Effects of Pulsed Pressure Curing on Beef Quality
Abstract
:1. Introduction
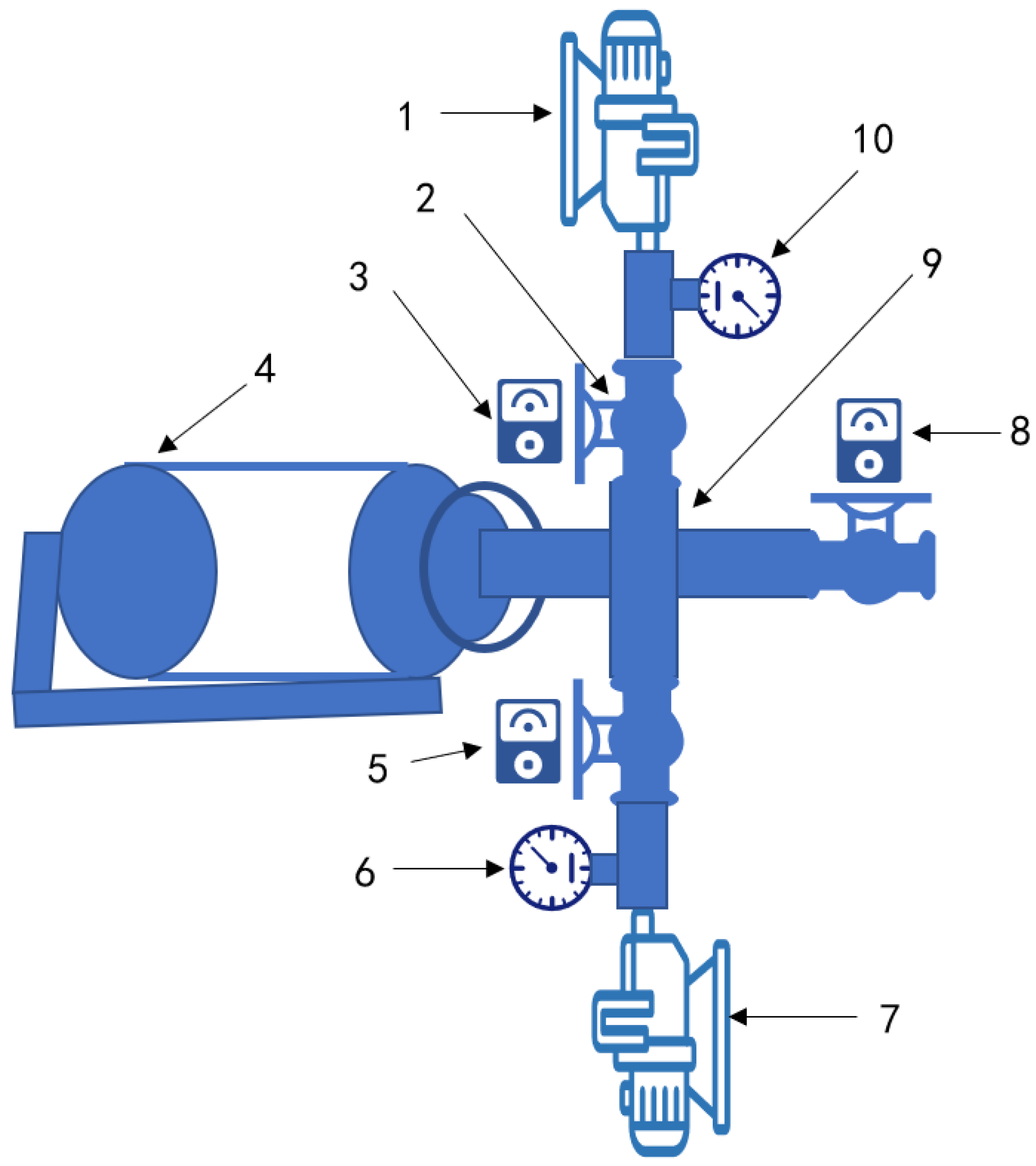
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation and Salting
2.3. Curing Absorption
2.4. Study of Physical and Chemical Parameters
2.5. Cooking Loss
2.6. Determination of Centrifugal Loss
2.7. Color
2.8. Determination of Texture
2.9. Sensory Analysis
2.9.1. Trained Analytical Panel
2.9.2. Consumer Acceptability
2.10. Scanning Electron Microscopy (SEM)
2.11. Volatile Compound Analysis
2.12. Statistical Analysis
3. Results and Discussion
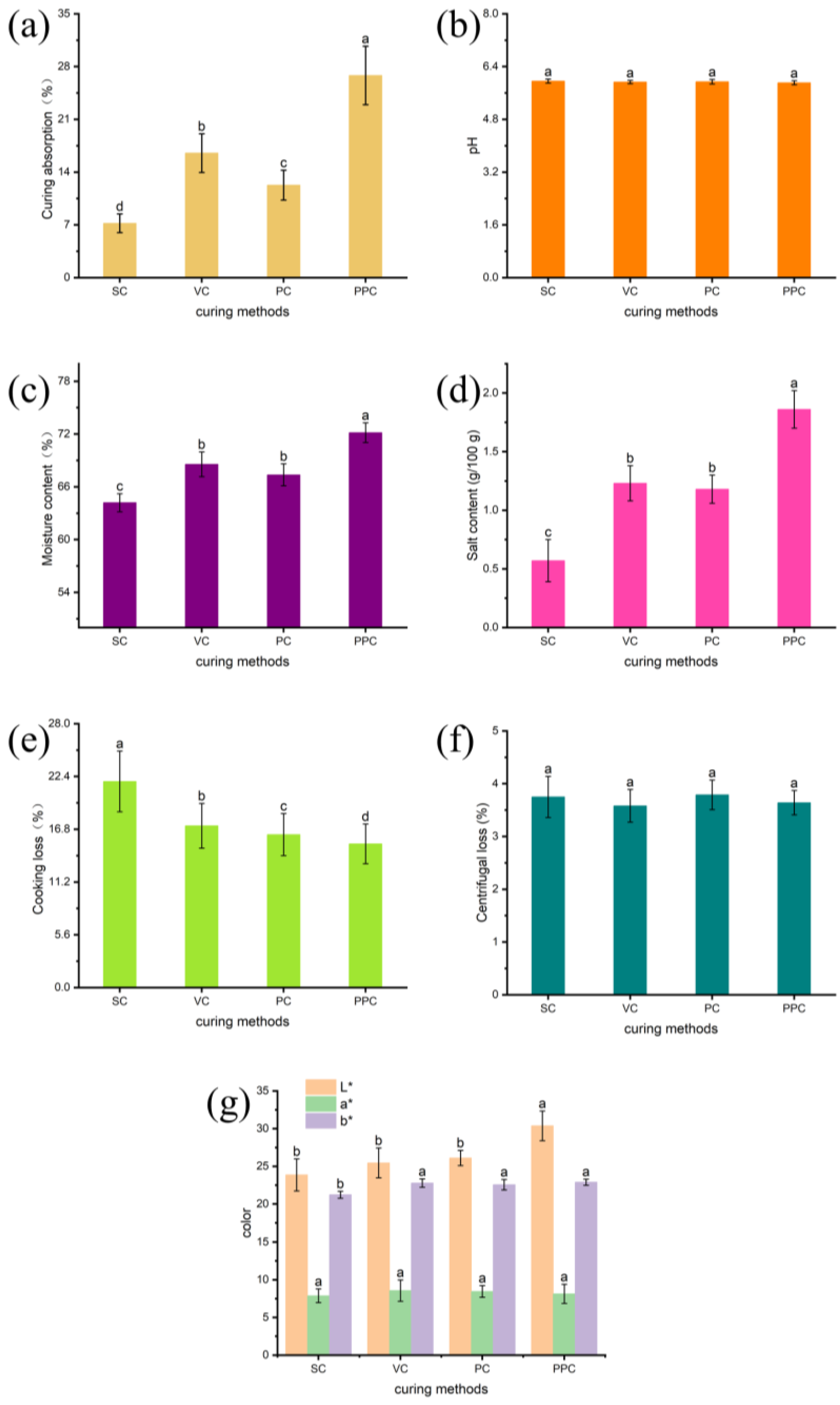
3.1. Curing Absorption
3.2. Study of Physical and Chemical Parameters
3.3. Cooking Loss
3.4. Centrifugal Loss
3.5. Color
3.6. Texture
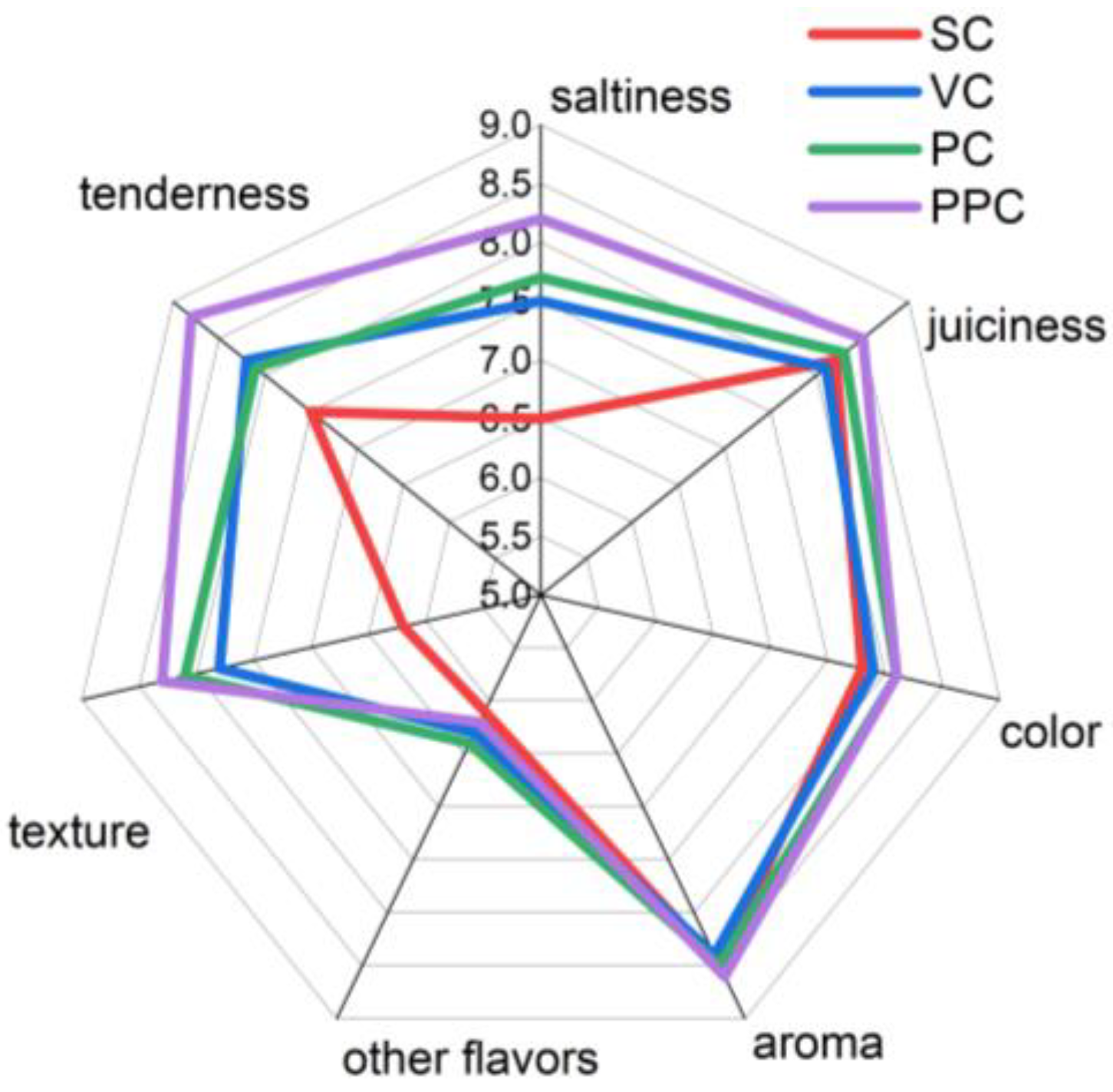
3.7. Sensory Evaluation: Trained and Consumer Panel
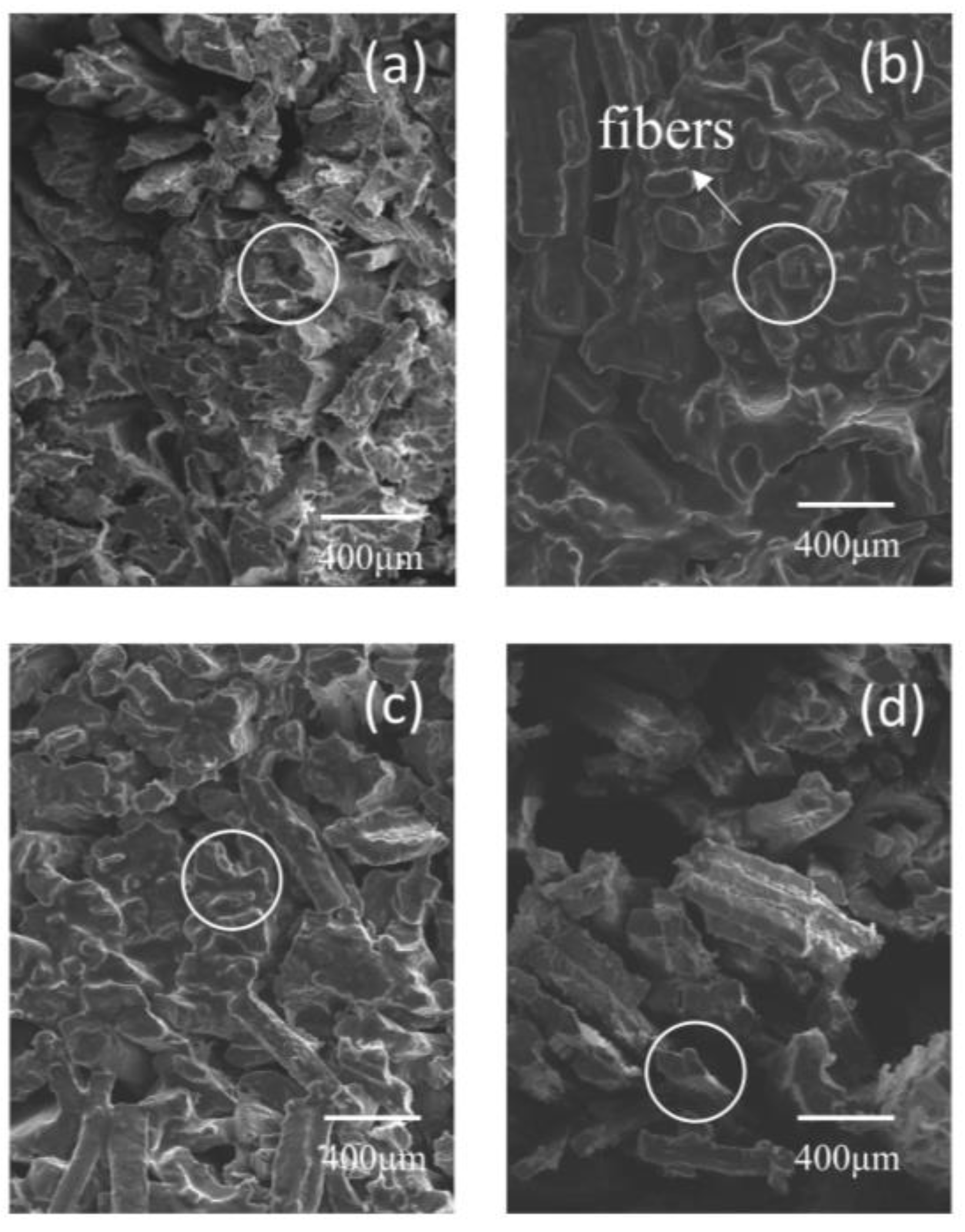
3.8. Scanning Electron Microscopy
3.9. Volatile Compound
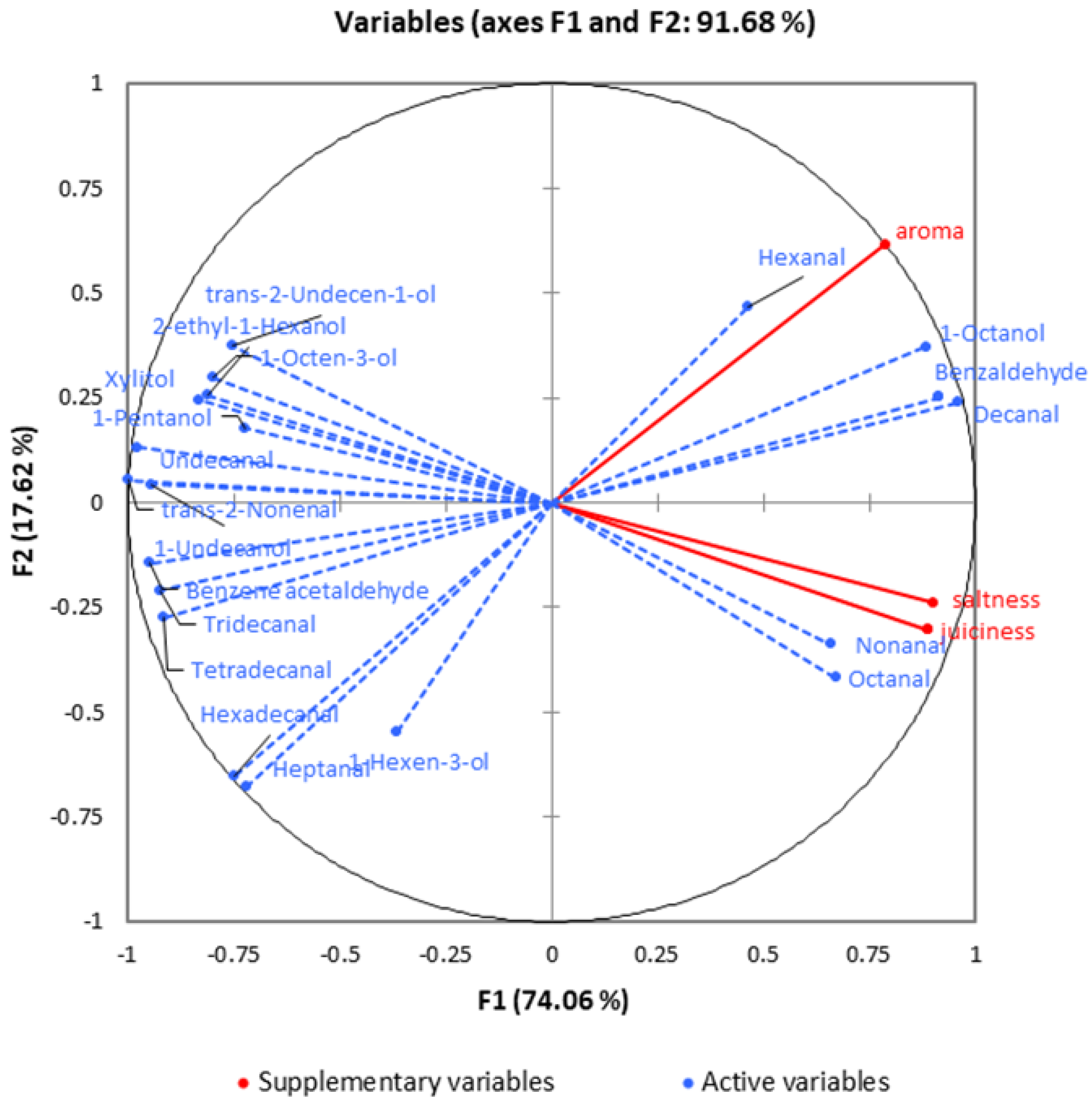
3.10. Multivariate Analysis of Sensory Attributes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cárcel, J.A.; Benedito, J.; Bon, J.; Mulet, A. High intensity ultrasound effects on meat brining. Meat Sci. 2007, 76, 611–619. [Google Scholar] [CrossRef]
- Boudhrioua, N.; Djendoubi, N.; Bellagha, S.; Kechaou, N. Study of moisture and salt transfers during salting of sardine fillets. J. Food Eng. 2009, 94, 83–89. [Google Scholar] [CrossRef]
- Yao, Y.; Han, R.; Li, F.; Tang, J.; Jiao, Y. Mass transfer enhancement of tuna brining with different NaCl concentrations assisted by ultrasound. Ultrason. Sonochem. 2022, 85, 105989. [Google Scholar] [CrossRef]
- Nagdalian, A.A.; Oboturova, N.P.; Budkevich, R.O.; Selimov, M.A.; Demchenkov, E.L. Study of the Influence of the Electrohydraulic Effect on the Structure and Mechanical Properties of Muscular Tissue Using Atomic-Force Microscopy. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 517–523. [Google Scholar]
