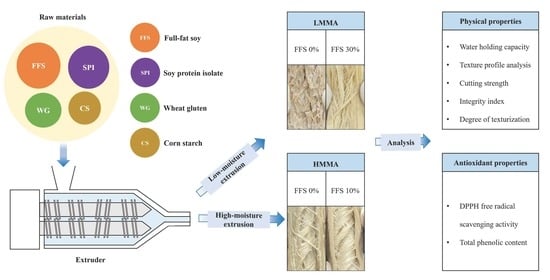
Investigating the Potential of Full-Fat Soy as an Alternative Ingredient in the Manufacture of Low- and High-Moisture Meat Analogs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
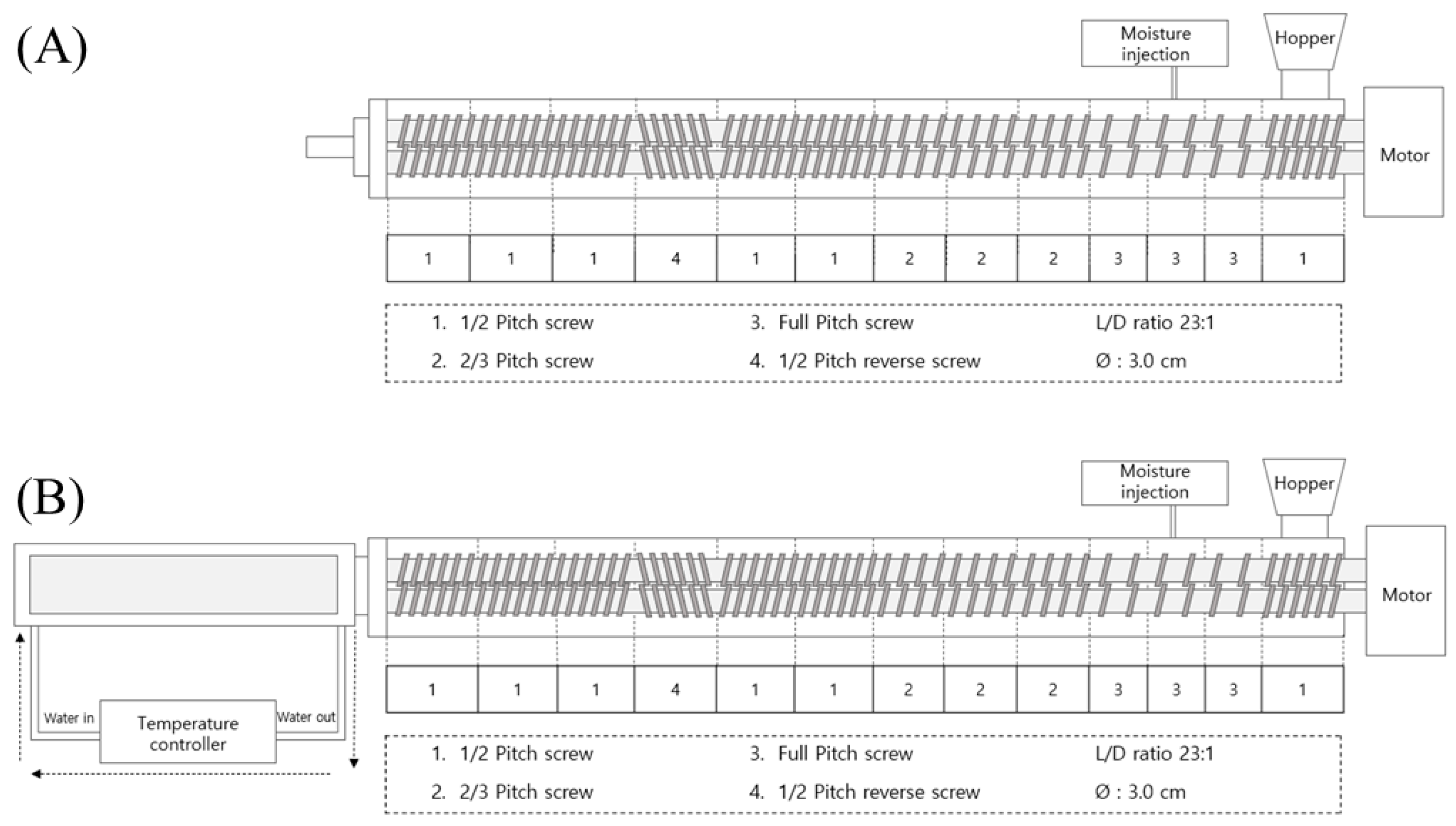
2.2. Manufacturing of Meat Analogs by Low- and High-Moisture Extrusion Cooking
2.3. Texture Profile Analysis and Cutting Strength
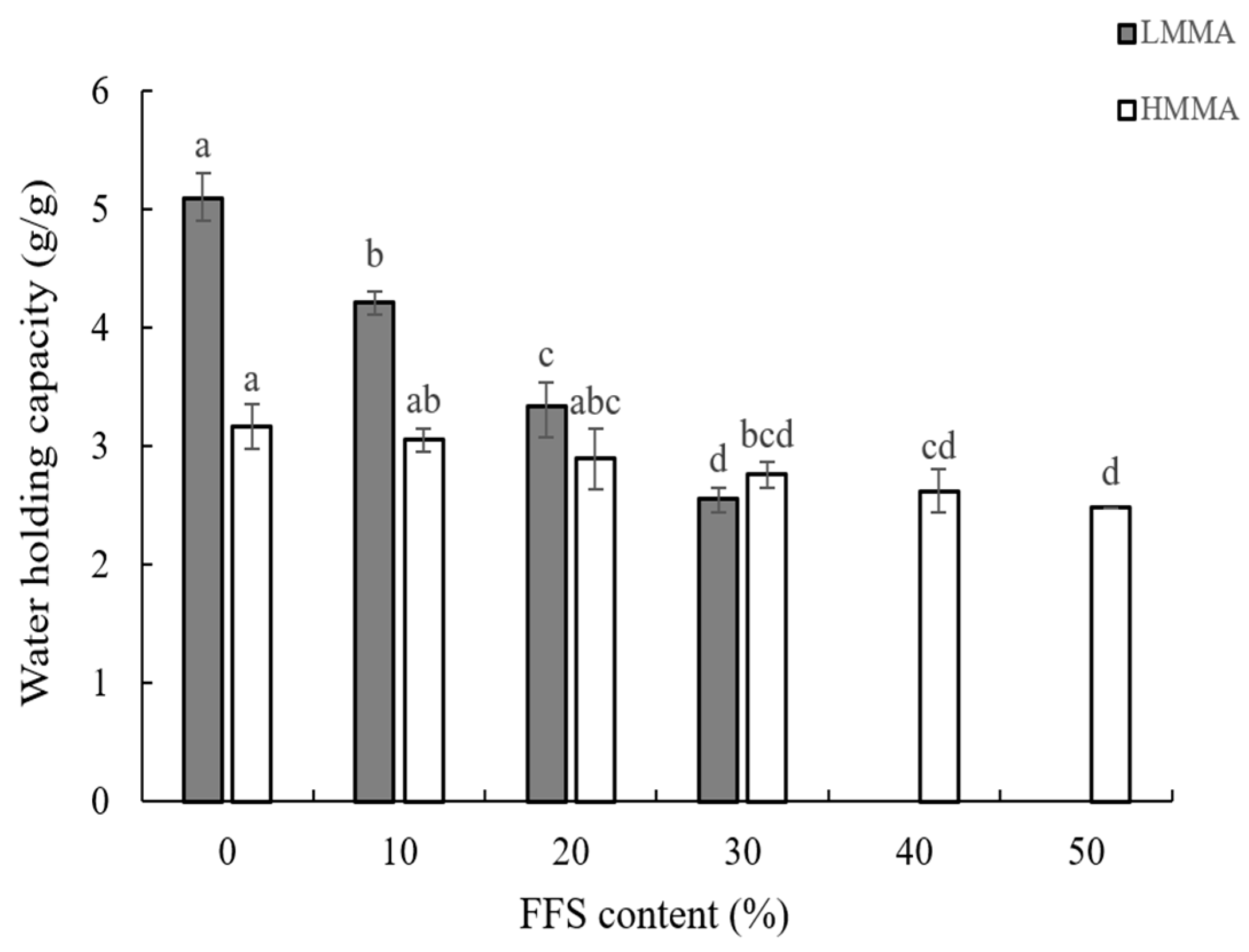
2.4. Water Holding Capacity
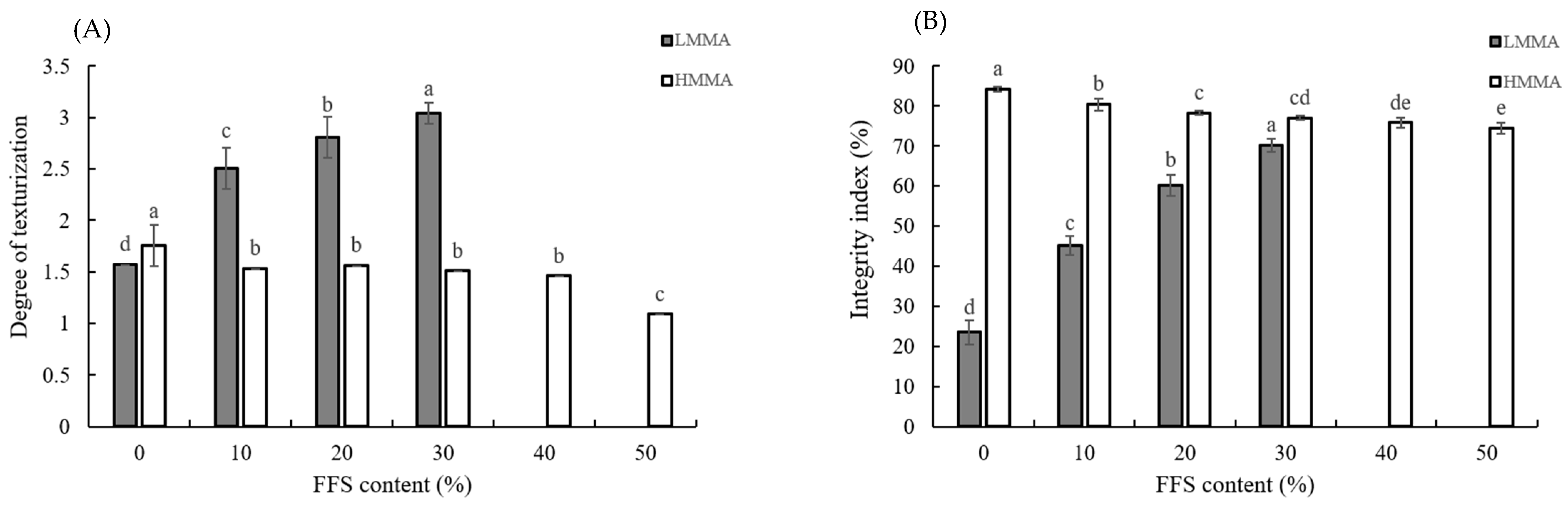
2.5. Degree of Texturization
2.6. Integrity Index
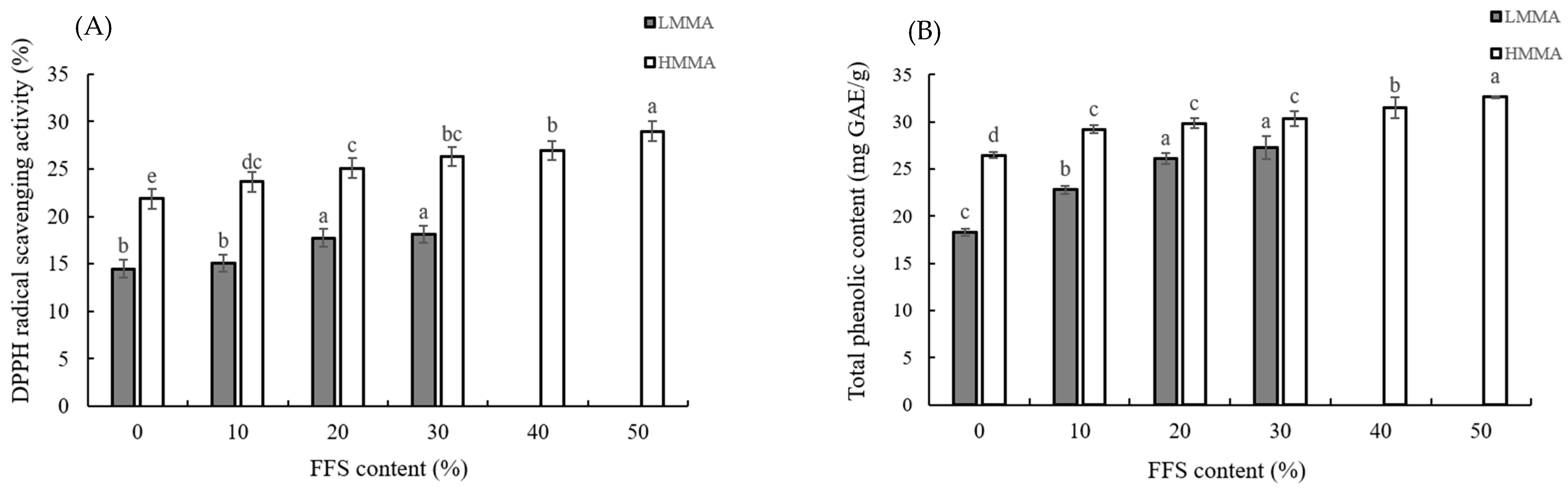
2.7. DPPH Free Radical Scavenging Activity
2.8. Total Phenolic Contents
2.9. Macrostructure
2.10. Statistical Analysis
3. Results and Discussion
3.1. Texture Profile Analysis, Cutting Strength, and Water Holding Capacity
3.2. Degree of Texturization and Integrity Index
3.3. DPPH Free Radical Scavenging Activity and Total Phenolic Contents
3.4. Macrostructure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bruinsma, J. World Agriculture: Towards 2015/2030: An FAO Study; Routledge: London, UK, 2017; p. 89. [Google Scholar]
- Errickson, F.; Kuruc, K.; McFadden, J. Animal-based foods have high social and climate costs. Nat. Food 2021, 2, 274–281. [Google Scholar] [CrossRef]
- Vatansever, S.; Tulbek, M.C.; Riaz, M.N. Low-and high-moisture extrusion of pulse proteins as plant-based meat ingredients: A review. Cereal. Foods World 2020, 65, 12–14. [Google Scholar]
- Davis, H.; Magistrali, A.; Butler, G.; Stergiadis, S. Nutritional benefits from fatty acids in organic and grass-fed beef. Foods 2022, 11, 646. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulou, K.; Dekkers, B.; Van Der Goot, A.J. Plant-Based Meat Analogues. In Sustainable Meat Production and Processing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–126. [Google Scholar]
