Abstract
Azara dentata Ruiz & Pav. is a small Chilean native plant from Patagonia, a producer of small white reddish berries. For the first time, the proximal analysis of the fruits, phenolic fingerprinting, the antioxidant activity, and the enzymatic inhibition and relaxation effects in rat aorta induced by the ethanolic extract of these fruits were investigated. The proximal composition and the mineral (Ca: 2434 ± 40 mg/kg; Mg: 702 ± 13 mg/kg; Fe: 117.1 ± 1.6 mg/kg; Zn: 16.1 ± 0.4 mg/kg) and heavy metal (As: 121 ± 11 µg/kg; Cd: 152 ± 5 µg/kg; Hg: 7.7 ± 1.3 µg/kg; Pb 294 ± 4 µg/kg) contents were analyzed. Anthocyanins, flavonoids, phenolic acids, and coumarins were identified using UHPLC-PDA-QTOF-MS. The ethanolic extracts showed a total phenolic content of 23.50 ± 0.93 mg GAE/g extract. In addition, the antioxidant activity was assessed using both DPPH and TEAC (28.64 ± 1.87 and 34.72 ± 2.33 mg Trolox/g of dry fruit, respectively), FRAP (25.32 ± 0.23 mg Trolox equivalent/g dry fruit), and ORAC (64.95 ± 1.23 mg Trolox equivalents/g dry fruit). The inhibition of enzymatic activities (acetylcholinesterase IC50: 2.87 + 0.23 µg extract/mL, butyrylcholinesterase IC50: 6.73 + 0.07 µg extract/mL, amylase IC50: 5.6 ± 0.0 µg extract/mL, lipase IC50: 30.8 ± 0.0 µg extract/mL, and tyrosinase IC50: 9.25 ± 0.15 µg extract/mL) was also assessed. The extract showed 50–60% relaxation in rat aorta (intact), mediated thorough the release of endothelial nitric oxide. Our results suggest that A. dentata is a good source of compounds with the capacity to inhibit important enzymes, can be hypotensive, and can thus have good potentiality as supplements in the amelioration of neurodegenerative diseases and could also have potential to be used to develop new functional foods. The study highlights the benefits of these neglected small fruits and could boost their consumption.
1. Introduction
In the last few years, the search for plant extracts rich in polyphenolic compounds from local plants in special ecosystems such as the Valdivian Forest or the Atacama Desert in Chile has been growing due to their potential benefits regarding the prevention of chronic—or noncommunicable—diseases (NCDs). The main types of chronic diseases include chronic respiratory disease, cardiovascular disease, diabetes, and cancer (WHO) [1]. These diseases, along with important degenerative diseases (such as Parkinson’s or Alzheimer’s), are principal causes of death in the world, and the search for alternatives in nature for the prevention of these diseases has become a priority [1,2]. On the other hand, antioxidant compounds coming from food sources can quench reactive oxygen species (ROS) in the body, the main responsible agents for functional impairment and neuronal damage in Alzheimer’s disease (AD), Parkinson’s disease (PD), and other neurodegenerative pathologies [3]. Several phenolic compounds studied previously by us coming from Chilean species were proved to be active against the inhibition of enzymes involved in these pathologies; for instance, from Gypothamnium pinifolium Phil., a plant from the Atacama desert, we could isolate 2-nor-1,2-secolycoserone coumarin, which proved to be active against cholinesterases (acetylcholinesterase (AChE) 1.21 ± 0.03 µg/mL, butyrylcholinesterase (BChE) IC50: 11.23 ± 0.02 µg/mL) and not active against tyrosinase (IC50: 103.43 ± 16.86 µg/mL). The G. pinifolium extracts also produced a good relaxation effect at 100 µg/mL in the pre-contracted intact aorta, so G. pinifolium could be helpful in hypertension [4]. Moreover, the diterpene ent-labda-8,13-E-diene-15-ol isolated from this source was highly cytotoxic (GI50, 4.5 ± 0.1 µM), more than the reference drug cisplatin (GI50, 4.9 ± 0.2 µM) [4]. Several extracts from Antarctic lichens also showed the good inhibition of those enzymes [5] and some of their isolated phenolic compounds such as usnic acid, barbatolic acid, 5,7-dihydroxy-6-methylphthalide, and atranol, [6], while from native fruits, we can mention the recent study of ungurahui palm datils extracts, which were potent in AChe inhibition (IC50 2.05 ± 0.03 μg/mL, a sample from the Jenaro Herrera location in Peru) and highly antioxidant (ABTS 1803.72 μmol Trolox/g, for the sample collected in Allpahuayo Mishana, Peru) [7]. In addition, a previous study by some of us demonstrated that samples from Geoffroea decorticans Burkart, fruits from a plant from the Atacama Desert, inhibited α-glucosidase (IC50 0.8–7.3 μg/mL) and lipase (9.9 to >100 μg/mL) [8].
Azara is a genus of evergreen shrubs local to subtropical and temperate places in South America, composed of ten species of plants in the Salicaceae family [9]. They are most often found at forest borders and lakesides, and in Chile, they can be found from Valdivia in the south (−40° S) to Coquimbo (−30° S) in the north. The small trees and shrubs can grow to a height of 1–8 m tall, and they show alternated leaves, or in some species, paired leaves, with 1–9 cm extension and being 0.5–5 cm wide. The fruits are reddish to blackish berries around 4–11 mm in diameter. Only a few research papers can be found on this genus from Chile. From Azara microphylla Hook.f., a new myricetin 3-O-α-L-dirhamnoside was reported a long time ago [10]; in honey and propolis made of Azara Petiolaris I.M.Johnst. and Azara integrifolia Ruiz & Pav, the presence of a variety of phenolic compounds has been recently reported, including caffeic and coumaric acids and three flavonoids (pinocembrin, chrysin, and luteolin) [11]. From Azara dentata Ruiz & Pav. (local name “corcolen”), its cytotoxic effect plus the presence of coumarins, sterols, and other components in the methanolic extract of leaves only via GC-MS was recently reported [12]. A. dentata is an endemic shrub with small berries (Figure 1) growing in southern Chile; however, only few reports are available about the chemistry and bioactivity of these neglected endemic berries; for this reason, it became interesting to perform a study that included the chemical and biological characterization of A. dentata, and it could be interesting to add a new value to an undervalued native Chilean fruit for the development of new food supplements or nutraceuticals.

Figure 1.
A. dentata plant and fruits from Calera de Tango, Santiago, Metropolitan Region, Chile (coin diameter:26 mm).
The aims of this study are to perform a proximal analysis; to determine the mineral content and the content of heavy metals in A. dentata fruits; to analyze the phenolic fingerprinting and antioxidant activity of crude ethanolic extracts of A. dentata fruits; to evaluate the enzymatic inhibitory potential against several enzymes, (cholinesterases, tyrosinase, lipase, amylase, and glucosidase), which are implicated in several NCDs such as neurodegenerative diseases (Alzheimer’s and Parkinson’s disease) and metabolic syndrome; and finally, to evaluate the hypotensive potential of the A. dentata fruits’ ethanolic extract in rat aorta obtained from a normotensive animal model.
The fruit extracts were studied using ultra-high-resolution chromatography (Ultra HPLC) coupled with mass spectrometry (Q- TOF-M-S), which is a rapid technique which provides precise information regarding the chemical profile of several phenolic compounds in food sources [13,14]. Using this technique, our group has explored the chemistry of Chilean plants, shrubs, and fruits combined with their antioxidant and enzyme inhibitory capacities [15,16].The aims of this study are: (i) to perform a proximal analysis and determine the mineral content and the content of heavy metals of A. dentata fruits; (ii) analyze the phenolic fingerprinting and the antioxidant activity of the crude ethanolic extracts of A. dentata fruits; (iii) evaluate the enzymatic inhibitory potential against several enzymes, such as cholinesterases, tyrosinase, lipase, amylase and glucosidase, which are implicated in several NCDs such as neurodegenerative diseases (Alzheimer’s and Parkinson’s disease) and metabolic syndrome; (iv) evaluate the hypotensive potential of the A. dentata fruits’ ethanolic extract in rat aorta obtained from a normotensive animal model. For the first time, the previously mentioned bioactivities for A. dentata fruits are reported.
