Abstract
Alzheimer’s disease (AD) is thought to be caused by the deposition of amyloid-β (Aβ) in the brain. Aβ begins to aggregate approximately 20 years before the expression of its symptoms. Previously, we developed a microliter-scale high-throughput screening (MSHTS) system for inhibitors against Aβ aggregation using quantum dot nanoprobes. Using this system, we also found that plants in the Lamiaceae, particularly Perilla frutescens var. crispa, have high activity. The cultivation environment has the potential to enhance Aβ aggregation inhibitory activity in plants by changing their metabolism. Here, we report on cultivation factors that affected the activity of P. frutescens var. crispa cultivated in three fields under different cultivation conditions. The results revealed that the activity of P. frutescens var. crispa harvested just before flowering was highest. Interestingly, the activity of wind-shielded plants that were cultivated to prevent exposure to wind, was reduced to 1/5th of plants just before flowering. Furthermore, activity just before flowering increased following appropriate nitrogen fertilization and at least one week of drying from the day before harvest. In addition, we confirmed that the P. frutescens var. crispa leaf extracts suppressed Aβ-induced toxicity in nerve growth factor-differentiated PC12 cells. In this study, we demonstrated that flowering, wind, soil water content, and soil nitrogen content affected Aβ aggregation inhibitory activity, necessary to suppress Aβ neurotoxicity, in P. frutescens var. crispa extracts. This study provides practical cultivation methods for P. frutescens var. crispa with high Aβ aggregation inhibitory activity for the prevention of AD.
1. Introduction
Alzheimer’s disease (AD) is the most common form of dementia and an acute medical issue due to the increasing number of AD patients worldwide. It is estimated that the number of people with dementia will increase from 57.4 million worldwide in 2019 to 152.8 million cases in 2050 [1]. Furthermore, the value of a statistical life-based global economic burden of Alzheimer’s disease and related dementias was estimated at USD 2.8 trillion in 2019 and was projected to increase to USD 16.9 trillion in 2050 [2]. AD progressively causes memory loss and cognitive dysfunction, eventually requiring full-time care [3]. Most studies target amyloid-β (Aβ) peptides that mainly consist of 40 or 42 amino residues [4]. Aβ grows into oligomers, fibrils, and aggregates and is observed as plaques in a patient’s brain [5]. Thus, Aβ aggregation is an initiating factor in AD that leads to the death of neurons [6,7]. Therefore, many studies have been performed aimed at decreasing Aβ aggregation [8].
Nevertheless, most clinical trials targeting AD patients failed [9]. These results might stem from Aβ aggregation that begins about two decades before the onset of symptoms [10], suggesting that treatment for patients in whom AD has developed is too late. Recently, a few clinical trials achieved better results using a treatment based on anti-Aβ antibodies in patients with mild cognitive impairment (MCI) and mild AD. Primarily, the US Food and Drug Administration approved aducanumab, which is a therapeutic medicine based on anti-Aβ fibril antibodies, via an accelerated approval pathway [11]. The treatment of patients with MCI and mild AD with aducanumab showed the removal of Aβ in the brain and reduced AD pathology [12]. In addition, treatment with lecanemab, an anti-Aβ protofibril antibody, showed an even greater reduction in cognitive decline in patients with MCI and mild AD [13]. These achievements support the concept that it is important to inhibit Aβ aggregation before the onset of AD symptoms. However, treatments with antibodies are very expensive [14] and are thus not accessible to most people.
Even if Aβ aggregates are removed after the neural network is severely damaged, it is expected that the network will not recover to its initial state due to its irreversibility. We believe that it is important to prevent neural networks from being damaged. In addition to treatment by removing Aβ aggregation with anti-Aβ antibody drugs, we suggest the importance of adding a preventive effect against Aβ aggregation to the daily diet. Since food enables us to take any substance affordably and permanently, we assume that food with Aβ aggregation inhibitory activity contributes greatly to AD prevention. Previously, we developed a microliter-scale high-throughput screening (MSHTS) system to evaluate Aβ42 aggregation inhibitors in vitro by using quantum dot (QD) nanoprobes [15]. The MSHTS system enables high-throughput screening and the accurate evaluation of samples, such as foods containing various contaminants [16,17,18,19]. Generally, atomic force microscopy and electron microscopy (EM) have been used to directly observe Aβ fibrils [20]. In particular, the latest cryo-EM provides high-resolution images of the structure of amyloid fibrils [21]. Although these methods are powerful tools for observing the structure of fibrils, they are not suited for quantification and high-throughput screening. Generally, Aβ aggregation is quantified using thioflavin T (ThT) [22], which measures the amount of fluorescence bound to the β-sheet. However, the ThT assay cannot accurately evaluate activity in contaminated samples because fluorescence is inhibited by many dyes in the samples [16]. In contrast, the MSHTS system allows the accurate evaluation of total Aβ inhibitory activity in various samples, such as crude extracts.
Using the MSHTS system, we have already evaluated the Aβ aggregation inhibitory activity of commercial dressings, which are processed foods, and reported those findings in this journal [16]. Furthermore, we applied this system to food plants and found that one family in particular, the Lamiaceae, displayed high activity [15]. In addition, we attempted to isolate active compounds from summer savory, a member of the Lamiaceae, revealing that the main active compound was rosmarinic acid (RA), a polyphenol. Subsequently, we found that Aβ aggregation inhibitory activity in leaf extracts from Perilla frutescens var. crispa was higher than from spearmint (Mentha spicata), and had the highest activity among all tested members of the Lamiaceae [15]. Presumably, this high activity derives from its rich polyphenol content [23,24,25]. However, we preliminarily confirmed that the activity differed, depending on harvest time and cultivation practice, even within P. frutescens var. crispa. Even in the same plants, the content of polyphenols changed due to the growth cycle and cultivation conditions, such as fertilization [26,27,28], including in perilla [29,30,31].
In this study, we investigated the cultivation factors that affected the Aβ aggregation inhibitory activity of P. frutescens var. crispa. We evaluate total activity in extracts using the automated MSHTS system, which allows for high-throughput screening [18]. While some papers have indicated cultivation methods for P. frutescens var. crispa to increase the content of polyphenols and other promising bioactive compounds [28,30], it is unclear whether those contents would contribute to total Aβ aggregation inhibitory activity. Here, we evaluated the activity of P. frutescens var. crispa cultivated in different fields and fertilization conditions. Consequently, we found that flowering, wind, nitrogen fertilization, and water content affected the activity of P. frutescens var. crispa. This study provides practical cultivation methods for enhancing Aβ aggregation inhibitory activity.