- Zhou, H.; Hu, Y.; Tan, Y.; Zhang, Z.; McClements, D.J. Digestibility and gastrointestinal fate of meat versus plant-based meat analogs: An in vitro comparison. Food Chem. 2021, 364, 130439. [Google Scholar] [CrossRef]
- Yang, C.; Chen, X.; Sun, J.; Gu, C. The Impact of Alternative Foods on Consumers’ Continuance Intention from an Innovation Perspective. Foods 2022, 11, 1167. [Google Scholar] [CrossRef]
- Pietsch, V.L.; Emin, M.A.; Schuchmann, H.P. Process conditions influencing wheat gluten polymerization during high moisture extrusion of meat analog products. J. Food Eng. 2017, 198, 28–35. [Google Scholar] [CrossRef]
- Smetana, S.; Larki, N.A.; Pernutz, C.; Franke, K.; Bindrich, U.; Toepfl, S.; Heinz, V. Structure design of insect-based meat analogs with high-moisture extrusion. J. Food Eng. 2018, 229, 83–85. [Google Scholar] [CrossRef]
- do Carmo, C.S.; Knutsen, S.H.; Malizia, G.; Dessev, T.; Geny, A.; Zobel, H.; Myhere, K.S.; Varela, P.; Sahlstrøm, S. Meat analogues from a faba bean concentrate can be generated by high moisture extrusion. Future Foods 2021, 3, 100014. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring processes for meat analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Nieuwland, M.; Geerdink, P.; Brier, P.; van den Eijnden, P.; Henket, J.T.M.M.; Langelaan, M.L.P.; Stroeks, N.; van Deventer, H.C.; Martin, A.H. Food-grade electrospinning of proteins. Innov. Food Sci. Emerg. Technol. 2013, 20, 269–275. [Google Scholar] [CrossRef]
- Chiang, J.H.; Tay, W.; Ong, D.S.M.; Liebl, D.; Ng, C.P.; Henry, C.J. Physicochemical, textural and structural characteristics of wheat gluten-soy protein composited meat analogues prepared with the mechanical elongation method. Food Struct. 2021, 28, 100183. [Google Scholar] [CrossRef]
- Langelaan, M.L.P.; Boonen, K.J.M.; Polak, R.B.; Baaijens, F.P.T.; Post, M.J.; van der Schaft, D.W.J. Meet the new meat: Tissue engineered skeletal muscle. Trends Food Sci. Technol. 2010, 21, 59–66. [Google Scholar] [CrossRef]