- Liu, H.; Xu, Y.; Zu, S.; Wu, X.; Shi, A.; Zhang, J.; Wang, Q.; He, N. Effects of High Hydrostatic Pressure on the Conformational Structure and Gel Properties of Myofibrillar Protein and Meat Quality: A Review. Foods 2021, 10, 1872. [Google Scholar] [CrossRef] [PubMed]
- Oboturova, N.P.; Evdokimov, I.A.; Nagdalian, A.A.; Kulikov, Y.I.; Gusevskaya, O.A. The study on the influence of the electrohydraulic effect on the diffusion coefficient and the penetration depth of salt into muscle tissues during salting. Foods Raw Mater. 2015, 3, 74–81. [Google Scholar]
- Bolumar, T.; Rohlik, B.A.; Stark, J.; Sikes, A.; Watkins, P.; Buckow, R. Investigation of Pulsed Electric Field Conditions at Low Field Strength for the Tenderisation of Beef Topside. Foods 2022, 11, 2803. [Google Scholar] [CrossRef]
- Bolumar, T.; Orlien, V.; Sikes, A.; Aganovic, K.; Bak, K.H.; Guyon, C.; Stübler, A.-S.; de Lamballerie, M.; Hertel, C.; Brüggemann, D.A. High-pressure processing of meat: Molecular impacts and industrial applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 332–368. [Google Scholar] [CrossRef]
- Jiao, D.; Zhang, D.; Chen, R.; Yang, X.; Shi, H.; Liu, H.; Wang, Z.; Lu, L.; Hui, T. A developed variable pressure-assisted salting process: Improving the textural, flavor, and sensory attributes in roasted duck breast. LWT 2022, 167, 113800. [Google Scholar] [CrossRef]
- Jin, G.; He, L.; Wang, Q.; Liu, C.; Jin, Y.; Huang, F.; Ma, M. Pulsed pressure assisted brining of porcine meat. Innov. Food Sci. Emerg. Technol. 2014, 22, 76–80. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, M.; Wang, L. Effects of different curing methods on edible quality and myofibrillar protein characteristics of pork. Food Chem. 2022, 387, 132872. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Yadav, S.; Pathera, A.; Sharma, D. Effect of vacuum tumbling and red beetroot juice incorporation on quality characteristics of marinated chicken breast and leg meats. Nutr. Food Sci. 2019, 50, 143–156. [Google Scholar] [CrossRef]
