Effects of Ultra-High Pressure Synergistic Enzymatic Hydrolysis on Flavor of Stropharia rugoso-annulata
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Determination of Volatile Flavor Compounds by HS-SPME-GC-MS
2.4. Determination of E-Nose
2.5. Determination of E-Tongue
2.6. Determination of Amino Acids
2.7. Determination of 5′-Nucleotides
2.8. Statistical Analysis
3. Results
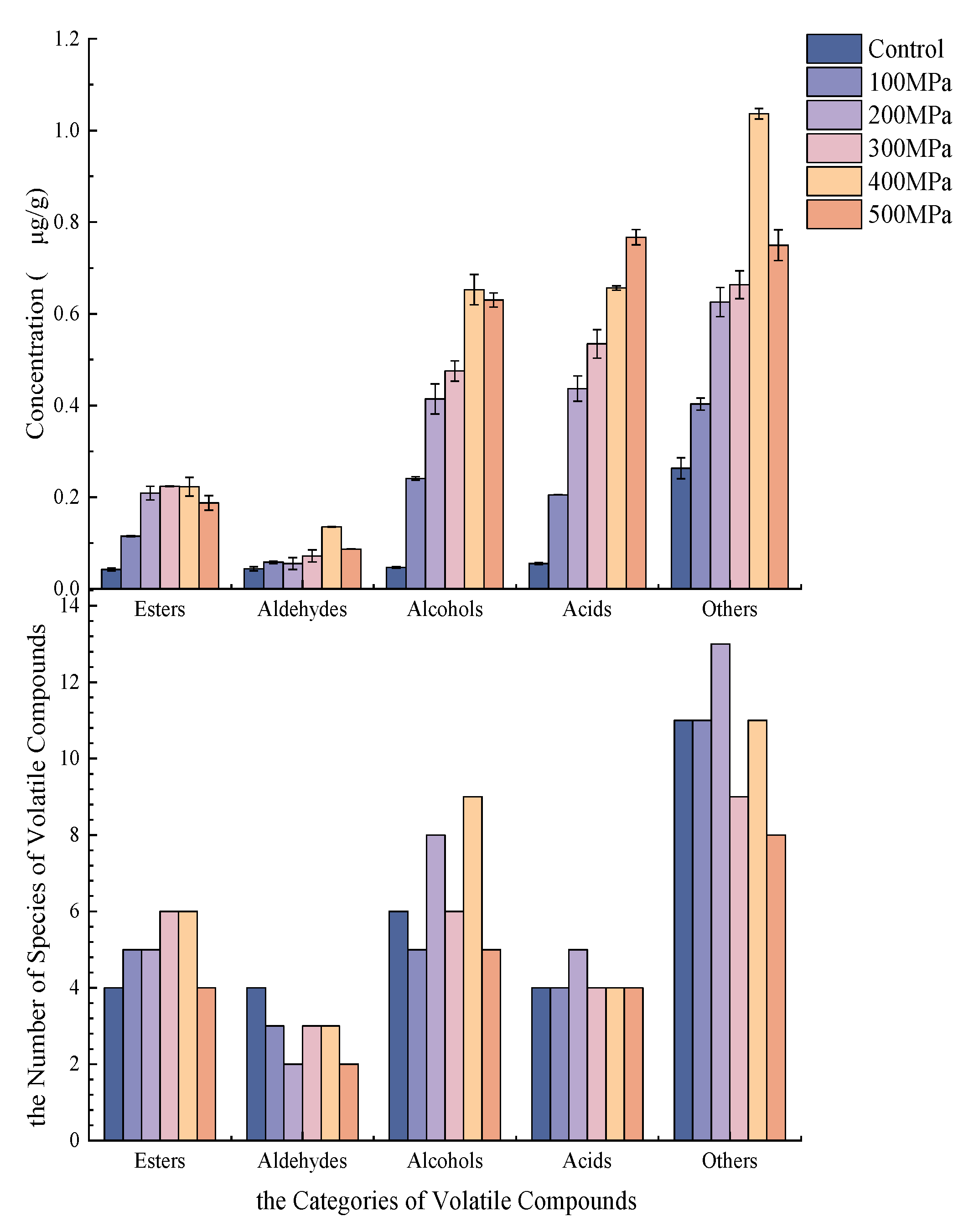
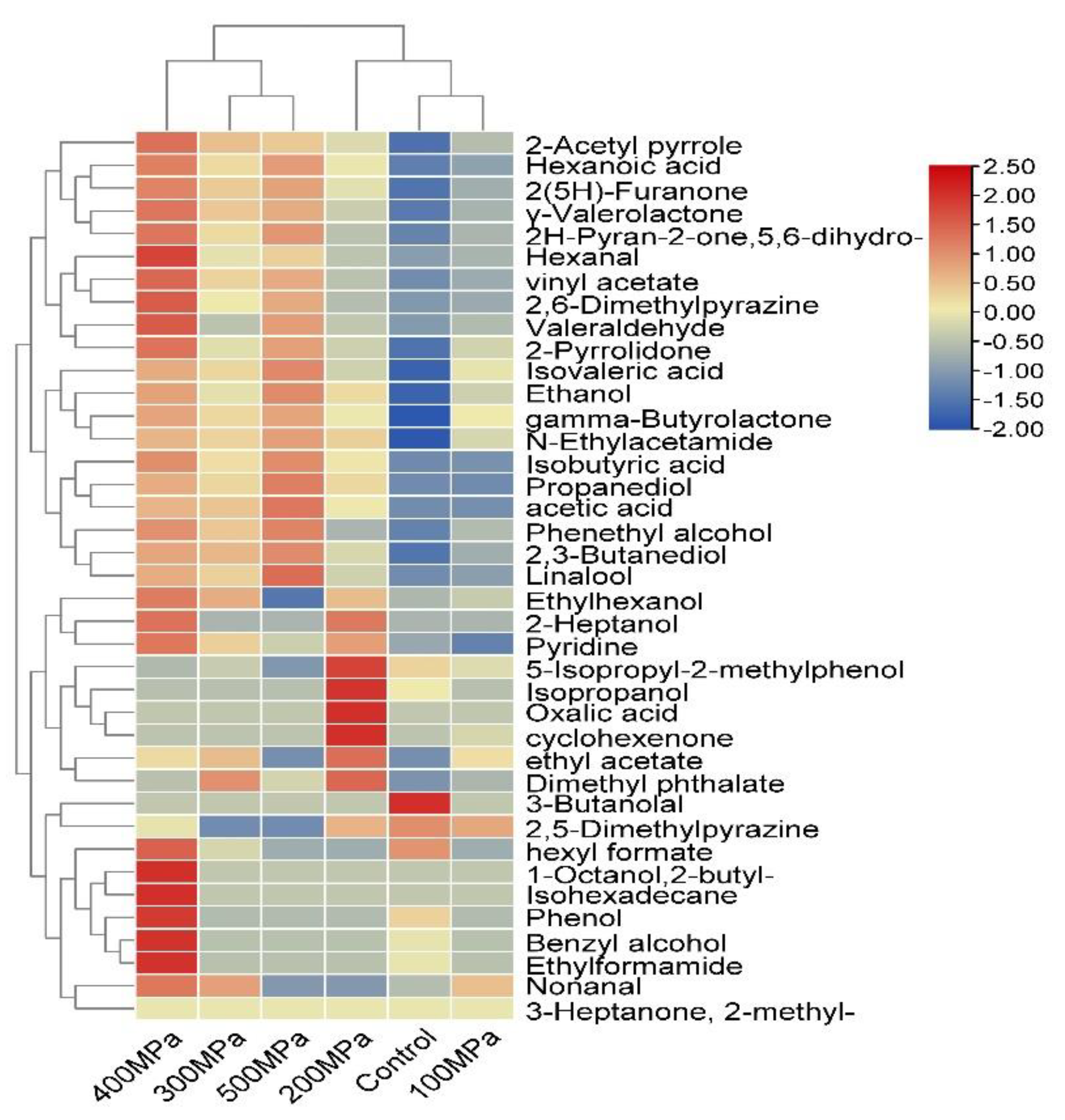
3.1. Analysis of Volatile Flavor Compounds
3.2. E-Nose Data Analysis
3.3. Correlation Analysis between E-Nose and GC-MS
3.4. E-Tongue Data Analysis
3.5. Amino Acids Analysis
3.6. 5′-Nucleotides Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Hu, C.-F.; Feng, X.; Cheng, L.; Ibrahim, S.A.; Wang, C.-T.; Huang, W. Isolation, characterization and antioxidant of polysaccharides from Stropharia rugosoannulata. Int. J. Biol. Macromol. 2020, 155, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, W.; Cui, Y.; Song, X.; Jia, L.; Zhang, J. Stropharia rugoso-annulata acetylated polysaccharides alleviate NAFLD via Nrf2/JNK1/AMPK signaling pathways. Int. J. Biol. Macromol. 2022, 215, 560–570. [Google Scholar] [CrossRef]
- Beluhan, S.; Ranogajec, A. Chemical composition and non-volatile components of Croatian wild edible mushrooms. Food Chem. 2011, 124, 1076–1082. [Google Scholar] [CrossRef]
- Yan, P.-S.; Jiang, J.-H.; Cui, W.-S. Characterization of protoplasts prepared from the edible fungus, Stropharia rugoso-annulata. World J. Microbiol. Biotechnol. 2004, 20, 173–177. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Sun, J.; Luo, Z.-Y.; Rao, S.-Q.; Su, Y.-J.; Xu, R.-R.; Yang, Y.-J. Chemical composition of five wild edible mushrooms collected from Southwest China and their antihyperglycemic and antioxidant activity. Food Chem. Toxicol. 2012, 50, 1238–1244. [Google Scholar] [CrossRef]
- Hu, S.; Feng, X.; Huang, W.; Ibrahim, S.A.; Liu, Y. Effects of drying methods on non-volatile taste components of Stropharia rugoso-annulata mushrooms. LWT 2020, 127, 109428. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, L.-L.; Zhang, H.; Zhang, Y.-Y.; Sun, B.-G. Effects of two sterilization methods on the taste compositions of sweet and sour spare ribs flavor. J. Food Compos. Anal. 2021, 104, 104143. [Google Scholar] [CrossRef]
- Chen, H.; Huang, Y.; Zhou, C.; Xu, T.; Chen, X.; Wu, Q.; Zhang, K.; Li, Y.; Li, D.; Chen, Y. Effects of ultra-high pressure treatment on structure and bioactivity of polysaccharides from large leaf yellow tea. Food Chem. 2022, 387, 132862. [Google Scholar] [CrossRef]