2. Materials and Methods
2.1. Chemicals
Purified water (TOC < 5 µg/L) was obtained from a reverse osmosis system (Arium 126 61316-RO and Arium 611 UV unit (Sartorius, Goettingen, Germany)). Methanol (HPLC grade) and formic acid (for mass spectrometry, puriss. P.a.) were obtained from J.T. Baker (Phillipsburg, NJ, USA). Folin–Ciocalteu (FC) reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric chloride hexahydrate, 2,4,6-tris(2-pyridyl)-s-triazine, Trolox, quercetin, gallic acid, Amberlite® resin (XAD4), dimethyl sulfoxide (DMSO), acetylcholinesterase (AchE), butyrylcholinesterase (BchE), phosphate buffer, L-DOPA, tyrosinase, kojic acid, and trichloroacetic acid were obtained (Merck, Darmstadt, Germany). HPLC standards (quercetin and gallic acid purified to 95% and up via HPLC) were purchased from Sigma-Aldrich Chem. Co. (St Louis, MO, USA) and Extrasynthese (Genay, France). Ultratrace nitric acid 69% ppb-trace Scharlab grade was employed for ICP determination.
2.2. Plant Material
A. dentata fruits were collected by hand in the Calera de Tango (−33,9687° S, −70.77500° W), Santiago, Metropolitan Region, Chile, in May 2021. The sample authentication was performed by Jorge Macaya, a botanist from the University of Chile, Santiago, Chile. The voucher specimen AS-012052022 was coded and maintained in the Natural Products lab of the Universidad Austral de Chile, in Valdivia, Chile.
2.3. Extraction Procedure
One hundred grams of sample was lyophilized (Labconco Freeze Dry Systems, Model 7670541 2.5 Liter Palo Alto, CA, USA), and then, the dehydrated fruit material was milled using a Grindomix blade mill (GM 200, Retsch, Haan, Germany); the fine powder was sieved trough a stainless steel sieve No. 100 (150 µm) and wrapped in plastic bags and stored at −20 °C. Afterward, 5 g of powder was disposed in a flask, and then, 20 mL of ethanol with 1% formic acid was added as an extraction solvent and protected from the light; to obtain the extract, the flask was placed in an ultrasonic water bath (UC-60A Biobase medical equipment, Shangai, China) at room temperature for 30 min. Three extractions were performed with the same material. The extraction was filtered, and the solvent was removed with a rotary evaporator at 36 °C to obtain the crude extract (125 mg).
2.4. Determination of Proximal Composition
AOAC procedures were employed for the physicochemical determinations [17,18]. The moisture content was obtained using the oven method, and the crude protein was obtained with the Kjeldahl (N × 6.25) method using a Kjelmaster k375 (Büchi AG, Flawil, Switzerland) apparatus. The fiber content was determined via the gravimetric method followed by the hydrolysis of the samples, and the total lipid content was obtained via Soxhlet methodology (Sigma-Aldrich, St. Louis, MO, USA) by performing extractions with petroleum ether. The ash content was analyzed by employing a muffle furnace for incineration at 550 ± 15 °C. Total carbohydrates were obtained by difference using the equation: 100 − (g fat +g water +g fiber + g protein + g ash). The results are indicated in grams per 100 g of fresh weight (g/100 g fw).
2.5. Determination of Heavy Metals and Multi-Element Determination via ICP-MS
The minerals and heavy metals were analyzed according to the methodology reported previously [19,20]. For the digestion of the fruit samples, approximately 10 mg of the samples was digested with 0.5–1 mL of HNO3 69% in a microwave oven, at a temperature of 180 °C, and taken to a final volume of 5 mL with purified water and then sent to the multielement determination. The ICP-MS equipment used was an Agilent 7900, equipped with a Micromist-type concentric nebulizer, a Scott-type spray chamber, platinum interface cones, an off-axis double lens system, a hyperbolic quadrupole as a mass filter and collision cell, and an octopolar reaction that uses He and H2 gases, with a 15-place rotor. The standards were 20 µg/g Ge, Rh, and Ir from ISC Science and Ca, Mg, Fe, Zn, As, Cd, Hg, and Pb certified to 10,000 µg/mL by High-Purity Standards.
2.6. LC Parameters and MS Parameters
The separation and identification of biological components from the corcolen berries were carried out on an UHPLC-ESI-Q-TOF-MS system, equipped with Ultimate 3000 RS controlled with Chromeleon 6.8 (Dionex GmbH, Idstein, Germany), and a Bruker maXis ESI-QTOF-MS controlled with Data Analysis 4.0 (all Bruker Daltonik GmbH, Bremen, Germany). First, 5 mg of each extract was dissolved in 2 mL of methanol for analysis, filtered with a polytetrafluoroethylene filter, and 20 µL was injected into the equipment. Elution was performed with a binary gradient system with eluent (A) 0.1% formic acid in water, eluent (B) 0.1% formic acid in acetonitrile, and following the gradient: 1% B isocratic (0–2 min), 1–5% B (2–3 min), 5% B isocratic (3–5 min), 5–10% B (5–12 min), 10–30% B (12–25 min), 30–95% B (26–40 min), and 1% B isocratic to 50 min. Separation was carried out using a Thermo 80 Å, 5 μm C-18 column (100 mm × 4.6 mm) at a flow rate of 0.8 mL/min. ESI-QTOF-MS experiments were recorded in negative and positive ion modes, and the scan range was between 100 and 1200 m/z. Electrospray ionization (ESI) consisted of a capillary temperature of 200 °C, a dry gas flow rate of 8 L/min, a capillary voltage of 2.0 kV, and a nebulizer pressure of 2 bar. The experiments were performed in the automatic MS/MS mode. The identification of secondary metabolites was based on HR full MS, in fragmentation patterns, and comparisons with data from the literature.
2.7. Determination of Total Phenolic and Flavonoid Content
The total phenolic content (TPC) was determined using the colorimetric method with Folin–Ciocalteu’ reagent, and the results are expressed as mg of gallic acid equivalents (GAE) per g of dry fruit (mg GAE/ g). The total flavonoids content (TFC) was determined using the aluminum chloride method, and the results are expressed as mg of quercetin equivalents (QE) per g of dry fruit (mg QE/ g) [21]. Both assays were carried out in a microplate and measured in triplicate using a Multiskan FC Microplate Photometer (Thermo Scientific, Waltham, MA, USA). The values are shown as the mean ± standard deviation (SD).
2.8. Antioxidant Capacity
2.8.1. DPPH Scavenging Activity
The potential of A. dentata extract dissolved in methanol as a free radical scavenger was determined using the DPPH assay in a microplate following the procedure previously reported [22]. This analysis was performed at 517 nm in triplicate using a FC Microplate Multiskan spectrophotometer (Thermo Scientific, Waltham, MA, USA), and the results are reported as the mean ± SD expressed in mg Trolox equivalent per gram of dry fruit.
2.8.2. Ferric-Reducing Antioxidant Power Assay (FRAP)
A FRAP assay of A. dentata extract was performed as previously described [23] and by considering details from other reports [24]. For the preparation of the FRAP solution, 10 mL of acetate buffer (300 mM, pH 3.6) was mixed with 1 mL of FeCl3 (20 mM) (dissolved in water) and 1 mL of 2,4,6-tris(2-pyridyl)-s-triazine (10 mM) dissolved in HCl 40 mM. Then, 10 µL of sample was mixed with 190 µL of FRAP solution. The results were obtained via linear regression from a calibration graph obtained with Trolox (0–1 mM). The assay was performed in triplicate, and the results are reported as the mean ± SD expressed as mg of Trolox equivalent (TE) per gram of dry fruit.
