Influence of Cultivar and Turbidity on Physicochemical Properties, Functional Characteristics and Volatile Flavor Substances of Pomelo Juices
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Water Purification
2.3. Preparation of the Pomelo Juices
2.4. Determination of Total Soluble Solid, pH and Juice Yield
2.5. Color Analysis
2.6. Determination of Total Flavonoids
2.7. Determination of Total Phenolics
2.8. HPLC Analysis of Sugar Component, Organic Acid and Flavonoid Composition
2.9. Determination of Viscosity
2.10. Analysis of Volatile Compounds
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of Pomelo Cultivars on Juice Yield, Total Soluble Solid, pH of Juices
3.2. Effect of Pomelo Cultivars on Organic Acids Composition of Pomelo Juices
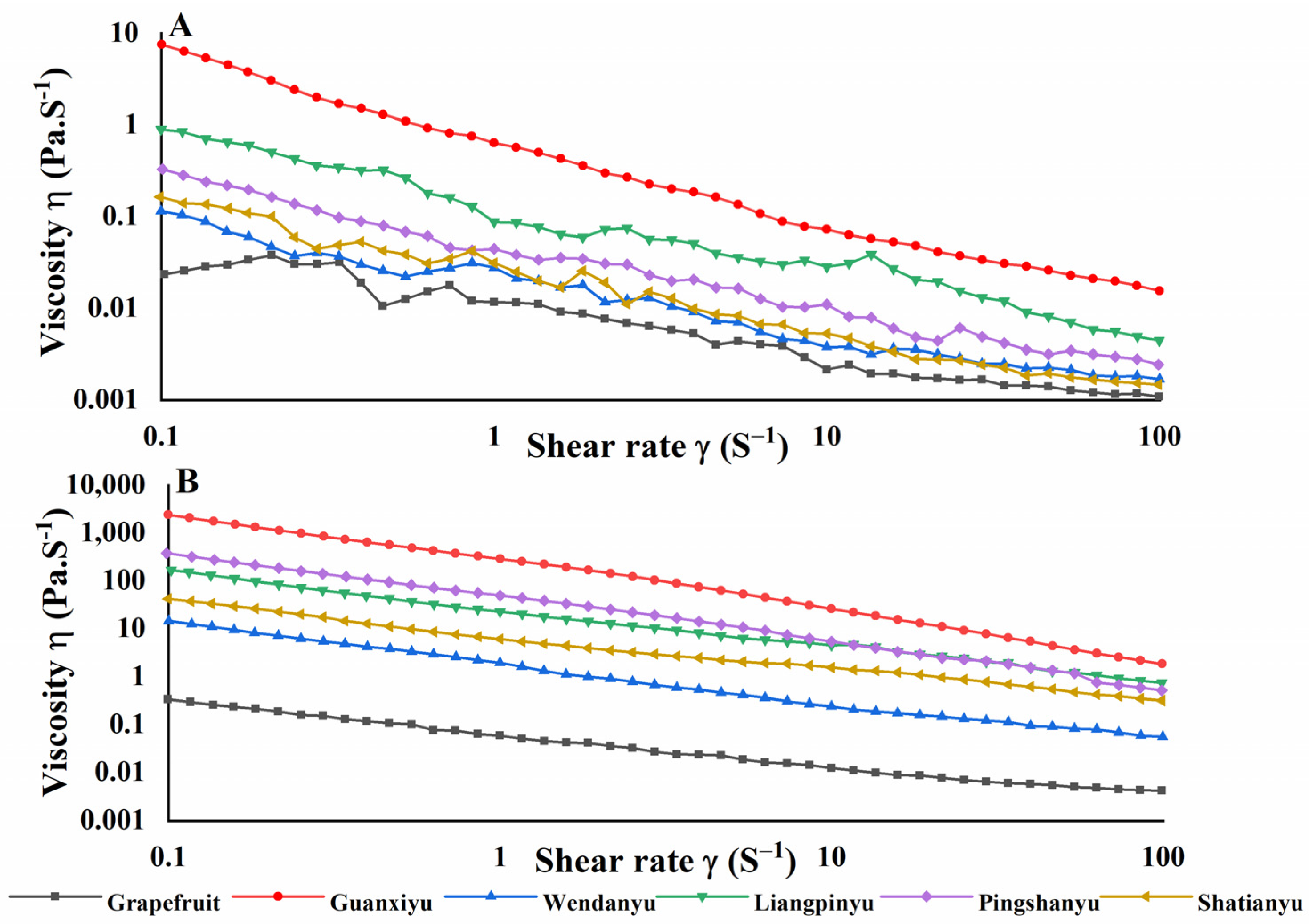
3.3. Effect of Pomelo Cultivars on Color and Viscosity of Juices
3.4. Effect of Pomelo Cultivars on the Contents of Total Phenolics (TP), Total Flavonoids (TF) and Ascorbic Acid of Juice
3.5. Effect of Pomelo Cultivars on Flavonoid Composition of the Juice
3.6. Effect of Pomelo Cultivars on Volatile Compounds of the Juice
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mapelli-Brahm, P.; Stinco, C.M.; Rodrigo, M.J.; Arías, L.Z.A.; Meléndez-Martínez, A. Impact of thermal treatments on the bioaccessibility of phytoene and phytofluene in relation to changes in the microstructure and size of orange juice particles. J. Funct. Foods 2018, 46, 38–47. [Google Scholar] [CrossRef]
- Sanja, G.; Marija, K.; Miroslav, L.; Katarina, M.Š.; Sonja, P.; Sonja, V.; Andrijana, R. Effect of Wheatgrass Juice on Nutritional Quality of Apple, Carrot, Beet, Orange and Lemon Juice. Foods 2022, 11, 445. [Google Scholar] [CrossRef]
- Yalcin, H.; Çapar, T.D. Bioactive compounds of fruits and vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Springer: Berlin/Heidelberg, Germany, 2017; pp. 723–745. [Google Scholar]
- Stinson, W. Clarity in juice classification. Pediatrics 2007, 120, 696–697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, H.; Sun, R.; Zhao, Y.; Xing, R.; Yu, N.; Deng, T.; Ni, X.; Chen, Y. Volatolomics approach for authentication of not-from-concentrate (NFC) orange juice based on characteristic volatile markers using headspace solid phase microextraction (HS-SPME) combined with GC-MS. Food Control 2022, 136, 108856. [Google Scholar] [CrossRef]
- Xu, L.; Xu, Z.; Kelly, S.; Liao, X. Integrating untargeted metabolomics and targeted analysis for not from concentrate and from concentrate orange juices discrimination and authentication. Food Chem. 2020, 329, 127130. [Google Scholar] [CrossRef]
