Development of Fermented Rice Water to Improve the Quality of Garaetteok, a Traditional Korean Rice Cake
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Lactic Acid Bacteria Strains
2.3. Preparation and Fermentation of WRW
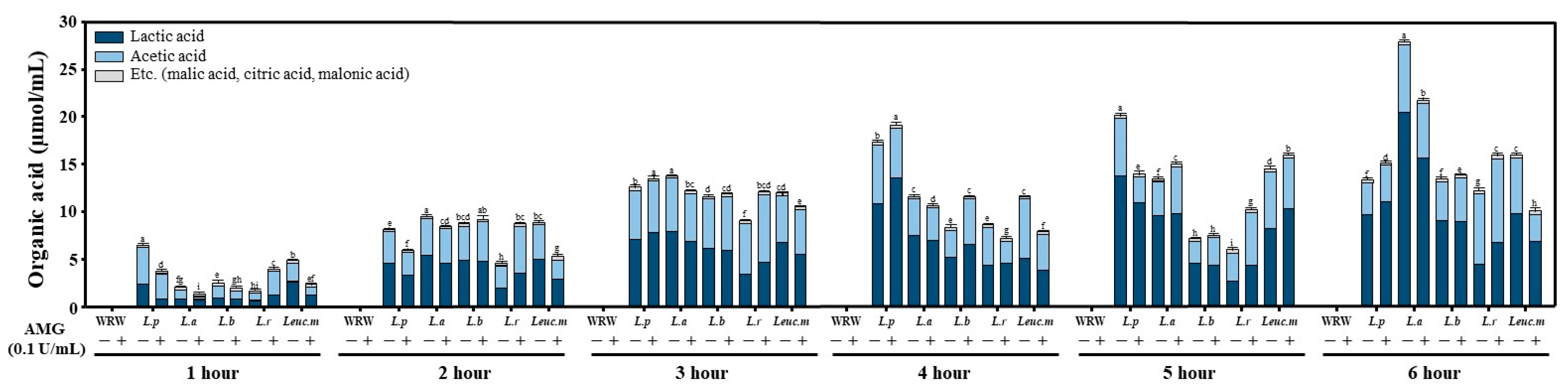
2.4. Determination of Organic Acid Content
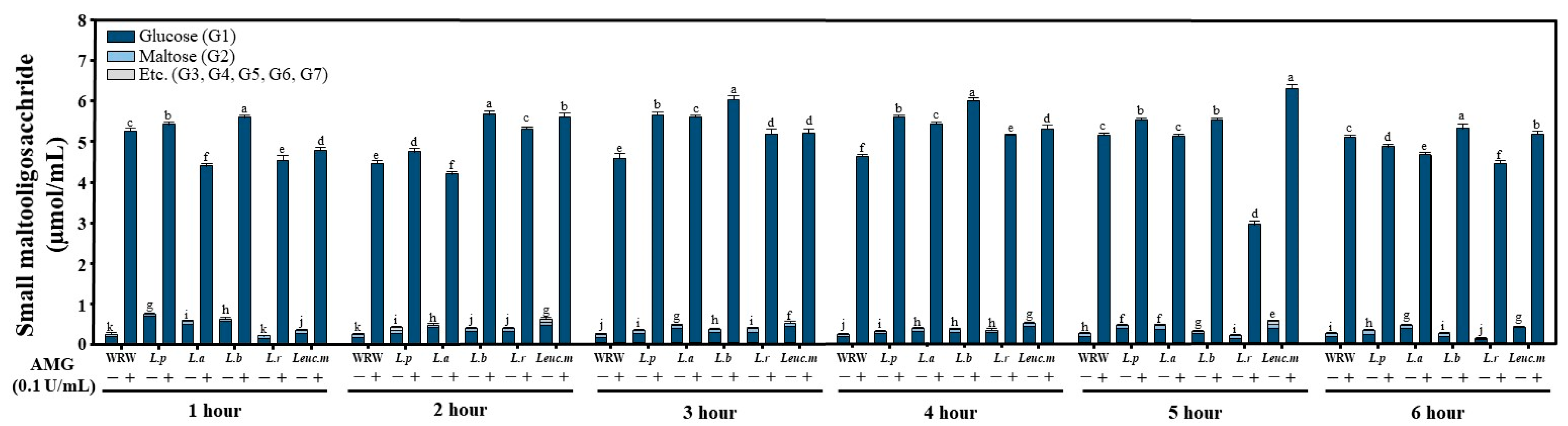
2.5. Analysis of Small Maltooligosaccharides (MOSs)
2.6. Garaetteok Preparation
2.7. Texture Analysis
2.8. Differential Scanning Calorimetry (DSC)
2.9. Microbiological Analysis
2.10. Physical Characteristics of Garaetteok
2.11. Statistical Analysis
3. Results
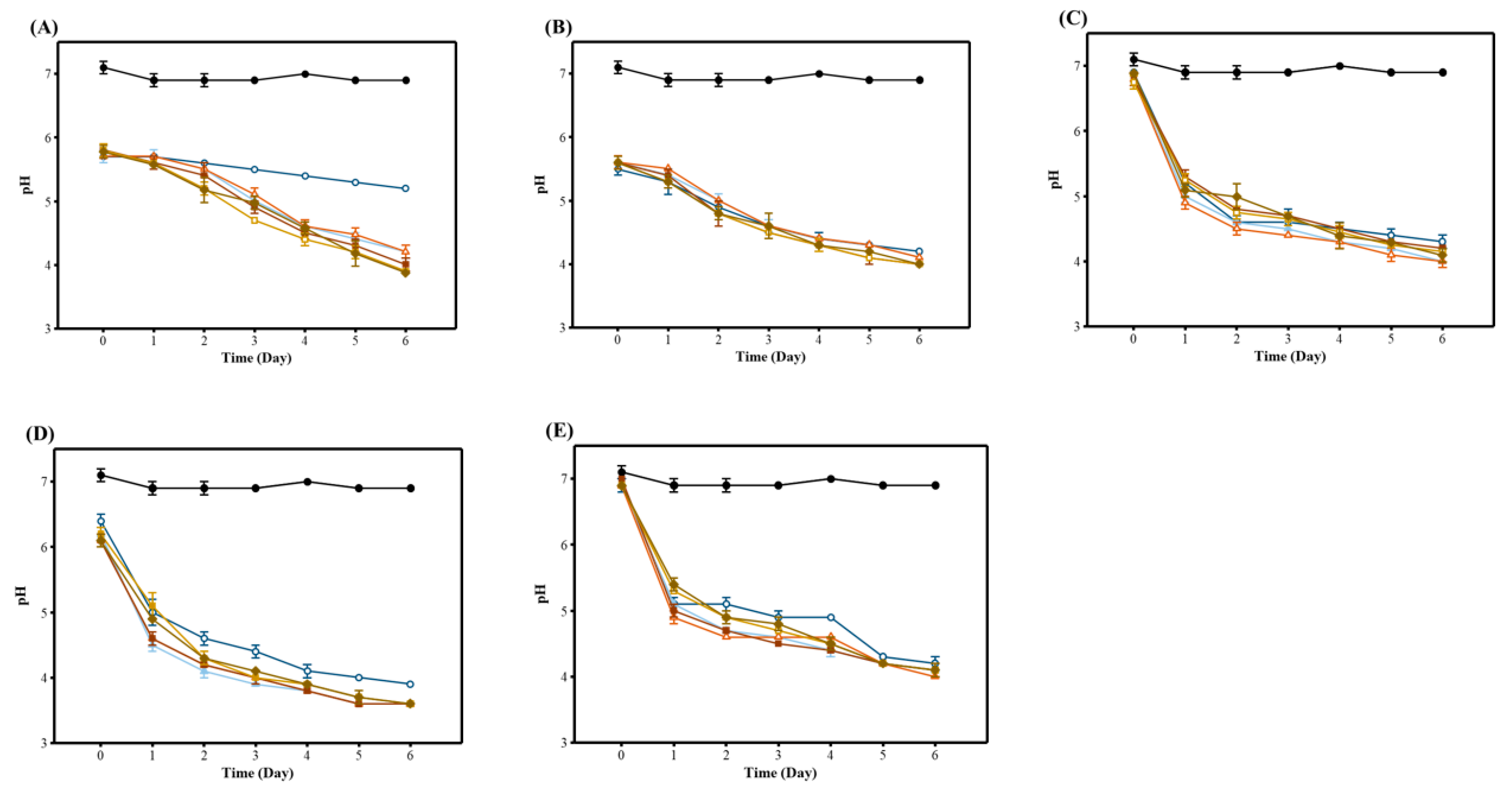
3.1. The pH Changes of WRW during Fermentation
3.2. Analysis of the Products of FWRW
3.3. Properties of FWRW as an Additive for Inhibiting Microbial Growth in Garaetteok
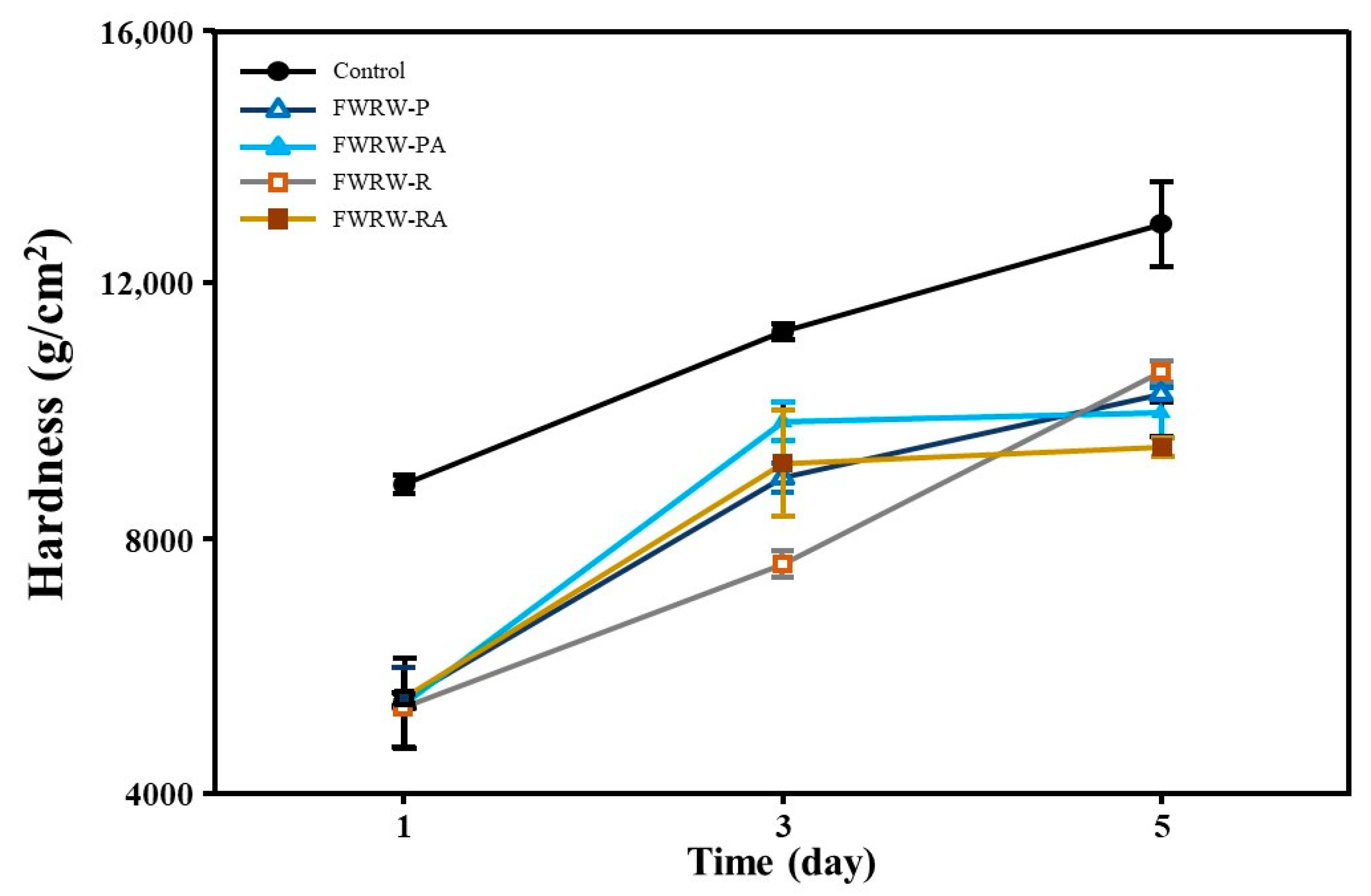
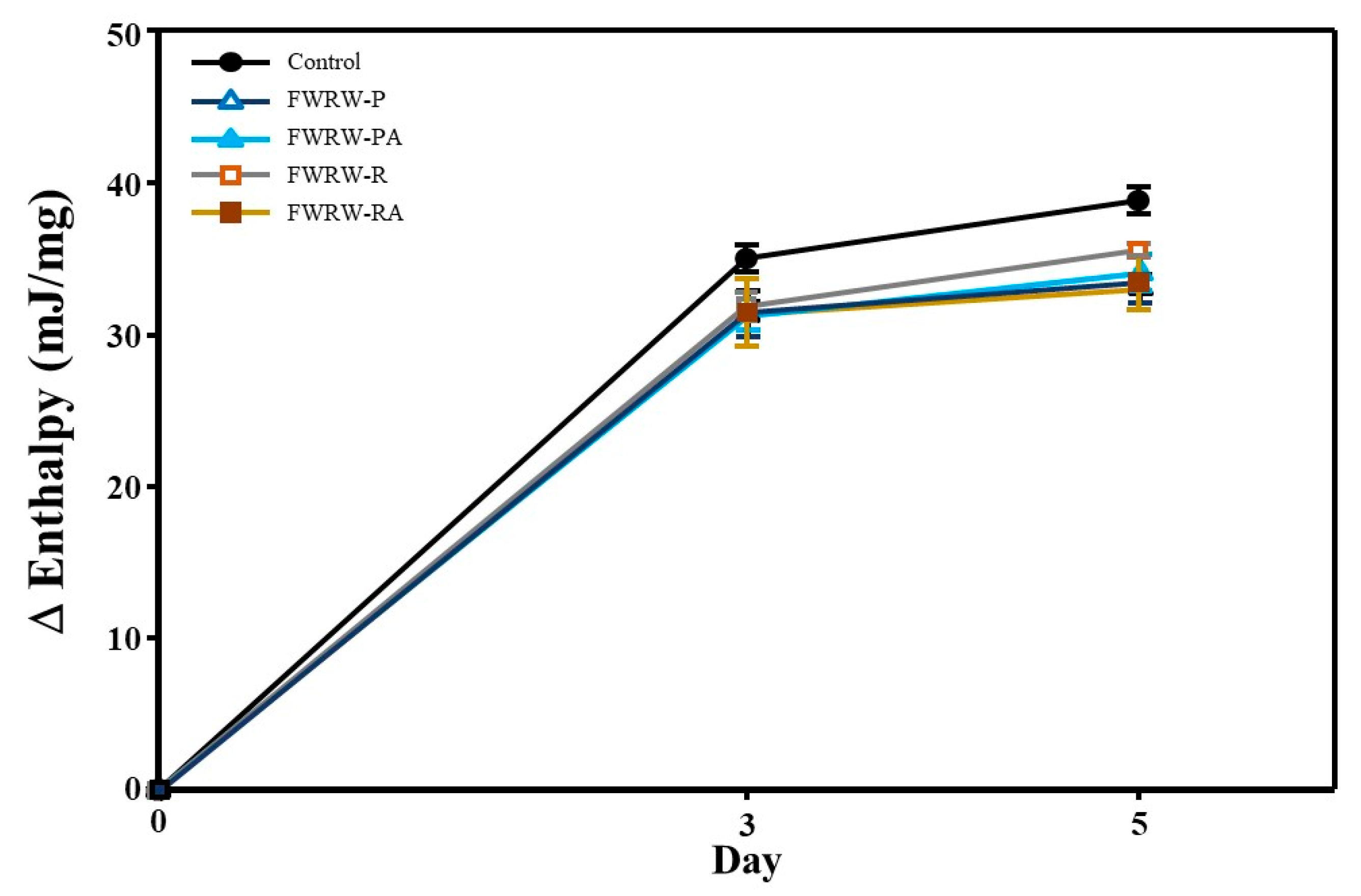
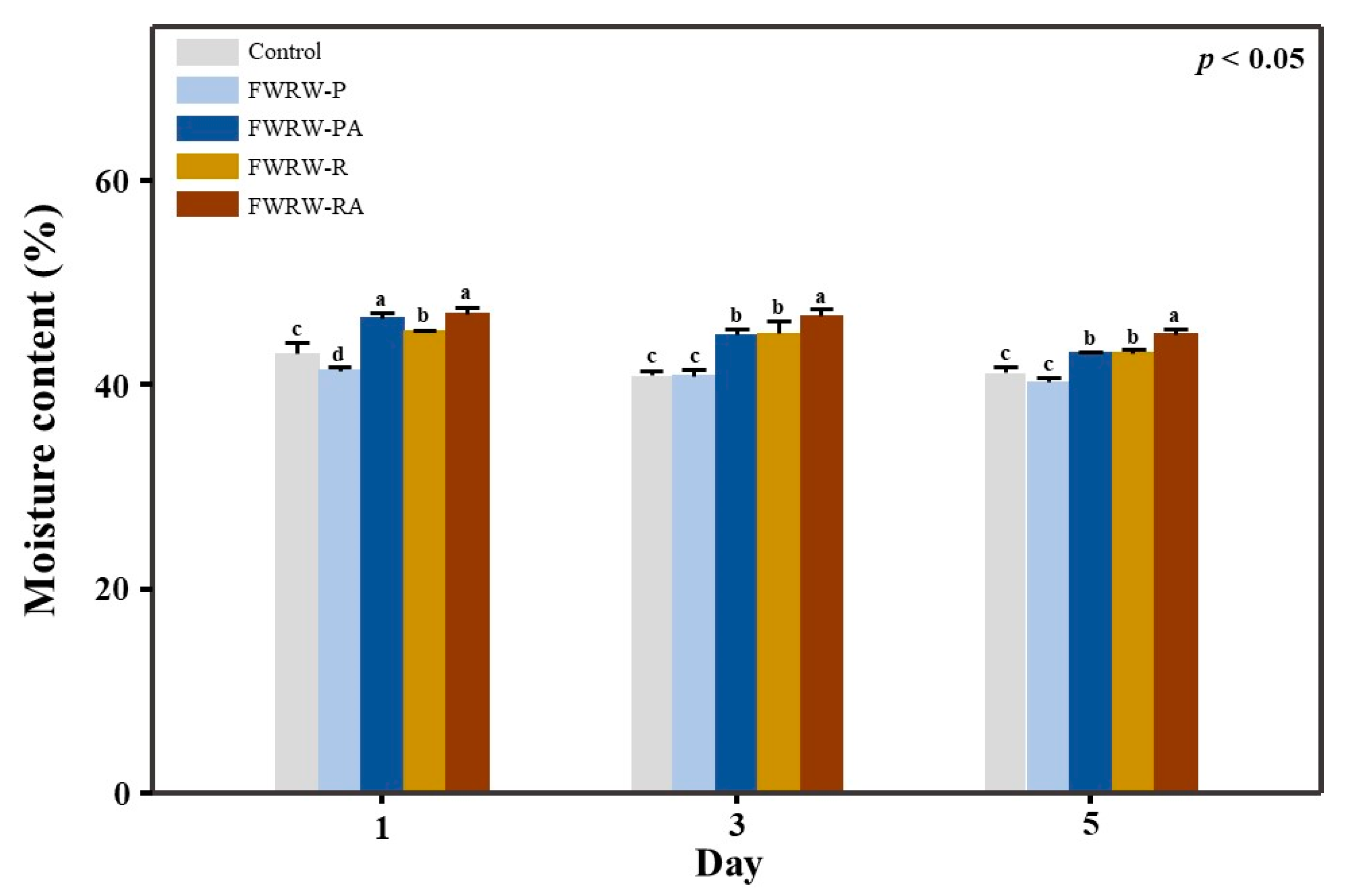
3.4. Properties of FWRW as an Antistaling Agent in Garaetteok
3.5. Preparation of Garaetteok Treated with FRWR and Analysis of Its Physical Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nabayi, A.; Teh, C.B.S.; Tan, A.K.Z.; Tan, N.P. Consecutive application effects of washed rice water on plant growth, soil chemical properties, nutrient leaching, and soil bacterial population on three different soil textures over three planting cycles. Agronomy 2022, 12, 2220. [Google Scholar] [CrossRef]
- Nabayi, A.; Sung, C.T.B.; Zuan, A.T.K.; Paing, T.N. Fermentation of washed rice water increases beneficial plant bacterial population and nutrient concentrations. Sustainability 2021, 13, 13437. [Google Scholar] [CrossRef]
- Abba, N.; Sung, C.T.B.; Paing, T.N.; Zuan, A.T.K. Wastewater from washed rice water as plant nutrient source: Current understanding and knowledge gaps. Pertanika J. Trop. Agric. Sci. 2021, 29, 1347–1369. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Zhong, Q.; Yun, Y.H.; Chen, W.; Chen, W. Determination of microbial diversity and community composition in unfermented and fermented washing rice water by high-throughput sequencing. Curr. Microbiol. 2021, 78, 1730–1740. [Google Scholar] [CrossRef]