- Dekkers, B.L.; de Kort, D.W.; Grabowska, K.J.; Tian, B.; Van As, H.; van der Goot, A.J. A combined rheology and time domain NMR approach for determining water distributions in protein blends. Food Hydrocoll. 2016, 60, 525–532. [Google Scholar] [CrossRef]
- Krintiras, G.A.; Göbel, J.; Van der Goot, A.J.; Stefanidis, G.D. Production of structured soy-based meat analogues using simple shear and heat in a Couette Cell. J. Food Eng. 2015, 160, 34–41. [Google Scholar] [CrossRef]
- Martín-Diana, A.B.; Blanco Espeso, B.; Jimenez Pulido, I.J.; Martínez, P.J.A.; Rico, D. Twin-Screw Extrusion as Hydrothermal Technology for the Development of Gluten-Free Teff Flours: Effect on Antioxidant, Glycaemic Index and Techno-Functional Properties. Foods 2022, 11, 3610. [Google Scholar] [CrossRef]
- Zahari, I.; Ferawati, F.; Purhagen, J.K.; Rayner, M.; Ahlström, C.; Helstad, A.; Östbring, K. Development and characterization of extrudates based on rapeseed and pea protein blends using high-moisture extrusion cooking. Foods 2021, 10, 2397. [Google Scholar] [CrossRef]
- Lin, S.; Huff, H.E.; Hsieh, F. Extrusion process parameters, sensory characteristics, and structural properties of a high moisture soy protein meat analog. J. Food Sci. 2002, 67, 1066–1072. [Google Scholar] [CrossRef]
- Kołodziejczak, K.; Onopiuk, A.; Szpicer, A.; Poltorak, A. Meat Analogues in the Perspective of Recent Scientific Research: A Review. Foods 2021, 11, 105. [Google Scholar] [CrossRef]
- Sun, C.; Ge, J.; He, J.; Gan, R.; Fang, Y. Processing, quality, safety, and acceptance of meat analogue products. Engineering 2021, 7, 674–678. [Google Scholar] [CrossRef]
- Zhang, T.; Dou, W.; Zhang, X.; Zhao, Y.; Zhang, Y.; Jiang, L.; Sui, X. The development history and recent updates on soy protein-based meat alternatives. Trends Food Sci. Technol. 2021, 109, 702–710. [Google Scholar] [CrossRef]