- Salihah, N.; Rosnah, S.; Norashikin, A.A. Mass modeling of Malaysian varieties Pomelo fruit (Citrus Grandis L. Osbeck) with some physical characteristics. Int. Food Res. J. 2015, 22, 488–493. [Google Scholar]
- Chonhenchob, V.; Singh, S.P. Packaging performance comparison for distribution and export of papaya fruit. Packag. Technol. Sci. 2005, 18, 125–131. [Google Scholar] [CrossRef]
- Deng, M.; Lin, Y.; Dong, L.; Jia, X.; Zhang, R. Physicochemical and functional properties of dietary fiber from pummelo (Citrus grandis L. Osbeck) and grapefruit (Citrus paradisi Mcfad) cultivars. Food Biosci. 2021, 40, 100890. [Google Scholar] [CrossRef]
- Tian, J.; Cao, Y.; Chen, S.; Fang, Z.; Chen, J.; Liu, D.; Ye, X. Juices processing characteristics of Chinese bayberry from different cultivars. Food Sci. Nutr. 2019, 7, 404–411. [Google Scholar] [CrossRef]
- Liu, Y.; He, C.; Song, H. Comparison of fresh watermelon juice aroma characteristics of five varieties based on gas chromatography-olfactometry-mass spectrometry. Food Res. Int. 2018, 107, 119–129. [Google Scholar] [CrossRef]
- Pan, T.; Ali, M.M.; Gong, J.; She, W.; Pan, D.; Guo, Z.; Yu, Y.; Chen, F. Fruit Physiology and Sugar-Acid Profile of 24 Pomelo (Citrus grandis (L.) Osbeck) Cultivars Grown in Subtropical Region of China. Agronomy 2021, 11, 2393. [Google Scholar] [CrossRef]
- Gamonpilas, C.; Buathongjan, C.; Kirdsawasd, T.; Rattanaprasert, M.; Klomtun, M.; Phonsatta, N.; Methacanon, P. Pomelo pectin and fiber: Some perspectives and applications in food industry. Food Hydrocoll. 2021, 120, 106981. [Google Scholar] [CrossRef]
- Ivanova, N.N.; Khomich, L.M.; Perova, I.B.; Eller, K.I. Grapefruit juice nutritional profile. Vopr. Pitan. 2018, 87, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hao, N.; Meng, Z.; Li, Y.; Zhao, Z. Identification, Comparison and Classification of Volatile Compounds in Peels of 40 Apple Cultivars by HS-SPME with GC-MS. Foods 2021, 10, 1051. [Google Scholar] [CrossRef] [PubMed]
- Teleszko, M.; Nowicka, P.; Wojdylo, A. Effect of cultivar and storage temperature on identification and stability of polyphenols in strawberry cloudy juices. J. Food Compos. Anal. 2016, 54, 10–19. [Google Scholar] [CrossRef]
- Basumatary, B.; Nayak, P.K.; Chandrasekar, C.M.; Nath, A.; Nayak, M.; Kesavan, R.K. Impact of thermo sonication and pasteurization on the physicochemical, microbiological and anti-oxidant properties of pomelo (Citrus maxima) juice. Int. J. Fruit Sci. 2020, 20, S2056–S2073. [Google Scholar] [CrossRef]