- Sun, L.; Lv, J.; Liu, Y.; Zang, M.; Li, P.; Wang, D.; Zhu, Y.; Xu, W. Effects of combined carnosine and ultra-high pressure on the inhibition of fishy off-odor of snakehead fillets and the possible mechanism. Food Chem. 2022, 395, 133615. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Qiao, Y.; Liao, L.; Shi, D.; Wang, J.; Shi, L. Effects of ultrasound and ultra-high pressure pretreatments on volatile and taste compounds of vacuum-freeze dried strawberry slice. LWT 2022, 160, 113232. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, M.; Fan, H.; Liu, Y. Effect of microwave combined with ultrasonic pretreatment on flavor and antioxidant activity of hydrolysates based on enzymatic hydrolysis of bovine bone. Food Biosci. 2021, 44, 101399. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Liu, S.; Wei, S.; Xia, Q.; Ji, H.; Deng, C.; Hao, J. Extraction of fish oil from fish heads using ultra-high pressure pre-treatment prior to enzymatic hydrolysis. Innov. Food Sci. Emerg. Technol. 2021, 70, 102670. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, X.; Luan, J.; Zhu, W.; Xu, Y.; Yi, S.; Li, J.; Wang, J.; Li, X. Effects of ultrasound pretreatment at different powers on flavor characteristics of enzymatic hydrolysates of cod (Gadus macrocephalus) head. Food Res. Int. 2022, 159, 111612. [Google Scholar] [CrossRef]
- Bai, T.-G.; Zhang, L.; Qian, J.-Y.; Jiang, W.; Wu, M.; Rao, S.-Q.; Li, Q.; Zhang, C.; Wu, C. Pulsed electric field pretreatment modifying digestion, texture, structure and flavor of rice. LWT 2021, 138, 110650. [Google Scholar] [CrossRef]
- Hou, H.; Liu, C.; Lu, X.; Fang, D.; Hu, Q.; Zhang, Y.; Zhao, L. Characterization of flavor frame in shiitake mushrooms (Lentinula edodes) detected by HS-GC-IMS coupled with electronic tongue and sensory analysis: Influence of drying techniques. LWT 2021, 146, 111402. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, M.; Ye, J.; Qian, M.; Li, X.; Zhao, W.; Bai, W. HS-SPME-GC-MS and OAV analyses of characteristic volatile flavour compounds in salt-baked drumstick. LWT 2022, 170, 114041. [Google Scholar] [CrossRef]
- Davila, M.; Muniz, A.; Du, X. The impact of roasting and steaming on savory flavors contributed by amino acids, 5′-nucleotides, and volatiles in Agaricus bisporus mushrooms. Int. J. Gastron. Food Sci. 2022, 30, 100590. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wang, Y.; Wang, J.; Xu, Y.; Yi, S.; Zhu, W.; Mi, H.; Li, T.; Li, J. Combined ultrasound and heat pretreatment improve the enzymatic hydrolysis of clam (Aloididae aloidi) and the flavor of hydrolysates. Innov. Food Sci. Emerg. Technol. 2021, 67, 102596. [Google Scholar] [CrossRef]