- Osen, R.; Schweiggert-Weisz, U. High-Moisture Extrusion: Meat Analogues. In Reference Module in Food Science; Smithers, G.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780081005965. [Google Scholar]
- Cho, S.Y.; Ryu, G.H. Effects of Oyster Mushroom Addition on Physicochemical Properties of Full Fat Soy-based Meat Analog by Extrusion Process. Food Eng. Prog. 2021, 25, 85–94. [Google Scholar] [CrossRef]
- Islam, M.; Huang, Y.; Islam, M.S.; Lei, N.; Shan, L.; Fan, B.; Tong, L.; Wang, F. Effect of high-moisture extrusion on soy meat analog: Study on its morphological and physiochemical properties. Ital. J. Food Sci. 2022, 34, 9–20. [Google Scholar] [CrossRef]
- Samard, S.; Gu, B.Y.; Ryu, G.H. Effects of extrusion types, screw speed and addition of wheat gluten on physicochemical characteristics and cooking stability of meat analogues. J. Sci. Food Agric. 2019, 99, 4922–4931. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Ryu, G.H. Effects of mushroom composition on the quality characteristics of extruded meat analog. Korean J. Food Sci. Technol. 2020, 52, 357–362. [Google Scholar]
- AOAC. AOAC Official Method 968.06: Protein (crude) in animal feed. Dumas method. In Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Danlami, J.M.; Arsad, A.; Zaini, M.A.A. Characterization and process optimization of castor oil (Ricinus communis L.) extracted by the soxhlet method using polar and non-polar solvents. J. Taiwan Inst. Chem. Eng. 2015, 47, 99–104. [Google Scholar] [CrossRef]
- Trinh, T.; Glasgow, S. On the Texture Profile Analysis Test. In Proceedings of the Chemeca 2012: Quality of Life through Chemical Engineering, Wellington, New Zealand, 23–26 September 2012; Available online: https://www.researchgate.net/publication/316093466 (accessed on 9 October 2020).
