Microbial Carotenoid Synthesis Optimization in Goat Cheese Whey Using the Robust Taguchi Method: A Sustainable Approach to Help Tackle Vitamin A Deficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Medium Based on GMW Preparation
2.2. Enzymatic Hydrolysis of Goat Milk Whey
2.3. Inoculum Preparation
2.4. Culture Conditions and Experimental Design
2.5. Spectrophotometric Quantification of Total Carotenoids
2.6. Biomass Quantification
2.7. Analysis of Carotenoids
2.7.1. Sample Preparation
2.7.2. HPLC Analysis
2.8. Data Analysis
3. Results
3.1. Enzymatic Hydrolysis of Goat Milk Whey
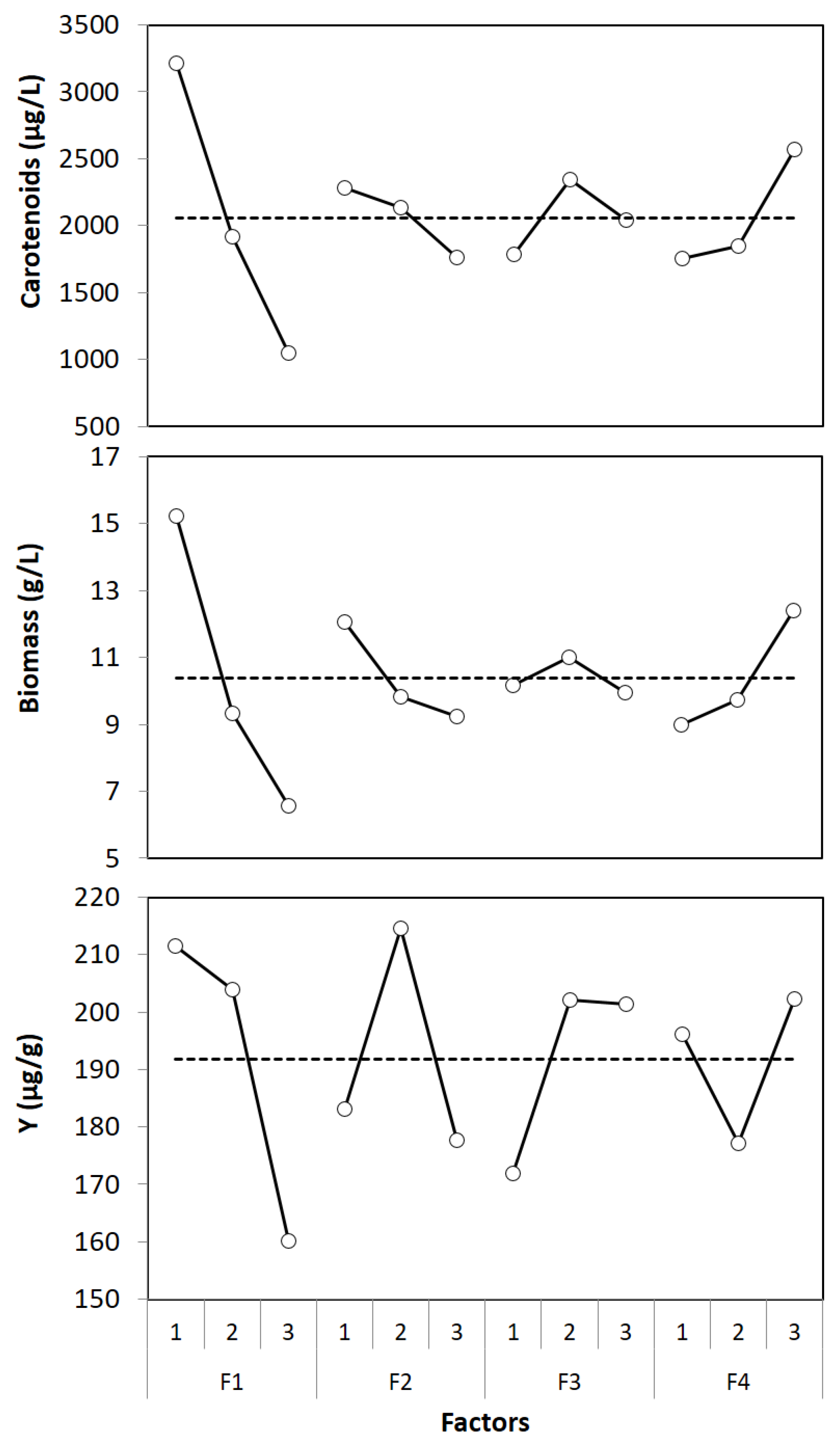
3.2. Optimization by Taguchi Method
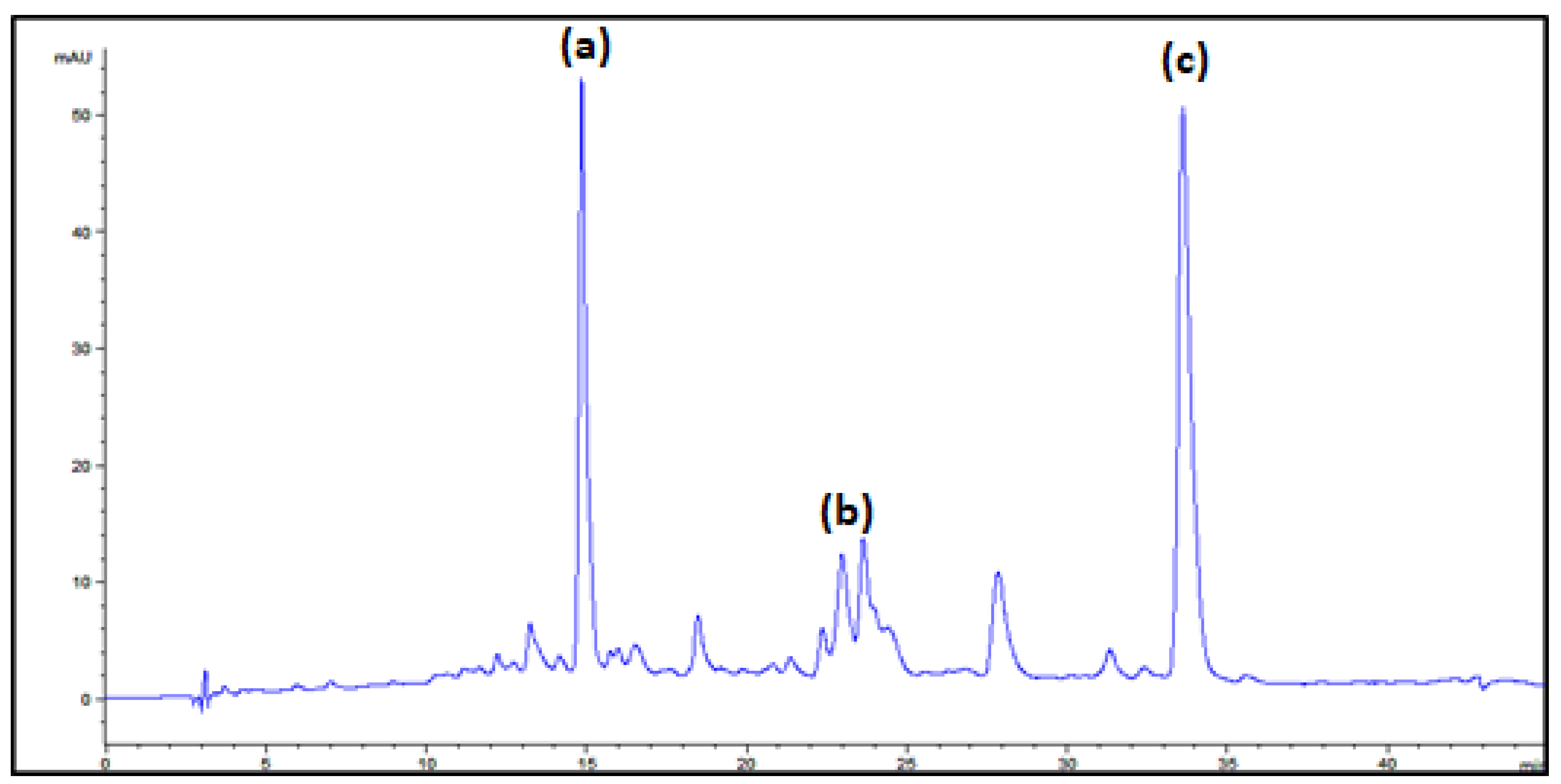
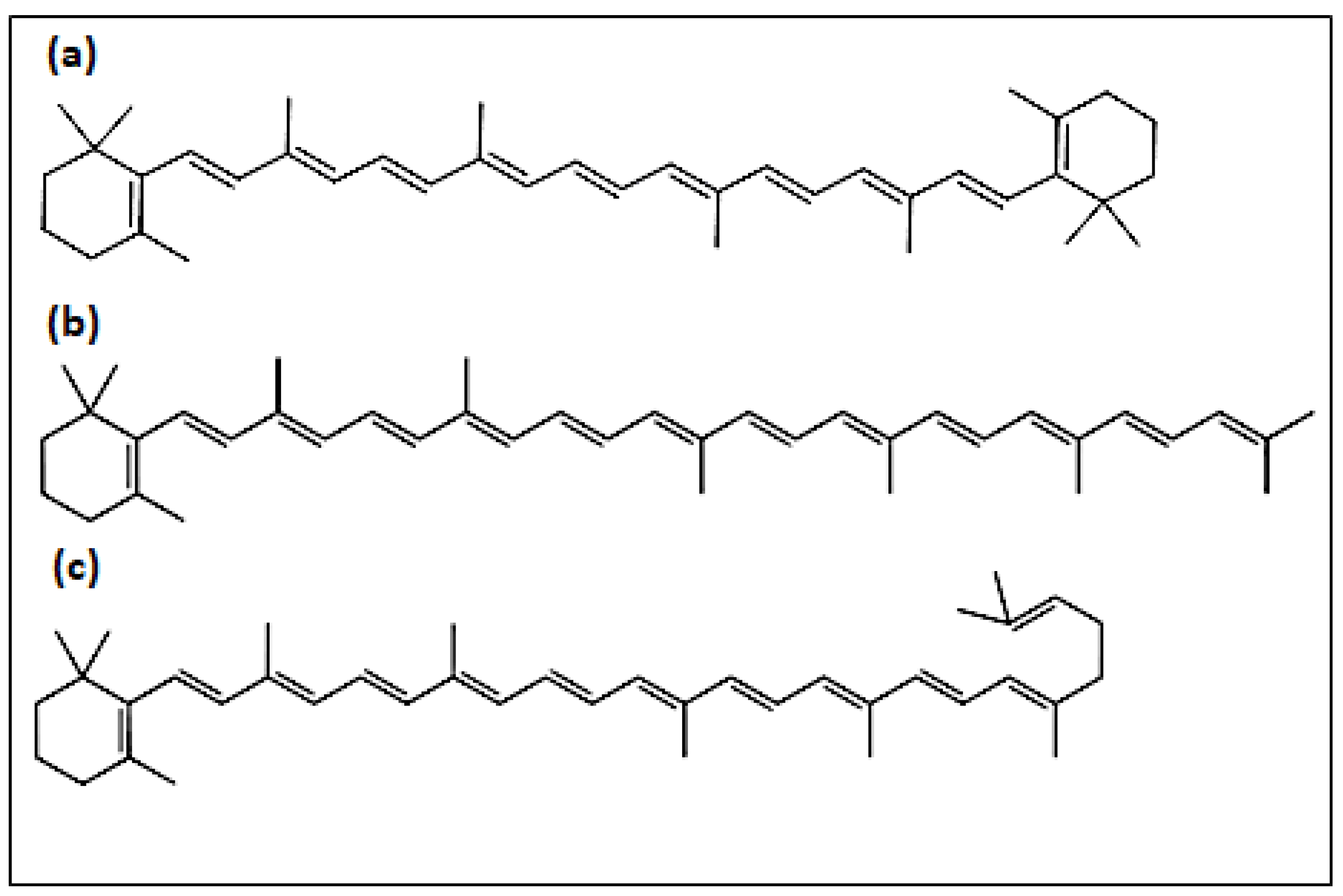
3.3. Carotenoid Identification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Melén-dez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carote-noids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef]
- BBC Publishing Staff. The Global Market for Carotenoids BBC Research Report; BBC Publishing: London, UK, 2022. [Google Scholar]
- Meléndez-Martínez, A.J. An Overview of Carotenoids, Apocarotenoids, and Vitamin A in Agro-Food, Nutrition, Health, and Disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar] [CrossRef]
