S.P.; Park, J.-S.; Cho, Y.-J.; Getachew, A.T.; Nguyen, T.-T.T.; Kim, G.-D.; Chun, B.-S. Green extraction of polyphenolic-polysaccharide conjugates from Pseuderanthemum palatiferum (Nees) Radlk.: Chemical profile and anticoagulant activity. Int. J. Biol. Macromol. 2020, 157, 484–493. [Google Scholar] [CrossRef]
- Wu, D.-T.; Liu, W.; Xian, M.-L.; Du, G.; Liu, X.; He, J.-J.; Wang, P.; Qin, W.; Zhao, L. Polyphenolic-Protein-Polysaccharide Complexes from Hovenia dulcis: Insights into Extraction Methods on Their Physicochemical Properties and In Vitro Bioactivities. Foods 2020, 9, 456. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Panda, S.K.; Navitha, M.; Asha, K.K.; Anandan, R.; Mathew, S. Vanillic acid and coumaric acid grafted chitosan derivatives: Improved grafting ratio and potential application in functional food. J. Food Sci. Technol. 2015, 52, 7153–7162. [Google Scholar] [CrossRef]
- Wen, Y.; Ye, F.; Zhu, J.; Zhao, G. Corn starch ferulates with antioxidant properties prepared by N,N′-carbonyldiimidazole-mediated grafting procedure. Food Chem. 2016, 208, 1–9. [Google Scholar] [CrossRef]
- Cai, W.-D.; Zhu, J.; Wu, L.-X.; Qiao, Z.-R.; Li, L.; Yan, J.-K. Preparation, characterization, rheological and antioxidant properties of ferulic acid-grafted curdlan conjugates. Food Chem. 2019, 300, 125221. [Google Scholar] [CrossRef]
- Wang, C.-K.; Cai, W.-D.; Yao, J.; Wu, L.-X.; Li, L.; Zhu, J.; Yan, J.-K. Conjugation of ferulic acid onto pectin affected the physicochemical, functional and antioxidant properties. J. Sci. Food Agric. 2020, 100, 5352–5362. [Google Scholar] [CrossRef] [PubMed]
- Vuillemin, M.E.; Michaux, F.; Adam, A.A.; Linder, M.; Muniglia, L.; Jasniewski, J. Physicochemical characterizations of gum Arabic modified with oxidation products of ferulic acid. Food Hydrocoll. 2020, 107, 105919. [Google Scholar] [CrossRef]
- Ahn, S.; Halake, K.; Lee, J. Antioxidant and ion-induced gelation functions of pectins enabled by polyphenol conjugation. Int. J. Biol. Macromol. 2017, 101, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Puoci, F.; Iemma, F.; Curcio, M.; Parisi, O.I.; Spizzirri, U.G.; Altimari, I.; Picci, N. Starch-quercetin conjugate by radical grafting: Synthesis and biological characterization. Pharm. Dev. Technol. 2012, 17, 466–476. [Google Scholar] [CrossRef]
- Mundlia, J.; Ahuja, M.; Kumar, P.; Pillay, V. Improved antioxidant, antimicrobial and anticancer activity of naringenin on conjugation with pectin. 3 Biotech 2019, 9, 312. [Google Scholar] [CrossRef]
- Mundlia, J.; Ahuja, M.; Kumar, P. Enhanced biological activity of polyphenols on conjugation with gellan gum. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 712–729. [Google Scholar] [CrossRef]
- Jing, Y.; Huang, J.; Yu, X. Preparation, characterization, and functional evaluation of proanthocyanidin-chitosan conjugate. Carbohydr. Polym. 2018, 194, 139–145. [Google Scholar] [CrossRef]
- Guo, Q.; Xiao, X.; Lu, L.; Ai, L.; Xu, M.; Liu, Y.; Goff, H.D. Polyphenol-Polysaccharide Complex: Preparation, Characterization, and Potential Utilization in Food and Health. Annu. Rev. Food Sci. Technol. 2022, 13, 59–87. [Google Scholar] [CrossRef] [PubMed]
- Doğan, E.; Gökmen, V. Mechanism of the interaction between insoluble wheat bran and polyphenols leading to increased antioxidant capacity. Food Res. Int. 2015, 69, 189–193. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.L.; Costa, C.M.; Nunes, J.; Vicente, A.A.; Pintado, M. Total and Sustainable Valorisation of Olive Pomace Using a Fractionation Approach. Appl. Sci. 2020, 10, 6785. [Google Scholar] [CrossRef]