- Aykın-Dinçer, E. Application of ultrasound-assisted vacuum impregnation for improving the diffusion of salt in beef cubes. Meat Sci. 2021, 176, 108469. [Google Scholar] [CrossRef]
- Singh, R.K.; Sharma, N. Physical and biochemical changes in ultra-rapid air chilled broiler muscle and quality of marinated chicken breast filets. Food Biosci. 2022, 46, 101524. [Google Scholar] [CrossRef]
- McDonnell, C.K.; Allen, P.; Chardonnereau, F.S.; Arimi, J.M.; Lyng, J.G. The use of pulsed electric fields for accelerating the salting of pork. LWT-Food Sci. Technol. 2014, 59, 1054–1060. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Xie, J. Evaluation of water dynamics and protein changes in bigeye tuna (Thunnus obesus) during cold storage. LWT 2019, 108, 289–296. [Google Scholar] [CrossRef]
- Djikeng, F.T.; Djikeng, C.F.T.; Womeni, H.M.; Ndefo, D.K.K.; Pougoué, A.A.N.; Tambo, S.T.; Esatbeyoglu, T. Effect of Different Processing Methods on the Chemical Composition, Antioxidant Activity and Lipid Quality of Tiger Nuts (Cyperus esculentus). Appl. Food Res. 2022, 2, 100124. [Google Scholar] [CrossRef]
- Knowles, S.O.; Grace, N.D.; Rounce, J.R.; Realini, C.E. Quality, nutrient and sensory characteristics of aged meat from lambs supplemented with selenomethionine. Food Res. Int. 2020, 137, 109655. [Google Scholar] [CrossRef]
- Macfie, H.J.; Bratchell, N.; Greenhoff, K.; Vallis, L.V. Designs to balance the effect of order of presentation and first-order carry-over effects in hall tests. J. Sens. Stud. 1989, 4, 129–148. [Google Scholar] [CrossRef]
- Peryam, D.R.; Pilgrim, F.J. Hedonic scale method of measuring food preferences. Food Technol. 1957, 11, 9–14. [Google Scholar]
- Guo, Q.; Kong, X.; Hu, C.; Zhou, B.; Wang, C.; Shen, Q.W. Fatty acid content, flavor compounds, and sensory quality of pork loin as affected by dietary supplementation with L-arginine and glutamic acid. J. Food Sci. 2019, 84, 3445–3453. [Google Scholar] [CrossRef]