- Maoka, T. Recent progress in structural studies of carotenoids in animals and plants. Arch. Biochem. Biophys. 2009, 483, 191–195. [Google Scholar] [CrossRef]
- da Costa Cardoso, L.A.; Kanno, K.Y.F.; Karp, S.G. Microbial production of carotenoids A review. Afr. J. Biotechnol. 2017, 16, 139–146. [Google Scholar] [CrossRef]
- Dyaa, A.; Soliman, H.; Abdelrazak, A.; Samra, B.N.; Khojah, E.; Ahmed, A.F.; El-Esawi, M.A.; Elsayed, A. Optimization of Carotenoids Production from Rhodotorula sp. Strain ATL72 for Enhancing Its Biotechnological Applications. J. Fungi 2022, 8, 160. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Khan, S.; Kot, A.M. Current developments on the application of microbial carotenoids as an alternative to syn-thetic pigments. Crit. Rev. Food Sci. Nutr. 2021, 62, 6932–6946. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Factories 2014, 13, 12. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Ribeiro, H.F.; Santos-Ebinuma, V.C.; Schuur, B.; Pereira, J.F.B. Rhodotorula sp.–based biorefinery: A source of valuable biomolecules. Appl. Microbiol. Biotechnol. 2022, 106, 7431–7447. [Google Scholar] [CrossRef]