- Kore, V.T.; Chakraborty, I. Efficacy of various techniques on biochemical characteristics and bitterness of pummelo juice. J. Food Sci. Technol. -Mysore 2015, 52, 6073–6077. [Google Scholar] [CrossRef]
- Cheong, M.W.; Shao, Q.L.; Zhou, W.; Curran, P.; Yu, B. Chemical composition and sensory profile of pomelo (Citrus grandis (L.) Osbeck) juice. Food Chem. 2012, 135, 2505–2513. [Google Scholar] [CrossRef]
- Ucan, F.; Agcam, E.; Akyildiz, A. Bioactive compounds and quality parameters of natural cloudy lemon juices. J. Food Sci. Technol. -Mysore 2016, 53, 1465–1474. [Google Scholar] [CrossRef]
- Harzallah, A.; Bhouri, A.M.; Amri, Z.; Soltana, H.; Hammami, M. Phytochemical content and antioxidant activity of different fruit parts juices of three figs (Ficus carica L.) varieties grown in Tunisia. Ind. Crops Prod. 2016, 83, 255–267. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, J.; Cai, S.; Chen, X.; Quan, X.; Zhang, G. Association mapping for total polyphenol content, total flavonoid content and antioxidant activity in barley. BMC Genom. 2018, 19, 81. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Juhaimi, F.A.; Özcan, M.M.; Uslu, N.; Babiker, E.E.; Ahmed, I.A.M. Total phenolics, total carotenoids, individual phenolics and antioxidant activity of ginger (Zingiber officinale) rhizome as affected by drying methods. LWT 2020, 126, 109354. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, G.; Xu, Y.; Yu, Y.; Wu, J.; Zou, B. High Hydrostatic Pressure and Co-Fermentation by Lactobacillus rhamnosus and Gluconacetobacter xylinus Improve Flavor of Yacon-Litchi-Longan Juice. Foods 2019, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, C.; Liu, H.; Liu, J.; Jiao, Z. Profiles of Sugar and Organic Acid of Fruit Juices: A Comparative Study and Implication for Authentication. J. Food Qual. 2020, 2020, 7236534. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wu, Z.; Wan, N.; Yang, M. Dehydration of hawthorn fruit juices using ultrasound-assisted vacuum drying. Ultrason. Sonochem. 2020, 68, 105219. [Google Scholar] [CrossRef]
- Juszczak, L.; Witczak, M.; Fortuna, T.; Solarz, B. Effect of temperature and soluble solids content on the viscosity of beetroot (Beta vulgaris) juice concentrate. Int. J. Food Prop. 2010, 13, 1364–1372. [Google Scholar] [CrossRef]
- Vivian Goh, R.M.; Lau, H.; Liu, S.Q.; Lassabliere, B.; Guervilly, R.; Sun, J.; Bian, Y.; Yu, B. Comparative analysis of pomelo volatiles using headspace-solid phase micro-extraction and solvent assisted flavour evaporation. LWT 2019, 99, 328–345. [Google Scholar] [CrossRef]