- Lee, S.H.; Jung, J.Y.; Jeon, C.O. Bacterial community dynamics and metabolite changes in myeolchi-aekjeot, a Korean traditional fermented fish sauce, during fermentation. Int. J. Food Microbiol. 2015, 203, 15–22. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, C.; Jiang, B.; Mo, X.; Wang, Z. Optimization of HS-SPME for GC-MS Analysis and Its Application in Characterization of Volatile Compounds in Sweet Potato. Molecules 2021, 26, 5808. [Google Scholar] [CrossRef]
- Cao, J.; Zou, X.-G.; Deng, L.; Fan, Y.-W.; Li, H.; Li, J.; Deng, Z.-Y. Analysis of nonpolar lipophilic aldehydes/ketones in oxidized edible oils using HPLC-QqQ-MS for the evaluation of their parent fatty acids. Food Res. Int. 2014, 64, 901–907. [Google Scholar] [CrossRef]
- Li, P.; Zhou, H.; Wang, Z.; Al-Dalali, S.; Nie, W.; Xu, F.; Li, C.; Li, P.; Cai, K.; Xu, B. Analysis of flavor formation during the production of Jinhua dry-cured ham using headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS). Meat Sci. 2022, 194, 108992. [Google Scholar] [CrossRef]
- Pongsetkul, J.; Benjakul, S.; Vongkamjan, K.; Sumpavapol, P.; Osako, K.; Faithong, N. Changes in volatile compounds, ATP-related compounds and antioxidative properties of Kapi, produced from Acetes vulgaris, during processing and fermentation. Food Biosci. 2017, 19, 49–56. [Google Scholar] [CrossRef]
- Yu, H.; Xie, T.; Xie, J.; Ai, L.; Tian, H. Characterization of key aroma compounds in Chinese rice wine using gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Chem. 2019, 293, 8–14. [Google Scholar] [CrossRef]
- Maggi, F.; Papa, F.; Cristalli, G.; Sagratini, G.; Vittori, S. Characterisation of the mushroom-like flavour of Melittis melissophyllum L. subsp. melissophyllum by headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography (GC–FID) and gas chromatography–mass spectrometry (GC–MS). Food Chem. 2010, 123, 983–992. [Google Scholar] [CrossRef]
- Kasote, D.; Singh, V.K.; Bollinedi, H.; Singh, A.K.; Sreenivasulu, N.; Regina, A. Profiling of 2-Acetyl-1-Pyrroline and Other Volatile Compounds in Raw and Cooked Rice of Traditional and Improved Varieties of India. Foods 2021, 10, 1917. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Li, L.; Wang, S.; Liu, Y.; Gao, M. Effect of enzymatic hydrolysis on volatile flavor compounds of Monascus-fermented tartary buckwheat based on headspace gas chromatography-ion mobility spectrometry. Food Res. Int. 2023, 163, 112180. [Google Scholar] [CrossRef]
- Weng, Z.; Sun, L.; Wang, F.; Sui, X.; Fang, Y.; Tang, X.; Shen, X. Assessment the flavor of soybean meal hydrolyzed with Alcalase enzyme under different hydrolysis conditions by E-nose, E-tongue and HS-SPME-GC–MS. Food Chem. X 2021, 12, 100141. [Google Scholar] [CrossRef]