- Roukas, T.; Varzakakou, M.; Kotzekidou, P. From Cheese Whey to Carotenes by Blakeslea trispora in a Bubble Column Reactor. Appl. Biochem. Biotechnol. 2015, 175, 182–193. [Google Scholar] [CrossRef]
- Colet, R.; Urnau, L.; Bampi, J.; Zeni, J.; Dias, B.B.; Rodrigues, E.; Jacques, R.A.; Di Luccio, M.; Valduga, E. Use of low-cost agro products as substrate in semi-continuous process to obtain carotenoids by Sporidiobolus salmonicolor. Biocatal. Agric. Biotechnol. 2017, 11, 268–274. [Google Scholar] [CrossRef]
- Bonadio, M.D.P.; de Freita, L.A.; Mutton, M.J.R. Carotenoid production in sugarcane juice and synthetic media supplemented with nutrients by Rhodotorula rubra l02. Braz. J. Microbiol. 2018, 49, 872–878. [Google Scholar] [CrossRef]
- Villegas-Méndez, M.A.; Aguilar-Machado, D.E.; Balagurusamy, N.; Montañez, J.; Morales-Oyervides, L. Agro-industrial wastes for the synthesis of carotenoids by Xanthophyllomyces dendrorhous: Mesquite pods-based medium design and optimization. Biochem. Eng. J. 2019, 150, 107260. [Google Scholar] [CrossRef]