- Prakash, V.; Veedu, A.; Babu, P.; Jothish, A.; Nair, S.S.; Suhail, A.; Prabhakar, M.; Rajan, R.; Priyanka, S.; Nair, B.G.; et al. Lactobacillus fermentum strains from rice water and lemon pickle with potential probiotic properties and wastewater treatment applications. Res. Sq. 2020; preprint. [Google Scholar] [CrossRef]
- Singh, N.; Goel, G.; Singh, N.; Kumar Pathak, B.; Kaushik, D. Modeling the red pigment production by monascus purpureus MTCC 369 by artificial neural network using rice water based medium. Food Biosci. 2015, 11, 17–22. [Google Scholar] [CrossRef]
- Park, M.K.; Jeong, J.S.; Kim, W.S. Antibacterial activity of Lactobacillus sakei on microorganisms isolated from chicken manure. J. Dairy Sci. Biotechnol. 2017, 35, 25–31. [Google Scholar] [CrossRef]
- Kim, Y.C.; Park, S.H. Recovery of useful components from rice-washing water using membranes. Membr. J. 2002, 12, 165–170. [Google Scholar]
- Kim, M.J.; Park, S.S.; Kim, D.H.; Kim, K.S. Proximate compositions changed before and after fermentation of rice spent water. J. Food Hyg. Saf. 2011, 26, 192–197. [Google Scholar]
- Marto, J.; Neves, Â.; Gonçalves, L.; Pinto, P.; Almeida, C.; Simões, S. Rice water: A traditional ingredient with anti-aging efficacy. Cosmetics 2018, 5, 26. [Google Scholar] [CrossRef]
- Ricci, A.; Diaz, A.B.; Caro, I.; Bernini, V.; Galaverna, G.; Lazzi, C.; Blandino, A. Orange peels: From by-product to resource through lactic acid fermentation. J. Sci. Food Agric. 2019, 99, 6761–6767. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; He, H.; Zhang, S.; Kong, J. Effects of inoculants Lactobacillus brevis and Lactobacillus parafarraginis on the fermentation characteristics and microbial communities of corn stover silage. Sci. Rep. 2017, 7, 13614. [Google Scholar] [CrossRef] [PubMed]
- Khalisanni, K.; Lee, H.K. Screening lactic acid bacteria for antimicrobial compound production. Nat. Preced. 2009. [Google Scholar] [CrossRef]
- Othman, M.; Ariff, A.B.; Rios-Solis, L.; Halim, M. Extractive fermentation of lactic acid in lactic acid bacteria cultivation: A review. Front. Microbiol. 2017, 8, 2285. [Google Scholar] [CrossRef]