- Singh, J.P.; Kaur, A.; Singh, N. Development of eggless gluten-free rice muffins utilizing black carrot dietary fibre concentrate and xanthan gum. J. Food Sci. Technol. 2016, 53, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Föste, M.; Verheyen, C.; Jekle, M.; Becker, T. Fibres of milling and fruit processing by-products in gluten-free bread making: A review of hydration properties, dough formation and quality-improving strategies. Food Chem. 2020, 306, 125451. [Google Scholar] [CrossRef]
- Angulo-López, J.E.; Flores-Gallegos, A.C.; Ascacio-Valdes, J.A.; Contreras Esquivel, J.C.; Torres-León, C.; Rúelas-Chácon, X.; Aguilar, C.N. Antioxidant Dietary Fiber Sourced from Agroindustrial Byproducts and Its Applications. Foods 2023, 12, 159. [Google Scholar] [CrossRef]
- Quiles, A.; Campbell, G.M.; Struck, S.; Rohm, H.; Hernando, I. Fiber from fruit pomace: A review of applications in cereal-based products. Food Rev. Int. 2018, 34, 162–181. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.A.; Ahmed, A.R. Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Ann. Agric. Sci. 2013, 58, 83–95. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Wang, L.-J.; Huber, G.M.; Pitts, N.L. Effect of baking on dietary fibre and phenolics of muffins incorporated with apple skin powder. Food Chem. 2008, 107, 1217–1224. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT-Food Sci. Technol. 2016, 65, 978–986. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef]

| By-Product | Total Dietary Fiber | Total Phenolic Content | Reference |
|---|---|---|---|
| (%) | (mg GAE/100 g) | ||
| Mango (Mangifera indica L.) | 54.2 | 2170 (TPC) | [167] |
| peel flour | |||
| Cabbage powder | 36.7 | 322 (TAE) | [168] |
| Guava (Psidium guajava) peel | 48.55 | 5871 (TEP) | [162] |
| Guava (Psidium acutangulum) peel | 51.5 | 5870 (TEP) | [162] |
| Açaí (Euterpe oleraceae) pulp | 71.2 | 1500 (TEP) | [169] |
| Red grape pomace. | 74.0 | 5.63 (TPC) | [170] |
| Apple pomace | 51.1 | 1016 (TPC) | [171] |
| Blueberry pomace powder | 26.2 | 28.514 (TPC) | [172] |
| Carrot (Daucus carota) peels | 45.4 | 1371 (TPC) | [164] |
| Banana (peels) | 41.6 | 7168.5 (TPC) | [173] |
| Coffee (pulp, husk, silver, skin, | 28.0–80.0 | 1020–1480 (TPC) | |
| and spent coffee) | |||
| Grape (Vitis vinifera L.) pomace | 74.5 | 2630 (TEP) | [174] |
| Orange (Citrus aurantium) peel | 33.1–36.5 | 0.51 (TPC) | [175] |
| Orange by-product | 71.6 | 40.7 * | [176] |
| (albedo,flavedo, and pulp) | |||
| Plantain peel flour | 37.6 | 771 (TEP) | [177] |
| Viburnum opulus (fruits) | 38.4 | 3730 (TPC) | [178] |
| Cacao pod husk products | 59.0 | 6893 (SD) | [161] |
| Mexican Blackberry | 44.3 | 4016 (TPC) | [179] |
| (Rubus fruticosus) residues | |||
| Passion fruit seeds (DCF) | 81.5 | 41.2 * | [180] |
| Pineapple (DFC) | 75.8 | 129 * | [181] |
| Papaya pulp (DFC) | 59.8 | 0.47 * | [182] |
| Tomato peel | 86.1 | 158.1 (TPC) | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, A.; Mateus, N.; de Freitas, V. Polyphenol-Dietary Fiber Conjugates from Fruits and Vegetables: Nature and Biological Fate in a Food and Nutrition Perspective. Foods 2023, 12, 1052. https://doi.org/10.3390/foods12051052
Fernandes A, Mateus N, de Freitas V. Polyphenol-Dietary Fiber Conjugates from Fruits and Vegetables: Nature and Biological Fate in a Food and Nutrition Perspective. Foods. 2023; 12(5):1052. https://doi.org/10.3390/foods12051052
Chicago/Turabian StyleFernandes, Ana, Nuno Mateus, and Victor de Freitas. 2023. "Polyphenol-Dietary Fiber Conjugates from Fruits and Vegetables: Nature and Biological Fate in a Food and Nutrition Perspective" Foods 12, no. 5: 1052. https://doi.org/10.3390/foods12051052
APA StyleFernandes, A., Mateus, N., & de Freitas, V. (2023). Polyphenol-Dietary Fiber Conjugates from Fruits and Vegetables: Nature and Biological Fate in a Food and Nutrition Perspective. Foods, 12(5), 1052. https://doi.org/10.3390/foods12051052