2.8.3. Trolox Equivalent Antioxidant Activity (TEAC) Assay
The TEAC assay was performed according to a previous study [25] with some modifications. First, 10 µL of the sample was mixed with 200 µL of ABTS•+. The absorbance was measured at 734 nm at 30 °C. The results were interpolated in a calibration curve of Trolox (0–1 mM). The results are expressed as mg of Trolox equivalents (TE) per gram of dry fruit; all the assays were performed in triplicate, and the values are reported as the average ± SD.
2.8.4. Assay of Oxygen Radical Absorbance Capacity (ORAC)
The ORAC capacity was determined as previously described [26]. The reaction was prepared with 50 µL of fluorescein, 50 µL of sample/Trolox/blank and 25 µL of AAPH (2,2′-Azobis(2-amidinopropane) dihydrochloride). Values were obtained using a regression equation between the sample concentration of Trolox (100 µM) and the area under the decay curve. Data are depicted as mg of Trolox equivalents (TE) per gram of dry fruit (mg of TE/g).
2.8.5. Lipid Peroxidation in Human Erythrocytes
The lipid peroxidation in human erythrocytes (LP) assay was performed as described in a previous study [22] with some modifications. Human blood cells (red) were used. After washing, the cells were suspended in phosphate-buffered saline (hemoglobin density of 1 mM). The final cell suspension with different concentrations of the test compounds was incubated and dissolved in DMSO and PBS for 10 min at 37 °C. After incubation, the cells were added to tert-butylhydroperoxide (1 mM) for 15 min at 37 °C. After this, the lipid peroxidation was obtained via the TBARs’ formation. The evaluation was performed in quadruplicate, and the results are shown as the inhibition percentage (IP) of lipid peroxidation in human erythrocytes (IPLP) contrasted to the control of catechin at 100 µg/mL and reported as the average ± SD. Healthy volunteer donors gave their informed consent for inclusion before participating in the study. The protocol assay was conducted in accordance with the Declaration of Helsinki and is included in the approved project by CICIT-CA-UNSJ-Argentina (Project code 80020190100277SJ, N 0591-20-R-UNSJ), referred to as an ethical and environmental safeguard and to preserve the hygiene and safety conditions in the activities to be carried out in laboratories.
2.9. Determination of Enzymatic Activity
The inhibition of AChE was obtained according to the Ellman method, as previously reported [27]. A solution of 2 mg of extract per milliliter was prepared. The assay was performed by mixing the sample or standard with the enzyme AChE or BChE dissolved in Tris-HCl buffer (50 mM, pH 8.0) and 5-dithiol-bis (2-nitrobenzoic acid) at a pH of 8.0; to start the reaction, the substrate acetyl-thiocholine iodide or butyryl-thiocholine chloride was added. The absorbance was read at 405 nm for 30 min at 37 °C. The experiments were carried out in triplicate, and the results are reported as the average ± SD. The results are expressed as IC50 (µg of extract per mL). Galantamine was used as the positive control.
Tyrosinase activity was assessed using the dopachrome method [28]; 20 µL of A. dentata extract dissolved in ethanol was mixed with 30 µL of pH 6.8 PBS, (67 mM), 40 µL of tyrosinase (100 U/mL), and 40 µL of the substrate L-DOPA (2.5 mM). The absorbance was measured at 492 nm. The results are expressed as IC50 (µg of extract per mL. The experiments were run in triplicate, and the results are reported as the average ± SD. Kojic acid was the positive control compound.
The amylase inhibition was implemented by agreeing to the method previously described by H. Ali, P.J. Houghton and A. Soumyanath [29]. The glucosidase inhibition assays were evaluated using the method described by L. Liu, M. Deseo, C. Morris et al. [30]. Finally, the lipase inhibition evaluation was performed according to D. Lewis and D.J. Liu [31].
2.10. Isolation of Rat Aorta-Vascular Reactivity Assays
This study included the use of male Sprague Dawley rats (6–8 weeks old; n = 3) weighing between 170 and 200 g. The experimental procedure was approved (CEIC #366/2022) by the local ethics research committee of Universidad de Antofagasta, and the research was performed in agreement with this. The rats were randomized and maintained at room temperature and a humidity of 45–51% with full access to tap water and food ad libitum.
After the euthanasia of animals via cervical dislocation, the aortas were taken, and aortic rings we placed in an organ bath with Krebs–Ringer bicarbonate solution. The integrity of the vascular endothelium was evaluated with 10−5 M acetylcholine (ACh); then, the assays were performed. For the evaluation of the relaxation capacity produced by the A. dentata ethanolic extract, the tissue was pre-contracted with 10−6 M phenylephrine (PE); then, increasing concentrations of the extract were added to the organ bath on the vascular plateau response, as reported previously [32,33]. In addition, other similar protocols were performed to evaluate the role of the endothelium in the relaxation effect produced by A. dentata ethanolic extract; for these, the removal of the endothelium of the aortic rings and experiments with the protocol which includes the use of Nω-Nitro-L-arginine methyl ester (L-NAME, 10−4 M) was performed.
2.11. Statistical Analysis
Statistical analysis of the data was carried out using one-way or two-way analysis of variance (ANOVA) followed by the post hoc Dunnett test where applicable. In addition, the sensitivity determination (EC50 or IC50) was performed using nonlinear regression (sigmoidal) via Graph Pad Prism version 5.0, software package. (GraphPad Software, Inc., La Jolla, CA, USA). Statistical significance was determined at p = 0.05.
3. Results and Discussion
3.1. Physico-Chemical Properties of A. dentata Fruits
Table 1 shows the proximal composition of A. dentata fruits such as the crude fiber, ashes, humidity, proteins, fatty matter, carbohydrates, and the mineral content of the fruits (Ca, Mg, Fe, and Zn). The results of proximal composition showed that A. dentata fruits are rich in carbohydrates (72.1%), have good protein content (4.3%)—which is similar to that in guava fruits [34] and more than that in kiwis (1.1%) [35]—and the mineral content showed that A. dentata fruits are rich in calcium. This mineral is most frequently associated with fruit firmness, is essential, and plays crucial roles in the metabolism, since it is used in muscle contraction, bone formation, blood clotting, and nerve function, among others, so it is very important to assess the amount of calcium intake in the diet [36,37]. Magnesium is very important for bone formation, cardiovascular and muscular function, and the synthesis of proteins and nucleic acids. The content of magnesium, iron, and zinc are connected at both the cellular and molecular levels and are important, because deficiencies can cause psychological and cognitive symptoms, as well as manifestations of fatigue and lack of energy [38]. Fruits with high magnesium and calcium contents include avocados, dried figs, bananas, guavas, kiwi fruit, blackberries, papayas, cantaloupes, raspberries, and grapefruit [39,40]. The daily value (DV) for magnesium is 420 mg per day, so 500 g of A. dentata fruit can provide the daily intake and can be helpful in hypertension. To consider food safety, we also report the heavy metal contents of A. dentata fruits.

Table 1.
Proximal composition and mineral and heavy metal contents of A. dentata fruits.
It is very important to check the full contents of metals in foods. According to the FAO/WHO, the maximum permissible values of heavy metals in vegetables were the following in the year 1989: Cd 0.2, Pb 0.3, Ni 67.9, Fe 425.5, Cu 73.5, and Zn 99.4 [41]. However, nowadays, permitted values are lower according to the EFSA [42] and Codex Alimentarius [43]. Regarding the content of heavy metals, the levels of arsenic, a harmful metal and one of the principal factors for public health risks [44], in A. dentata fruits were 121 ± 11 µg per kilogram of fruit. On the other hand, cadmium values were 152 ± 5 µg per kilogram of fruit. Cadmium is an industrial and environmental pollutant that has harmful effects on human health, being a carcinogenic agent [45]. The mercury content in A. dentata fruits was 7.7 ± 1.3 µg/kg, while the values of lead, a harmful pollutant that has high toxic effects on many body organs [44], in A. dentata fruits were 294 ± 4 µg per kilogram of fruit. The values for arsenic, mercury, and lead were below or equal to the maximum limits set by the EFSA [42] and Codex Alimentarius [43]: 0.1mg/kg (in fruits), 0.5 mg/kg (in fishery products), and 0.20 mg/kg (in berries), respectively. Cadmium was the only case in which the concentration found in A. dentata fruits was higher than the maximum limit (0.050 mg/kg). However, further analysis must be carried out to estimate the daily intake and carcinogenic risk due to the consumption of this berry.