- Li, R.; Guo, M.; Liao, E.; Wang, Q.; Peng, L.; Jin, W.; Wang, H. Effects of repeated freezing and thawing on myofibrillar protein and quality characteristics of marinated Enshi black pork. Food Chem. 2022, 378, 131994. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, N.K.; Niranjan, K. Enhanced Mass Transfer During Osmotic Dehydration of High Pressure Treated Pineapple. J. Food Sci. 1998, 63, 508–511. [Google Scholar] [CrossRef]
- Villacís, M.F.; Rastogi, N.K.; Balasubramaniam, V.M. Effect of high pressure on moisture and NaCl diffusion into turkey breast. LWT-Food Sci. Technol. 2008, 41, 836–844. [Google Scholar] [CrossRef]
- Medyński, A.; Pospiech, E.; Kniat, R. Effect of various concentrations of lactic acid and sodium chloride on selected physico-chemical meat traits. Meat Sci. 2000, 55, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, A.; Graiver, N.; Califano, A.; Zaritzky, N. Diffusion of nitrite and nitrate salts in pork tissue in the presence of sodium chloride. J. Food Sci. 2002, 67, 2165–2171. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.-T.; Cao, J.-X.; Chen, Y.-J.; Sun, Y.-Y.; Zeng, X.-Q.; Pan, D.-D.; Ou, C.-R.; Gan, N. Study on lipolysis-oxidation and volatile flavour compounds of dry-cured goose with different curing salt content during production. Food Chem. 2016, 190, 33–40. [Google Scholar]
- Contreras-Lopez, G.; Carnero-Hernandez, A.; Huerta-Jimenez, M.; Alarcon-Rojo, A.D.; Garcia-Galicia, I.; Carrillo-López, L. High-intensity ultrasound applied on cured pork: Sensory and physicochemical characteristics. Food Sci. Nutr. 2020, 8, 786–795. [Google Scholar] [CrossRef]
- Sanches, M.A.R.; Colombo Silva, P.M.O.; Barretto, T.L.; Darros-Barbosa, R.; da Silva-Barretto, A.C.; Telis-Romero, J. Technological and diffusion properties in the wet salting of beef assisted by ultrasound. LWT 2021, 149, 112036. [Google Scholar] [CrossRef]
- Macfarlane, J. Pre-rigor pressurization of muscle: Effects on pH, shear value and taste panel assessment. J. Food Sci. 1973, 38, 294–298. [Google Scholar] [CrossRef]