- Garbacz, K. Anticancer activity of lactic acid bacteria. Semin. Cancer Biol. 2022, 86, 356–366. [Google Scholar] [CrossRef]
- Saadat, Y.R.; Khosroushahi, A.Y.; Gargari, B.P. A comprehensive review of anticancer, immunomodulatory and health beneficial effects of the lactic acid bacteria exopolysaccharides. Carbohydr. Polym. 2019, 217, 79–89. [Google Scholar] [CrossRef]
- Harutoshi, T. Exopolysaccharides of lactic acid bacteria for food and colon health applications. In Lactic acid Bacteria-R & D for Food, Health and Livestock Purposes; IntechOpen: London, UK, 2013. [Google Scholar]
- Cui, F.; Li, Y.; Wan, C. Lactic acid production from corn stover using mixed cultures of Lactobacillus rhamnosus and Lactobacillus brevis. Bioresour. Technol. 2011, 102, 1831–1836. [Google Scholar] [CrossRef]
- Barcenilla, C.; Ducic, M.; Lopez, M.; Prieto, M.; Alvarez-Ordonez, A. Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Sci. 2022, 183, 108661. [Google Scholar] [CrossRef]
- Mygdalia, A.S.; Nouska, C.; Hatzikamari, M.; Biliaderis, C.G.; Lazaridou, A. A sourdough process based on fermented chickpea extract as leavening and anti-staling agent for improving the quality of gluten-free breads. Food Res. Int. 2022, 159, 111593. [Google Scholar] [CrossRef]
- Tejedor-Sanz, S.; Stevens, E.T.; Li, S.; Finnegan, P.; Nelson, J.; Knoesen, A.; Light, S.H.; Ajo-Franklin, C.M.; Marco, M.L. Extracellular electron transfer increases fermentation in lactic acid bacteria via a hybrid metabolism. eLife 2022, 11, e70684. [Google Scholar] [CrossRef]
- Lidon, F.; Silva, M. An overview on applications and side effects of antioxidant food additives. Emir. J. Food Agric. 2016, 28, 823. [Google Scholar] [CrossRef]
- Ryssel, H.; Kloeters, O.; Germann, G.; Schafer, T.; Wiedemann, G.; Oehlbauer, M. The antimicrobial effect of acetic acid-an alternative to common local antiseptics? Burns 2009, 35, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.I.; Oh, B.M.; Oh, H.H.; Jeong, D.Y.; Kim, Y.S.; Song, G.S. Quality characteristics and shelf-life establishment of tteokbokki tteok added with sikhye fermented by lactic acid bacteria. J. Korean Soc. Food Sci. Nutr. 2020, 49, 883–892. [Google Scholar] [CrossRef]
