Effect of High-Pressure Micro-Fluidization on the Inactivation of Staphylococcus aureus in Liquid Food
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Growth Conditions
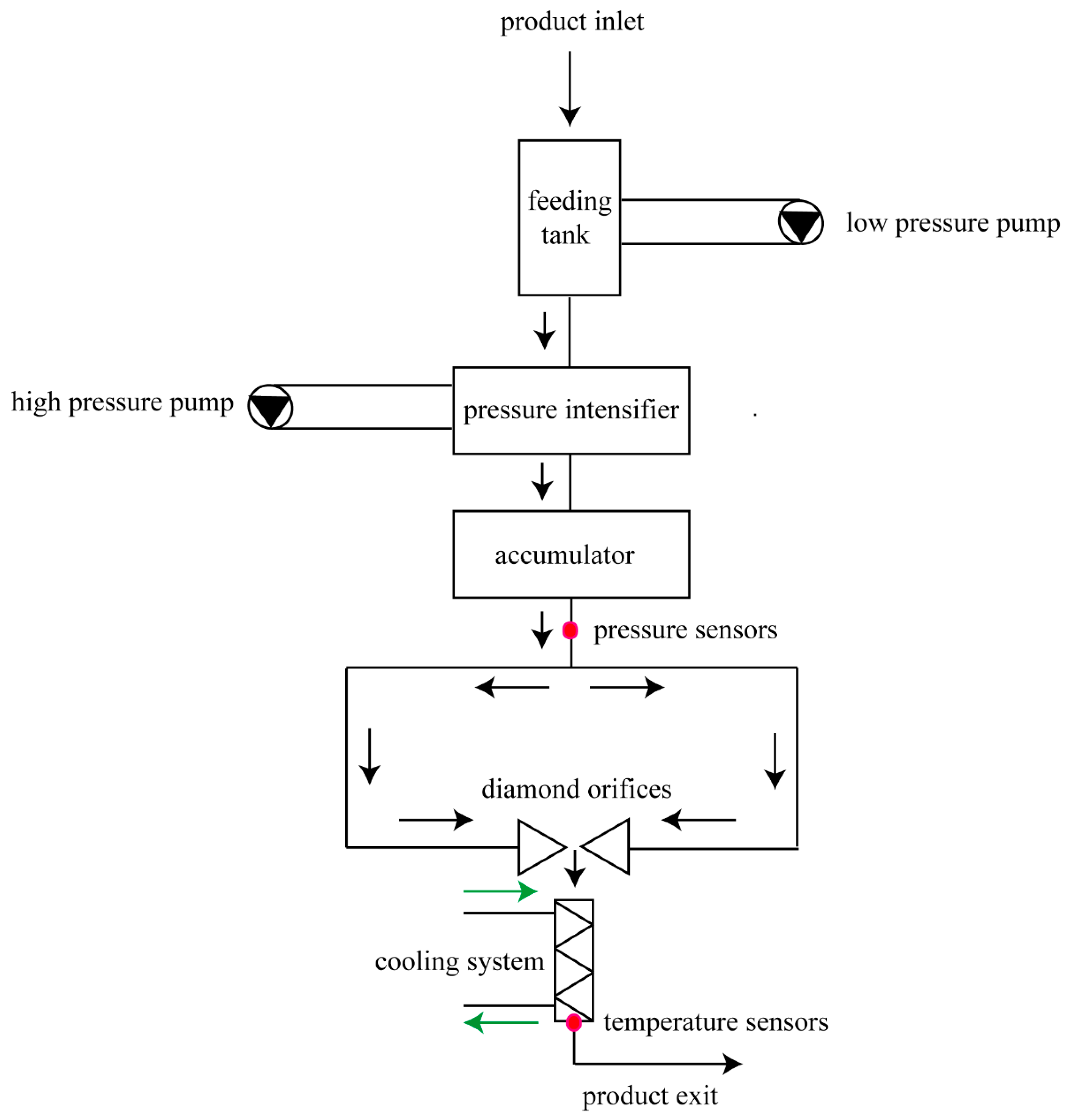