- Matteo, A.D.; Simeone, G.D.R.; Cirillo, A.; Rao, M.A.; Vaio, C.D. Morphological characteristics, ascorbic acid and antioxidant activity during fruit ripening of four lemon (Citrus limon (L.) Burm. F.) cultivars. Sci. Hortic. 2021, 276, 109741. [Google Scholar] [CrossRef]
- Chen, L.; Caballero, B.; Mitchell, D.C.; Loria, C.; Lin, P.H.; Champagne, C.M.; Elmer, P.J.; Ard, J.D.; Batch, B.C.; Anderson, C.A.; et al. Reducing Consumption of Sugar-Sweetened Beverages Is Associated With Reduced Blood Pressure: A Prospective Study Among United States Adults (vol 121, pg 2398, 2010). Circulation 2010, 122, E408. [Google Scholar] [CrossRef]
- Yu, K.; Xu, Q.; Da, X.; Guo, F.; Ding, Y.; Deng, X. Transcriptome changes during fruit development and ripening of sweet orange (Citrus sinensis). BMC Genom. 2012, 13, 10. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Q.; Quan, J.; Zheng, Q.; Xi, W. Determination of sugars, organic acids, aroma components, and carotenoids in grapefruit pulps. Food Chem. 2016, 205, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Cendres, A.; Chemat, F.; Page, D.; Le Bourvellec, C.; Markowski, J.; Zbrzezniak, M.; Renard, C.M.G.C.; Plocharski, W. Comparison between microwave hydrodiffusion and pressing for plum juice extraction. LWT-Food Sci. Technol. 2012, 49, 229–237. [Google Scholar] [CrossRef]
- Wawrzynczak, A.; Rutkowski, K.P.; Kruczynska, D.E. Ripening of ‘radana’ and ‘conference’ pears as influenced by cold storage duration. Acta Hortic. 2008, 800, 1091–1098. [Google Scholar] [CrossRef]
- Albertini, M.V.; Carcouet, E.; Pailly, O.; Gambotti, C.; Luro, F.O.; Berti, L. Changes in Organic Acids and Sugars during Early Stages of Development of Acidic and Acidless Citrus Fruit. J. Agric. Food Chem. 2006, 54, 8335–8339. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Puoci, F.; Clodoveo, M.L.; Picci, N. LC with Evaporative Light-Scattering Detection for Quantitative Analysis of Organic Acids in Juices. Food Anal. Methods 2017, 10, 704–712. [Google Scholar] [CrossRef]
- Sun, X.; Baldwin, E.A.; Plotto, A.; Manthey, J.A.; Duan, Y.; Bai, J. Effects of thermal processing and pulp filtration on physical, chemical and sensory properties of winter melon juice. J. Sci. Food Agric. 2017, 97, 543–550. [Google Scholar] [CrossRef]
- Aghajanzadeh, S.; Ziaiifar, A.M.; Verkerk, R. Effect of thermal and non-thermal treatments on the color of citrus juice: A review. Food Rev. Int. 2022. [Google Scholar] [CrossRef]