3.2. UHPLC–MS Analysis of Extracts
The fingerprint analysis via UHPLC MS (Figure 2) of the crude extracts of corcolen (A. dentata) was investigated through high-resolution mass spectrometric analysis. A negative mode of detection was used. Some metabolites have been pointed out for the first time in this species. Twenty-nine compounds were detected and twenty-seven were tentatively identified based on UV absorption and HR-MSn patterns (Table 2), and the major compounds of this plant are presented in Figure 3. Eleven detected compounds were assigned to phenolic acids, (peak 1–3, 6–8, 12, 15, 17, 18, and 24), seven to flavonoids (peaks 4, 11, 14, 18, 19, and 20), one flavanol (peak 13), two coumarins (peaks 5 and 27), five cyanidins (peaks 21–26), and one quinone (peak 9). The detailed fingerprinting analysis is explained below.
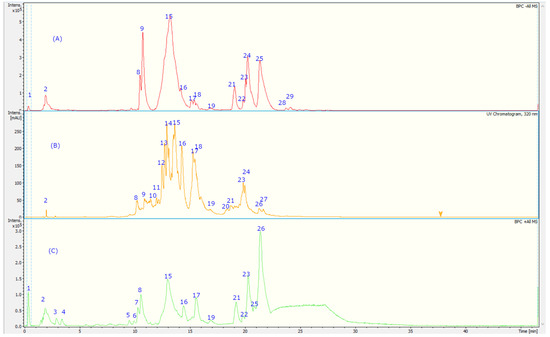
Figure 2.
UHPLC-TIC chromatogram of A. dentata extract with (A) negative MS mode, (B) UV at 320 nm, and (C) positive mode. The peak numbers refer to the compounds identified in Table 2.

Table 2.
Identification of compounds via UHPLC MS of A. dentata extracts.
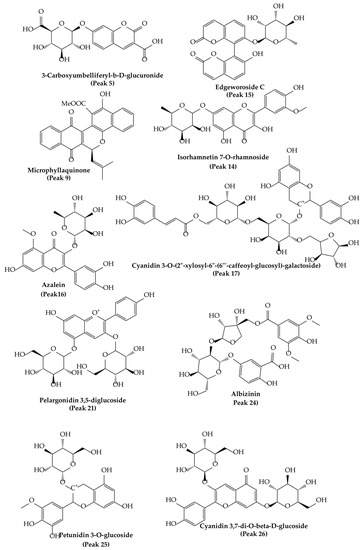
Figure 3.
Molecular structures of some main compounds detected via HPLC-MS on A. dentata fruits.
Peaks 17 and 21–26 showed PDA spectra characteristics of anthocyanidins (around 520 nm) [46], peak 17 with a positive ion at m/z: 904.2266 was identified as cyanidin 3-O-(-xylosyl-(6-caffeoyl-glucosyl)-galactoside) (C41H45O23), while peak 21 was identified as diglucosylated pelargonidin 3, 5 diglucoside, (C27H31O5) [47]. In the same fashion, peak 26 was identified as cyanidin 3, 7-di-O-glucoside, (MS daughter ion at m/z: 287.0554 after the loss of diglucosyl moieties) and a prominent ion at m/z: 449.1083 (M-glucose moiety); peak 22 was identified as delphinidin 3-O-glucoside (C27H31O5); peak 23, with a parent cation ion at m/z: 463.1124 and daughter ion at m/z: 301.2740 (peonidin), was identified as peonidin 3-O-glucoside [48]; and peak 25 was identified as petunidin 3-O-glucoside (C27H31O5).
On the other hand, peak 10 was tentatively identified as daphnotin B (C26H25O9) [49]. Peak 11 showed UV max characteristic of kaempferol feruloyl derivative (UV max 265-325–365 nm) and molecular anion at m/z: 947.2457, and losses of sugar derivatives leaving kaempferol moiety (285.0399 m/z) and was identified as kaempferol 3-O-feruloyl-sophorotrioside. Peak 13 with a parent molecular anion at m/z: 467.1160 and adduct (2M-H-) at m/z: 935.2375 was identified as the flavanol derivative catechin-4-ol 3-O-glucoside (C21H23O12); peak 14 with UV data at flavonol range and parent anion at m/z: 461.1085 was identified as isorhamnetin 7-O-rhamnoside (C22H22O11); peak 19 was identified as rutin (C27H31O17); and peak 20 was identified as isorhoifolin (C27H30O14). Peak 18 with UV vis data coincident with flavanone (281 nm) and a parent anion at m/z: 447.1235 was identified as sakuranin, the -O-glucoside of sakuranetin, and finally, peak 16 was identified as azalein, the 3-O-α-L-rhamnoside of azaleatin (C22H21O11) [50].
Peaks 1 and 2 with pseudomolecular ions at m/z: 355.1029 and 367.1044 were identified as ferulic acid 3-O-glucoside (C16H20O9) [48] and 3-O-feruloylquinic acid (C17H20O9), respectively, peak 3 with an M-H ion at m/z: 353.0847 and daughter ions at m/z: 179.0338 and 191.0468 was identified as chlorogenic acid (C16H18O9) [48], and peak 8 was identified as trans-p-coumaric acid (C9H8O3). Peak 6 with a M-H- ion at m/z: 301.0883 was identified as tachioside (C13H18O8) [51], and peak 7 as its isomer isotachioside; and finally, peak 12 was identified as grandisin (C24H32O7) and peak 24 as Albizinin (C27H31O17). Peak 27 with a pseudo molecular ion at m/z: 145.0299 was identified as coumarin (C9H6O2), peak 5 was identified as carboxyumbelliferyl glucuronide (C16H13O11) [52], and peak 15 was identified as dicoumarin edgeworoside C (C24H19O10) [53]. Finally, peak 9 with a M-H ion at m/z: 439.1212 was identified as microphyllaquinone (C27H19O6) [54].
3.3. Total Phenolic and Flavonoid Contents and Antioxidant Activity
The results are depicted in Table 3; the phenolics contents (23.50 ± 0.93 mg gallic acid equivalent/g of dry fruit) and flavonoids 10.75 ± 0.62 mg of quercetin/ g dry fruit) support the antioxidant potential of the ethanol extracts which was measured using several methods, namely DPPH (28.64 ± 1.87 mg TE/g dry fruit) methods, FRAP (25.32 ± 0.23 mg Trolox equivalents (TE)/g dry fruit),TEAC (34.72 ± 2.33 mg TE/g dry fruit), and ORAC (64.95 ± 1.23mg TE/g dry fruit). Additionally, A. dentata extract exhibited a strong inhibition of lipoperoxidation in human erythrocytes with an IPLP of 98% at 50 µg of A. dentata extract/mL compared to catechin, which showed 70% of IPLP at 100 µg/mL. These results are in the range of superfruit berries from Chile such as maqui berries (DPPH 28.18 ± 0.37 mg TE/g, TEAC 18.66 ± 0.26 mg TE/g, and FRAP 25.22 ± 0.38 mg TE/g), [55] as well as other Chilean berries: for instance, the reported TFC of murta, highbush blueberries, arrayán, calafate, chequén, and meli were in the range of 2.57–45.72 mg quercetin equivalent/g [46], and the TEAC from the popular goji berries was reported as 24.86 ± 2.15 mg Trolox/g, and its FRAP was 16.91 ± 1.95 mg Trolox/g (Licium barbarum cv. Erma) [56].

Table 3.
Total phenolic and antioxidant activity of A. dentata ethanolic extract.