- Gu, B.Y.; Ryu, G.H. Effects of moisture content and screw speed on physical properties of extruded soy protein isolate. J. Korean Soc. Food Sci. Nutr. 2017, 46, 751–758. [Google Scholar]
- Diaz, J.R.; Kantanen, K.; Edelmann, J.M.; Suhonen, H.; Sontag-Strohm, T.; Jouppila, K.; Piironen, V. Fibrous meat analogues containing oat fiber concentrate and pea protein isolate: Mechanical and physicochemical characterization. Innov. Food Sci. Emerg. Technol. 2022, 77, 102954. [Google Scholar] [CrossRef]
- Chen, F.L.; Wei, Y.M.; Zhang, B.; Ojokoh, A.O. System parameters and product properties response of soybean protein extruded at wide moisture range. J. Food Eng. 2010, 96, 208–213. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Wee, M.S.M.; Goh, A.T.; Stieger, M.; Forde, C.G. Correlation of instrumental texture properties from textural profile analysis (TPA) with eating behaviours and macronutrient composition for a wide range of solid foods. Food Funct. 2018, 9, 5301–5312. [Google Scholar] [CrossRef]
- Chandra, M.V.; Shamasundar, B.A. Texture profile analysis and functional properties of gelatin from the skin of three species of fresh water fish. Int. J. Food Prop. 2015, 18, 572–584. [Google Scholar] [CrossRef]
- Ma, X.; Ryu, G.H. Effects of green tea contents on the quality and antioxidant properties of textured vegetable protein by extrusion-cooking. Food Sci. Biotechnol. 2019, 28, 67–74. [Google Scholar] [CrossRef]
- Yao, G.; Liu, K.S.; Hsieh, F. A new method for characterizing fiber formation in meat analogs during high-moisture extrusion. J. Food Sci. 2004, 69, 303–307. [Google Scholar] [CrossRef]
- Chen, F.L.; Wei, Y.M.; Zhang, B. Chemical cross-linking and molecular aggregation of soybean protein during extrusion cooking at low and high moisture content. LWT 2011, 44, 957–962. [Google Scholar] [CrossRef]
- Liu, K.S.; Hsieh, H.S. Protein–protein interactions during high-moisture extrusion for fibrous meat analogues and comparison of protein solubility methods using different solvent systems. J. Agric. Food Chem. 2008, 56, 2681–2687. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Areas, J.A.G. Structural changes in biopolymers during extrusion. In Food Extrusion Science and Technology; Kokini, J.L., Ho, C.T., Karwe, M.V., Eds.; Marcel Dekker: New York, NY, USA, 1992; pp. 345–371. [Google Scholar]
- Choton, S.; Gupta, N.; Bandral, J.; Anjum, N.; Choudary, A. Extrusion technology and its application in food processing: A review. Pharma. Innov. 2020, 9, 162–168. [Google Scholar] [CrossRef]