2.2. High-Pressure Micro-Fluidization Treatment
2.3. Enumeration of Viable Cells and Injured Cells
2.4. Scanning Electron Microscopy Analysis
2.5. Determination of Cell Membrane Permeability
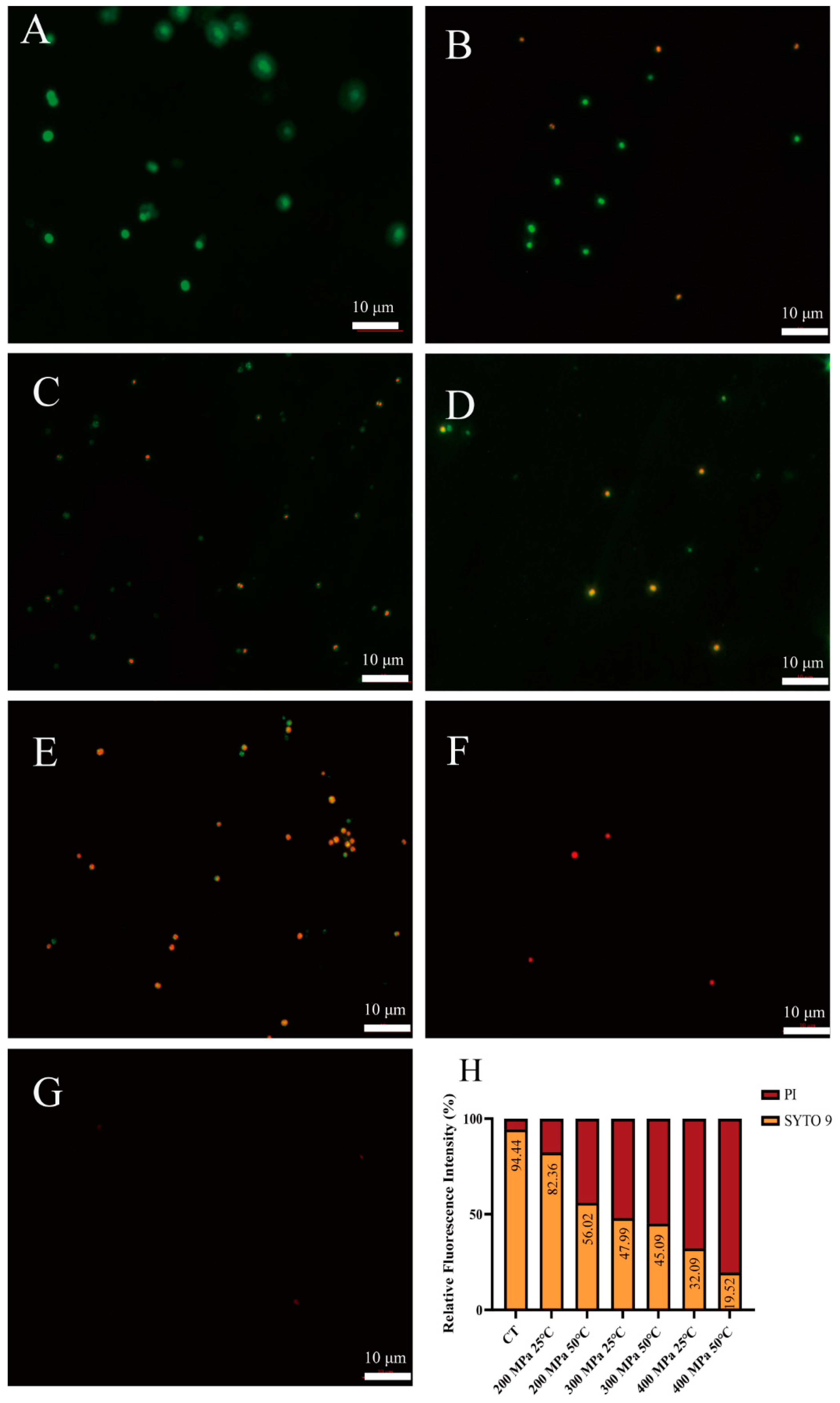