2. Materials and Methods
2.1. Materials
Human Aβ42 (4349-v, Peptide Institute Inc., Osaka, Japan) and Cys-conjugated Aβ40 (23519, Anaspec Inc., Fremont, CA, USA) were purchased commercially. Thiazolyl blue tetrazolium bromide (MTT)(M5655) and poly-D-lysine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nerve growth factor (NGF) was purchased from Cosmo Bio (Toyo, Japan).
2.2. Preparation of QDAβ Nanoprobe
The QDAβ nanoprobe was prepared using QD-PEG-NH2 (QdotTM 605 ITKTM Amino (PEG) Quantum dot; Q21501MP, Waltham, MA, USA, Thermo Fisher Scientific) according to our previous reports [15,18,32]. QDAβ concentration was determined by comparing absorbance at 350 nm to unlabeled QD-PEG-NH2.
2.3. Evaluation Using the Automated MSHTS System
The half maximal effective concentration (EC50) values of all plant extracts were determined by a modified automated MSHTS system, as was described in our previous report [18]. Specifically, mixed solutions were prepared with six concentrations of extracts, 25 nM QDAβ, and 25 μM Aβ42 in PBS containing 5% ethanol (EtOH) and 2.5% dimethyl sulfoxide (DMSO) and incubated in a 1536-well plate (782096, Greiner, Kremsmünster, Austria) at 37 °C for 24 h. The images of each well were captured pre- and post-incubation by an inverted fluorescence microscope (see Section 2.4.). Standard deviation (SD) values of the central images of the region of interest (432 × 432 pixels) in each well were measured by the General Analysis program of NIS-Elements (Nikon, Tokyo, Japan). The images on the highest extract concentration, including insoluble substances, were eliminated because these affect SD values and disrupt accurate evaluation. EC50 was estimated from the SD values by Prism software (GraphPad software, San Diego, CA, USA) using an EC50 shift by global fitting (asymmetric sigmoidal, five-parameter logistic) [15].
2.4. Fluorescence Microscopy
Fluorescence images in the 1536-well plate were observed by an inverted fluorescence microscope system (ECLIPSE Ti-E, Nikon) equipped with a color CMOS camera (DS-Ri2, Nikon). QD fluorescence was imaged using a 4× objective lens (MRD00045, Nikon) and a TRITC filter set (TRITC- A-Basic-NTE, Semrock, NY, USA).
2.5. MTT Assay
Rat adrenal pheochromocytoma, PC12 cells, were obtained from the JCRB Cell Bank (Osaka, Japan). PC12 cells were maintained in dulbecco’s modified eagle medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin, as was described in our previous report [33]. Cells were cultured at 37 °C in humidified air containing 5% CO2 with CO2 incubator. PC12 cells were plated at 1.2 × 104 cells in poly-D-lysine-coated 96-well plates (3860-096, AGC TECHNO GLASS, Shizuoka, Japan) and incubated for 24 h. After incubation, cells were treated with 50 ng/mL NGF for 24 h for neuronal differentiation. After this step, cells were cultured in a medium without FBS but containing 50 ng/mL NGF. Cells were treated with extracts and 25 μM Aβ42 for 24 h by replacing the medium with 1% EtOH/1% DMSO. Cells were then treated with 0.5 mM MTT for 4 h by medium replacement in reference to the MTT assay [34]. After incubation, the supernatant of each well was removed, and formazan crystals were dissolved in a 10% SDS/0.01 M HCl solution. After overnight incubation, absorbance (570 nm) was measured in each well with (SH-9000Lab, CORONA ELECTRIC, Ibaraki, Japan). Cell viability was calculated as a percentage relative to cells treated with the control medium (1% EtOH/1% DMSO).
2.6. Cultivation of Perilla frutescens var. crispa
P. frutescens var. crispa seedlings were purchased from AW Farm Chitose Co., Ltd. (Hokkaido, Japan). Seeds were planted around May and harvested regularly. In 2018, plants were cultivated in Chitose (42°53′24.5″ N 141°41′41.5″ E), Watenbetsu (42°58′08.8″ N 144°00′02.9″ E), and Tomaribetsu (43°01′48.1″ N 144°07′55.2″ E) in Hokkaido, and in 2019, on the field on a college campus (42°22′38.1″ N 141°02′12.1″ E). In 2020, plants were cultivated in a plastic greenhouse on a college campus. In 2018, meteorological data was measured with meteorological equipment installed in each field. In 2019 and 2020, soil from the plot was sampled at the same time as the harvest from the field on the college campus. The water content of the soil was calculated from pre- and post-drying weights.
2.7. Preparation of Extracts
Extracts were prepared by extraction with 5 mL of 95% EtOH per gram of fresh leaf weight at room temperature for seven days in 2018. In 2019/2020, perilla leaf extracts were prepared by extraction for one day to prepare many samples efficiently. After the EtOH solution was filtered and concentrated in vacuo, samples were prepared as a 10 mg/mL extract with EtOH.
2.8. Statistical Analysis
All statistical analyses were performed in Excel (version 16; Microsoft, Redmond, WA, USA). A two-tailed Welch’s t-test was performed for comparisons between groups. Soil water content and EC50 were evaluated by linear regression using least squares in Excel. p < 0.05 was considered statistically significant (*: p < 0.05, **: p < 0.01, ***: p < 0.005).
3. Results
3.1. Principle of the MSHTS System
We evaluated more than a hundred samples of P. frutescens var. crispa extracts for Aβ aggregation inhibitory activity using the automated MSHTS System [18] (representative; Figure 1B,C). This system’s uniqueness is in its use of QDs, which are nanoscale crystals with an overwhelmingly non-photobleaching property (Figure 1A). After incubation of a mixture of the fluorescent probe QDAβ and Aβ42, QDAβ is incorporated into Aβ42 fibrils. From the fluorescence images of the mixtures, the variation in brightness was measured (Figure 1B) as an SD value, which can be considered as the progress of aggregation. Aβ aggregation inhibitory activity was evaluated by developing an inhibition curve using SD values (Figure 1C) and calculating the EC50. Lower EC50 values indicate higher activity. If the inhibition curve did not reach 50% of the percentage of SD values, then a sample was determined to be not detectable (ND).
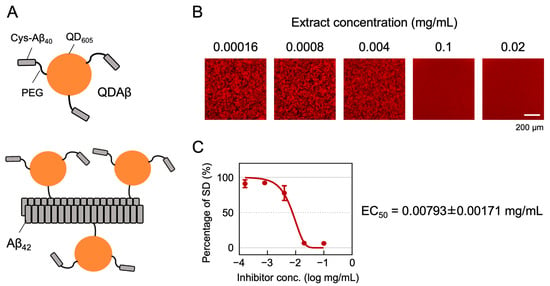
Figure 1.