- Wang, L.; Wu, J.; Huang, H.; Huang, W.; Wang, P.; Chen, J. Coloration mechanisms of fresh sugarcane juice: Investigating the critical components and enzyme activity. J. Food Sci. 2022. [Google Scholar] [CrossRef]
- Genovese, D.B.; Lozano, J.E. Effect of Cloud Particle Characteristics on the Viscosity of Cloudy Apple Juice. J. Food Sci. 2000, 65, 641–645. [Google Scholar] [CrossRef]
- Szczepanska, J.; Skapska, S.; Lorenzo, J.M.; Marszalek, K. The Influence of Static and Multi-Pulsed Pressure Processing on the Enzymatic and Physico-Chemical Quality, and Antioxidant Potential of Carrot Juice During Refrigerated Storage. Food Bioprocess Technol. 2021, 14, 52–64. [Google Scholar] [CrossRef]
- Zhang, H.; Xi, W.; Yang, Y.; Zhou, X.; Liu, X.; Yin, S.; Zhang, J.; Zhou, Z. An on-line HPLC-FRSD system for rapid evaluation of the total antioxidant capacity of Citrus fruits. Food Chem. 2015, 172, 622–629. [Google Scholar] [CrossRef]
- Stos, K.; Przygoda, B.; Jarosz, M.; Matczuk, E.; Markowski, J. Oranges as fruit and as juice. Proc. Nutr. Soc. 2020, 79, 1815. [Google Scholar] [CrossRef]
- Oszmianski, J.; Wolniak, M.; Wojdylo, A.; Wawer, I. Comparative study of polyphenolic content and antiradical activity of cloudy and clear apple juices. J. Sci. Food Agric. 2010, 87, 573–579. [Google Scholar] [CrossRef]
- Difonzo, G.; Vollmer, K.; Caponio, F.; Pasqualone, A.; Carle, R.; Steingass, C.B. Characterisation and classification of pineapple (Ananas comosus [L.] Merr.) juice from pulp and peel. Food Control 2019, 96, 260–270. [Google Scholar] [CrossRef]
- Stinco, C.M.; Sentandreu, E.; Mapelli-Brahm, P.; Navarro, J.L.; Vicario, I.M.; Melendez-Martinez, A.J. Influence of high pressure homogenization and pasteurization on the in vitro bioaccessibility of carotenoids and flavonoids in orange juice. Food Chem. 2020, 331, 127259. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lu, Y.-J.; Guo, C.; Zuo, S.; Zhou, J.-L.; Wong, W.-L.; Huang, B. The study of citrus-derived flavonoids as effective bitter taste inhibitors. J. Sci. Food Agric. 2021, 101, 5163–5171. [Google Scholar] [CrossRef]
- Ji, K.; Min, K. The potential use of citrus juice waste as sources of natural phenolic antioxidants. J. Appl. Pharm. Sci. 2016, 6, 202–205. [Google Scholar]
- Wibowo, S.; Essel, E.A.; De Man, S.; Bernaert, N.; Van Droogenbroeck, B.; Grauwet, T.; Van Loey, A.; Hendrickx, M. Comparing the impact of high pressure, pulsed electric field and thermal pasteurization on quality attributes of cloudy apple juice using targeted and untargeted analyses. Innov. Food Sci. Emerg. Technol. 2019, 54, 64–77. [Google Scholar] [CrossRef]