2.5.1. Fluorescence Microscopy Analysis
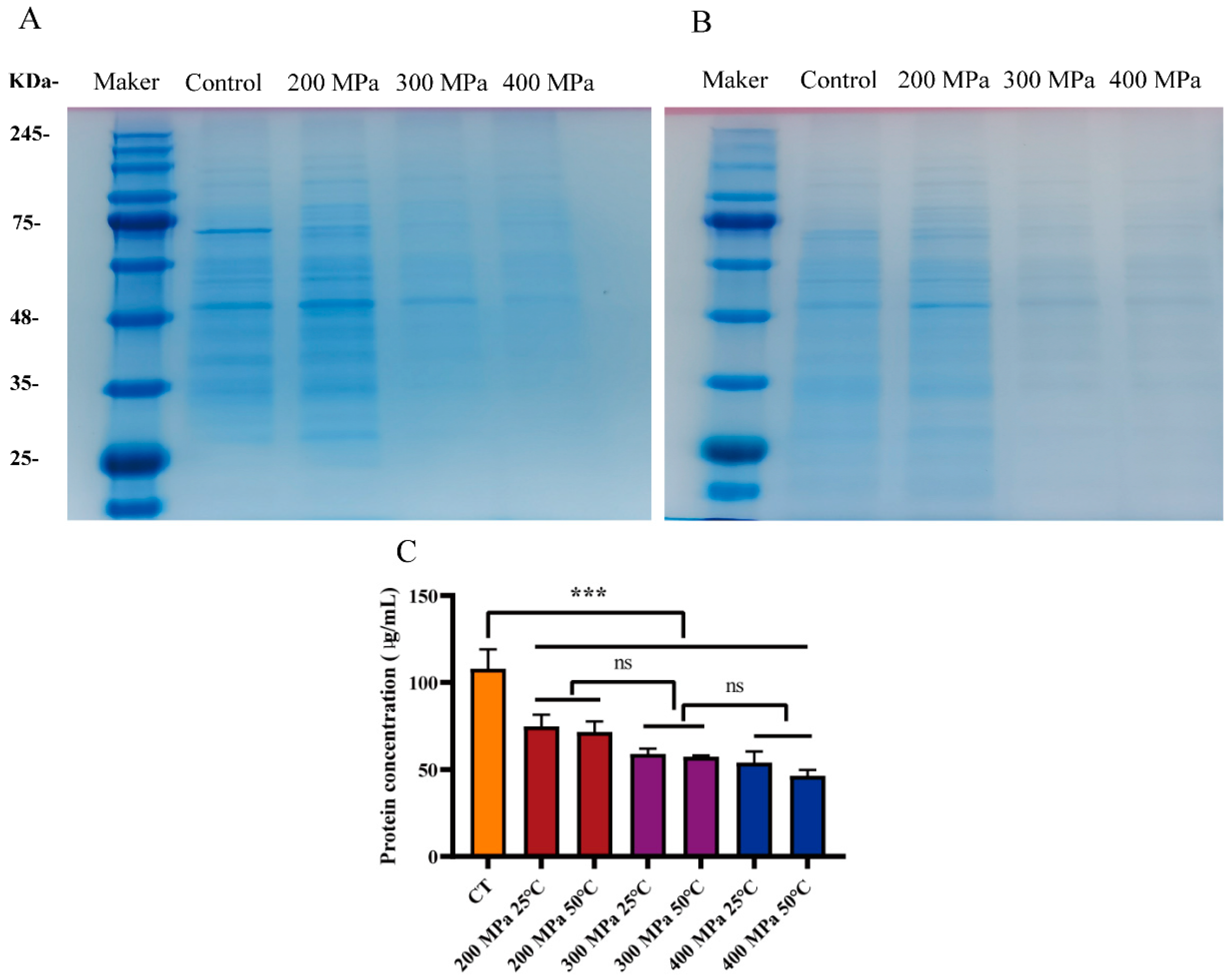
2.5.2. Measurement of Bacterial Intracellular Protein Loss
2.6. Flow Cytometry Analysis
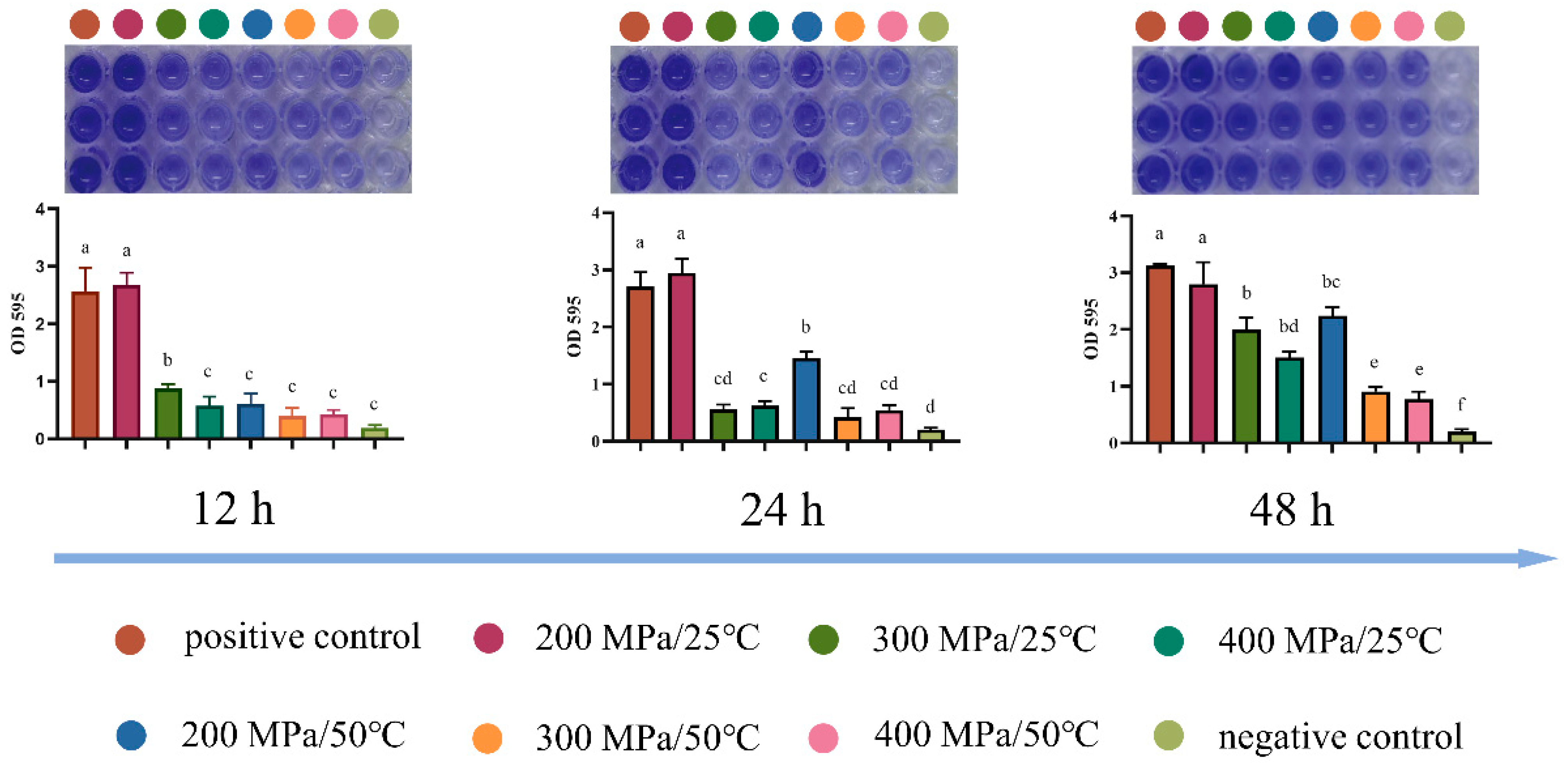
2.7. Analysis of the Biofilm Formation Ability