Principle of the MSHTS system for evaluating Aβ42 aggregation inhibitory activity. (A) QDAβ is a fluorescent probe prepared by cross-linking Cys-Aβ40 and QD605 via amino polyethylene glycol (PEG)(top). QDAβ co-aggregates with unlabeled Aβ42, allowing Aβ42 fibrils to be visualized by fluorescence microscopy (bottom). (B,C) Representative analysis of Aβ aggregation inhibitory activity of Perilla frutescens var. crispa leaf extract. Mixed solutions of 25 μM Aβ42 and extract were added to 1536-well plates and imaged by fluorescence microscopy before and after incubation at 37 °C for 24 h. (B) The fluorescence images were trimmed to 432 × 432 pixels, and the SD values, meaning the variation in brightness, were measured. (C) The inhibition curve was drawn from SD values, and EC50 was calculated using Prism GraphPad software.
3.2. Aβ Aggregation Inhibitory Activity of Perilla frutescens var. crispa and Meteorological Data in Three Fields
At first, we cultivated P. frutescens var. crispa plants in three fields in Hokkaido, Japan, in 2018 to investigate regional differences in Aβ aggregation inhibitory activity and installed meteorological equipment in each field in late August (Figure 2). Plants were harvested on three dates (24 and 25 July, 28 and 29 August, and 5 and 10 October). Extracts from leaves, stems, and roots were compared in late July. Leaf extracts showed high activity in all fields, while stem extracts in Tomaribetsu and root extracts in Chitose and Watenbetsu showed no activity (i.e., ND) (Figure 3A). In Chitose and Watenbetsu, roots showed no activity, and leaves showed significantly higher activity than stems. Therefore, we compared the activity of extracts of leaves harvested on the three dates (Figure 3B); the extracts from plants growing in Chitose and Tomaribetsu fields showed higher activity on October 5 and 10 than on July 24 and 25. Furthermore, we compared the activity of leaves harvested on October 5 and 10 in plants growing in different fields: the highest activity was observed in extracts from plants grown in the Chitose field, followed by the Tomaribetsu and Watenbetsu fields.
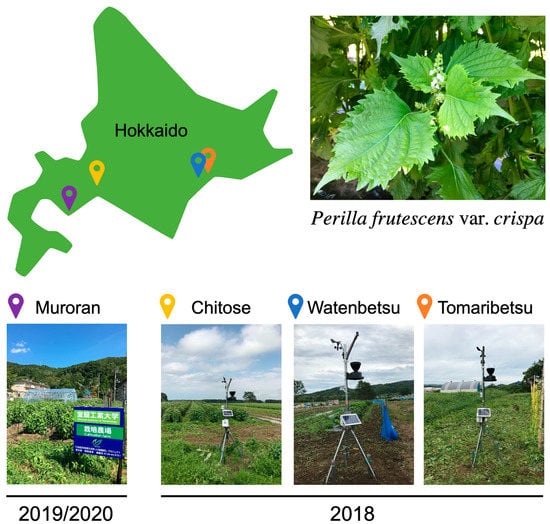
Figure 2.
Cultivation of Perilla frutescens var. crispa in four fields in Hokkaido. In 2018, plants were cultivated in three fields (Chitose, Watenbetsu, and Tomaribetsu) in Hokkaido, Japan, where meteorological equipment was installed. In 2019 and 2020, plants were cultivated under multiple conditions in only Muroran.
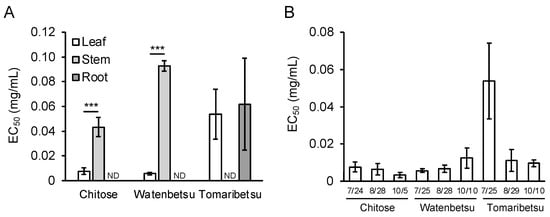
Figure 3.
Aβ aggregation inhibitory activity of Perilla frutescens var. crispa leaves differed depending on fields and harvest time. (A) EC50 in leaf, stem, and root extracts. Plants were cultivated in three fields in 2018 and harvested in late July. (B) The activity of Perilla frutescens var. crispa leaves was harvested in late July, late August, and early October. The activity was determined to be not detectable (ND) in samples if the inhibitory curve did not reach 50% of the percentage of SD values. Data are represented as mean ± SD (n = 4 separate experiments). Comparison of EC50 values (***: p < 0.005, Welch’s t-test).
We also investigated the correlation between meteorological data and Aβ aggregation inhibitory activity. Meteorological data from August 24 to October 4 were acquired from each field and were plotted as a daily average (Figure 4). These data appear to show that low soil water content (Figure 4G) was correlated with the above-mentioned plot-related rank in activity (Chitose < Tomaribetsu < Watenbetsu). Wind speed was highest at Chitose (Figure 4E,F), similar to the activity trend. Thus, we speculated that wind and water content affect Aβ aggregation inhibitory activity.
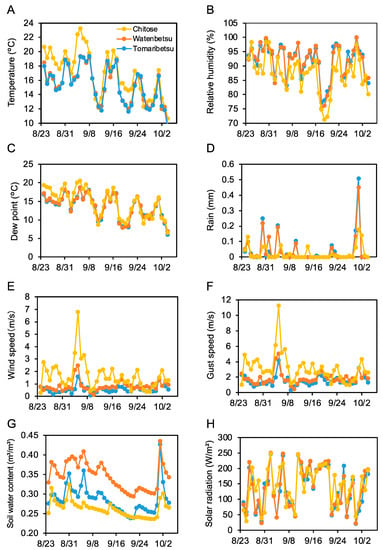
Figure 4.
Wind speed and soil water content correlate with regional differences in Aβ aggregation inhibition activity. Meteorological data was measured in three fields from August 24 to October 4, shown as daily averages. Yellow, red, and blue show Chitose, Watenbetsu, and Tomaribetsu, respectively. Temperature (A), relative humidity (B), dew point (C), rain (D), wind speed (E), gust speed (F), soil water content (G), and solar radiation (H). Data represent an average of each day from every 10 min measurement.