- Urnau, L.; Colet, R.; Reato, P.T.; Fernandes de Medeiros Burkert, J.; Rodrigues, E.; Gomes, R.D.P.; Jacques, R.; Valduga, E.; Steffens, C. Use of Low-Cost Agro-Industrial Substrate to Obtain Carotenoids from Phaffia rhodozyma in a Bioreactor. Ind. Biotechnol. 2019, 15, 25–34. [Google Scholar] [CrossRef]
- Liu, Z.; Feist, A.M.; Dragone, G.; Mussatto, S.I. Lipid and carotenoid production from wheat straw hydrolysates by different oleaginous yeasts. J. Clean. Prod. 2020, 249, 119308. [Google Scholar] [CrossRef]
- Villegas-Méndez, M.Á.; Papadaki, A.; Pateraki, C.; Balagurusamy, N.; Montañez, J.; Koutinas, A.A.; Morales-Oyervides, L. Fed-batch bioprocess development for astaxanthin production by Xanthophyllomyces dendrorhous based on the utilization of Prosopis sp. pods extract. Biochem. Eng. J. 2021, 166, 107844. [Google Scholar] [CrossRef]
- Lakshmidevi, R.; Ramakrishnan, B.; Ratha, S.K.; Bhaskar, S.; Chinnasamy, S. Valorisation of molasses by oleaginous yeasts for single cell oil (SCO) and carotenoids production. Environ. Technol. Innov. 2021, 21, 101281. [Google Scholar] [CrossRef]
- SIAP-SAGARPA Avance de la Producción Pecuaria por Producto. Leche de Caprino. Available online: http://infosiap.siap.gob.mx/repoAvance_siap_gb/pecAvanceProd.jsp (accessed on 21 November 2022).