- Chaudhary, P.R.; Jayaprakasha, G.K.; Patil, B.S. Identification of volatile profiles of Rio Red grapefruit at various developmental to maturity stages. J. Essent. Oil Res. 2018, 30, 77–83. [Google Scholar] [CrossRef]
- Kranz, P.; Adler, P.; Kunz, B. Sorption of citrus flavour compounds on XAD-7HP resin during the debittering of grapefruit juice. Int. J. Food Sci. Technol. 2011, 46, 30–36. [Google Scholar] [CrossRef]
- Sheng, J.; Shan, C.; Liu, Y.; Zhang, P.; Li, J.; Cai, W.; Tang, F. Comparative evaluation of the quality of red globe grape juice fermented by Lactobacillus acidophilus and Lactobacillus plantarum. Int. J. Food Sci. Technol. 2022, 57, 2235–2248. [Google Scholar] [CrossRef]
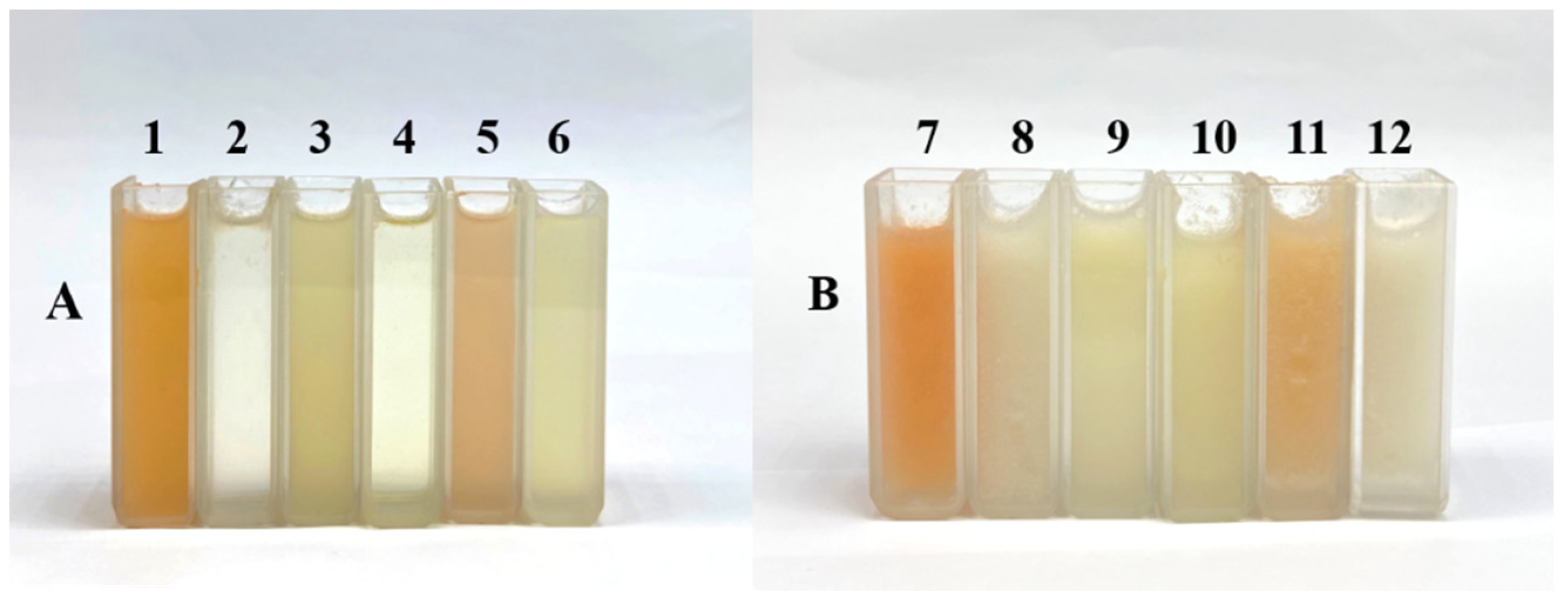




| Cultivars | Grapefruit | Guanxiyu | Wendanyu | Liangpinyu | Pingshanyu | Shatianyu |
|---|---|---|---|---|---|---|
| Juice yield (%) | 73.22 ± 2.01 a | 52.69 ± 3.09 c | 64.63 ± 1.32 b | 56.98 ± 3.66 c | 53.92 ± 3.51 c | 45.35 ± 3.11 d |
| Types | Cultivars | Sugar Component (g L−1) | pH | Total Soluble Solids (‘Brix’) | ||
|---|---|---|---|---|---|---|
| Fructose y = 791724x – 56967 (R² = 0.998) | Glucose y = 520381x – 123387 (R² = 0.991) | Sucrose y = 573278x − 77121 (R² = 0.995) | ||||
| LP juices | Grapefruit | 14.02 ± 0.41 a | 27.15 ± 0.55 b | 40.49 ± 0.98 j | 3.24 ± 0.00 j | 11.4 ± 0.00 h |
| Guanxiyu | 7.74 ± 0.26 c | 17.64 ± 0.15 c | 81.01 ± 1.74 d | 3.39 ± 0.01 h | 12.26 ± 0.04 f | |
| Wendanyu | 6.77 ± 0.28 d | 17.11 ± 0.69 c | 57.76 ± 0.73 h | 3.58 ± 0.01 f | 11.75 ± 0.00 j | |
| Liangpinyu | 3.15 ± 0.20 f | 9.91 ± 0.48 f | 62.28 ± 0.80 g | 4.74 ± 0.011 b | 12.49 ± 0.00 l | |
| Pingshanyu | 5.35 ± 0.026 e | 14.33 ± 0.34 e | 87.14 ± 0.39 b | 3.75 ± 0.01 e | 12.49 ± 0.03 e | |
| Shatianyu | 1.47 ± 0.12 g | 5.22 ± 0.045 g | 83.12 ± 1.73 c | 4.74 ± 0.011 b | 11.86 ± 0.00 g | |
| HP juices | Grapefruit | 12.88 ± 0.81 b | 28.57 ± 1.39 a | 53.18 ± 0.15 i | 3.31 ± 0.00 i | 11.7 ± 0.00 i |
| Guanxiyu | 7.19 ± 0.21 d | 17.45 ± 0.42 c | 84.14 ± 0.84 c | 3.52 ± 0.01 g | 13.18 ± 0.021 b | |
| Wendanyu | 5.66 ± 0.081 e | 15.48 ± 0.31 d | 76.87 ± 0.31 e | 3.85 ± 0.01 d | 12.68 ± 0.00 c | |
| Liangpinyu | 2.93 ± 0.10 f | 9.12 ± 0.76 f | 69.95 ± 0.86 f | 4.75 ± 0.01 a | 10.55 ± 0.00 k | |
| Pingshanyu | 5.22 ± 0.27 e | 13.56 ± 1.02 e | 97.69 ± 1.06 a | 3.96 ± 0.01 c | 13.30 ± 0.01 a | |
| Shatianyu | 1.53 ± 0.18 g | 4.59 ± 0.078 g | 82.92 ± 0.94 c | 4.75 ± 0.01 a | 12.59 ± 0.00 d | |