3.3. Aβ Aggregation Inhibitory Activity of Perilla frutescens var. crispa Was Highest Just before Flowering and Was Enhanced by the Wind
In 2019/2020, we prepared extracts efficiently with a change in extraction time from seven days to one day and evaluated many samples to investigate the details of cultivation conditions. We confirmed no change of Aβ aggregation inhibitory activity by extraction time (Supplemental Figure S1). In 2019, plots of various conditions with additional fertilization were made after laying compost on the field in Muroran, and P. frutescens var. crispa was cultivated (Figure 2). Plants cultivated without additional fertilizer were referred to as the standard (Std) and harvested every four weeks. Activity in the leaves of Std plants was highest just before the flowering, on 12 September, decreasing thereafter (Figure 5A). Plants were cultivated in the same soil conditions as Std, but a windbreak was created, enclosing plants with a plastic sheet (Standard with windbreak; Sw)(Figure 5B). In Sw plants, activity just before flowering was significantly lower than that of Std plants, with an EC50 that was five-fold higher (Figure 5A). The activity of Sw plants harvested on October 3 was highest, but EC50 was still three-fold higher than Std plants harvested on September 5. These results indicate that wind exposure enhanced Aβ aggregation inhibition activity in P. frutescens var. crispa.
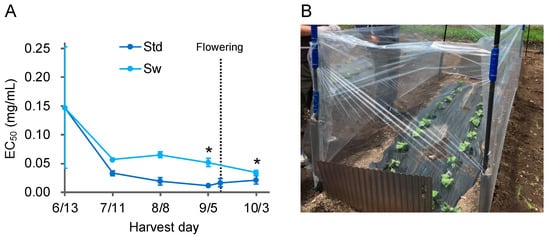
Figure 5.
Aβ aggregation inhibitory activity showed the highest just before flowering, with wind enhancement. (A) Aβ aggregation inhibitory activity of leaves in regularly harvested Perilla frutescens var. crispa in 2019. P. frutescens var. crispa was planted on June 13, and whole plants were harvested every 4 weeks. Plants flowered on 12 September. The Std is the condition with no additional fertilizer under all conditions. Plants from Std were also harvested on September 12, during flowering. (B) Plants in the standard with windbreak (Sw) were cultivated in the same soil as Std and enclosed with a plastic sheet. Data represent the mean ± SD (n = 3, separate plant). Comparison of the EC50 values vs. Std harvested on September 5 (*: p < 0.05, Welch’s t-test).
3.4. Adjustment of Soil Nitrogen and Soil Water Content Enhanced Aβ Aggregation Inhibitory Activity of Perilla frutescens var. crispa Just before Flowering
In 2019, P. frutescens var. crispa plants were cultivated under fertilization conditions other than Std, as shown in Table 1. Specifically, the activities of four major types of fertilizers were compared: slaked lime and calcium carbonate powder, scallop shell powder (Sp) and granular (Sg) with zeolite, each superphosphate of lime (SPL), ammonium sulfate (AMS), potassium sulfate (SOP), and a combination of Sp, SPL, AMS, and SOP (Figure 6A–D). There were no significant differences between any treatments in the activity of leaves of plants harvested just before flowering. On the other hand, the activity of plants cultivated on soil enhanced with SPL, AMS, and SOP fertilizer, just before flowering, was highest in AMS-fertilized conditions (Figure 6E).

Table 1.
Multiple Fertilization Conditions in 2019.
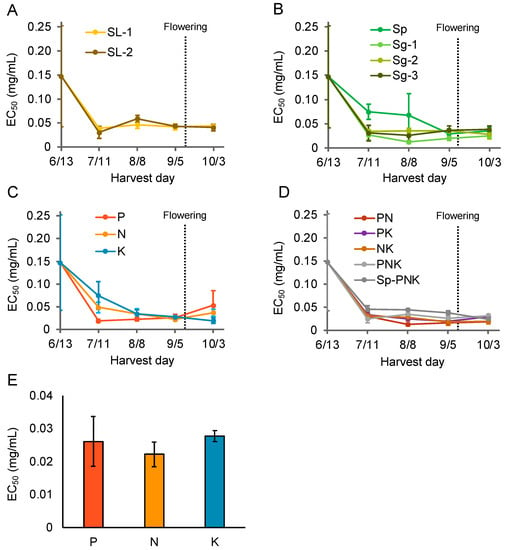
Figure 6.
Nitrogen fertilization enhanced the Aβ aggregation inhibitory activity of Perilla frutescens var. crispa leaves. Plants were cultivated under multiple fertilization conditions and harvested every 4 weeks. (A–D) Aβ aggregation inhibitory activity of leaves harvested in 2019. Activity in leaves of plants cultivated in soil with varying amounts of (A) slaked lime (SL), (B) shell powder (Sp), and shell granular (Sg) fertilizer (Table 1). (C,D) Activity in leaves of plants cultivated in soil with a combination of ammonium sulfate, lime superphosphate lime, and potassium sulfate fertilizer. (E) The activity of (C) was compared on September 5, just before flowering. Data represent the mean ± SD (n = 3, separate plants).
In 2020, to identify an appropriate amount of AMS fertilizer, P. frutescens var. crispa plants cultivated with six levels of AMS fertilization were harvested every four weeks (Table 2). Activity in all six fertilization conditions increased until September 9, just before flowering on September 10 (Figure 7A). Just before flowering, Aβ aggregation inhibitory activity and total fresh leaf weight from each plant were the highest in the N3 treatment (Figure 7B,C). In particular, total leaf weight improved from Std to N3 but decreased above N4. These results show that appropriate nitrogen fertilization increased Aβ aggregation inhibitory activity and total leaf.

Table 2.
Fertilizer conditions of nitrogen in 2020.
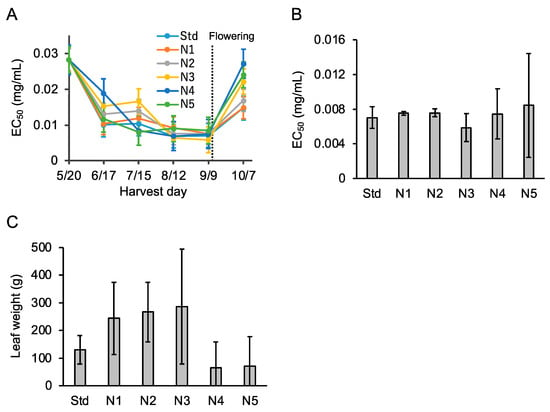
Figure 7.
Nitrogen fertilization enhanced Aβ aggregation inhibitory activity and leaf weight. (A) Aβ aggregation inhibitory activity in leaves of Perilla frutescens var. crispa cultivated in a plastic greenhouse with six levels of ammonium sulfate fertilization (Table 2) and harvested every four weeks in 2020. (B,C) Activity (B) and leaf weight (C) just before flowering on September 9 in (A). Data represent as mean ± SD (n = 3, separate plants).