2.8. Application of HPM in Apple Juice
2.9. Statistical Analysis
3. Results
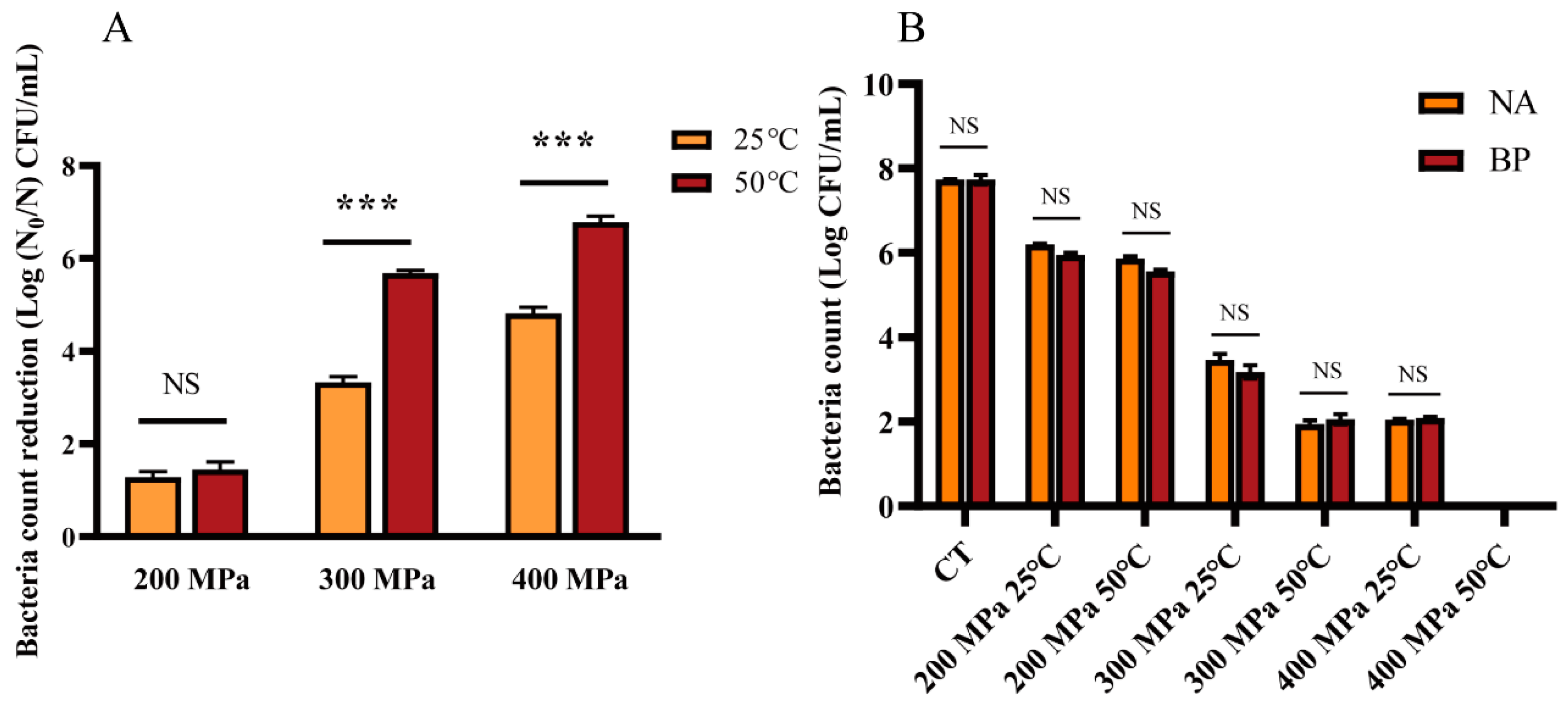
3.1. Reduction Effect of HPM on S. aureus subsp. aureus
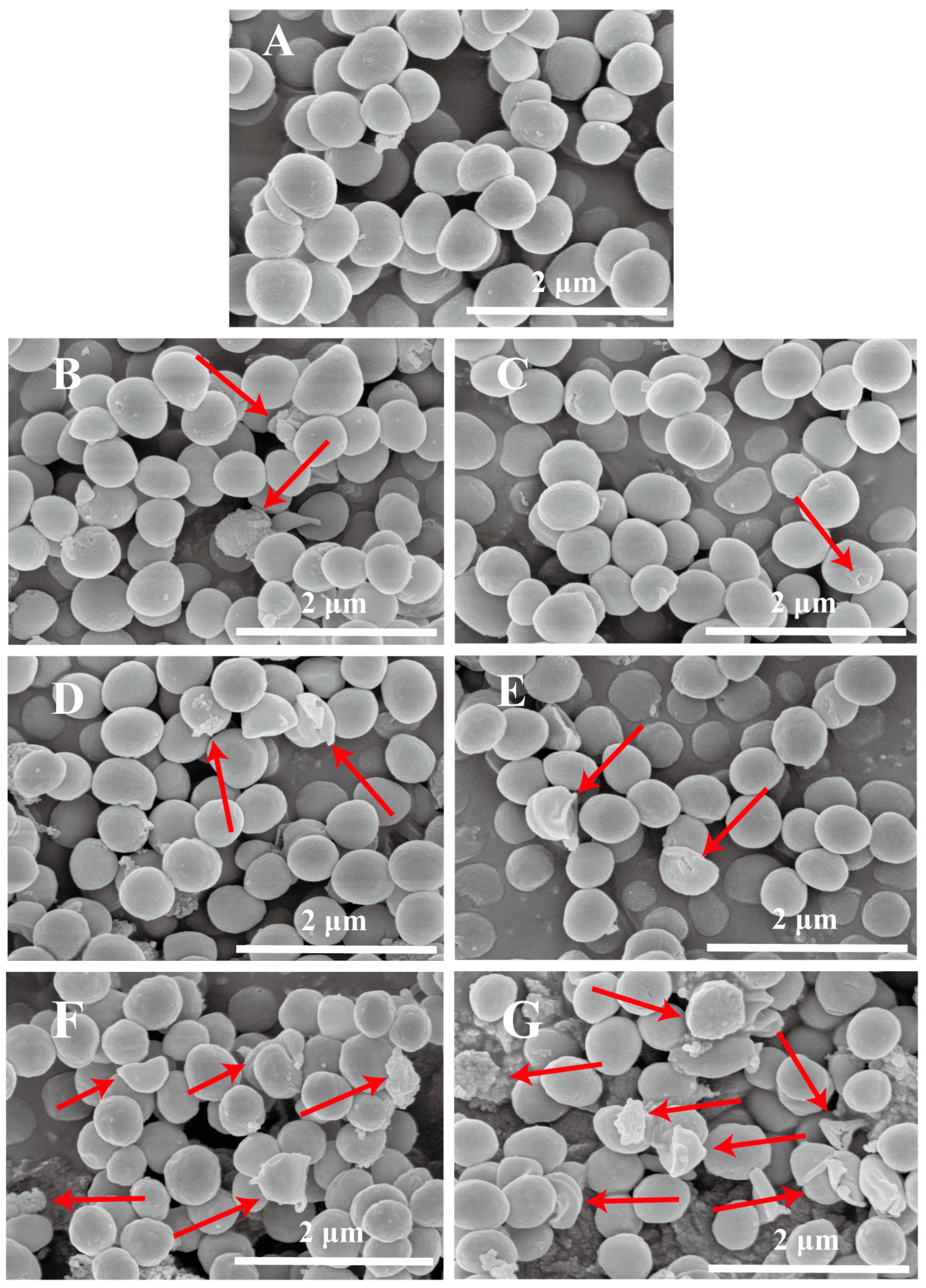