- Kumar, L.; Brennan, M.A.; Mason, S.L.; Zheng, H.; Brennan, C.S. Rheological, pasting and microstructural studies of dairy protein-starch interactions and their application in extrusion-based products: A review. Starch-Stärke 2017, 69, 1600273. [Google Scholar] [CrossRef]
- Alam, M.S.; Kaur, J.; Khaira, H.; Gupta, K. Extrusion and extruded products: Changes in quality attributes as affected by extrusion process parameters: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 445–473. [Google Scholar] [CrossRef]
- de Mesa, N.J.E.; Alavi, S.; Singh, N.; Shi, Y.C.; Dogan, H.; Sang, Y. Soy protein-fortified expanded extrudates: Baseline study using normal corn starch. J. Food Eng. 2009, 90, 262–270. [Google Scholar] [CrossRef]
- Van Hoan, N.; Mouquet-Rivier, C.; Trèche, S. Effects of starch, lipid and moisture contents on extrusion behavior and extrudate characteristics of rice-based blends prepared with a very-low-cost extruder. J. Food Process Eng. 2010, 33, 519–539. [Google Scholar] [CrossRef]
- Alzagtat, A.A.; Alli, I. Protein-lipid interactions in food systems: A review. Int. J. Food Sci. Nutr. 2002, 53, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Villota, R. Influence of 7S and 11S globulins on the extrusion performance of soy protein concentrates. J. Food Proc. Preserv. 1994, 18, 421. [Google Scholar] [CrossRef]
- Lee, J.S.; Choi, I.; Han, J. Construction of rice protein-based meat analogues by extruding process: Effect of substitution of soy protein with rice protein on dynamic energy, appearance, physicochemical, and textural properties of meat analogues. Food Res. Int. 2022, 161, 111840. [Google Scholar] [CrossRef] [PubMed]
- Ottoboni, M.; Spranghers, T.; Pinotti, L.; Baldi, A.; De Jaeghere, W.; Eeckhout, M. Inclusion of Hermetia Illucens larvae or prepupae in an experimental extruded feed: Process optimisation and impact on in vitro digestibility. Ital. J. Anim. Sci. 2018, 17, 418–427. [Google Scholar] [CrossRef]
- Gu, B.Y.; Ryu, G.H. Effects of extrusion variables and die configuration on physicochemical characteristics of texturized soy protein isolate. J. Korean Soc. Food Sci. Nutr. 2019, 48, 1405–1411. [Google Scholar] [CrossRef]
- Palanisamy, M.; Töpfl, S.; Aganovic, K.; Berger, R.G. Influence of iota carrageenan addition on the properties of soya protein meat analogues. LWT 2018, 87, 546–552. [Google Scholar] [CrossRef]