When P. frutescens var. crispa plants cultivated in all conditions in 2019 were harvested, soil samples were drawn to analyze soil water content (Table 1). We created a scatter plot between soil water content and the activity of leaves and performed linear regression bordering on 20% water content (Figure 8A). The linear regression shows that activity was high at 20% but decreased markedly when water content exceeded 20%. Therefore, to test whether the cessation of watering just before harvest would enhance activity, plants were cultivated in greenhouses in 2020 and watering was stopped every week starting before the day of harvest. Predictably, soil water content decreased when watering was stopped (Figure 8C). On the other hand, Aβ aggregation inhibition activity was enhanced when watering was stopped for one week and higher than on the day before the harvest date. A scatter plot for this data was created, as shown in Figure 8A, although a linear regression was not performed due to too few samples (Figure 8D). The plots with water content exceeding 20% had highest activity. These results indicate that Aβ aggregation inhibitory activity was enhanced by stopping watering before harvest to reduce soil water content.
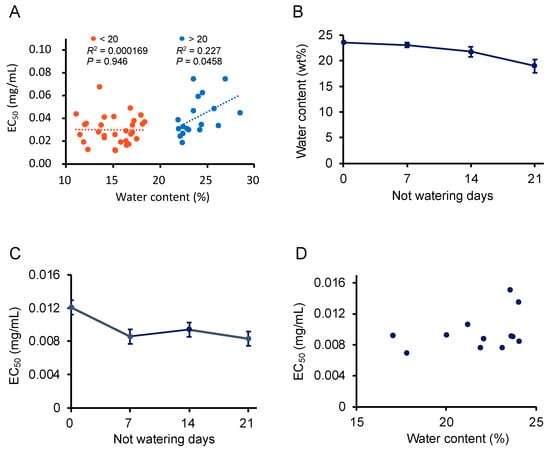
Figure 8.
Drying enhanced the Aβ aggregation inhibitory activity of Perilla frutescens var. crispa leaves. (A) Scatter plot of soil water content at harvesting and EC50 of leaf extracts from P. frutescens var. crispa under conditions described in Table 1 harvested on 11 July, 8 August, and 5 September in 2019. Linear regression was performed on more and less than 20% s water content. (B–D) Plants were cultivated in greenhouses in 2020, and watering was stopped every week from the day before the harvest date (15 September) to determine their Aβ aggregation inhibitory activity (C) and soil water content (B). A scatter plot was created in (D) about water content and EC50 similar to (A). (A,D) represented as mean, and (B,C) represented as mean ± SD (n = 3, separate plants).
3.5. Suppression Effect of Perilla frutescens var. crispa on Aβ-Induced Neurocytotoxicity
We tested whether the P. frutescens var. crispa extract could suppress Aβ-induced neurocytotoxicity by the MTT assay using NGF-differentiated PC12 cells (Figure 9). The data show that 25 μM Aβ42 reduced the viability of PC12 cells by 40% relative to the control. The extract significantly restored cell viability and suppressed Aβ-induced neurocytotoxicity the most at 0.781 μg/mL. In contrast, the extracts with more than 25 μg/mL concentrations reduced cell viability to less than 25 μM Aβ and significantly when the concentration was 100 μg/mL.
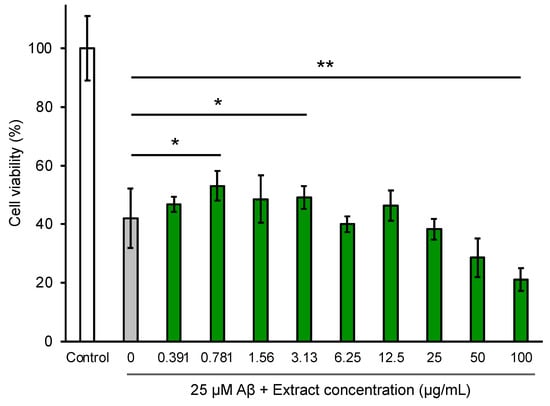
Figure 9.
Extracts from Perilla frutescens var. crispa leaves suppressed Aβ-induced cytotoxicity in NGF-differentiated PC12 cells. PC12 cells were differentiated by NGF for 24 h and treated with 25 μM Aβ42 and leaf extract for 24 h. Cell viability was measured by the MTT assay and shown as the relative percentage of absorbance of treated samples compared to the control without Aβ and extract. Each plot and bar graph represent the mean ± SD (n = 3 separate experiments with extracts, 6 separate experiments in control, and 25 μM Aβ only). Comparison of the values (*: p < 0.05, **: p < 0.01, Welch’s t-test).
4. Discussion
In this study, we evaluated the Aβ aggregation inhibitory activity of P. frutescens var. crispa cultivated under multiple conditions using the MSHTS system, which has the advantage of directly assessing this activity. At first, we evaluated the activity of plants cultivated in three fields (Figure 3) and found that leaves showed the highest activity among all plant parts that were assessed. In particular, this study’s highest activity of N3 (EC50 = 0.0059 mg/mL; Figure 7B) was the second highest in the 504 natural plant extracts previously evaluated [18]. We speculate that this activity is due to polyphenols having Aβ inhibitory effect because perilla leaves are rich in polyphenols [23,24,25]. Several compounds have been reported to show Aβ inhibitory activity among the polyphenols that are found in perilla. In particular, RA is the most promising compound since it is abundant in perilla [23] and has high Aβ aggregation inhibitory activity [15]. Similarly, chalcone [35] and asaron derivatives [36] isolated from perilla have also been reported as having this activity. Since perilla has a wide variety of compounds, total activity is considered by their accumulation and synergistic effect. These secondary metabolites are biosynthesized in response to environmental stress to protect against their oxidation [29,30].
Overall, the Aβ aggregation inhibitory activity of P. frutescens var. crispa increased until early September, until flowering, and then decreased after flowering (Figure 5A and Figure 7A). In perilla, during flowering, the contents of RA and total phenolic compounds become reduced [28,31]. These contents may have been altered by a change in metabolic pathways. The amount of total phenolic compounds in plants decreases with flowering due to the oxidation caused by the action of polyphenol oxidase and peroxidase [37]. Therefore, perilla should be harvested just before flowering to avoid a loss of active compounds.
The Aβ aggregation inhibitory activity showed the same trend as wind speed in Perilla frutescens var. crispa cultivated in the three fields in 2018 (Figure 3 and Figure 4). Furthermore, Perilla frutescens var. crispa cultivated without wind exposure showed significantly lower activity (Figure 5). Wind, as an environmental stressor, may promote plant growth and affect secondary metabolites. Wind promotes heat exchange, gas trade, and photosynthesis on plant leaves [38]. It was reported that leaf morphology is altered when plants are exposed to wind, increasing the total phenolics in the leaves [39]. Cultivation with more wind may enhance the activity of plants.