3.2. Analysis of Cell Morphology
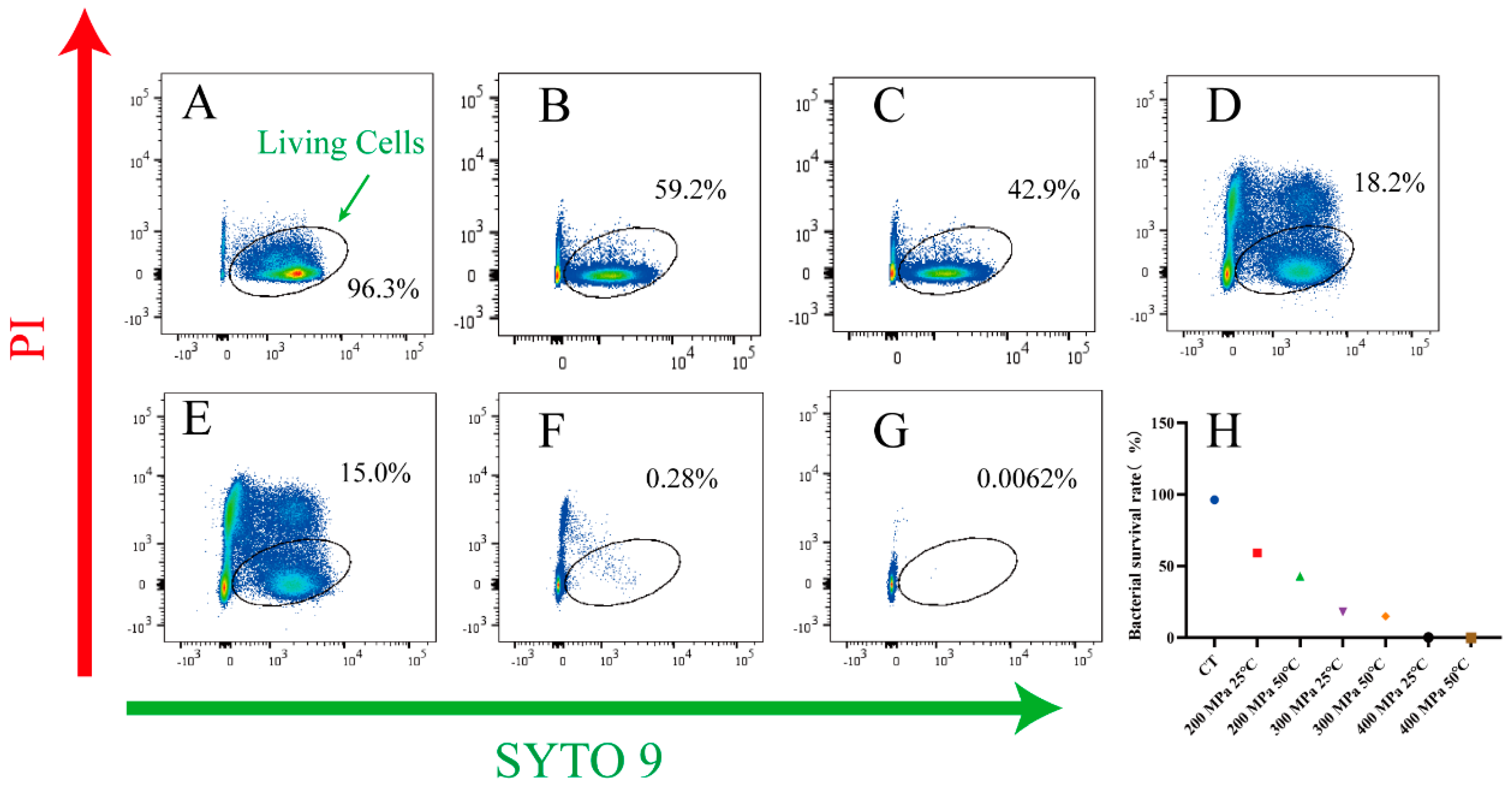
3.3. Analysis of Cell Membrane Integrity
3.4. Analysis of Intracellular Protein
3.5. Analysis of Resistance
3.6. The Antibacterial Effect of HPM in Apple Juice
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shao, L.; Liu, Y.; Tian, X.; Yu, Q.; Wang, H.; Li, X.; Dai, R. Inactivation and recovery of Staphylococcus aureus in milk, apple juice and broth treated with ohmic heating. LWT 2021, 139, 110545. [Google Scholar] [CrossRef]
- World Health Organization. Assessing Microbiological Risks in Food. 2015. Available online: https://www.who.int/activities/assessing-microbiological-risks-in-food (accessed on 18 April 2023).