| Types | Cultivars | Oxalic Acid (g L−1) y = 10499663.84x + 88080.34247 (R2 = 0.999) | Tartaric Acid (g L−1) y = 1068533.055x − 32284.32329 (R2 = 0.999) | Malic Acid (g L−1) y = 454821x + 1516.4 (R2 = 0.999) | Acetic Acid (g L−1) y = 376758x + 5878.5 (R2 = 0.999) | Citric Acid (g L−1) y = 619376x + 11163 (R2 = 0.999) | Succinic Acid (g L−1) y = 353408x − 26483 (R2 = 0.999) |
|---|---|---|---|---|---|---|---|
| LP juices | Grapefruit | - | 0.85 ± 0.55 a | 0.57 ± 0.01 g | 0.66 ± 0.03 g | 14.49 ± 0.01 a | - |
| Guanxiyu | 0.16 ± 0.04 e | 0.45 ± 0.15 e | 0.69 ± 0.05 f | 0.77 ± 0.04 efg | 10.96 ± 1.59 b | - | |
| Wendanyu | - | 0.48 ± 0.69 e | 0.98 ± 0.05 cd | 1.47 ± 0.06 ab | 7.71 ± 0.13 c | 0.71 ± 0.08 b | |
| Liangpinyu | - | 0.28 ± 0.48 f | 0.81 ± 0.041 e | 1.51 ± 0.01 ab | 5.57 ± 0.41 d | 0.64 ± 0.09 b | |
| Pingshanyu | 0.22 ± 0.22 c | - | 1.11 ± 0.06 a | 0.91 ± 0.39 efg | 7.85 ± 0.72 c | - | |
| Shatianyu | 0.21 ± 0.00 d | - | 0.91 ± 0.03 d | 1.30 ± 0.27 bcd | 5.78 ± 0.39 d | 0.94 ± 0.02 a | |
| HP juices | Grapefruit | - | 0.73 ± 0.029 b | 0.45 ± 0.03 h | 0.69 ± 0.18 fg | 13.7 ± 2.15 a | - |
| Guanxiyu | 0.21 ± 0.01 d | 0.56 ± 0.00 d | 0.71 ± 0.01 f | 1.09 ± 0.13 cde | 10.32 ± 1.36 b | - | |
| Wendanyu | - | 0.61 ± 0.01 c | 1.08 ± 0.03 ab | 1.39 ± 0.1 bc | 6.94 ± 0.03 cd | 0.70 ± 0.02 b | |
| Liangpinyu | - | 0.47 ± 0.04 e | 0.53 ± 0.11 g | 1.78 ± 0.23 a | 5.61 ± 0.1 d | 0.44 ± 0.02 c | |
| Pingshanyu | 0.29 ± 0.01 a | - | 1.02 ± 0.01 bc | 1.01 ± 0.16 def | 6.78 ± 0.41 cd | - | |
| Shatianyu | 0.27 ± 0.01 b | - | 0.71 ± 0.01 f | 1.61 ± 0.06 ab | 6.31 ± 0.04 cd | 0.65 ± 0.06 b |
| Types | Cultivars | L | a | b |
|---|---|---|---|---|
| LP juices | Grapefruit | 33.73 ± 0.05 h | 1.64 ± 0.02 b | 3.43 ± 0.02 a |
| Guanxiyu | 33.24 ± 0.05 i | −0.42 ± 0.02 e | −0.82 ± 0.02 f | |
| Wendanyu | 37.10 ± 0.02 f | −1.55 ± 0.02 j | 0.42 ± 0.02 e | |
| Liangpinyu | 37.92 ± 0.02 e | −2.13 ± 0.02 k | 1.68 ± 0.03 b | |
| Pingshanyu | 31.86 ± 0.02 k | 1.52 ± 0.03 c | 0.98 ± 0.04 d | |
| Shatianyu | 33.24 ± 0.01 i | −0.5 ± 0.01 f | 1.48 ± 0.01 c | |
| HP juices | Grapefruit | 38.40 ± 0.02 c | 4.31 ± 0.02 a | 7.55 ± 0.03 a |
| Guanxiyu | 39.47 ± 0.02 b | −1.23 ± 0.02 h | −0.053 ± 0.01 f | |
| Wendanyu | 35.81 ± 0.02 g | −1.48 ± 0.03 i | 1.56 ± 0.02 d | |
| Liangpinyu | 32.84 ± 0.02 j | −0.73 ± 0.02 g | 2.66 ± 0.04 b | |
| Pingshanyu | 37.98 ± 0.01 d | 0.85 ± 0.01 d | 1.89 ± 0.01 c | |
| Shatianyu | 45.27 ± 0.01 a | −1.57 ± 0.02 j | 0.93 ± 0.02 e |