In 2019, Aβ aggregation inhibitory activity increased in soil with less than 20% water content in plants cultivated under multiple fertilization conditions (Figure 8A). Furthermore, watering of plants was ceased just before harvest to induce drought stress, and the cessation of watering for more than one week enhanced the activity (Figure 8C). Phenolic acids increase in response to drought stress in plants [40]. Plants biosynthesize polyphenols and flavonoids to defend themselves from drought [41,42]. Abscisic acid (ABA), a plant hormone, is synthesized in response to drought stress in plants [43]. ABA is involved in the accumulation of phenolic acids, especially anthocyanin, in plants under drought stress [44]. Based on the above, we speculate that drought stress promoted the biosynthesis of compounds that contribute to the Aβ aggregation inhibitory activity in P. frutescens var. crispa via the ABA response pathway.
Aβ aggregation inhibitory activity in P. frutescens var. crispa was highest with appropriate nitrogen fertilization as N3, and lower activity when excessive fertilization was applied (Figure 7B). In general, plants contain high levels of polyphenols when cultivated under low levels of nitrogen fertilization [45]. It may be essential to adjust fertilization according to nutrient availability in the field. Furthermore, the choice of nitrogen supply reduced the total phenolic content of leaves in strawberries, and the influence is compound-specific [46]. Perilla contains not only the main active compounds, such as RA, but also other active compounds [23]. Nitrogen fertilization might alter the composition of compounds to enhance total activity.
We showed that P. frutescens var. crispa leaf extracts suppressed Aβ-induced neurocytotoxicity in neuronally differentiated PC12 cells (Figure 9). The methanolic extracts of Perilla frutescens (L.) Britton similarly rescued the Aβ-induced cytotoxicity of PC12 cells [47]. Furthermore, compounds isolated from a Perilla frutescens (L.) Britton extract also suppressed Aβ-induced cytotoxicity and exhibited toxicity at high concentrations of 100 μg/mL [36]. In this study, the leaf extract at a high concentration of more than 25 μg/mL reduced cell viability but was lower than Aβ toxicity (Figure 9). Thus, an appropriate concentration of perilla extracts is essential so as not to exhibit toxicity.
This study has a limitation: we did not find other compounds that contribute to the Aβ inhibitory activity of P. frutescens var. crispa. A promising compound seems to be RA because the extracts from fresh perilla leaves prepared similarly in this study contain a high content of RA [48]. Furthermore, we previously reported that RA is the active component of the extract from summer savory (Satureja hortensis) in the Lamiaceae [15]. Even though we confirmed the presence of RA in the perilla extracts, the content was not enough to assess total activity. Previously, we did not obtain a fraction even equal to total activity, although we attempted to isolate active compounds from P. frutescens var. crispa extracts. This result shows that the activity in the extracts may be due to the synergistic effect of multiple compounds. Total activity is important when assessing the intake of substances such as food. A known example of this synergistic effect is the co-administration of epigallocatechin and gallic acid to improve learning ability in a mouse model of brain senescence [49]. We speculate that multiple compounds show a total activity in P. frutescens var. crispa extracts through synergistic effects and are trying to identify those compounds that contribute to this activity. In addition, it is necessary to research whether these compounds have blood–brain barrier permeability. In a rat model, RA reached the brain via the intraperitoneal administration of extracts from Plectranthus barbatus (Lamiaceae) herbal tea, including RA [50]. Moreover, perilla leaf extracts improved blocked Aβ aggregates-induced memory impairment and reduced Aβ deposits in the hippocampus with systemic administration in 5XFAD mice with AD-linked mutations [51]. In addition to treatment with anti-Aβ antibody drugs (e.g., aducanumab, lecanemab), prevention with foods such as Perilla may protect many people from AD risk. We expect that this study may contribute to preparing the extracts to show improvement against AD-like pathology.
5. Conclusions
In this study, we found several cultivating and harvesting factors of Perilla frutescens var. crispa that enhanced its Aβ aggregation inhibitory activity. The best harvesting time was just before flowering, and the following cultivation methods were effective: wind, appropriate nitrogen fertilization, and pre-harvest soil drying. These factors may alter the biosynthesis of polyphenols that exhibit this activity, although the active compounds were not identified. Furthermore, we successfully used the MSHTS system to assess total Aβ aggregation inhibitory activity. We consider this method to be a powerful tool for identifying substances with activity in plant-derived food. We hope that the active substances will be discovered and become a preventive and therapeutic agent against AD, for which no cure has yet been found.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods12030486/s1. Figure S1: Amyloid-β aggregation inhibitory activity is not changed on perilla leaf extracts within one day and seven days of extraction time
Author Contributions
Conceptualization, S.Y., K.U. and K.T.; Methodology, S.Y., K.U. and K.T.; Software, K.S., T.N., D.K. and M.K.; Formal Analysis, K.S., T.N., D.K., N.N. and M.K.; Investigation, K.S., T.N., D.K., M.K. and K.U.; Resources, S.Y., K.U. and K.T.; Data Curation, K.S. and T.N.; Writing—Original Draft Preparation, K.S.; Writing—Review & Editing, M.K., N.N., T.I., S.Y., K.U. and K.T.; Visualization, K.S. and T.N.; Supervision, K.T.; Funding Acquisition, K.S, S.Y., K.U. and K.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by JSPS KAKENHI Grant Number JP16H03288 (K.T.), JP25350974 (K.T.), and JP16K01909 (K.U.) and supported by JST SPRING Grant Number JPMJSP2153.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available upon request from the corresponding authors.
Acknowledgments
We are grateful to AW Farm Chitose Co., Ltd., Ohmae Giken Industry Co., Ltd., and Shiranuka town office for the cultivation of Perilla frutescens var. crispa in each field (Chitose, Watenbetsu, and Tomaribetsu, respectively).
Conflicts of Interest
Naoki Nishishita is an employee of KANEKA Corporation. Kaneka Corporation provides joint research funding to Koji Uwai and Kiyotaka Tokuraku for another study. Therefore, the funding source had no role in the design of the experiment and in providing any materials and equipment. Naoki Nishishita supported the analysis and writing of the paper as an expert on this research area.