- Yu, M.; He, S.; Tang, M.; Zhang, Z.; Zhu, Y.; Sun, H. Antioxidant activity and sensory characteristics of Maillard reaction products derived from different peptide fractions of soybean meal hydrolysate. Food Chem. 2018, 243, 249–257. [Google Scholar] [CrossRef]
- Shahneela, M.; Kieran, N.K.; Colin, H.; Olivia, M. A Systems-Wide Analysis of Proteolytic and Lipolytic Pathways Uncovers The Flavor-Forming Potential of The Gram-Positive Bacterium Macrococcus caseolyticus subsp. Caseolyticus. Front. Microbiol. 2020, 11, 1533. [Google Scholar]
- Harada-Padermo, S.D.S.; Dias-Faceto, L.S.; Selani, M.M.; Alvim, I.D.; Floh, E.I.S.; Macedo, A.F.; Bogusz, S.; Dias, C.T.D.S.; Conti-Silva, A.C.; Vieira, T.M.F.S. Umami Ingredient: Flavor enhancer from shiitake (Lentinula edodes) byproducts. Food Res. Int. 2020, 137, 109540. [Google Scholar] [CrossRef]
- Moerdijk-Poortvliet, T.C.W.; De Jong, D.L.C.; Fremouw, R.; De Reu, S.; De Winter, J.M.; Timmermans, K.; Mol, G.; Reuter, N.; Derksen, G.C.H. Extraction and analysis of free amino acids and 5′-nucleotides, the key contributors to the umami taste of seaweed. Food Chem. 2022, 370, 131352. [Google Scholar] [CrossRef]
- Gao, J.; Fang, D.; Kimatu, B.M.; Chen, X.; Wu, X.; Du, J.; Yang, Q.; Chen, H.; Zheng, H.; An, X.; et al. Analysis of umami taste substances of morel mushroom (Morchella sextelata) hydrolysates derived from different enzymatic systems. Food Chem. 2021, 362, 130192. [Google Scholar] [CrossRef]
- Kawai, M.; Okiyama, A.; Ueda, Y. Taste Enhancements Between Various Amino Acids and IMP. Chem. Senses 2002, 27, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Liu, W.; Li, L.; Cao, W.; Zhao, M.; Dong, J.; Ren, G.; Bhandari, B.; Duan, X. Dynamic changes of non-volatile compounds and evaluation on umami during infrared assisted spouted bed drying of shiitake mushrooms. Food Control 2022, 142, 109245. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, H.; Wu, W.; Chen, H.; Fang, X.; Han, Y.; Mu, H. Effects of fermentation with different microbial species on the umami taste of Shiitake mushroom (Lentinus edodes). LWT 2021, 141, 110889. [Google Scholar] [CrossRef]
- Yue, J.; Zhang, Y.; Jin, Y.; Deng, Y.; Zhao, Y. Impact of high hydrostatic pressure on non-volatile and volatile compounds of squid muscles. Food Chem. 2016, 194, 12–19. [Google Scholar] [CrossRef]
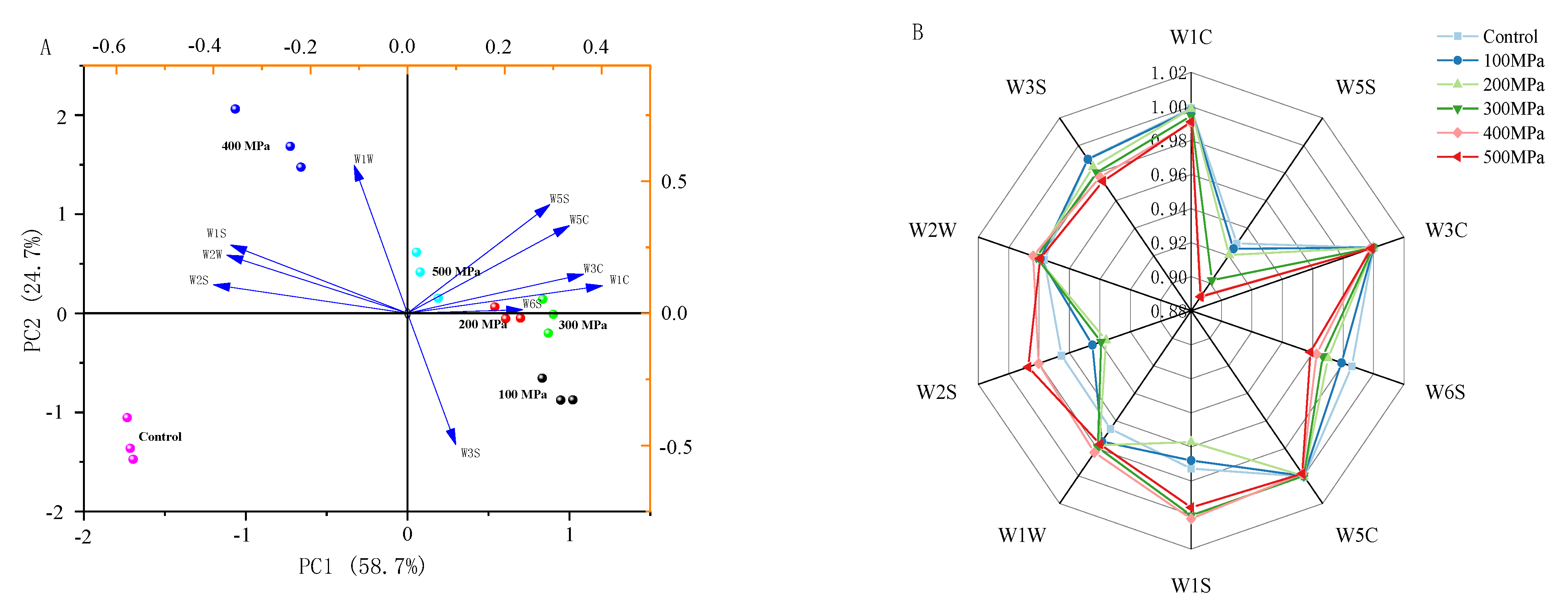
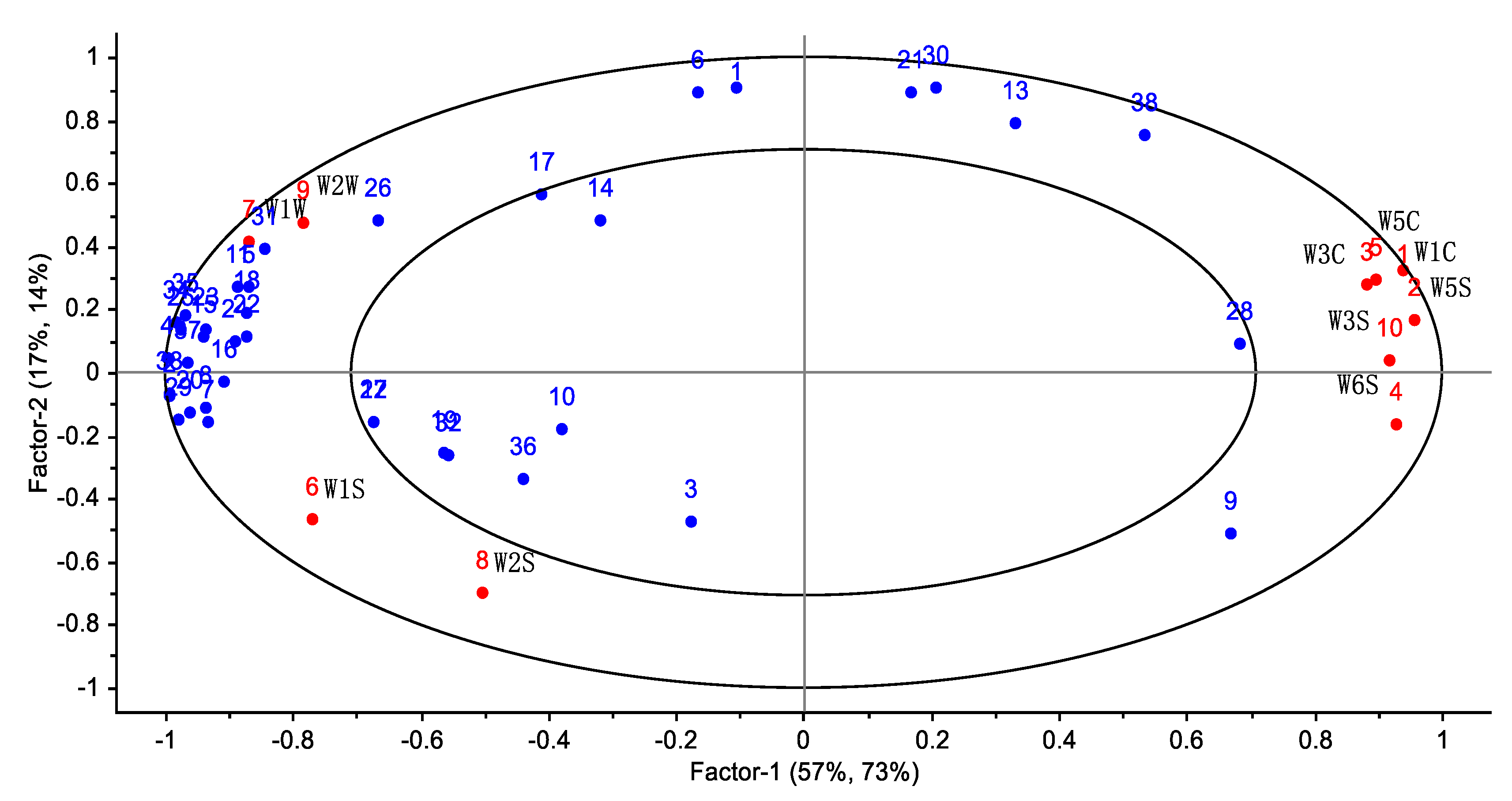
| Array Serial Number | Sensor | Sensing Substance |
|---|---|---|
| 1 | W1C | Sensitive to aromatic compounds, benzene |
| 2 | W5S | High sensitivity to nitrogen oxides |
| 3 | W3C | Sensitive to ammonia and aromatic compounds |