3.4. Enzymatic Inhibitory Activity
The enzymatic inhibitory properties (cholinesterases, amylase, lipase, glucosidase, and tyrosinase inhibitory potential) of A. dentata extracts were evaluated for the first time; it is important to mention that no previous research exists related to the anti-enzymatic potential of this species reported. The enzymes that were included in this study are important in the treatment of noncommunicable diseases such as neurodegenerative diseases and metabolic syndrome. The results are outlined in Table 4 and are depicted as IC50 values (µg/mL). The employment of vegetables and shrubs has been important through the years to prevent neurodegenerative diseases due to the high amount of phenolics [57]. Neurodegenerative diseases, such as Alzheimer’s disease or Parkinson’s disease, are the NCDs linked to prolonged disability in the older adult population. For the treatment of Alzheimer’s Disease, usually, anticholinergic agents are employed; however, it is known that this treatment can produce many adverse effects that reduce the quality of life of the patient, despite collaborating with the delay of the progression of the disease. Parkinson’s disease (PD) is the second most common neurodegenerative disorder after AD and remains essentially untreatable. Experimental evidence suggests that it is a significant contributor to dopaminergic neuronal loss and that targeting tyrosinase is a frequent strategy in the prevention of neuronal degeneration in this NCD [58]. In the AChE assay, A. dentata extract showed an IC50 of 4.58 ± 0.04 µg/mL, while in the BChE assays, the IC50 was 23.44 ± 0.03 µg/mL (Table 4), and in the tyrosinase assays the inhibition was IC50 9.25 ± 0.15 µg/ml. Regarding the metabolites identified in the extract of A. dentata (Table 2), we can highlight the importance of some biologically active components such as anthocyanin flavonoids, coumarins, and phenolic compounds in this species, which could be worthy for use in the prevention of chronic noncommunicable or neurodegenerative diseases. On the other hand, A. dentata ethanolic extract showed good inhibition capacities against amylase (IC50: 5.6 + 0.0 µg/mL) and lipase (IC50: 30.8 ± 0.0 µg/mL), enzymes, which are relevant in the study of NCDs because they are related to the treatment of metabolic syndrome (Table 4). They describe the association between many chronic pathologies such as obesity, diabetes mellitus type 2, and CVDs, among others. Indeed, natural products can be inhibitors of the enzymes α-glucosidase and α-amylase and can be used to control post-prandial hyperglycemia [8]. Some fruits from Chañar from northern Chile—which were studied by some of us—containing flavonoids such as glycosides of kaempferol, isorhamnetin plus procyanidin dimers, etc., were able to inhibit those enzymes [8]. Some other berry extracts with phenolic compounds have been reported to inhibit α-amylase in vitro, but the most potent were from rowanberry and raspberry (IC50 values of 4.5 and 21.0 μg/mL, respectively) [59], the latter was similar to our corcolen berries and was also similar to the standard compound, acarbose. On the other hand, corcolen berries did not show good anti-glucosidase activity (360.9 µg/mL) as compared to other berries, for instance, rowanberry and blackcurrant, which inhibited α-glucosidase with IC50 values of 20 and 30 μg GAE/mL and were as effective as the current inhibitor, acarbose [60]. Meanwhile, some berries were able to inhibit pancreatic lipase activity: blueberry showed slight inhibition, whilst arctic bramble, strawberry, lingonberry, cloudberry, and raspberry were considerably more potent (the most active EC50 below 20 µg/mL) [61]. However, the enzymatic inhibitory activity of A. dentata ethanolic extract has demonstrated its capacity to inhibit enzymes that are linked to NCDs. The ability of phenolic compounds to modulate the activity of various enzymes, and therefore to interfere with cell signaling mechanisms, can be attributed in part to their chemical structures, while the antioxidant properties of the compounds may explain some of their beneficial effects [32]. Regarding the bioactive compounds, it was proposed that anthocyanins, such as peaks 21–26, may attenuate the symptoms of metabolic syndrome via improving impaired glucose tolerance, insulin resistance, dyslipidemia, blood glucose, hypertension and cholesterol levels; protecting β cells; and preventing ROS production [62]. Catechins such as peak 13 (catechin 4-ol-3-O-glucoside) plus other phenolic compounds and flavonoids were indicated as beneficial in the amelioration of chronic diseases, such as cancer and metabolic syndrome [63]. Rutin, a main flavonoid detected in this study, has been shown to cause the significant inhibition of α-amylase (p < 0.001; 53.66%) and α-glucosidase (p < 0.001; 52.56%) [64]. On the other hand, it was found that chlorogenic acid has a great inhibitory effect on lipase (IC50: 0.647 mg/mL) [65] and amylase (IC50 0.498 ± 0.013 mg/mL) [66]. It also inhibited cholinesterases (IC50: 8.01 ± 0.01 and 6.30 ± 0.02 for AChE and BChE, respectively [67].

Table 4.
Enzymatic inhibitory activity (IC50, in µg/mL) of A. dentata ethanolic extract against AChE, BChE, tyrosinase, amylase, glucosidase, and lipase.
3.5. Effect of A. dentata on Vascular Relaxation: Role of the Endothelium
The search for new vasodilatory functions of natural products is a main topic, since only 40 percent of the hypotensive or vasoactive plants from traditional medicine have been investigated so far [68]. Traditional medicines used for vascular relaxation, such as Vernonia amygdalina Del., Tridax procuinbens L., Mikania glomerata Spreng., and Kaempferia galanga L., have significant vasodilatory bioactivities, found via tests in vitro, through acting on eNOS and Ca2+ channels, while others, such as Gaultheria procumbens L., H. cere, Bidens pilosa Linn., etc. have vasodilatory activity which were investigated in vivo; they also showed their activity based upon other mechanisms, including sGC-cGMP, potassium channels, cyclooxygenase pathways, muscarinic receptors, prostaglandin I2, etc. [68]. In this study, the role of the endothelium in vascular relaxation shown by A. dentata fruits was investigated. The endothelium denudation in aortic rings produced a drastic decrease in relaxation for the A. dentata extract. Thus, the relaxation was approximately 20-50% lower in endothelium-rubbed rat aorta (rubbed); 32 ± 8% intact aorta (control) versus 6 ± 2% (rubbed) at 2 log μg/mL or 100 μg/mL (p < 0.001); and 78 ± 1% intact aorta (control) versus 27 ± 3% (rubbed) at 3 log μg/mL or 1000 μg/mL (p < 0.001) (Figure 4). The negative logarithm of the half-maximal effective concentration (pEC50) was significantly (p < 0.05) lower in rubbed aortic rings in the presence of the extract (Table 5).
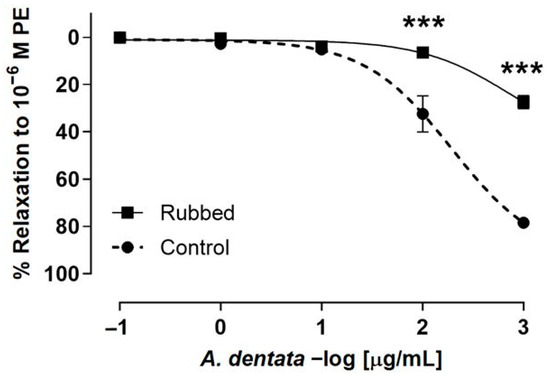
Figure 4.
A. dentata extract caused relaxation in rat aorta depending on the vascular endothelium. Effect the extract on the intact aortic rings (control) versus endothelium denuded (rubbed). Data are reported as the average SE of the mean (SEM) of 5–7 independent experiments. *** p < 0.001.

Table 5.
Relaxation effect of A. dentata in pre-contracted aortic rings with PE in the absence of endothelium (rubbed), presence (control), and pre-incubated with 10−4 M L-NAME.
The aortic ring tissue was pre-incubated with L-NAME, an endothelial nitric oxide synthase inhibitor, to evaluate whether endothelial factors were released. The results show that the use of 10−4 M L-NAME for pre-incubation with the aortic rings significantly decreased the vascular relaxation to 100 μg/mL A. dentata extract concentration (Figure 5A,B). The values for 2 log μg extract/mL (100 μg/mL) were 44 ± 4% control versus 5 ± 3% L-NAME, p < 0.001. In addition, the values for 3 log μg/mL (1000 μg/mL) extract were 77 ± 7% control vs. 14 ± 3% L-NAME, p < 0.001 (Figure 5C). The pIC50 of L-NAME vs. control was not significantly different (Table 5).