- Jung, S.; Ghoul, M.; de Lamballerie-Anton, M. Changes in lysosomal enzyme activities and shear values of high pressure treated meat during ageing. Meat Sci. 2000, 56, 239–246. [Google Scholar] [CrossRef]
- Martinez, O.; Salmerón, J.; Guillén, M.D.; Casas, C. Texture profile analysis of meat products treated with commercial liquid smoke flavourings. Food Control 2004, 15, 457–461. [Google Scholar] [CrossRef]
- Bao, G.; Niu, J.; Li, S.; Zhang, L.; Luo, Y. Effects of ultrasound pretreatment on the quality, nutrients and volatile compounds of dry-cured yak meat. Ultrason. Sonochem. 2022, 82, 105864. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, J.; Lou, A.; Wang, Y.; Yang, D.; Shen, Q.W. Duck breast muscle proteins, free fatty acids and volatile compounds as affected by curing methods. Food Chem. 2021, 338, 128138. [Google Scholar] [CrossRef] [PubMed]
- Bak, K.H.; Richards, M.P. Hexanal as a Predictor of Development of Oxidation Flavor in Cured and Uncured Deli Meat Products as Affected by Natural Antioxidants. Foods 2021, 10, 152. [Google Scholar] [CrossRef]
- Jin, G.; He, L.; Li, C.; Zhao, Y.; Chen, C.; Zhang, Y.; Zhang, J.; Ma, M. Effect of pulsed pressure-assisted brining on lipid oxidation and volatiles development in pork bacon during salting and drying-ripening. LWT-Food Sci. Technol. 2015, 64, 1099–1106. [Google Scholar] [CrossRef]
- Xu, C.-C.; Yu, H.; Xie, P.; Sun, B.-Z.; Wang, X.-Y.; Zhang, S.-S. Influence of Electrostatic Field on the Quality Attributes and Volatile Flavor Compounds of Dry-Cured Beef during Chill Storage. Foods 2020, 9, 478. [Google Scholar] [CrossRef]
- Pu, D.; Zhang, Y.; Zhang, H.; Sun, B.; Ren, F.; Chen, H.; Tang, Y. Characterization of the Key Aroma Compounds in Traditional Hunan Smoke-Cured Pork Leg (Larou, THSL) by Aroma Extract Dilution Analysis (AEDA), Odor Activity Value (OAV), and Sensory Evaluation Experiments. Foods 2020, 9, 413. [Google Scholar] [CrossRef]
- Feng, H.; Timira, V.; Zhao, J.; Lin, H.; Wang, H.; Li, Z. Insight into the Characterization of Volatile Compounds in Smoke-Flavored Sea Bass (Lateolabrax maculatus) during Processing via HS-SPME-GC-MS and HS-GC-IMS. Foods 2022, 11, 2614. [Google Scholar] [CrossRef]