- Bai, J.S.; Han, J.A. Characterization of rice cake with ramie (Boehmeria nivea L.) leaf extract. J. Korean Soc. Food Natr. 2018, 47, 1014–1020. [Google Scholar] [CrossRef]
- Lee, E.S.; Song, H.G.; Choi, I.; Lee, J.S.; Han, J. Effects of mung bean starch/guar gum-based edible emulsion coatings on the staling and safety of rice cakes. Carbohydr. Polym. 2020, 247, 116696. [Google Scholar] [CrossRef]
- Kang, H.J.; Park, J.D.; Lee, H.Y.; Kum, J.S. Effect of grapefruit seed extracts and acid regulation agents on the qualities of topokkidduk. J. Korean Soc. Food Sci. Nutr. 2013, 42, 948–956. [Google Scholar] [CrossRef]
- Shin, D.S.; Yoo, Y.M.; Han, G.J.; Oh, S.G. Quality of tteokbokki tteok prepared by adding various concentration of brown rice. Korean J. Food Preserv. 2016, 23, 194–203. [Google Scholar] [CrossRef]
- Pack, S.Y.; Sim, K.H. Quality characteristics and antioxidant activity of tteokbokkidduk supplemented with wheat bran powder. Korean J. Food Nutr. 2022, 35, 16–33. [Google Scholar] [CrossRef]
- Choi, H.Y.; Kim, J.S.; Go, E.S.; Woo, H.E.; Park, J.D.; Sung, J.M. Quality characteristics of tteokbokki tteok after ethanol and heat moisture treatments during the storage periods. J. Korean Soc. Food Cult. 2021, 36, 325–332. [Google Scholar] [CrossRef]
- Jung, K.I.; Bang, H.J.; Boo, H.J.; Choi, Y.J. Quality characteristics of topokkidduk added with enteromorpha intestinalispowder. J. Life Sci. 2019, 29, 588–595. [Google Scholar] [CrossRef]
- Kim, S.S.; Jung, H.Y. Retarding retrogradation of Korean rice cakes (Karedduk) with a mixture of trehalose and modified starch analyzed by Avrami kinetics. J. Korean Soc. Food Sci. Nutr. 2010, 23, 39–44. [Google Scholar]
- Kim, S.S.; Jung, H.Y. Texture properties of a Korean rice cake (Karedduk) with addition of carbohydrate materials. J. Korean Soc. Food Nutr. 2007, 36, 1205–1210. [Google Scholar] [CrossRef]
- Cheon, H.S.; Cho, W.I.; Lee, S.J.; Chung, M.S.; Choi, J.B. Acidic and steaming treatments of tteokbokki rice cake to improve its microbial and textural properties. Korean J. Food Sci. Technol. 2017, 49, 502–506. [Google Scholar] [CrossRef]
- Lee, J.K.; Jeong, J.H.; Lim, J.K. Quality characteristics of Topokki garaedduk added with ginseng powder. J. Korean Soc. Food Sci. Nutr. 2011, 40, 426–434. [Google Scholar] [CrossRef]