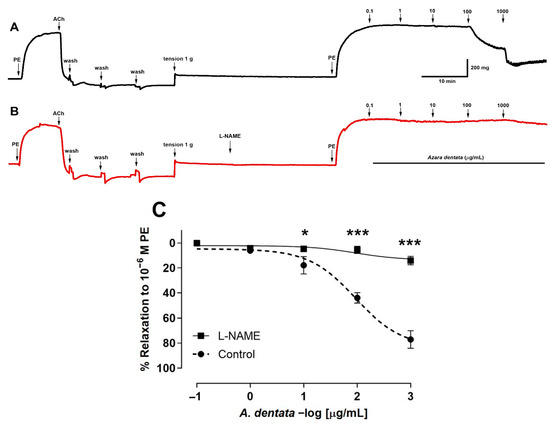
Figure 5.
Original record of the vascular response to A. Dentata extract in intact rat aorta (A,B). Previously, vascular endothelium was tested with 10−5 M acetylcholine in pre-contracted aortic rings with 10−6 M phenylephrine (PE). Effect of the extract on the aortic ring in absence of L-NAME (black line) (A) versus aortic ring pre-incubated with 10−4 M L-NAME for 20 min (red line) (B). Aortic rings pre-incubated with 10−4 M L-NAME or absence (Control) (C). Rat aorta was pre-contracted with 10−6 M phenylephrine (PE) for 10 min, and then, rising concentrations of A. dentata (0.1 to 1000 µg/mL) were added in organ bath at 7 min intervals. Data are presented as the average standard error of the mean (SEM) of 5–7 independent experiments. * p < 0.05, *** p < 0.001.
The denudation of the endothelium in aortic rings reduced the vascular relaxation induced by A. dentata in the vascular tissue pre-contracted with PE. These results suggest that the extract produces relaxation in intact rat aorta mediated by the release of factors, such as NO, from the vascular endothelium and vascular smooth muscle [69]. Certainly, the pre-incubation of the rat aorta with an inhibitor of endothelial nitric oxide synthase (L-NAME) reduced the relaxation induced by A. dentata, strongly suggesting that the nitric oxide (NO) pathway produced the response in some way. Therefore, these findings suggest that A. dentata shows a potential hypotensive effect.
4. Conclusions
In this study, the proximal analysis, the metal content, plus the chemical profile of the native berry Azara dentata is reported. The phenolic fingerprinting was investigated using UHPLC-PDA-Q-TOF-MS, and twenty-seven metabolites were detected, and of those, eleven detected compounds were assigned to phenolic acids, (peak 1–3, 6–8, 12, 15, 17, 18, and 24), seven to flavonoids (peaks 4, 11, 14, 16, 18, 19, and 20), one to a flavanol (peak 13), two to coumarins (peaks 5 and 27), five to cyanidins (peaks 21–26), and one to quinone (peak 9) for the first time. In addition, the content of phenolic and flavonoid compounds plus the antioxidant activity using different methods, including a strong inhibition of lipoperoxidation in human erythrocytes with an IPLP of 98% at 50 µg of A. dentata extract/mL compared to catechin, and the inhibition of different enzymes related to noncommunicable diseases such as metabolic syndrome and Alzheimer´ disease (AChE, BChE, tyrosinase, amylase, glucosidase, and lipase) are reported for the first time. The amylase inhibition was IC50 5.6 ± 0.0, more potent than standard acarbose, while for tyrosinase, it was found to be a good inhibitor, with IC50 9.25 ± 0.15, as well as for cholinesterases (IC50 2.87± 0.23 and IC50 6.73 ± 0.07 for AChE and BChE, respectively). Furthermore, the A. dentata extract showed 50-60% relaxation in rat aorta mediated thorough the release of endothelial nitric oxide, so it has potential for use in the treatment of hypertension as a dietary supplement. These findings are important to position these neglected fruits as interesting sources of phenolic compounds and as fruits with nutritional and health-promoting properties. The study highlights the nutritional benefits of these fruits and could boost their consumption.
Author Contributions
M.J.S. and F.J.B. conceived the research idea. Conceptualization, M.J.S., J.P. and F.J.B.; fruit collections, M.J.S., R.E.B. and L.C.R.; methodology, formal HPLC-MS analysis, A.T., A.P., F.C., J.M.C. and M.J.S.; in vitro antioxidant assays, L.C.R., J.M.C., J.G. and A.T.; resources, F.J.B. and M.J.S.; animal tests, R.E.B., J.P., A.P. and F.C.; writing—original draft preparation, A.T., J.M.C., F.J.B. and J.P.; writing—review and editing, J.G., R.E.B., J.P., M.J.S. and F.J.B.; project administration.; M.J.S. and F.J.B.; funding acquisition, M.J.S., J.P. and F.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by FONDECYT 1220075 to M.J.S. and FONDECYT 1200610 to J.P.; R.B. received funding from ANID PFCHA/Beca Doctorado Nacional/2019-21191978. J.G. and A.T. thank CICITCA-UNSJ and CONICET, Argentina for the doctoral scholarship.
Data Availability Statement
Raw HPLC-MS data and other experimental data are available from authors ‘request.
Acknowledgments
Juan Manuel Castagnini is a beneficiary of the grant (ZA21-028) for the requalification of the Spanish university system from the Ministry of Universities of the Government of Spain, modality “Maria Zambrano”, financed by the European Union, Next Generation EU through the project “Extraction of bioactive compounds from food matrices using innovative and sustainable technologies (EXTRABIO)”. The authors would also like to acknowledge the University of Valencia, Spain through the project OTR2021-22219SERVI supported by the Universidad Austral de Chile, Chile. Francisco J. Barba and Mario J. Simirgiotis are members of the CYTED network “P320RT0186—Aprovechamiento sostenible de recursos biomásicos vegetales iberoamericanos en cosmética (BIOLATES)”.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Siddiqi, K. Noncommunicable diseases. In Public Health: An Action Guide to Improving Health.; Walley, J., Wright, J., Eds.; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- WHO. Noncommunicable Diseases; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, R.E.; Ibáñez, E.; Puerta, A.; Padrón, J.M.; Paredes, A.; Cifuentes, F.; Romero-Parra, J.; Palacios, J.; Bórquez, J.; Simirgiotis, M.J. Phenolic Fingerprinting and Bioactivity Profiling of Extracts and Isolated Compounds from Gypothamnium pinifolium Phil. Antioxidants 2022, 11, 2313. [Google Scholar] [CrossRef] [PubMed]
- Torres-Benítez, A.; Ortega-Valencia, J.E.; Sanchez, M.; Divakar, P.K.; Simirgiotis, M.J.; Gómez-Serranillos, M.P. Metabolomic Profiling, Antioxidant and Enzyme Inhibition Properties and Molecular Docking Analysis of Antarctic Lichens. Molecules 2022, 27, 8086. [Google Scholar] [CrossRef] [PubMed]
- Areche, C.; Parra, J.R.; Sepulveda, B.; Garc, O.; Simirgiotis, M.J. UHPLC-MS Metabolomic Fingerpringint, Antioxidant, and Enzyme Inhibition Activities of Himantormia lugubris from Antarctica. Metabolites 2022, 12, 560. [Google Scholar] [CrossRef]
- Vargas-Arana, G.; Merino-Zegarra, C.; Del-Castillo, Á.M.R.; Quispe, C.; Viveros-Valdez, E.; Simirgiotis, M.J. Antioxidant, Antiproliferative and Anti-Enzymatic Capacities, Nutritional Analysis and UHPLC-PDA-MS Characterization of Ungurahui Palm Fruits (Oenocarpus bataua Mart) from the Peruvian Amazon. Antioxidants 2022, 11, 1598. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Theoduloz, C.; Soriano, M.D.P.C.; Ugalde-Arbizu, M.; Alberto, M.R.; Zampini, I.C.; Isla, M.I.; Simirgiotis, M.J.; Schmeda-Hirschmann, G. The Native Fruit Geoffroea decorticans from Arid Northern Chile: Phenolic Composition, Antioxidant Activities and In Vitro Inhibition of Pro-Inflammatory and Metabolic Syndrome-Associated Enzymes. Molecules 2017, 22, 1565. [Google Scholar] [CrossRef]
- Fundación, R.A. Philippi. Available online: https://fundacionphilippi.cl/ (accessed on 30 November 2022).