- Kerth, C.R.; Miller, R.K. Beef flavor: A review from chemistry to consumer. J. Sci. Food Agric. 2015, 95, 2783–2798. [Google Scholar] [CrossRef]
| Curing Methods | Hardness (g) | Springiness (mm) | Chewiness (g) | Cohesiveness |
|---|---|---|---|---|
| SC | 5112.74 ± 236.78 a | 0.76 ± 0.04 b | 1448.58 ± 121.21 a | 0.54 ± 0.02 c |
| VC | 4125.45 ± 212.67 c | 0.76 ± 0.03 b | 1227.26 ± 113.37 b | 0.59 ± 0.02 b |
| PC | 4556.94 ± 196 b | 0.78 ± 0.04 b | 1328.43 ± 98.51 b | 0.63 ± 0.02 b |
| PPC | 3892.97 ± 158.77 c | 0.85 ± 0.05 a | 987.56 ± 57.51 c | 0.67 ± 0.04 a |
| Acceptability | SC | VC | PC | PPC |
|---|---|---|---|---|
| aroma | 8.4 ± 0.3 a | 8.3 ± 0.3 a | 8.4 ± 0.2 a | 8.5 ± 0.3 a |
| saltness | 6.4 ± 0.2 c | 7.3 ± 0.3 b | 7.4 ± 0.2 b | 8.4 ± 0.2 a |
| tenderness | 7.4 ± 0.3 c | 8.2 ± 0.2 b | 8.1 ± 0.2 b | 8.7 ± 0.2 a |
| juiciness | 7.8 ± 0.3 b | 8.0 ± 0.2 b | 7.9 ± 0.2 b | 8.0 ± 0.2 a |
| overall | 7.3 ± 0.1 c | 7.9 ± 0.2 b | 7.8 ± 0.2 b | 8.4 ± 0.2 a |
| No | Volatile Compounds | Content (μg·kg−1) | |||
|---|---|---|---|---|---|
| SC | VC | PC | PPC | ||
| 1 | 1-Hexanol | 0.82 ± 0.08 a | 0.54 ± 0.02 b | 0.55 ± 0.02 b | 0.42 ± 0.03 c |
| 2 | 1-Octen-3-ol | 3.76 ± 0.83 a | 2.63 ± 0.82 b | 2.15 ± 0.05 c | 2.13 ± 0.07 c |
| 3 | 2-ethyl-1-Hexanol | 9.87 ± 0.82 b | 12.06 ± 1.01 a | 12.16 ± 1.22 a | 12.24 ± 1.61 a |
| 4 | Xylitol | 6.65 ± 1.03 b | 6.41 ± 0.92 b | 8.64 ± 0.82 a | 8.44 ± 0.62 a |
| 5 | 1-Pentanol | 5.95 ± 1.02 c | 9.71 ± 1.12 b | 9.95 ± 1.05 b | 12.95 ± 1.92 a |
| 6 | 1-Hexen-3-ol | 9.16 ± 1.23 b | 13.16 ± 1.82 a | 8.86 ± 1.65 b | 13.77 ± 1.54 a |
| 7 | 1-Octanol | 8.11 ± 0.82 b | 8.52 ± 0.69 b | 8.23 ± 0.57 b | 12.26 ± 0.62 a |
| 8 | 1-Undecanol | 5.77 ± 0.83 b | 5.37 ± 0.96 b | 8.94 ± 1.32 a | 9.57 ± 1.53 a |
| 9 | trans-2-Undecen-1-ol | 5.59 ± 0.58 b | 8.56 ± 0.68 a | 8.84 ± 0.65 a | 8.67 ± 0.98 a |