- Ahn, J.W. Properties of rice cakes for topokki with curry powder. Korean J. Food Cook. Sci. 2009, 25, 467–473. [Google Scholar]
- Honh, E.J.; Son, H.J.; Kang, J.H.; Noh, B.S. Analysis of binding trimethylamine with rice washed solution using electronic nose based on mass spectrometer. Korean J. Food Technol. 2009, 41, 509–514. [Google Scholar]
- Kim, H.J.; Otyinloye, T.M.; Yoon, W.B. Rice cake shelf life extension using acid-soaking and the chlorine dioxide drying process. J. Agric. Life Environ. Sci. 2020, 32, 90–98. [Google Scholar] [CrossRef]
- Freese, E.; Sheu, C.W.; Galliers, E. Function of lipophilic acids as antimicrobial food additives. Nature 1973, 241, 321–325. [Google Scholar] [CrossRef]
- Choi, J.Y.; Park, J.H.; Kim, J.Y.; Kim, J.S.; Jeong, S.U.; Kim, M.H.; Park, S.H.; Moon, K.D. Quality characteristics of lotus root (Nelumbo nucifera G.) snacks according to heat treatment methods and conditions. Korean J. Food Preserv. 2021, 28, 344–355. [Google Scholar] [CrossRef]
- Woo, S.H.; Kim, J.S.; Jeong, H.M.; Shin, Y.J.; Hong, J.S.; Choi, H.D.; Shim, J.H. Development of freeze-thaw stable starch through enzymatic modification. Foods 2021, 10, 2269. [Google Scholar] [CrossRef]
- Ha, J.H.; Lee, S.J.; Hwang, S.S.; Ha, S.D. Antibacterial effect of fermented rice water against food-borne bacteria in kitchen towel. J. Food Hyg. Saf. 2007, 22, 365–369. [Google Scholar]
- Ahn, G.N.; Lee, W.W.; Jeon, Y.J. Effect of different molecular weights of chitosans on the growth of lactic acid bacteria from the traditional fermented foods. J. Chitin Chitosan 2014, 19, 194–200. [Google Scholar]
- Ann, Y.G. Probiotic lactic acid bacteria. Korean J. Food Nutr. 2011, 24, 817–832. [Google Scholar] [CrossRef]
- Kwon, N.H.; Kim, S.H.; Bae, W.K.; Kim, J.Y.; Lim, J.Y. Antimicrobial activity of Lactobacillus reuteri against major food-borne pathogens. J. Food Hyg. Saf. 2001, 16, 264–273. [Google Scholar]
- Babić, J.; Šubarić, D.; Milicevic, B.; Ačkar, D.; Kopjar, M.; Nedic Tiban, N. Influence of trehalose, glucose, fructose, and sucrose on gelatinisation and retrogradation of corn and tapioca starches. Czech J. Food Sci. 2009, 27, 151–157. [Google Scholar] [CrossRef]