| Types | Cultivars | Narirutin (mg L−1) y = 16191678.7116x + 296198.99829 (R2 = 0.999) | Naringin (mg L−1) y = 13268006.79 + 190986.41 (R2 = 0.999) | Hydroxynaringenin (mg L−1) y = 34294757.82x + 1610605.07 (R2 = 0.999) | Naringenin (g L−1) y = 23910089.51x + 1094130.87 (R2 = 0.999) | Sinensetin (g L−1) y = 15534697.16x + 398342.37 (R2 = 0.999) | Hesperidin (g L−1) y = 18934028.40x + 3184162.71 (R2 = 0.999) |
|---|---|---|---|---|---|---|---|
| LP juices | Grapefruit | 140.11 ± 1.23 d | 519.10 ± 1.26 b | - | 1.19 ± 0.00 d | 0.23 ± 0.025 | - |
| Guanxiyu | 25.70 ± 1.88 j | 75.90 ± 5.72 e | 427.52 ± 1.13 g | 1.17 ± 0.00 f | - | - | |
| Wendanyu | 121.55 ± 0.68 g | - | 452.36 ± 0.00 e | 1.08 ± 0.00 h | 1.30 ± 0.27 | - | |
| Liangpinyu | 200.10 ± 9.07 b | - | 452.36 ± 0.00 e | 1.18 ± 0.00 e | - | 1.53 ± 0.0018 | |
| Pingshanyu | 107.69 ± 0.00 h | - | 426.24 ± 0.00 g | 1.06 ± 0.00 i | - | - | |
| Shatianyu | 43.37 ± 4.99 i | 75.70 ± 4.18 e | 443.03 ± 0.00 f | 1.20 ± 0.00 c | - | - | |
| HP juices | Grapefruit | 150.79 ± 1.85 c | 1109.4 ± 9.72 a | - | 1.20 ± 0.00 c | 0.22 ± 0.00 | - |
| Guanxiyu | 107.68 ± 0.00 h | 133.3 ± 1.91 c | 462.35 ± 1.18 b | 1.22 ± 0.00 a | - | - | |
| Wendanyu | 142.88 ± 0.00 d | - | 465.22 ± 0.00 a | 1.19 ± 0.00 d | 1.30 ± 0.27 | - | |
| Liangpinyu | 689.32 ± 2.16 a | - | 464.48 ± 0.00 c | 1.21 ± 0.00 b | - | 1.61 ± 0.00 | |
| Pingshanyu | 138.12 ± 0.00 e | - | 451.58 ± 1.65 e | 1.15 ± 0.01 g | - | - | |
| Shatianyu | 131.57 ± 3.77 f | 100.3 ± 9.48 d | 459.52 ± 2.52 d | 1.21 ± 0.00 b | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Luo, W.; Cheng, L.; Wu, J.; Yu, Y.; Li, L.; Xu, Y. Influence of Cultivar and Turbidity on Physicochemical Properties, Functional Characteristics and Volatile Flavor Substances of Pomelo Juices. Foods 2023, 12, 1028. https://doi.org/10.3390/foods12051028
Chen J, Luo W, Cheng L, Wu J, Yu Y, Li L, Xu Y. Influence of Cultivar and Turbidity on Physicochemical Properties, Functional Characteristics and Volatile Flavor Substances of Pomelo Juices. Foods. 2023; 12(5):1028. https://doi.org/10.3390/foods12051028
Chicago/Turabian StyleChen, Jiajia, Wenshan Luo, Lina Cheng, Jijun Wu, Yuanshan Yu, Lu Li, and Yujuan Xu. 2023. "Influence of Cultivar and Turbidity on Physicochemical Properties, Functional Characteristics and Volatile Flavor Substances of Pomelo Juices" Foods 12, no. 5: 1028. https://doi.org/10.3390/foods12051028
APA StyleChen, J., Luo, W., Cheng, L., Wu, J., Yu, Y., Li, L., & Xu, Y. (2023). Influence of Cultivar and Turbidity on Physicochemical Properties, Functional Characteristics and Volatile Flavor Substances of Pomelo Juices. Foods, 12(5), 1028. https://doi.org/10.3390/foods12051028