| 10 | 3,7,11-trimethyl-1-Dodecanol | 6.11 ± 0.67 a | 6.52 ± 0.86 a | 6.28 ± 0.69 a | 6.09 ± 0.89 a |
| 11 | Hexanal | 117.46 ± 9.51 c | 137.36 ± 8.62 b | 139.61 ± 9.12 b | 167.24 ± 10.23 a |
| 12 | Heptanal | 28.69 ± 2.2 b | 29.71 ± 2.1 b | 28.66 ± 1.45 b | 35.74 ± 3.15 a |
| 13 | Benzaldehyde | 141.66 ± 6.58 b | 167.82 ± 9.52 a | 169.81 ± 8.46 a | 172.58 ± 8.45 a |
| 14 | Octanal | 58.82 ± 4.1 b | 60.07 ± 3.12 b | 67.92 ± 4.15 a | 68.93 ± 5.12 a |
| 15 | Benzene acetaldehyde | 36.98 ± 1.8 c | 43.36 ± 2.02 b | 45.1 ± 3.45 b | 51.43 ± 3.48 a |
| 16 | Nonanal | 170.33 ± 7.52 b | 196.62 ± 9.54 a | 199.76 ± 8.41 a | 202.92 ± 10.56 a |
| 17 | trans-2-Nonenal | 47.4 ± 3.1 b | 49.59 ± 4.31 b | 55.44 ± 3.81 a | 57.59 ± 4.21 a |
| 18 | Decanal | 60.94 ± 4.5 b | 69.67 ± 3.2 a | 68.37 ± 4.1 a | 70.06 ± 3.02 a |
| 19 | Undecanal | 56.32 ± 5.82 b | 54.48 ± 6.32 b | 54.59 ± 7.41 b | 71.86 ± 6.22 a |
| 20 | Dodecanal | 23.74 ± 4.9 a | 22.8 ± 5.8 a | 23.76 ± 6.03 a | 21.59 ± 6.61 a |
| 21 | Tridecanal | 34.46 ± 5.3 b | 34.42 ± 5.48 b | 33.33 ± 6.14 b | 44.47 ± 3.45 a |
| 22 | Tetradecanal | 68.08 ± 8.1 b | 93.94 ± 7.47 a | 72.5 ± 7.26 b | 96.67 ± 8.75 a |
| 23 | Pentadecanal | 79.44 ± 4.8 a | 81.99 ± 5.58 a | 82.72 ± 5.48 a | 83.1 ± 6.46 a |
| 24 | Hexadecanal | 104.16 ± 2.5 c | 124.12 ± 3.58 b | 123.83 ± 3.47 b | 135.09 ± 3.74 a |
| 25 | Acetophenone | 24.34 ± 3.11 c | 34.89 ± 4.41 b | 32.15 ± 4.78 b | 51.8 ± 5.44 a |
| 26 | 2-Nonanone | 42.27 ± 5.46 b | 55.36 ± 8.11 a | 59.47 ± 7.46 a | 56.54 ± 6.12 a |
| 27 | Geranyl acetone | 43.34 ± 5.15 b | 46.88 ± 7.45 b | 48.93 ± 6.78 b | 69.58 ± 7.98 a |
| 28 | 2,5-Octanedione | 32.12 ± 4.02 b | 35.91 ± 5.32 b | 31.23 ± 4.26 b | 46.74 ± 6.49 a |
| 29 | 3-methyl-Butanoic acid | 102.26 ± 10.13 a | 106.63 ± 8.52 a | 103.38 ± 9.44 a | 110.18 ± 12.14 a |
| 30 | 2-methyl-Butanoic acid | 89.82 ± 8.01 a | 90.41 ± 5.11 a | 87.21 ± 9.61 a | 92.98 ± 8.71 a |
| 31 | 2,2-dimethylpropyl acetate | 46.96 ± 5.16 a | 47.05 ± 3.12 a | 45.89 ± 4.12 a | 45.83 ± 6.26 a |