| 4 | W6S | Selective for hydrogenation |
| 5 | W5C | Sensitive to short-chain alkanes, aromatic compounds |
| 6 | W1S | Sensitive to methyl groups |
| 7 | W1W | Sensitive to sulfides, pyrazines |
| 8 | W2S | Sensitive to alcohols, aldehydes and ketones |
| 9 | W2W | Sensitive to aromatic components, organic sulfides |
| 10 | W3S | Sensitive to long-chain alkanes |
| Count | RT | Volatile Compounds | RI | Content (μg/g) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 100 MPa | 200 MPa | 300 MPa | 400 MPa | 500 MPa | ||||
| 1 | 2.75 | ethyl acetate | 611.34 | ND | 0.0098 ± 0.0001 c | 0.0184 ± 0.0008 a | 0.0123 ± 0.0022 b | 0.0101 ± 0.0003 c | ND |
| 2 | 4.30 | vinyl acetate | 972.28 | ND | 0.0054 ± 0.0002 c | 0.0093 ± 0.0013 c | 0.0194 ± 0.0037 b | 0.0342 ± 0.0056 a | 0.0248 ± 0.0097 b |
| 3 | 15.69 | hexyl formate | 918.62 | 0.0069 ± 0.0012 b | ND | ND | 0.0022 ± 0.0008 c | 0.0090 ± 0.0010 a | ND |
| 4 | 22.38 | γ-Valerolactone | 958.24 | 0.0109 ± 0.0006 d | 0.0328 ± 0.0030 c | 0.0424 ± 0.0077 c | 0.0637 ± 0.0039 b | 0.0876 ± 0.0048 a | 0.0720 ± 0.0170 b |
| 5 | 22.85 | gamma-Butyrolactone | 915.25 | 0.0175 ± 0.0003 c | 0.0437 ± 0.0005 b | 0.0433 ± 0.0005 b | 0.0464 ± 0.0063 ab | 0.0536 ± 0.0062 a | 0.0535 ± 0.0038 a |
| 6 | 36.43 | Dimethyl phthalate | 1454.58 | 0.0069 ± 0.0020 e | 0.0234 ± 0.0011 d | 0.0953 ± 0.0046 a | 0.0798 ± 0.0044 b | 0.0282 ± 0.0030 d | 0.0372 ± 0.0074 c |
| 7 | 3.11 | Valeraldehyde | 715.01 | 0.0044 ± 0.0005 d | 0.0108 ± 0.0011 c | 0.0129 ± 0.0049 c | 0.0121 ± 0.0013 c | 0.0404 ± 0.0026 a | 0.0305 ± 0.0060 b |
| 8 | 7.19 | Hexanal | 1070.32 | 0.0331 ± 0.0043 d | 0.0387 ± 0.0008 cd | 0.0422 ± 0.0080 cd | 0.0493 ± 0.0028 bc | 0.0822 ± 0.0034 a | 0.0563 ± 0.0058 b |
| 9 | 12.56 | 3-Butanolal | 895.21 | 0.0035 ± 0.0005 a | ND | ND | ND | ND | ND |
| 10 | 16.73 | Nonanal | 1104.74 | 0.0028 ± 0.0005 bc | 0.0087 ± 0.0008 abc | ND | 0.0105 ± 0.0092 ab | 0.0129 ± 0.0069 a | ND |
| 11 | 3.40 | Ethanol | 545.36 | ND | 0.1109 ± 0.0015 b | 0.1509 ± 0.0112 ab | 0.1279 ± 0.0725 b | 0.1946 ± 0.0158 b | 0.2127 ± 0.0341 a |
| 12 | 7.68 | 1-Octanol,2-butyl- | 452.01 | ND | ND | ND | ND | 0.0159 ± 0.0075 a | ND |
| 13 | 13.72 | Isopropanol | 934.65 | 0.0030 ± 0.0002 b | ND | 0.0130 ± 0.0068 a | ND | ND | ND |
| 14 | 19.39 | Ethylhexanol | 1030.87 | 0.0117 ± 0.0004 b | 0.0153 ± 0.0025 b | 0.0270 ± 0.0080 a | 0.0292 ± 0.0091 a | 0.0362 ± 0.0050 a | ND |
| 15 | 20.74 | 2,3-Butanediol | 789.46 | 0.0102 ± 0.0014 d | 0.0757 ± 0.0011 c | 0.1218 ± 0.0231 c | 0.1888 ± 0.0324 b | 0.2035 ± 0.0057 ab | 0.2264 ± 0.0266 a |
| 16 | 20.86 | Linalool | 1099.77 | 0.0092 ± 0.0002 b | 0.0132 ± 0.0013 b | 0.0245 ± 0.1535 a | 0.0344 ± 0.0058 b | 0.0406 ± 0.0028 b | 0.0516 ± 0.0104 b |