- Cheng, W.; Sun, Y.; Xia, X.; Yang, L.; Fan, M.; Li, Y.; Wang, L.; Qian, H. Effects of β-amylase treatment conditions on the gelatinization and retrogradation characteristics of wheat starch. Food Hydrocoll. 2022, 124, 107286. [Google Scholar] [CrossRef]
- Woo, S.H.; Shin, Y.J.; Jeong, H.M.; Kim, J.S.; Ko, D.S.; Hong, J.S.; Choi, H.W.; Shim, J.H. Effects of maltogenic amylase from Lactobacillus plantarum on retrogradation of bread. J. Cereal Sci. 2020, 93, 102976. [Google Scholar] [CrossRef]
- Tolve, R.; Bianchi, F.; Lomuscio, E.; Sportiello, L.; Simonato, B. Current advantages in the application of microencapsulation in functional bread development. Foods 2022, 12, 96. [Google Scholar] [CrossRef]
- Jeon, H.Y.; Kim, N.R.; Lee, H.W.; Choi, H.J.; Choung, W.J.; Koo, Y.S.; Ko, D.S.; Shim, J.H. Characterization of a novel maltose-forming alpha-amylase from Lactobacillus plantarum subsp. plantarum ST-III. J. Agric. Food Chem. 2016, 64, 2307–2314. [Google Scholar] [CrossRef]
| Enzyme (1) | Fermentation Time | ||||||
|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | ||
| Lactobacillus acidophilus | N | 5.7 ± 0.1 aB | 5.4 ± 0.0 bA | 4.9 ± 0.1 cA | 4.5 ± 0.1 dB | 4.3 ± 0.1 eA | 4.3 ± 0.0 eA |
| AMG | 5.8 ± 0.0 aB | 5.6 ± 0.1 bA | 5.0 ± 0.2 cA | 4.5 ± 0.1 dB | 4.3 ± 0.1 dA | 4.1 ± 0.1 eB | |
| Termamyl (2) | 5.9 ± 0.1 aA | 5.6 ± 0.1 bA | 5.2 ± 0.1 cA | 4.6 ± 0.0 dA | 4.4 ± 0.0 eA | 4.3 ± 0.0 fA | |
| Lactobacillus brevis | NA | 5.4 ± 0.0 aA | 5.4 ± 0.1 bA | 5.3 ± 0.0 bA | 4.8 ± 0.0 cA | 4.6 ± 0.0 dA | 4.3 ± 0.0 eA |
| AMG | 5.4 ± 0.0 aA | 5.4 ± 0.1 aA | 5.3 ± 0.0 bA | 4.8 ± 0.0 cA | 4.4 ± 0.0 dB | 4.2 ± 0.0 eB | |
| Termamyl | 5.4 ± 0.1 aA | 5.4 ± 0.0 aA | 5.3 ± 0.1 bA | 4.9 ± 0.1 cA | 4.6 ± 0.0 dA | 4.3 ± 0.0 eA | |
| Lactobacillus casei | NA | 5.6 ± 0.1 aB | 5.5 ± 0.1 aA | 5.0 ± 0.1 bA | 4.9 ± 0.1 bcA | 4.7 ± 0.1 cdA | 4.6 ± 0.1 eA |
| AMG | 5.9 ± 0.1 aA | 5.6 ± 0.1 bA | 5.1 ± 0.1 cA | 4.9 ± 0.1 dA | 4.6 ± 0.1 eA | 4.4 ± 0.0 fB | |
| Termamyl | 5.8 ± 0.0 aA | 5.6 ± 0.0 bA | 5.1 ± 0.0 cA | 4.9 ± 0.1 dA | 4.7 ± 0.1 eA | 4.6 ± 0.0 fA | |