References
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the Global Prevalence of Dementia in 2019 and Forecasted Prevalence in 2050: An Analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Counts, N.; Chen, S.; Seligman, B.; Tortorice, D.; Vigo, D.; Bloom, D.E. Global and Regional Projections of the Economic Burden of Alzheimer’s Disease and Related Dementias from 2019 to 2050: A Value of Statistical Life Approach. eClinicalMedicine 2022, 51, 101580. [Google Scholar] [CrossRef] [PubMed]
- Brodaty, H.; Donkin, M. Family Caregivers of People with Dementia. Dialogues Clin. Neurosci. 2009, 11, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaup, G.; McDonald, B.L.; Beyreuther, K. Amyloid Plaque Core Protein in Alzheimer Disease and Down Syndrome. Proc. Natl. Acad. Sci. USA 1985, 82, 4245–4249. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A Critical Appraisal of Amyloid-β-Targeting Therapies for Alzheimer Disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef]
- Yiannopoulou, K.G.; Anastasiou, A.I.; Zachariou, V.; Pelidou, S.-H. Reasons for Failed Trials of Disease-Modifying Treatments for Alzheimer Disease and Their Contribution in Recent Research. Biomedicines 2019, 7, 97. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical Model of Dynamic Biomarkers of the Alzheimer’s Pathological Cascade. Lancet Neurol. 2010, 9, 119–128. [Google Scholar] [CrossRef]
- Dunn, B.; Stein, P.; Cavazzoni, P. Approval of Aducanumab for Alzheimer Disease-The FDA’s Perspective. JAMA Intern. Med. 2021, 181, 1276–1278. [Google Scholar] [CrossRef] [PubMed]
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2022, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Fleming, W.K.; Brown, C.R.; Shrank, W.H. Costly New Alzheimer Disease Medications on the Horizon-Financing Alternatives for Medicare. JAMA Health Forum 2020, 1, e201148. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, Y.; Tanaka, H.; Akama, H.; Ogara, T.; Uwai, K.; Tokuraku, K. A Microliter-Scale High-Throughput Screening System with Quantum-Dot Nanoprobes for Amyloid-β Aggregation Inhibitors. PLoS ONE 2013, 8, e72992. [Google Scholar] [CrossRef]
- Kuragano, M.; Yoshinari, W.; Lin, X.; Shimamori, K.; Uwai, K.; Tokuraku, K. Evaluation of Amyloid Β42 Aggregation Inhibitory Activity of Commercial Dressings by A Microliter-Scale High-Throughput Screening System Using Quantum-Dot Nanoprobes. Foods 2020, 9, 825. [Google Scholar] [CrossRef]
- Lin, X.; Watanabe, K.; Kuragano, M.; Kurotaki, Y.; Nakanishi, U.; Tokuraku, K. Dietary Intake of Rosmarinic Acid Increases Serum Inhibitory Activity in Amyloid A Aggregation and Suppresses Deposition in the Organs of Mice. Int. J. Mol. Sci. 2020, 21, 6031. [Google Scholar] [CrossRef]
- Sasaki, R.; Tainaka, R.; Ando, Y.; Hashi, Y.; Deepak, H.V.; Suga, Y.; Murai, Y.; Anetai, M.; Monde, K.; Ohta, K.; et al. An Automated Microliter-Scale High-Throughput Screening System (MSHTS) for Real-Time Monitoring of Protein Aggregation Using Quantum-Dot Nanoprobes. Sci. Rep. 2019, 9, 2587. [Google Scholar] [CrossRef]
- Ogara, T.; Takahashi, T.; Yasui, H.; Uwai, K.; Tokuraku, K. Evaluation of the Effects of Amyloid β Aggregation from Seaweed Extracts by a Microliter-Scale High-Throughput Screening System with a Quantum Dot Nanoprobe. J. Biosci. Bioeng. 2015, 120, 45–50. [Google Scholar] [CrossRef]
- Adamcik, J.; Mezzenga, R. Study of Amyloid Fibrils via Atomic Force Microscopy. Curr. Opin. Colloid Interface Sci. 2012, 17, 369–376. [Google Scholar] [CrossRef]
- Yang, Y.; Arseni, D.; Zhang, W.; Huang, M.; Lövestam, S.; Schweighauser, M.; Kotecha, A.; Murzin, A.G.; Peak-Chew, S.Y.; Macdonald, J.; et al. Cryo-EM Structures of Amyloid-β 42 Filaments from Human Brains. Science 2022, 375, 167–172. [Google Scholar] [CrossRef]
- LeVine, H. [18] Quantification of β-Sheet Amyloid Fibril Structures with Thioflavin T. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 309, pp. 274–284. [Google Scholar]
- Wang, Z.; Tu, Z.; Xie, X.; Cui, H.; Kong, K.W.; Zhang, L. Perilla Frutescens Leaf Extract and Fractions: Polyphenol Composition, Antioxidant, Enzymes (α-Glucosidase, Acetylcholinesterase, and Tyrosinase) Inhibitory, Anticancer, and Antidiabetic Activities. Foods 2021, 10, 315. [Google Scholar] [CrossRef]
- Tipsuwan, W.; Chaiwangyen, W. Preventive Effects of Polyphenol-Rich Perilla Leaves on Oxidative Stress and Haemolysis. Sci. Asia. 2018, 44, 162–169. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Yoo, J.H.; Yu, C.Y.; Kim, S.-H.; Chung, I.-M. Profiling Volatile and Phenolic Compound Composition and Characterization of the Morphological and Biological Activities of Perilla Frutescence Britton Var. Japonica Accessions. Acta Physiol. Plant. 2019, 41, 108. [Google Scholar] [CrossRef]
- Luță, E.A.; Biță, A.; Moroșan, A.; Mihaiescu, D.E.; Ghica, M.; Mihai, D.P.; Olaru, O.T.; Deculescu-Ioniță, T.; Duțu, L.E.; Popescu, M.L.; et al. The Influence of Phytosociological Cultivation and Fertilization on Polyphenolic Content of Menthae and Melissae Folium and Evaluation of Antioxidant Properties through In Vitro and In Silico Methods. Plants 2022, 11, 2398. [Google Scholar] [CrossRef] [PubMed]
- Suwa, R.; Tajima, H.; Gima, S.; Uehara, N.; Watanabe, K.; Yabuta, S.; Tominaga, J.; Kawamitsu, Y. Polyphenol Production in Peucedanum Japonicum Thunb. Varies with Soil Type and Growth Stage. Hortic. J. 2018, 87, 382–388. [Google Scholar] [CrossRef]
- Gai, F.; Peiretti, P.G.; Karamać, M.; Amarowicz, R. Changes in the Total Polyphenolic Content and Antioxidant Capacities of Perilla (Perilla frutescens L.) Plant Extracts during the Growth Cycle. J. Food Qual. 2017, 2017, 7214747. [Google Scholar] [CrossRef]