- Ma, X.; Gu, B.Y.; Ryu, G.H. Optimization of extrusion variables for improving the qualities of textured vegetable protein with green tea using response surface methodology. Food Eng. Prog. 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Rajendra, A.; Ying, D.; Warner, R.D.; Ha, M.; Fang, Z. Effect of Extrusion on the Functional, Textural and Colour Characteristics of Texturized Hempseed Protein. Food Bioproc. Tech. 2022, 16, 98–110. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Olszowy, M. On practical problems in estimation of antioxidant activity of compounds by DPPH method (Problems in estimation of antioxidant activity). Food Chem. 2012, 131, 1037–1043. [Google Scholar] [CrossRef]
- Rahman, M.A.A.; Moon, S.S. Antioxidant polyphenol glycosides from the Plant Draba nemorosa. Bull. Korean Chem. Soc. 2007, 28, 827–831. [Google Scholar] [CrossRef]
- Aludatt, M.H.; Rababah, T.; Ereifej, K.; Alli, I. Distribution, antioxidant, and characterisation of phenolic compounds in soybeans, flaxseed and olives. Food Chem. 2019, 139, 93–99. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhao, X.; Sun, P.; Zhao, D.; Jiang, L.; Sui, X. The texture of plant protein-based meat analogs by high moisture extrusion: A review. J. Texture Stud. 2022, 1–14. [Google Scholar] [CrossRef]
| Extrusion Type | Full-Fat Soy (%) | Soy Protein Isolate (%) | Wheat Gluten (%) | Corn Starch (%) |
|---|---|---|---|---|
| LMMA | 0 | 50 | 40 | 10 |
| 10 | 40 | 40 | 10 | |
| 20 | 30 | 40 | 10 | |
| 30 | 20 | 40 | 10 | |
| HMMA | 0 | 50 | 40 | 10 |
| 10 | 40 | 40 | 10 | |
| 20 | 30 | 40 | 10 | |
| 30 | 20 | 40 | 10 | |
| 40 | 10 | 40 | 10 | |
| 50 | 0 | 40 | 10 |
| Extrusion Type | FFS Content (%) | Springiness (%) | Cohesiveness (%) | Chewiness (g) | Cutting Strength (g/cm2) | |
|---|---|---|---|---|---|---|
| Vertical | Parallel | |||||
| LMMA | 0 | 97.43 ± 3.4 a | 94.15 ± 3.5 a | 263.42 ± 98.6 c | 551.3 ± 38.9 d | 351.2 ± 30.4 c |
| 10 | 84.57 ± 1.3 b | 79.74 ± 2.3 b | 399.16 ± 82.2 b | 999.7 ± 69.3 c | 403.4 ± 64.7 bc | |
| 20 | 83.87 ± 5.3 bc | 74.95 ± 3.5 b | 436.72 ± 43.5 b | 1193.4 ± 68.2 b | 427.5 ± 45.1 b | |
| 30 | 79.32 ± 5.1 c | 67.58 ± 6.0 c | 537.67 ± 34.5 a | 1677.5 ± 66.2 a | 552.0 ± 31.1 a | |
| HMMA | 0 | 93.03 ± 0.8 a | 79.07 ± 1.2 a | 4295.64 ± 130.9 a | 1236.8 ± 128.8 a | 711.7 ± 114.8 a |
| 10 | 93.78 ± 0.6 a | 79.15 ± 0.7 a | 3967.45 ± 114.2 b | 1020.7 ± 52.8 b | 667.9 ± 30.1 ab | |
| 20 | 90.01 ± 1.0 b | 74.45 ± 2.0 b | 3559.24 ± 175.4 c | 977.7 ± 57.5 b | 625.5 ± 37.5 bc | |
| 30 | 88.58 ± 1.2 b | 73.11 ± 1.8 b | 3170.47 ± 172.5 d | 947.2 ± 63.8 b | 626.0 ± 41.6 bc | |
| 40 | 86.26 ± 1.5 c | 69.73 ± 4.1 c | 2550.08 ± 206.1 e | 821.1 ± 70.6 c | 562.3 ± 47.4 c | |
| 50 | 75.09 ± 3.6 d | 54.51 ± 2.8 d | 1183.40 ± 115.6 f | 477.9 ± 35.0 d | 438.6 ± 39.3 d | |
| WHC | Springiness | Cohesiveness | Chewiness | V-CS | P-CS | DT | Integrity Index | |
|---|---|---|---|---|---|---|---|---|