- Tajonar, K.; Díaz, C.A.L.; Ibarra, L.E.S.; Chay-Canul, A.J.; Gonzalez-Ronquillo, M.; Vargas-Bello-Pérez, E. A Brief Update on the Challenges and Prospects for Goat Production in Mexico. Animals 2022, 12, 837. [Google Scholar] [CrossRef]
- Cabral, C.F.S.; Veiga, L.B.E.; Araújo, M.G.; de Souza, S.L.Q. Environmental Life Cycle Assessment of goat cheese production in Brazil: A path towards sustainability. Lwt 2020, 129, 109550. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Tan, X.; Show, P.L.; Rambabu, K.; Banat, F.; Veeramuthu, A.; Lau, B.F.; Ng, E.P.; Ling, T.C. Incorporating biowaste into circular bioeconomy: A critical review of current trend and scaling up feasibility. Environ. Technol. Innov. 2020, 19, 101034. [Google Scholar] [CrossRef]
- Villegas-Méndez, M.Á.; Montañez, J.; Contreras-Esquivel, J.C.; Salmerón, I.; Koutinas, A.; Morales-Oyervides, L. Coproduction of Microbial Oil and Carotenoids within the Circular Bioeconomy Concept: A Sequential Solid-State and Submerged Fermentation Approach. Fermentation 2022, 8, 258. [Google Scholar] [CrossRef]
- Khodaiyan, F.; Razavi, S.H.; Mousavi, S.M. Optimization of canthaxanthin production by Dietzia natronolimnaea HS-1 from cheese whey using statistical experimental methods. Biochem. Eng. J. 2008, 40, 415–422. [Google Scholar] [CrossRef]
- Marova, I.; Carnecka, M.; Halienova, A.; Certik, M.; Dvorakova, T.; Haronikova, A. Use of several waste substrates for carotenoid-rich yeast biomass production. J. Environ. Manag. 2012, 95, S338–S342. [Google Scholar] [CrossRef]
- Valduga, E.; Tatsch, P.; Vanzo, L.T.; Rauber, F.; Di Luccio, M.; Treichel, H. Assessment of hydrolysis of cheese whey and use of hydrolysate for bioproduction of carotenoids by Sporidiobolus salmonicolor CBS 2636. J. Sci. Food Agric. 2009, 89, 1060–1065. [Google Scholar] [CrossRef]
- Varzakakou, M.; Roukas, T. Identification of carotenoids produced from cheese whey by blakeslea trispora in submerged fermentation. Prep. Biochem. Biotechnol. 2009, 40, 76–82. [Google Scholar] [CrossRef]
- Nasrabadi, M.R.N.; Razavi, S.H. Optimization of β-carotene production by a mutant of the lactose-positive yeast Rhodotorula acheniorum from whey ultrafiltrate. Food Sci. Biotechnol. 2011, 20, 445–454. [Google Scholar] [CrossRef]
- Rao, R.S.; Kumar, C.G.; Prakasham, R.S.; Hobbs, P.J. The Taguchi methodology as a statistical tool for biotechnological applications: A critical appraisal. Biotechnol. J. 2008, 3, 510–523. [Google Scholar] [CrossRef]
- Shinde, S.D.; Lele, S.S. Statistical media optimization for lutein production from microalgae Auxenochlorella protothecoides SAG 211-7A. Int. J. Adv. Biotechnol. Res. 2010, 1, 104–114. [Google Scholar]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Roy, R.K. Design of Experiment Using the Taguchi approach. In Taguchi’s Quality Engineering Handbook; John Wiley & Son: Hoboken, NJ, USA, 2001. [Google Scholar]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids: Handbook; Birkhäuser: Basel, Switzerland, 2004. [Google Scholar]
- Sousa, Y.R.F.; Araújo, D.F.S.; Pulido, J.O.; Pintado, M.M.E.; Martínez-Férez, A.; Queiroga, R.C.R.E. Composition and isolation of goat cheese whey oligosaccharides by membrane technology. Int. J. Biol. Macromol. 2019, 139, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Bury, D.; Jelen, P. Lactose hydrolysis using a disrupted dairy culture: Evaluation of technical and economical feasibility. Can. Biosyst. Eng. Genie Biosyst. Canada 2000, 42, 75–80. [Google Scholar]