- Sagareishvili, T.G.; Alaniya, M.D.; Kemertelidze, E.P. A new flavonol glycoside from Azara microphylla. Chem. Nat. Compd. 1983, 19, 275–278. [Google Scholar] [CrossRef]
- Giordano, A.; Retamal, M.; Leyton, F.; Martínez, P.; Bridi, R.; Velásquez, P.; Montenegro, G. Bioactive polyphenols and antioxidant capacity of Azara petiolaris and Azara integrifolia honeys. CyTA J. Food 2018, 16, 484–489. [Google Scholar] [CrossRef]
- Paz, C.; González-Chavarría, I.; Freire, E.; Ortiz, L.; Karpiński, T.M.; Duprat, F.; Baggio, R. Phytochemical and crystallographic studies of azara dentata extracts and its cytotoxic effects on human breast cancer cell, MCF-7. J. Chil. Chem. Soc. 2020, 65, 4891–4894. [Google Scholar] [CrossRef]
- Rocchetti, G.; Chiodelli, G.; Giuberti, G.; Ghisoni, S.; Baccolo, G.; Blasi, F.; Montesano, D.; Trevisan, M.; Lucini, L. UHPLC-ESI-QTOF-MS profile of polyphenols in Goji berries (Lycium barbarum L.) and its dynamics during in vitro gastrointestinal digestion and fermentation. J. Funct. Foods 2018, 40, 564–572. [Google Scholar] [CrossRef]
- Liu, S.; Marsol-Vall, A.; Laaksonen, O.; Kortesniemi, M.; Yang, B. Characterization and Quantification of Nonanthocyanin Phenolic Compounds in White and Blue Bilberry (Vaccinium myrtillus) Juices and Wines Using UHPLC-DAD-ESI-QTOF-MS and UHPLC-DAD. J. Agric. Food Chem. 2020, 68, 7734–7744. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, R.; Fernández-Galleguillos, C.; Pastene, E.; Simirgiotis, M.; Romero-Parra, J.; Ahmed, S.; Echeverría, J. Metabolomic Analysis, Fast Isolation of Phenolic Compounds, and Evaluation of Biological Activities of the Bark From Weinmannia trichosperma Cav. (Cunoniaceae). Front. Pharmacol. 2020, 11, 780. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, R.E.; Ahmed, S.; Cortés, C.; Fernández-Galleguillos, C.; Romero-Parra, J.; Simirgiotis, M.J.; Echeverría, J. Chemical Fingerprinting and Biological Evaluation of the Endemic Chilean Fruit Greigia sphacelata (Ruiz and Pav.) Regel (Bromeliaceae) by UHPLC-PDA-Orbitrap-Mass Spectrometry. Molecules 2020, 25, 3750. [Google Scholar] [CrossRef] [PubMed]
- AOAC International; Horwitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International—18th Edition, Revision 3; AOAC International: Gaitherburg, MD, USA, 2005. [Google Scholar]
- Vargas-Arana, G.; Merino-Zegarra, C.; Riquelme-Penaherrera, M.; Nonato-Ramirez, L.; Delgado-Wong, H.; Pertino, M.W.; Parra, C.; Simirgiotis, M.J. Antihyperlipidemic and antioxidant capacities, nutritional analysis and uhplc-pda-ms characterization of cocona fruits (Solanum sessiliflorum dunal) from the peruvian amazon. Antioxidants 2021, 10, 1566. [Google Scholar] [CrossRef]
- De la Fuente, B.; López-García, G.; Mañez, V.; Alegría, A.; Barberá, R.; Cilla, A. Evaluation of the Bioaccessibility of Antioxidant Bioactive Compounds and Minerals of Four Genotypes of Brassicaceae Microgreens. Foods 2019, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Calleja-Gómez, M.; Castagnini, J.M.; Carbó, E.; Ferrer, E.; Berrada, H.; Barba, F.J. Evaluation of Pulsed Electric Field-Assisted Extraction on the Microstructure and Recovery of Nutrients and Bioactive Compounds from Mushroom (Agaricus bisporus). Separations 2022, 9, 302. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Tapia, A.; Rodriguez, J.; Theoduloz, C.; Lopez, S.; Schmeda-Hirschmann, G.; Feresin, G.E. Free radical scavengers and antioxidants from Baccharis grisebachii. J. Ethnopharmacol. 2004, 95, 155–161. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Hao, Z.; Liang, L.; Liu, H.; Yan, Y.; Zhang, Y. Exploring the Extraction Methods of Phenolic Compounds in Daylily (Hemerocallis citrina Baroni) and Its Antioxidant Activity. Molecules 2022, 27, 2964. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Larrazábal-Fuentes, M.J.; Fernández-Galleguillos, C.; Palma-Ramírez, J.; Romero-Parra, J.; Sepúlveda, K.; Galetovic, A.; González, J.; Paredes, A.; Bórquez, J.; Simirgiotis, M.J.; et al. Chemical Profiling, Antioxidant, Anticholinesterase, and Antiprotozoal Potentials of Artemisia copa Phil. (Asteraceae). Front. Pharmacol. 2020, 11, 594174. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Uysal, S.; Ceylan, R.; Aktumsek, A. Phenolic constituent, antioxidative and tyrosinase inhibitory activity of Ornithogalum narbonense L. from Turkey: A phytochemical study. Ind. Crops Prod. 2015, 70, 1–6. [Google Scholar] [CrossRef]
- Ali, H.; Houghton, P.J.; Soumyanath, A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef]
- Liu, L.; Deseo, M.A.; Morris, C.; Winter, K.M.; Leach, D.N. Investigation of α-glucosidase inhibitory activity of wheat bran and germ. Food Chem. 2011, 126, 553–561. [Google Scholar] [CrossRef]
- Lewis, D.; Liu, D. Direct Measurement of Lipase Inhibition by Orlistat Using a Dissolution Linked In Vitro Assay. Clin. Pharmacol. Biopharm. 2012, 1, 1–3. [Google Scholar] [CrossRef]
- Paredes, A.; Palacios, J.; Quispe, C.; Nwokocha, C.R.; Morales, G.; Kuzmicic, J.; Cifuentes, F. Hydroalcoholic extract and pure compounds from Senecio nutans Sch. Bip (Compositae) induce vasodilation in rat aorta through endothelium-dependent and independent mechanisms. J. Ethnopharmacol. 2016, 192, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Palacios, J.; Paredes, A.; Cifuentes, F.; Catalán, M.A.; García-Villalón, A.L.; Borquez, J.; Simirgiotis, M.J.; Jones, M.; Foster, A.; Greensmith, D.J. A hydroalcoholic extract of Senecio nutans SCh. Bip (Asteraceae); Its effects on cardiac function and chemical characterization. J. Ethnopharmacol. 2023, 300, 115747. [Google Scholar] [CrossRef]
- Bashir, H.A.; Abu-Goukh, A.B.A. Compositional changes during guava fruit ripening. Food Chem. 2003, 80, 557–563. [Google Scholar] [CrossRef]
- Boland, M. Kiwifruit Proteins. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 68, pp. 59–80. [Google Scholar]
- Rato, A.E.; Agulheiro, A.C.; Barroso, J.M.; Riquelme, F. Effect of different calcium fruit content in physical and chemical properties of european plum. J. Plant Nutr. 2010, 33, 391–404. [Google Scholar] [CrossRef]