| Lactobacillus curvatus | NA | 6.0 ± 0.1 aA | 5.9 ± 0.0 abA | 5.8 ± 0.0 bA | 5.6 ± 0.1 cA | 5.6 ± 0.0 dA | 5.5 ± 0.0 eA |
| AMG | 6.0 ± 0.0 aA | 6.0 ± 0.0 aA | 5.9 ± 0.1 aA | 5.6 ± 0.1 bA | 5.5 ± 0.1 bcA | 5.5 ± 0.0 cA | |
| Termamyl | 6.1 ± 0.1 aA | 6.0 ± 0.1 abA | 5.9 ± 0.1 bA | 5.5 ± 0.0 cA | 5.6 ± 0.1 cA | 5.5 ± 0.1 cA | |
| Lactobacillus reuteri | NA | 5.8 ± 0.1 aB | 5.6 ± 0.1 bA | 5.4 ± 0.1 cA | 5.3 ± 0.1 dA | 5.2 ± 0.0 dA | 5.2 ± 0.0 dA |
| AMG | 5.9 ± 0.0 aB | 5.6 ± 0.1 bA | 5.2 ± 0.0 cB | 4.9 ± 0.1 dB | 4.6 ± 0.1 eC | 4.1 ± 0.1 fC | |
| Termamyl | 6.0 ± 0.1 aA | 5.6 ± 0.1 bA | 5.3 ± 0.1 cAB | 5.0 ± 0.1 dB | 4.7 ± 0.1 eB | 4.6 ± 0.0 fB | |
| Lactobacillus plantarum | NA | 5.8 ± 0.0 aA | 5.4 ± 0.1 bA | 5.0 ± 0.0 cA | 4.6 ± 0.0 dA | 4.3 ± 0.0 eA | 4.1 ± 0.0 fA |
| AMG | 5.5 ± 0.0 aB | 5.1 ± 0.0 bC | 4.6 ± 0.0 cC | 4.3 ± 0.0 dC | 4.3 ± 0.0 dA | 3.9 ± 0.0 eA | |
| Termamyl | 5.6 ± 0.0 aB | 5.2 ± 0.0 bB | 4.8 ± 0.0 cB | 4.5 ± 0.0 dB | 4.2 ±0.0 eA | 4.0 ± 0.0 fA | |
| Leuconostoc mesenteroides | NA | 5.6 ± 0.1 aA | 5.5 ± 0.0 aA | 5.4 ± 0.1 aA | 5.0 ± 0.2 bA | 4.7 ± 0.1 cA | 4.4 ± 0.1 dA |
| AMG | 5.6 ± 0.0 aA | 5.5 ± 0.0 aA | 5.4 ± 0.1 bA | 4.8 ± 0.0 cB | 4.4 ± 0.0 dB | 4.2 ± 0.1 eB | |
| Termamyl | 5.5 ± 0.0 aA | 5.5 ± 0.1 aA | 5.4 ± 0.0 bA | 4.8 ± 0.0 cAB | 4.6 ± 0.0 dA | 4.3 ± 0.0 eA | |
| Microbe | Day | Control (1) | FWRW-P | FWRW-PA | FWRW-R | FWRW-RA |
|---|---|---|---|---|---|---|
| Total plate count (log CFU/g) | 1 | 5.87 ± 0.05 aA | 4.60 ± 0.12 aB | 3.36 ± 0.10 aC | 1.90 ± 0.07 aD | ND bE |
| 2 | 7.77 ± 0.04 bA | 7.62 ± 0.05 bB | 6.83 ± 0.08 bC | 6.61 ± 0.03 bD | ND bE | |
| 3 | 8.92 ± 0.09 cA | 8.24 ± 0.08 cB | 7.65 ± 0.05 cC | 7.66 ± 0.04 cC | ND bD | |
| 4 | 9.14 ± 0.03 dA | 8.81 ± 0.07 dB | 7.99 ± 0.09 dC | 8.75 ± 0.09 dB | ND bD | |
| 5 | 9.41 ± 0.03 eA | 8.98 ± 0.02 eB | 9.00 ± 0.02 eB | 8.96 ± 0.03 eB | 5.41 ± 0.05 aC | |
| E. coli (log CFU/g) | 1 | ND | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND | ND | |
| 3 | ND | ND | ND | ND | ND | |
| 4 | ND | ND | ND | ND | ND | |
| 5 | ND | ND | ND | ND | ND | |
| Yeast & Mold (log CFU/g) | 1 | ND | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND | ND | |
| 3 | ND | ND | ND | ND | ND | |
| 4 | ND | ND | ND | ND | ND | |