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Gientka, I.; Kieliszek, M. Rhodotorula glutinis—Potential source of lipids, carotenoids, and enzymes for use in industries. Appl. Microbiol. Biotechnol. 2016, 100, 6103–6117. [Google Scholar] [CrossRef]
- Braunwald, T.; Schwemmlein, L.; Graeff-Hönninger, S.; French, W.T.; Hernandez, R.; Holmes, W.E.; Claupein, W. Effect of different C/N ratios on carotenoid and lipid production by Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2013, 97, 6581–6588. [Google Scholar] [CrossRef]
- Park, P.K.; Cho, D.H.; Kim, E.Y.; Chu, K.H. Optimization of carotenoid production by Rhodotorula glutinis using statistical experimental design. World, J. Microbiol. Biotechnol. 2005, 21, 429–434. [Google Scholar] [CrossRef]
- Tkáčová, J.; Čaplová, J.; Klempová, T.; Čertík, M. Correlation between lipid and carotenoid synthesis in torularhodin-producing Rhodotorula glutinis. Ann. Microbiol. 2017, 67, 541–551. [Google Scholar] [CrossRef]
- Bhosale, P. Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl. Microbiol. Biotechnol. 2004, 63, 351–361. [Google Scholar] [CrossRef]
- Marcoleta, A.; Niklitschek, M.; Wozniak, A.; Lozano, C.; Alcaíno, J.; Baeza, M.; Cifuentes, V. "Glucose and ethanol-dependent transcriptional regulation of the astaxanthin biosynthesis pathway in Xanthophyllomyces dendrorhous". BMC Microbiol. 2011, 11, 190. [Google Scholar] [CrossRef]
- Valduga, E.; Valério, A.; Treichel, H.; Di Luccio, M.; Furigo Júnior, A. Study of the bio-production of carotenoids by Sporidiobolus salmonicolor (CBS 2636) using pre-treated agro-industrial substrates. J. Chem. Technol. Biotechnol. 2008, 83, 1267–1274. [Google Scholar] [CrossRef]
- Husseiny, S.M.; Abdelhafez, A.A.; Ali, A.A.-A.; Sand, H.M. Optimization of β-Carotene Production from Rhodotorula glutinis ATCC 4054 Growing on Agro-industrial Substrate Using Plackett–Burman Design. Proc. Natl. Acad. Sci. India B Biol. Sci. 2018, 88, 1637–1646. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Bryś, J.; Gientka, I.; Bzducha-Wróbel, A.; Maliszewska, M.; Reczek, L. Effect of initial pH of medium with potato wastewater and glycerol on protein, lipid and carotenoid biosynthesis by Rhodotorula glutinis. Electron. J. Biotechnol. 2017, 27, 25–31. [Google Scholar] [CrossRef]
- Sar, T.; Harirchi, S.; Ramezani, M.; Bulkan, G.; Akbas, M.Y.; Pandey, A.; Taherzadeh, M.J. Potential utilization of dairy industries by-products and wastes through microbial processes: A critical review. Sci. Total Environ. 2022, 810, 152253. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Relationship between the colour and the chemical structure of carotenoid pigments. Food Chem. 2007, 101, 1145–1150. [Google Scholar] [CrossRef]
| Factors | Levels | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Urea (F1, % w/v) | 0.3 | 0.5 | 0.7 |
| pH (F2) | 4.5 | 5.5 | 6.5 |
| Ethanol (F3, % v/v) | 0.1 | 1.0 | 1.9 |
| Glucose (F4, % w/v) | 3.5 | 4.5 | 6.0 |
| Run | F1 | F2 | F3 | F4 | Total Carotenoids (µg/L) | Biomass (g/L) | Yield (µg/g) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 2860.6 ± 111.1 | 15.3 ± 1.4 | 187.2 ± 10.5 |