| WHC | 1 | |||||||
| Springiness | 0.934 ** | 1 | ||||||
| Cohesiveness | 0.950 ** | 0.987 ** | 1 | |||||
| Chewiness | −0.910 ** | −0.905 ** | −0.917 ** | 1 | ||||
| V-CS | −0.972 ** | −0.949 ** | −0.961 ** | 0.928 ** | 1 | |||
| P-CS | −0.911 ** | −0.875 ** | −0.891 ** | 0.917 ** | 0.968 ** | 1 | ||
| DT | −0.925 ** | −0.964 ** | −0.957 ** | 0.841 ** | 0.910 ** | 0.790 ** | 1 | |
| Integrity index | −0.962 ** | −0.949 ** | −0.952 ** | 0.866 ** | 0.965 ** | 0.881 ** | 0.955 ** | 1 |
| WHC | Springiness | Cohesiveness | Chewiness | V-CS | P-CS | DT | Integrity Index | |
|---|---|---|---|---|---|---|---|---|
| WHC | 1 | |||||||
| Springiness | 0.778 ** | 1 | ||||||
| Cohesiveness | 0.764 ** | 0.990 ** | 1 | |||||
| Chewiness | 0.821 ** | 0.976 ** | 0.964 ** | 1 | ||||
| V-CS | 0.807 ** | 0.916 ** | 0.900 ** | 0.950 ** | 1 | |||
| P-CS | 0.767 ** | 0.864 ** | 0.840 ** | 0.889 ** | 0.958 ** | 1 | ||
| DT | 0.715 ** | 0.889 ** | 0.882 ** | 0.907 ** | 0.902 ** | 0.752 ** | 1 | |
| Integrity index | 0.777 ** | 0.749 ** | 0.739 ** | 0.837 ** | 0.809 ** | 0.720 ** | 0.768 ** | 1 |
| Extrusion Types | Structure | Full-Fat Soy Content (%) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | ||
| LMMA | Cross-sectional | 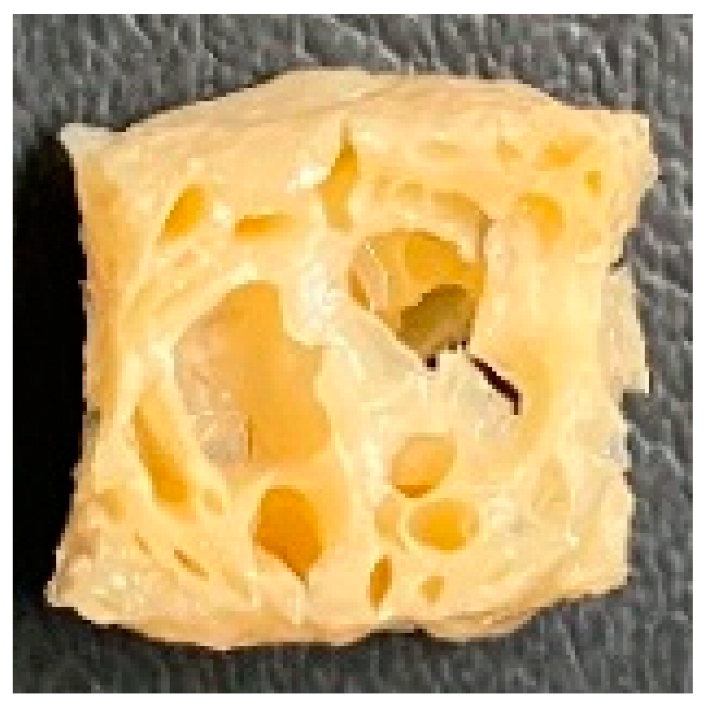 | 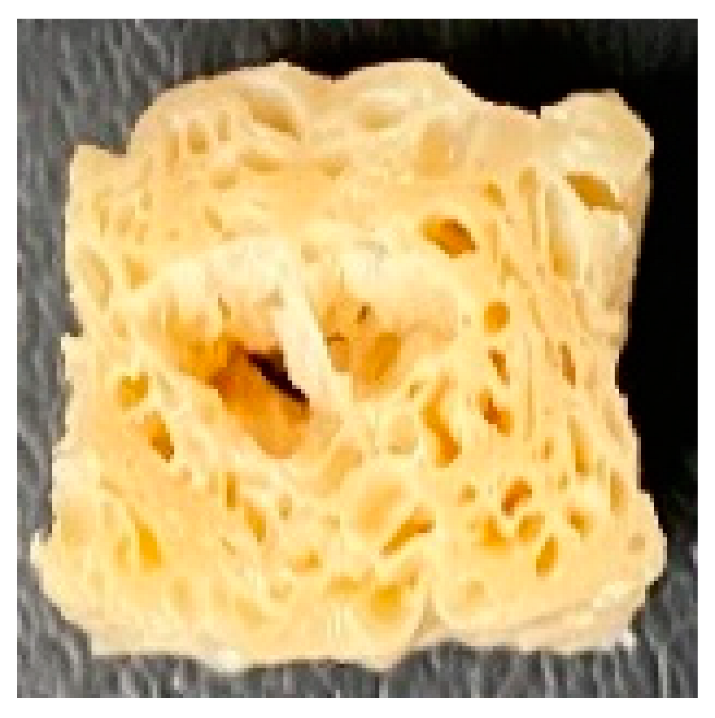 |  |  | ||
| Fibrous | 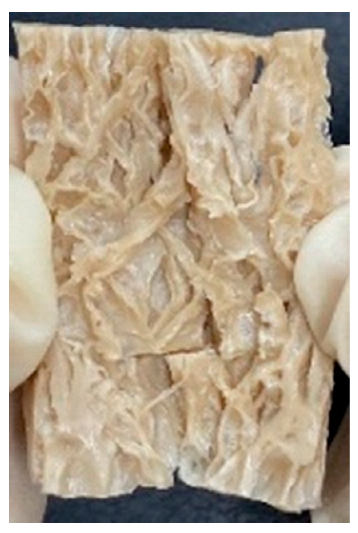 | 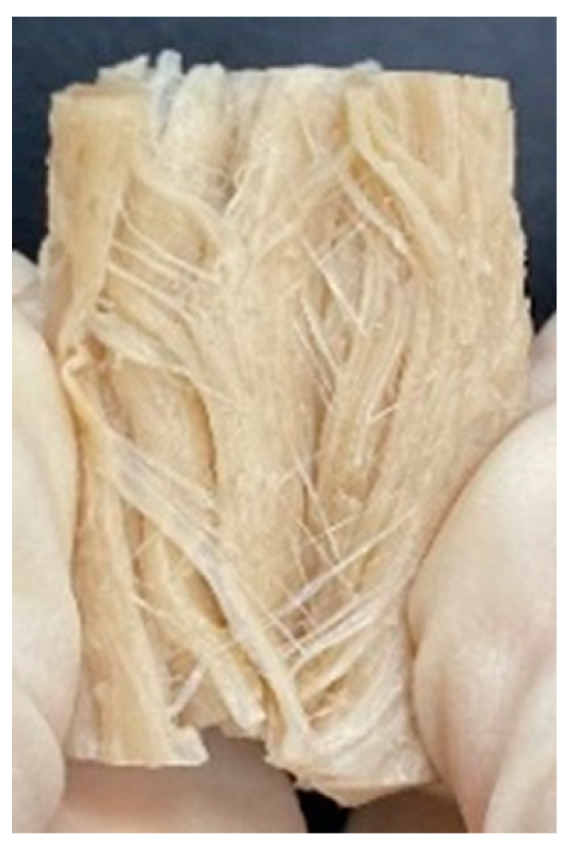 |  |  | |||
| HMMA | Cross-sectional |  |  |  |  |  |  |
| Fibrous |  |  |  | 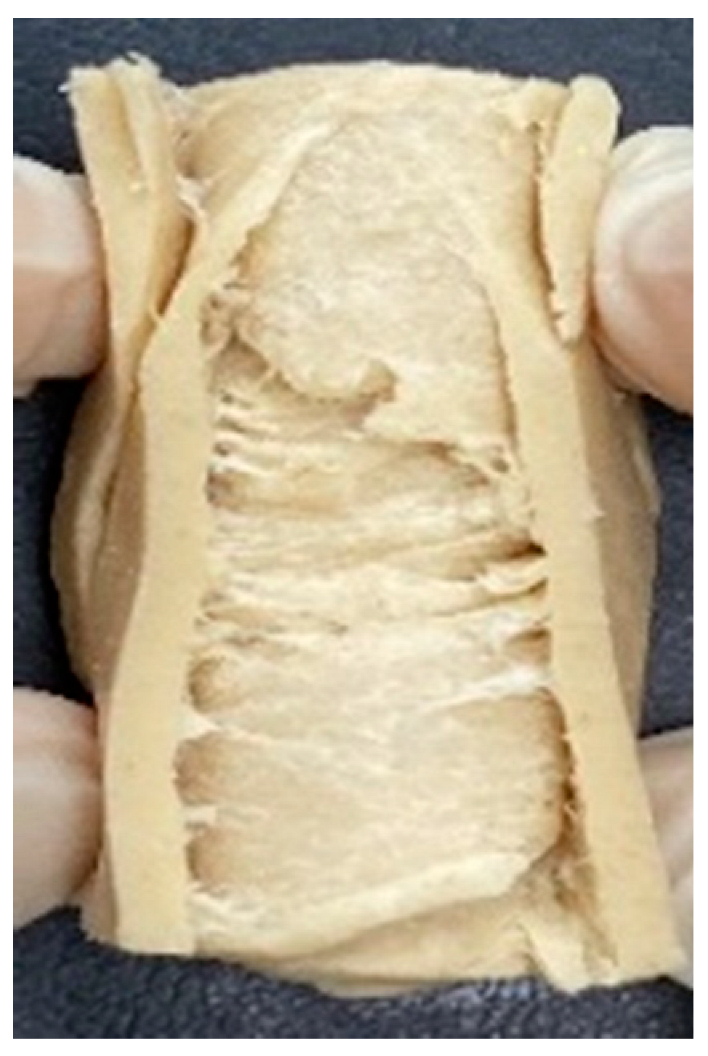 |  | 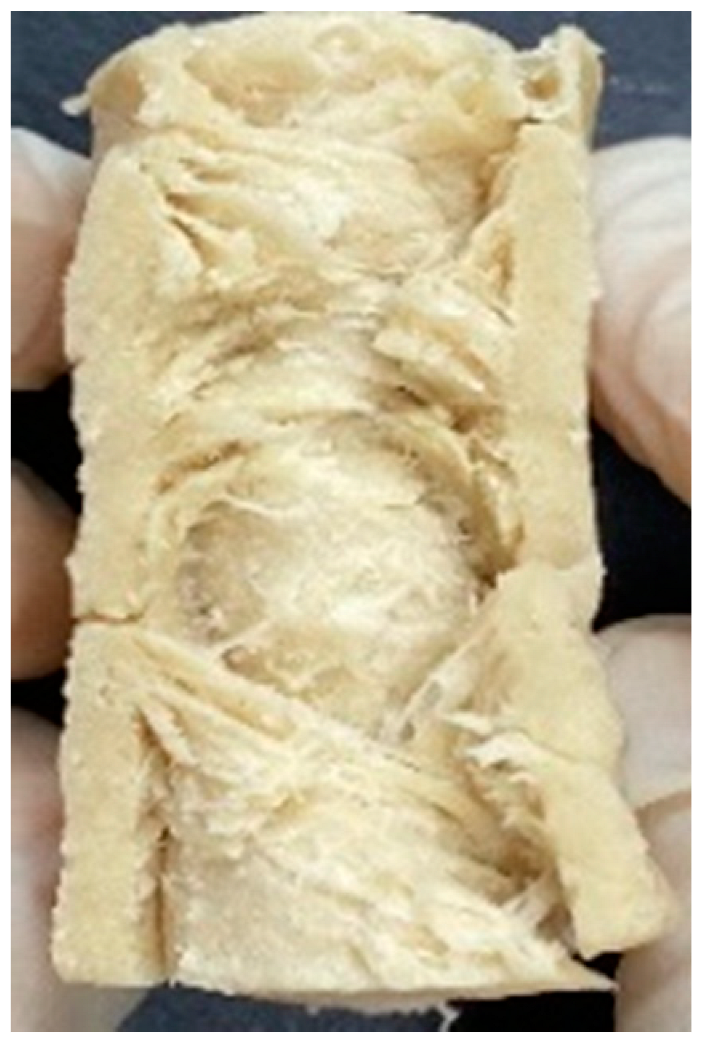 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, Y.-H.; Gu, B.-J.; Ryu, G.-H. Investigating the Potential of Full-Fat Soy as an Alternative Ingredient in the Manufacture of Low- and High-Moisture Meat Analogs. Foods 2023, 12, 1011. https://doi.org/10.3390/foods12051011
Jeon Y-H, Gu B-J, Ryu G-H. Investigating the Potential of Full-Fat Soy as an Alternative Ingredient in the Manufacture of Low- and High-Moisture Meat Analogs. Foods. 2023; 12(5):1011. https://doi.org/10.3390/foods12051011
Chicago/Turabian StyleJeon, Yung-Hee, Bon-Jae Gu, and Gi-Hyung Ryu. 2023. "Investigating the Potential of Full-Fat Soy as an Alternative Ingredient in the Manufacture of Low- and High-Moisture Meat Analogs" Foods 12, no. 5: 1011. https://doi.org/10.3390/foods12051011
APA StyleJeon, Y.-H., Gu, B.-J., & Ryu, G.-H. (2023). Investigating the Potential of Full-Fat Soy as an Alternative Ingredient in the Manufacture of Low- and High-Moisture Meat Analogs. Foods, 12(5), 1011. https://doi.org/10.3390/foods12051011