| 17 | 21.21 | 2-Heptanol | 900.26 | ND | ND | 0.0087 ± 0.0012 a | ND | 0.0090 ± 0.0030 a | ND |
| 18 | 22.04 | Propanediol | 740.33 | ND | ND | 0.0447 ± 0.0077 b | 0.0452 ± 0.0054 b | 0.0587 ± 0.0034 ab | 0.0730 ± 0.0250 a |
| 19 | 28.54 | Benzyl alcohol | 1036.78 | 0.0057 ± 0.0008 b | ND | ND | ND | 0.0309 ± 0.0011 a | ND |
| 20 | 29.30 | Phenethyl alcohol | 1116.46 | 0.0070 ± 0.0008 d | 0.0257 ± 0.0025 c | 0.0238 ± 0.0040 c | 0.0497 ± 0.0095 b | 0.0633 ± 0.0079 a | 0.0664 ± 0.0066 a |
| 21 | 11.67 | Oxalic acid | 1023.01 | ND | ND | 0.0024 ± 0.0021 a | ND | ND | ND |
| 22 | 18.70 | acetic acid | 612.58 | 0.0241 ± 0.0003 d | 0.0287 ± 0.0016 d | 0.1841 ± 0.0110 c | 0.2354 ± 0.0369 bc | 0.2570 ± 0.0440 b | 0.3410 ± 0.0440 a |
| 23 | 21.60 | Isobutyric acid | 774.38 | 0.0087 ± 0.007 c | 0.0148 ± 0.0004 c | 0.1002 ± 0.0118 b | 0.1048 ± 0.0004 b | 0.1617 ± 0.0400 a | 0.1624 ± 0.0388 a |
| 24 | 23.98 | Isovaleric acid | 863.34 | 0.0176 ± 0.0007 d | 0.1527 ± 0.0005 bc | 0.1339 ± 0.0012 c | 0.1757 ± 0.0063 b | 0.2114 ± 0.0231 a | 0.2401 ± 0.0400 a |
| 25 | 27.89 | Hexanoic acid | 990.12 | 0.0049 ± 0.0020 b | 0.0087 ± 0.0008 b | 0.0165 ± 0.0013 ab | 0.0184 ± 0.0018 ab | 0.0262 ± 0.0026 a | 0.0237 ± 0.0184 a |
| 26 | 10.35 | Pyridine | 819.76 | 0.1750 ± 0.0060 cd | 0.1384 ± 0.0011 d | 0.2939 ± 0.0073 ab | 0.2580 ± 0.1100 abc | 0.3235 ± 0.0231 a | 0.2127 ± 0.0332 bcd |
| 27 | 13.52 | Isohexadecane | 326.15 | ND | ND | ND | ND | 0.0875 ± 0.0037 a | ND |
| 28 | 14.61 | 2,5-Dimethylpyrazine | 923.12 | 0.0034 ± 0.0005 a | 0.0030 ± 0.0020 a | 0.0028 ± 0.0013 a | ND | 0.0018 ± 0.0003 ab | ND |
| 29 | 14.81 | 2,6-Dimethylpyrazine | 917.03 | 0.0065 ± 0.0023 d | 0.0102 ± 0.0023 d | 0.0143 ± 0.0016 d | 0.0236 ± 0.0038 c | 0.0464 ± 0.0063 a | 0.0338 ± 0.0089 b |
| 30 | 17.69 | cyclohexenone | 920.51 | ND | 0.0012 ± 0.0012 b | 0.0121 ± 0.0013 a | ND | ND | ND |
| 31 | 23.02 | N-Ethylacetamide | 877.47 | ND | 0.0483 ± 0.0013 c | 0.0639 ± 0.0050 b | 0.0629 ± 0.0083 b | 0.0720 ± 0.0090 ab | 0.0786 ± 0.0026 a |
| 32 | 23.38 | Ethylformamide | 947.51 | 0.0028 ± 0.007 b | ND | ND | ND | 0.0145 ± 0.0126 a | ND |
| 33 | 24.39 | 2 H-Pyran-2-one,5,6-dihydro- | 916.41 | 0.0287 ± 0.0008 e | 0.0931 ± 0.0034 d | 0.1089 ± 0.0087 d | 0.1741 ± 0.0091 c | 0.2738 ± 0.0263 a | 0.2417 ± 0.0289 b |
| 34 | 25.79 | 2(5 H)-Furanone | 918.52 | 0.0140 ± 0.0019 e | 0.0321 ± 0.0028 d | 0.0476 ± 0.0074 c | 0.0583 ± 0.0038 b | 0.0752 ± 0.0041 a | 0.0681 ± 0.0061 a |
| 35 | 30.65 | 2-Acetyl pyrrole | 1064.65 | 0.0151 ± 0.0002 a | 0.0201 ± 0.0008 a | 0.0219 ± 0.0022 a | 0.0250 ± 0.0170 a | 0.0289 ± 0.0058 a | 0.0245 ± 0.0030 a |