- Salomone, V.N.; Riera, M. Proximal Composition of Undaria pinnatifida from San Jorge Gulf (Patagonia, Argentina). Biol. Trace Elem. Res. 2020, 196, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Tardy, A.L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and minerals for energy, fatigue and cognition: A narrative review of the biochemical and clinical evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Harmankaya, M.; Gezgin, S. Mineral and heavy metal contents of the outer and inner tissues of commonly used fruits. Environ. Monit. Assess. 2012, 184, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Castillo, C.P.; Dewey, P.J.S.; Aguirre, A.; Lara, J.J.; Vaca, R.; De La Barra, P.L.; Ortiz, M.; Escamilla, I.; James, W.P.T. The mineral content of Mexican fruits and vegetables. J. Food Compos. Anal. 1998, 11, 340–356. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives; World Health Organization & Food and Agriculture Organization of the United Nations. Toxicological evaluation of certain food additives and contaminants. In Proceedings of the 33rd Meeting of the Joint FAO/WHO Expert Committee on Food Additives, Geneva, Switzerland, 21–30 March 1989; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Commission of the European Communities. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- FAO; WHO. Codex Alimentarius—Joint FAO/WHO Food Standards Programme; World Health Organization Food and Agriculture Organization of the United Nations: Rome, Italy, 2001. [Google Scholar]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Castro-González, M.I.; Méndez-Armenta, M. Heavy metals: Implications associated to fish consumption. Environ. Toxicol. Pharmacol. 2008, 26, 263–271. [Google Scholar] [CrossRef]
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC-HR-ESI-ToF-MS. Food Chem. 2015, 176, 106–114. [Google Scholar] [CrossRef]
- Sabando, C.; Rodríguez-Díaz, M.; Ide, W.; Pastene, E.; Avello, M.; Simirgiotis, M.; Rojas, S.; Villarroel, E.; Silva-Grecchi, T.; Gutiérrez, C.; et al. Improvement of endothelial function by Gunnera tinctoria extract with antioxidant properties. Biol. Res. 2020, 53, 1–14. [Google Scholar] [CrossRef]
- Fernández-Galleguillos, C.; Quesada-Romero, L.; Puerta, A.; Padrón, J.M.; Souza, E.; Romero-Parra, J.; Simirgiotis, M.J. Uhplc-ms chemical fingerprinting and antioxidant, antiproliferative, and enzyme inhibition potential of gaultheria pumila berries. Metabolites 2021, 11, 523. [Google Scholar] [CrossRef]
- Liang, S.; Tian, J.-M.; Feng, Y.; Liu, X.-H.; Xiong, Z.; Zhang, W.-D. Flavonoids from Daphne aurantiaca and Their Inhibitory Activities against Nitric Oxide Production. Chem. Pharm. Bull. 2011, 59, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Naliwajski, M.R.; Wileńska, B.; Misicka, A.; Pietrosiuk, A.; Sykłowska-Baranek, K. HPLC-PDA-ESI-HRMS-Based Profiling of Secondary Metabolites of Rindera graeca Anatomical and Hairy Roots Treated with Drought and Cold Stress. Cells 2022, 11, 931. [Google Scholar] [CrossRef]
- Garba Koffi, J.; Keumoe, R.; Ngansop, C.A.N.; Kagho, D.U.K.; Tchegnitegni, B.T.; Fotsing, Y.S.F.; Bankeu, J.J.K.; Boyom, F.F.; Sewald, N.; Lenta, B.N. Constituents of Endodesmia calophylloides Benth. and Adenia lobata (Jacq.) Engl. with antileihsmanial activities. Chem. Data Collect. 2021, 35, 100751. [Google Scholar] [CrossRef]
- Raineri, D.M.; Bottino, P.; Gordon, M.P.; Nester, E.W. Agrobacterium-mediated transformation of rice (Oryza sauva L.). Biotechnology 1990, 8, 33–38. [Google Scholar] [CrossRef]
- Yan, J.; Tong, S.; Chu, J.; Sheng, L.; Chen, G. Preparative isolation and purification of syringin and edgeworoside C from Edgeworthia chrysantha Lindl by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1043, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.S.; Costa, S.M.O.; Pessoa, O.D.L.; Moraes, M.O.; Pessoa, C.; Fortier, S.; Silveira, E.R.; Lemos, T.L.G. Cytotoxic naphthoquinones from roots of Lippia microphylla. Z. Für Nat. Sect. C J. Biosci. 2003, 58, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Determination of polyphenolic profile, antioxidant activity and antibacterial properties of maqui [Aristotelia chilensi s (Molina) Stuntz] a Chilean blackberry. J. Sci. Food Agric. 2016, 96, 4235–4242. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Moldovan, C.; Zengin, G.; Bender, O.; Locatelli, M.; Simirgiotis, M.; Atalay, A.; Vodnar, D.C.; Rohn, S.; Crișan, G. UHPLC-QTOF-MS analysis of bioactive constituents from two Romanian Goji (Lycium barbarum L.) berries cultivars and their antioxidant, enzyme inhibitory, and real-time cytotoxicological evaluation. Food Chem. Toxicol. 2018, 115, 414–424. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Bórquez, J.; Schmeda-Hirschmann, G. Antioxidant capacity, polyphenolic content and tandem HPLC-DAD-ESI/MS profiling of phenolic compounds from the South American berries Luma apiculata and L. chequén. Food Chem. 2013, 139, 289–299. [Google Scholar] [CrossRef]
- Khan, M.S.; Tabrez, S.; Priyadarshini, M.; Priyamvada, S. Targeting Parkinson’s—Tyrosine Hydroxylase and Oxidative Stress as Points of Interventions. CNS Neurol. Disord. Drug Targets 2012, 11, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Grussu, D.; Stewart, D.; McDougall, G.J. Berry polyphenols inhibit α-amylase in vitro: Identifying active components in rowanberry and raspberry. J. Agric. Food Chem. 2011, 59, 2324–2331. [Google Scholar] [CrossRef]
- Boath, A.S.; Stewart, D.; McDougall, G.J. Berry components inhibit α-glucosidase in vitro: Synergies between acarbose and polyphenols from black currant and rowanberry. Food Chem. 2012, 135, 929–936. [Google Scholar] [CrossRef]
- McDougall, G.J.; Kulkarni, N.N.; Stewart, D. Berry polyphenols inhibit pancreatic lipase activity in vitro. Food Chem. 2009, 115, 193–199. [Google Scholar] [CrossRef]
- Naseri, R.; Farzaei, F.; Haratipour, P.; Nabavi, S.F.; Habtemariam, S.; Farzaei, M.H.; Khodarahmi, R.; Tewari, D.; Momtaz, S. Anthocyanins in the Management of Metabolic Syndrome: A Pharmacological and Biopharmaceutical Review. Front. Pharmacol. 2018, 9, 1310. [Google Scholar] [CrossRef]
- Shabbir, U.; Rubab, M.; Daliri, E.B.M.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, quercetin, catechins and metabolic diseases: The role of gut microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Ganeshpurkar, A.; Ganeshpurkar, A.; Bansal, D.; Dubey, N. Glycolytic enzyme inhibitory and antiglycation potential of rutin. Futur. J. Pharm. Sci. 2017, 3, 158–162. [Google Scholar] [CrossRef]
- Xu, Z.; Cao, Q.; Manyande, A.; Xiong, S.; Du, H. Analysis of the binding selectivity and inhibiting mechanism of chlorogenic acid isomers and their interaction with grass carp endogenous lipase using multi-spectroscopic, inhibition kinetics and modeling methods. Food Chem. 2022, 382, 132106. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J. Funct. Foods 2020, 64, 103587. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef]
- Tang, F.; Yan, H.L.; Wang, L.X.; Xu, J.F.; Peng, C.; Ao, H.; Tan, Y.Z. Review of Natural Resources With Vasodilation: Traditional Medicinal Plants, Natural Products, and Their Mechanism and Clinical Efficacy. Front. Pharmacol. 2021, 12, 627458. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Schroeder, H.; Power, G.G.; Blood, A.B. A physiologically relevant role for NO stored in vascular smooth muscle cells: A novel theory of vascular NO signaling. Redox Biol. 2022, 53, 102327. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).