| 32 | n-Propyl propionate | 38.63 ± 6.12 a | 39.45 ± 5.87 a | 36.11 ± 7.14 a | 39.65 ± 5.37 a |
| 33 | Ethyl caprylate | 25.97 ± 3.65 a | 24.71 ± 3.97 a | 27.28 ± 2.89 a | 26.42 ± 2.15 a |
| 34 | Methyl nonoate | 36.99 ± 3.97 a | 39.45 ± 3.24 a | 37.14 ± 2.77 a | 38.85 ± 3.45 a |
| 35 | Methyl tetradecanoate | 44.34 ± 5.46 a | 43.45 ± 3.11 a | 41.87 ± 5.68 a | 45.46 ± 3.62 a |
| 36 | Dodecane | 79.17 ± 7.43 a | 78.22 ± 9.87 a | 81.47 ± 8.86 a | 80.79 ± 8.25 a |
| 37 | Tridecane | 78.82 ± 7.89 a | 81.46 ± 8.86 a | 82.78 ± 9.23 a | 79.85 ± 6.85 a |
| 38 | 2-cyclohexyl-Dodecane | 52.32 ± 2.41 b | 58.48 ± 2.41 a | 57.98 ± 3.96 a | 59.78 ± 2.98 a |
| 39 | Tetradecane | 60.41 ± 5.87 a | 62.45 ± 4.65 a | 60.54 ± 4.58 a | 61.42 ± 3.87 a |
| 40 | 1-Pentadecene | 53.54 ± 4.86 a | 51.13 ± 3.65 a | 54.54 ± 2.85 a | 52.89 ± 3.89 a |
| 41 | Pentadecane | 66 ± 7.79 a | 61.14 ± 9.76 a | 62.46 ± 6.85 a | 64.78 ± 6.46 a |
| 42 | Heptadecane | 36.64 ± 7.65 a | 31.46 ± 6.34 a | 32.74 ± 5.82 a | 34.12 ± 6.18 a |
| 43 | 2-Methyl-3-furanthiol | 22.98 ± 3.84 a | 23.64 ± 3.68 a | 21.45 ± 2.75 a | 21.28 ± 2.54 a |
| 44 | 2-pentyl-Furan | 18.64 ± 3.58 a | 16.14 ± 4.38 a | 17.24 ± 3.89 a | 16.37 ± 3.61 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Shi, J.; Zhai, X.; Yang, Z.; Huang, X.; Li, Z.; Li, Y.; Zou, X. Effects of Pulsed Pressure Curing on Beef Quality. Foods 2023, 12, 656. https://doi.org/10.3390/foods12030656
Li C, Shi J, Zhai X, Yang Z, Huang X, Li Z, Li Y, Zou X. Effects of Pulsed Pressure Curing on Beef Quality. Foods. 2023; 12(3):656. https://doi.org/10.3390/foods12030656
Chicago/Turabian StyleLi, Chuang, Jiyong Shi, Xiaodong Zhai, Zhikun Yang, Xiaowei Huang, Zhihua Li, Yanxiao Li, and Xiaobo Zou. 2023. "Effects of Pulsed Pressure Curing on Beef Quality" Foods 12, no. 3: 656. https://doi.org/10.3390/foods12030656
APA StyleLi, C., Shi, J., Zhai, X., Yang, Z., Huang, X., Li, Z., Li, Y., & Zou, X. (2023). Effects of Pulsed Pressure Curing on Beef Quality. Foods, 12(3), 656. https://doi.org/10.3390/foods12030656