| 36 | 31.44 | Phenol | 980.38 | 0.0021 ± 0.0003 b | ND | ND | ND | 0.0059 ± 0.0064 a | ND |
| 37 | 31.87 | 2-Pyrrolidone | 1076.53 | 0.0121 ± 0.0009 d | 0.0548 ± 0.0033 c | 0.0533 ± 0.0035 c | 0.0599 ± 0.0011 c | 0.1061 ± 0.002 a | 0.0905 ± 0.0097 b |
| 38 | 34.65 | 5-Isopropyl-2-methylphenol | 1299.34 | 0.0032 ± 0.0003 b | 0.0022 ± 0.0010 bc | 0.0068 ± 0.0019 a | 0.0017 ± 0.0007 bc | 0.0011 ± 0.0018 bc | ND |
| Sensors | Taste Value | |||||
|---|---|---|---|---|---|---|
| Control | 100 MPa | 200 MPa | 300 MPa | 400 MPa | 500 MPa | |
| Bitterness | 4.090 ± 0.030 d | 4.737 ± 0.078 b | 5.140 ± 0.133 a | 4.577 ± 0.091 c | 3.613 ± 0.101 e | 4.197 ± 0.004 d |
| Aftertaste-B | −0.190 ± 0.050 b | −0.167 ± 0.018 b | −0.330 ± 0.011 c | −0.197 ± 0.013 b | −0.577 ± 0.079 d | 0.023 ± 0.011 a |
| Umami | 11.007 ± 0.343 d | 13.120 ± 0.015 bc | 13.267 ± 0.035 bc | 13.283 ± 0.119 b | 13.930 ± 0.051 a | 12.987 ± 0.016 c |
| Richness | 1.740 ± 0.110 e | 2.360 ± 0.061 b | 2.600 ± 0.016 a | 2.240 ± 0.080 c | 1.940 ± 0.041 d | 2.207 ± 0.006 c |
| Saltiness | 6.570 ± 0.080 a | 6.030 ± 0.036 b | 5.940 ± 0.004 b | 5.120 ± 0.118 c | 4.210 ± 0.020 e | 4.550 ± 0.095 d |
| Sweetness | 8.790 ± 0.070 b | 9.120 ± 0.022 a | 9.200 ± 0.253 a | 9.210 ± 0.205 a | 9.350 ± 0.052 a | 9.320 ± 0.085 a |
| Amino Acid | Content (g/100 g) | |||||
|---|---|---|---|---|---|---|
| Control | 100 MPa | 200 MPa | 300 MPa | 400 MPa | 500 MPa | |
| Aspartic acid (Asp) | 2.54 ± 0.18 a | 2.53 ± 0.05 a | 2.54 ± 0.09 a | 2.55 ± 0.08 a | 2.59 ± 0.03 a | 2.59 ± 0.05 a |
| Glutamic acid (Glu) | 5.21 ± 0.23 b | 5.36 ± 0.10 ab | 5.44 ± 0.58 ab | 5.49 ± 0.04 ab | 5.89 ± 0.28 a | 5.61 ± 0.12 ab |
| Threonine (Thr) | 1.48 ± 0.10 a | 1.51 ± 0.06 a | 1.50 ± 0.20 a | 1.52 ± 0.12 a | 1.53 ± 0.09 a | 1.52 ± 0.02 a |
| Serine (Ser) | 1.52 ± 0.06 a | 1.58 ± 0.06 a | 1.62 ± 0.06 a | 1.65 ± 0.07 a | 1.67 ± 0.04 a | 1.62 ± 0.16 a |
| Glycine (Gly) | 1.48 ± 0.09 a | 1.52 ± 0.10 a | 1.54 ± 0.06 a | 1.48 ± 0.05 a | 1.49 ± 0.03 a | 1.51 ± 0.13 a |
| Proline (Pro) | 1.20 ± 0.16 b | 1.19 ± 0.17 b | 1.21 ± 0.12 b | 1.23 ± 0.11 b | 1.24 ± 0.05 b | 1.56 ± 0.06 a |
| Alanine (Ala) | 2.54 ± 0.08 c | 2.72 ± 0.13 ab | 2.77 ± 0.21 ab | 2.78 ± 0.24 ab | 2.91 ± 0.11 a | 2.93 ± 0.06 a |
| Phenylalanine (Phe) | 1.45 ± 0.08 a | 1.39 ± 0.03 ab | 1.34 ± 0.06 abc | 1.28 ± 0.04 bc | 1.23 ± 0.08 c | 1.26 ± 0.06 c |
| Histidine (His) | 1.35 ± 0.14 a | 1.29 ± 0.01 a | 1.25 ± 0.06 a | 0.98 ± 0.14 b | 0.97 ± 0.05 b | 1.03 ± 0.09 b |
| Valine (Val) | 1.85 ± 0.07 a | 1.82 ± 0.14 ab | 1.75 ± 0.06 abc | 1.62 ± 0.03 c | 1.60 ± 0.11 c | 1.66 ± 0.12 bc |