- Meng, L.; Lozano, Y.; Bombarda, I.; Gaydou, E.M.; Li, B. Polyphenol Extraction from Eight Perilla Frutescens Cultivars. C. R. Chim. 2009, 12, 602–611. [Google Scholar] [CrossRef]
- Lu, N.; Bernardo, E.L.; Tippayadarapanich, C.; Takagaki, M.; Kagawa, N.; Yamori, W. Growth and Accumulation of Secondary Metabolites in Perilla as Affected by Photosynthetic Photon Flux Density and Electrical Conductivity of the Nutrient Solution. Front. Plant Sci. 2017, 8, 708. [Google Scholar] [CrossRef]
- Gaihre, Y.R.; Tsuge, K.; Hamajima, H.; Nagata, Y.; Yanagita, T. The Contents of Polyphenols in Perilla Frutescens (L.) Britton Var. Frutescens (Egoma) Leaves Are Determined by Vegetative Stage, Spatial Leaf Position, and Timing of Harvesting during the Day. J. Oleo Sci. 2021, 70, 855–859. [Google Scholar]
- Tokuraku, K.; Marquardt, M.; Ikezu, T. Real-Time Imaging and Quantification of Amyloid-Beta Peptide Aggregates by Novel Quantum-Dot Nanoprobes. PLoS ONE 2009, 4, e8492. [Google Scholar] [CrossRef] [PubMed]
- Kuragano, M.; Yamashita, R.; Chikai, Y.; Kitamura, R.; Tokuraku, K. Three-Dimensional Real Time Imaging of Amyloid β Aggregation on Living Cells. Sci. Rep. 2020, 10, 9742. [Google Scholar] [CrossRef] [PubMed]
- Mairuae, N.; Connor, J.R.; Buranrat, B.; Lee, S.Y. Oroxylum indicum (L.) Extract Protects Human Neuroblastoma SH-SY5Y Cells against Β-amyloid-induced Cell Injury. Mol. Med. Rep. 2019, 20, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Sakaguchi, Y.; Taniwa, K.; Izuo, N.; Hanaki, M.; Kawase, T.; Hirose, K.; Shimizu, T.; Irie, K. Lysine-Targeting Inhibition of Amyloid β Oligomerization by a Green Perilla-Derived Metastable Chalcone in Vitro and in Vivo. RSC Chem. Biol. 2022, 3, 1380–1396. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, N.; Yeo, J.Y.; Seo, D.-G.; Kim, S.; Lee, J.-S.; Hwang, K.W.; Park, S.-Y. Anti-Amyloidogenic Effects of Asarone Derivatives From Perilla Frutescens Leaves against Beta-Amyloid Aggregation and Nitric Oxide Production. Molecules 2019, 24, 4297. [Google Scholar] [CrossRef]
- André, A.; Leupin, M.; Kneubühl, M.; Pedan, V.; Chetschik, I. Evolution of the Polyphenol and Terpene Content, Antioxidant Activity and Plant Morphology of Eight Different Fiber-Type Cultivars of Cannabis sativa L. Cultivated at Three Sowing Densities. Plants 2020, 9, 1740. [Google Scholar] [CrossRef]
- Mezour, S. Forests Phenolic Compounds Instigate Cloud Precipitation. Res. Sq. 2020; preprint. [Google Scholar] [CrossRef]
- McArthur, C.; Bradshaw, O.S.; Jordan, G.J.; Clissold, F.J.; Pile, A.J. Wind Affects Morphology, Function, and Chemistry of Eucalypt Tree Seedlings. Int. J. Plant Sci. 2010, 171, 73–80. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Stress Enhances the Synthesis of Secondary Plant Products: The Impact of Stress-Related over-Reduction on the Accumulation of Natural Products. Plant Cell Physiol. 2013, 54, 817–826. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of Oxidative and Drought Tolerance in Arabidopsis by Overaccumulation of Antioxidant Flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- González-Villagra, J.; Kurepin, L.V.; Reyes-Díaz, M.M. Evaluating the Involvement and Interaction of Abscisic Acid and MiRNA156 in the Induction of Anthocyanin Biosynthesis in Drought-Stressed Plants. Planta 2017, 246, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Sauter, A.; Davies, W.J.; Hartung, W. The Long-Distance Abscisic Acid Signal in the Droughted Plant: The Fate of the Hormone on Its Way from Root to Shoot. J. Exp. Bot. 2001, 52, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- González-Villagra, J.; Cohen, J.D.; Reyes-Díaz, M.M. Abscisic Acid Is Involved in Phenolic Compounds Biosynthesis, Mainly Anthocyanins, in Leaves OfAristotelia Chilensisplants (Mol.) Subjected to Drought Stress. Physiol. Plant. 2019, 165, 855–866. [Google Scholar] [CrossRef]
- Heimler, D.; Romani, A.; Ieri, F. Plant Polyphenol Content, Soil Fertilization and Agricultural Management: A Review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Narvekar, A.S.; Tharayil, N. Nitrogen Fertilization Influences the Quantity, Composition, and Tissue Association of Foliar Phenolics in Strawberries. Front. Plant Sci. 2021, 12, 613839. [Google Scholar] [CrossRef]
- Kim, D.-J.; Kim, M.-S.; Kim, S.; Hwang, K.-W.; Park, S.-Y. Anti-Amyloidogenic Effects of Perilla Frutescens Var. Acuta on Beta-Amyloid Aggregation and Disaggregation. J. Food Biochem. 2017, 41, e12393. [Google Scholar] [CrossRef]
- Kagawa, N.; Iguchi, H.; Henzan, M.; Hanaoka, M. Drying the Leaves of Perilla Frutescens Increases Their Content of Anticancer Nutraceuticals. Food Sci. Nutr. 2019, 7, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Unno, K.; Nakagawa, A.; Takahashi, Y.; Iguchi, K.; Yamamoto, H.; Hoshino, M.; Hara, A.; Takagaki, A.; Nanjo, F.; et al. Blood Brain Barrier Permeability of (-)-Epigallocatechin Gallate, Its Proliferation-Enhancing Activity of Human Neuroblastoma SH-SY5Y Cells, and Its Preventive Effect on Age-Related Cognitive Dysfunction in Mice. Biochem. Biophys. Rep. 2017, 9, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Falé, P.L.V.; Madeira, P.J.A.; Florêncio, M.H.; Ascensão, L.; Serralheiro, M.L.M. Function of Plectranthus barbatus Herbal Tea as Neuronal Acetylcholinesterase Inhibitor. Food Funct. 2011, 2, 130–136. [Google Scholar] [CrossRef]
- Cho, E.; Lee, J.; Sin, J.S.; Kim, S.-K.; Kim, C.J.; Park, M.H.; Cho, W.-S.; Moon, M.; Kim, D.H.; Jung, J.W. Effects of Perilla Frutescens Var. Acuta in Amyloid β Toxicity and Alzheimer’s Disease-like Pathology in 5XFAD Mice. Food Chem. Toxicol. 2022, 161, 112847. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).