| 5 | ND | ND | ND | ND | ND | |
| S. aureus (log CFU/g) | 1 | ND | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND | ND | |
| 3 | ND | ND | ND | ND | ND | |
| 4 | ND | ND | ND | ND | ND | |
| 5 | ND | ND | ND | ND | ND |
| Storage Time (Days) | Control | FWRW-P | FWRW-PA | FWRW-R | FWRW-RA | |
|---|---|---|---|---|---|---|
| L* (1) | 0 | 69.06 ± 0.03 c | 71.25 ± 0.66 b | 72.40 ± 0.83 a | 67.73 ± 0.59 d | 68.14 ± 0.10 d |
| 3 | 63.57 ± 0.05 d | 66.93 ± 0.05 bc | 62.78 ± 0.21 e | 66.64 ± 0.13 c | 70.62 ± 0.01 a | |
| 5 | 64.25 ± 0.90 b | 65.64 ± 0.03 a | 61.66 ± 0.12 c | 66.65 ± 0.58 a | 62.57 ± 0.64 c | |
| a* (2) | 0 | −1.28 ± 0.05 c | −1.24 ± 0.01 c | −1.76 ± 0.02 a | −1.13 ± 0.02 d | −1.56 ± 0.02 b |
| 3 | −2.15 ± 0.01 a | −1.44 ± 0.00 c | −1.12 ± 0.03 e | −1.37 ± 0.04 d | −1.92 ± 0.01 b | |
| 5 | −2.02 ± 0.02 a | −1.19 ± 0.00 e | −1.51 ± 0.02 b | −1.30 ± 0.01 d | −1.34 ± 0.00 c | |
| b* (3) | 0 | 11.71 ± 0.01 c | 12.82 ± 0.02 d | 11.07 ± 0.03 b | 11.04 ± 0.03 b | 10.42 ± 0.36 a |
| 3 | 10.06 ± 0.04 a | 13.13 ± 0.06 d | 11.71 ± 0.06 b | 11.91 ± 0.06 c | 11.84 ± 0.02 c | |
| 5 | 8.48 ± 0.02 a | 11.26 ± 0.03 d | 10.54 ± 0.02 b | 10.76 ± 0.02 c | 11.69 ± 0.12 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.-H.; Jeong, H.-M.; Kim, E.-A.; Lee, Y.-R.; Shim, J.-H. Development of Fermented Rice Water to Improve the Quality of Garaetteok, a Traditional Korean Rice Cake. Foods 2023, 12, 642. https://doi.org/10.3390/foods12030642
Lee E-H, Jeong H-M, Kim E-A, Lee Y-R, Shim J-H. Development of Fermented Rice Water to Improve the Quality of Garaetteok, a Traditional Korean Rice Cake. Foods. 2023; 12(3):642. https://doi.org/10.3390/foods12030642
Chicago/Turabian StyleLee, Eun-Hyeong, Hyun-Mo Jeong, Eun-A Kim, Ye-Rim Lee, and Jae-Hoon Shim. 2023. "Development of Fermented Rice Water to Improve the Quality of Garaetteok, a Traditional Korean Rice Cake" Foods 12, no. 3: 642. https://doi.org/10.3390/foods12030642
APA StyleLee, E.-H., Jeong, H.-M., Kim, E.-A., Lee, Y.-R., & Shim, J.-H. (2023). Development of Fermented Rice Water to Improve the Quality of Garaetteok, a Traditional Korean Rice Cake. Foods, 12(3), 642. https://doi.org/10.3390/foods12030642