| Methionine (Met) | 0.38 ± 0.14 a | 0.38 ± 0.07 a | 0.36 ± 0.09 a | 0.29 ± 0.07 a | 0.25 ± 0.09 a | 0.24 ± 0.08 a |
| Isoleucine (Ile) | 1.61 ± 0.02 a | 1.59 ± 0.11 a | 1.35 ± 0.06 bc | 1.42 ± 0.03 b | 1.25 ± 0.09 c | 1.27 ± 0.08 c |
| Leucine (Leu) | 2.49 ± 0.12 a | 2.22 ± 0.19 ab | 2.09 ± 0.03 b | 1.98 ± 0.22 b | 1.99 ± 0.13 b | 2.04 ± 0.17 b |
| Arginine (Arg) | 1.02 ± 0.01 a | 0.84 ± 0.12 a | 0.39 ± 0.12 b | 0.42 ± 0.11 b | 0.36 ± 0.09 b | 0.30 ± 0.20 b |
| Tyrosine (Tyr) | 0.98 ± 0.11 a | 0.99 ± 0.23 a | 1.11 ± 0.10 a | 1.08 ± 0.76 a | 1.00 ± 0.22 a | 1.00 ± 0.02 a |
| Lysine (Lys) | 1.29 ± 0.10 c | 1.32 ± 0.02 bc | 1.52 ± 0.04 a | 1.48 ± 1.04 ab | 1.31 ± 0.01 bc | 1.41 ± 0.15 abc |
| Cysteine (Cys) | 1.21 ± 0.15 bc | 1.34 ± 0.06 ab | 1.47 ± 0.06 a | 1.02 ± 0.72 cd | 0.96 ± 0.15 d | 1.06 ± 0.06 cd |
| Umami | 7.75 ± 0.09 c | 7.89 ± 0.11 c | 7.98 ± 0.05 b | 8.04 ± 0.06 b | 8.48 ± 0.25 a | 8.20 ± 0.07 ab |
| Sweet | 8.22 ± 0.12 d | 8.52 ± 0.02 d | 8.64 ± 0.02 c | 8.66 ± 0.15 c | 8.84 ± 0.16 b | 9.14 ± 0.12 a |
| Bitter | 10.15 ± 0.16 a | 9.53 ± 0.07 ab | 8.53 ± 0.03 b | 7.99 ± 0.34 b | 7.65 ± 0.05 c | 7.8 ± 0.09 bc |
| 5′-Nucleotides | Content (mg/100 g) | |||||
|---|---|---|---|---|---|---|
| Control | 100 MPa | 200 MPa | 300 MPa | 400 MPa | 500 MPa | |
| CMP | 411.753 ± 0.251 c | 412.024 ± 0.288 c | 395.293 ± 0.331 d | 413.162 ± 0.398 b | 414.409 ± 0.594 a | 412.711 ± 0.147 b |
| GMP | 4.785 ± 0.126 b | 4.896 ± 0.327 b | 4.823 ± 0.279 b | 5.027 ± 0.041 ab | 5.364 ± 0.162 a | 5.032 ± 0.181 ab |
| IMP | 37.497 ± 0.495 f | 39.243 ± 0.757 d | 40.075 ± 0.206 c | 40.891 ± 0.132 b | 43.098 ± 0.027 a | 38.298 ± 0.123 e |
| UMP | 23.419 ± 0.477 b | 23.512 ± 0.015 a | 23.579 ± 0.149 a | 23.075 ± 0.046 bc | 21.914 ± 0.096 c | 22.379 ± 0.516 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, C.; Xin, M.; Su, K.; Guan, C.; Wang, D. Effects of Ultra-High Pressure Synergistic Enzymatic Hydrolysis on Flavor of Stropharia rugoso-annulata. Foods 2023, 12, 848. https://doi.org/10.3390/foods12040848
Bao C, Xin M, Su K, Guan C, Wang D. Effects of Ultra-High Pressure Synergistic Enzymatic Hydrolysis on Flavor of Stropharia rugoso-annulata. Foods. 2023; 12(4):848. https://doi.org/10.3390/foods12040848
Chicago/Turabian StyleBao, Chenligen, Minghang Xin, Keyu Su, Chunbo Guan, and Dawei Wang. 2023. "Effects of Ultra-High Pressure Synergistic Enzymatic Hydrolysis on Flavor of Stropharia rugoso-annulata" Foods 12, no. 4: 848. https://doi.org/10.3390/foods12040848
APA StyleBao, C., Xin, M., Su, K., Guan, C., & Wang, D. (2023). Effects of Ultra-High Pressure Synergistic Enzymatic Hydrolysis on Flavor of Stropharia rugoso-annulata. Foods, 12(4), 848. https://doi.org/10.3390/foods12040848