| 2 | 1 | 2 | 2 | 2 | 3369.7 ± 147.0 | 14.6 ± 0.2 | 229.7 ± 8.8 |
| 3 | 1 | 3 | 3 | 3 | 3406.1 ± 75.7 | 15.6 ± 0.2 | 217.5 ± 8.1 |
| 4 | 2 | 1 | 2 | 3 | 2933.3 ± 111.1 | 13.6 ± 1.0 | 215.9 ± 24.7 |
| 5 | 2 | 2 | 3 | 1 | 1672.7 ± 109.1 | 7.0 ± 0.5 | 240.5 ± 32.8 |
| 6 | 2 | 3 | 1 | 2 | 1139.4 ± 137.7 | 7.3 ± 0.7 | 155.2 ± 4.5 |
| 7 | 3 | 1 | 3 | 2 | 1042.4 ± 105.0 | 7.1 ± 1.1 | 146.3 ± 8.2 |
| 8 | 3 | 2 | 1 | 3 | 1357.6 ± 42.0 | 7.8 ± 0.2 | 173.5 ± 10.3 |
| 9 | 3 | 3 | 2 | 1 | 739.4 ± 42.0 | 4.6 ±0.5 | 160.6 ± 27.2 |
| Factors | Variance | F-Ratio | Relative Influence (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TC | B | Y | TC | B | Y | TC | B | Y | |
| F1 | 10,690,223.4 | 176.3 | 6903.4 | 987.7 | 268.1 | 21.6 | 76.9 | 75.4 | 39.3 |
| F2 | 640,195.9 | 20.0 | 3563.8 | 59.1 | 30.4 | 11.1 | 4.6 | 8.6 | 20.3 |
| F3 | 711,747.8 | 2.8 | 2661.9 | 65.8 | 4.2 | 8.3 | 5.1 | 1.2 | 15.2 |
| F4 | 1,759,608.2 | 28.7 | 1553.1 | 162.6 | 43.6 | 4.9 | 12.7 | 12.3 | 8.8 |
| Error | 10,823.4 | 0.7 | 319.8 | 0.7 | 2.5 | 16.4 | |||
| Total | 1,069,168.1 | 18.0 | 1350.8 | 76.9 | 75.4 | 39.3 | |||
| Factors | Optimum Levels | Contribution | ||
|---|---|---|---|---|
| P (µg/L) | B (g/L) | Yx (µg/g) | ||
| F1 | 1 | 1154.2 | 4.9 | 19.6 |
| F2 | 1 | 220.9 | 1.7 | -8.7 |
| F3 | 2 | 289.6 | 0.6 | 10.2 |
| F4 | 3 | 507.7 | 2.0 | 10.5 |
| Current grand average of performance | 2057.9 | 10.4 | 191.8 | |
| Contribution of all factors | 2172.4 | 9.2 | 31.6 | |
| Expected result | 4230.3 | 19.6 | 223.5 | |
| Confidence interval | 154.2 | 0.8 | 17.2 | |
| Peak | Assigned Carotenoid | tR/min | λmax a | λmax b |
|---|---|---|---|---|
| a | β-carotene | 14.7 | 426, 450, 476 | 425, 450, 477 |
| b | γ-carotene | 22.7 | 438, 460, 490 | 437, 462, 494 |
| c | torulene | 33.1 | 460, 486, 518 | 460, 484, 520 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mata-Gómez, L.C.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J.; Méndez-Zavala, A.; Morales-Oyervides, L.; Montañez, J. Microbial Carotenoid Synthesis Optimization in Goat Cheese Whey Using the Robust Taguchi Method: A Sustainable Approach to Help Tackle Vitamin A Deficiency. Foods 2023, 12, 658. https://doi.org/10.3390/foods12030658
Mata-Gómez LC, Mapelli-Brahm P, Meléndez-Martínez AJ, Méndez-Zavala A, Morales-Oyervides L, Montañez J. Microbial Carotenoid Synthesis Optimization in Goat Cheese Whey Using the Robust Taguchi Method: A Sustainable Approach to Help Tackle Vitamin A Deficiency. Foods. 2023; 12(3):658. https://doi.org/10.3390/foods12030658
Chicago/Turabian StyleMata-Gómez, Luis Carlos, Paula Mapelli-Brahm, Antonio J. Meléndez-Martínez, Alejandro Méndez-Zavala, Lourdes Morales-Oyervides, and Julio Montañez. 2023. "Microbial Carotenoid Synthesis Optimization in Goat Cheese Whey Using the Robust Taguchi Method: A Sustainable Approach to Help Tackle Vitamin A Deficiency" Foods 12, no. 3: 658. https://doi.org/10.3390/foods12030658
APA StyleMata-Gómez, L. C., Mapelli-Brahm, P., Meléndez-Martínez, A. J., Méndez-Zavala, A., Morales-Oyervides, L., & Montañez, J. (2023). Microbial Carotenoid Synthesis Optimization in Goat Cheese Whey Using the Robust Taguchi Method: A Sustainable Approach to Help Tackle Vitamin A Deficiency. Foods, 12(3), 658. https://doi.org/10.3390/foods12030658