- Shi, C.; Che, M.; Zhang, X.; Liu, Z.; Meng, R.; Bu, X.; Ye, H.; Guo, N. Antibacterial activity and mode of action of totarol against Staphylococcus aureus in carrot juice. J. Food Sci. Technol. 2018, 55, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Bae, Y.-M.; Lee, S.-Y.; Lee, S.-Y. Biofilm Formation of Staphylococcus aureus on Various Surfaces and Their Resistance to Chlorine Sanitizer. J. Food Sci. 2015, 80, M2279–M2286. [Google Scholar] [CrossRef] [PubMed]
- San Martín, M.F.; Barbosa-Cánovas, G.V.; Swanson, B.G. Food processing by high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 2002, 42, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, L.; Geeraerd, A.H.; Spilimbergo, S.; Elst, K.; Van Ginneken, L.; Debevere, J.; Van Impe, J.F.; Devlieghere, F. High pressure carbon dioxide inactivation of microorganisms in foods: The past, the present and the future. Int. J. Food Microbiol. 2007, 117, 1–28. [Google Scholar] [CrossRef]
- Martínez-Monteagudo, S.I.; Yan, B.; Balasubramaniam, V.M. Engineering Process Characterization of High-Pressure Homogenization—From Laboratory to Industrial Scale. Food Eng. Rev. 2016, 9, 143–169. [Google Scholar] [CrossRef]
- Donsì, F.; Wang, Y.; Li, J.; Huang, Q. Preparation of Curcumin Sub-micrometer Dispersions by High-Pressure Homogenization. J. Agric. Food Chem. 2010, 58, 2848–2853. [Google Scholar] [CrossRef]
- Dong, P.; Zhou, B.; Zou, H.; Wang, Y.; Liao, X.; Hu, X.; Zhang, Y. High pressure homogenization inactivation of Escherichia coli and Staphylococcus aureus in phosphate buffered saline, milk and apple juice. Lett. Appl. Microbiol. 2021, 73, 159–167. [Google Scholar] [CrossRef]
- Taylor, T.M.; Roach, A.; Black, D.G.; Davidson, P.M.; Harte, F. Inactivation of Escherichia coli K-12 exposed to pressures in excess of 300 MPa in a high-pressure homogenizer. J. Food Prot. 2007, 70, 1007–1010. [Google Scholar] [CrossRef]
- Diels, A.M.J.; Callewaert, L.; Wuytack, E.Y.; Masschalck, B.; Michiels, C.W. Inactivation of Escherichia coli by high-pressure homogenisation is influenced by fluid viscosity but not by water activity and product composition. Int. J. Food Microbiol. 2005, 101, 281–291. [Google Scholar] [CrossRef]
- Donsì, F.; Esposito, L.; Lenza, E.; Senatore, B.; Ferrari, G. Production of Shelf-Stable Annurca Apple Juice with Pulp by High Pressure Homogenization. Int. J. Food Eng. 2009, 5. [Google Scholar] [CrossRef]
- Saldo, J.; Suárez-Jacobo, Á.; Gervilla, R.; Guamis, B.; Roig-Sagués, A.X. Use of ultra-high-pressure homogenization to preserve apple juice without heat damage. High Press. Res. 2009, 29, 52–56. [Google Scholar] [CrossRef]
- Szczepańska, J.; Skąpska, S.; Marszałek, K. Continuous High-pressure Cooling-Assisted Homogenization Process for Stabilization of Apple Juice. Food Bioprocess Technol. 2021, 14, 1101–1117. [Google Scholar] [CrossRef]
- Patrignani, F.; Mannozzi, C.; Tappi, S.; Tylewicz, U.; Pasini, F.; Castellone, V.; Riciputi, Y.; Rocculi, P.; Romani, S.; Caboni, M.F.; et al. (Ultra) High Pressure Homogenization Potential on the Shelf-Life and Functionality of Kiwifruit Juice. Front. Microbiol. 2019, 10, 246. [Google Scholar] [CrossRef]
- Szczepanska, J.; Skapska, S.; Polaska, M.; Marszalek, K. High pressure homogenization with a cooling circulating system: The effect on physiochemical and rheological properties, enzymes, and carotenoid profile of carrot juice. Food Chem. 2022, 370, 131023. [Google Scholar] [CrossRef]
- Baird-Parker, A.C. An Improved Diagnostic and Selective Medium for Isolating Coagulase Positive Staphylococci. J. Appl. Bacteriol. 1962, 25, 12–19. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; He, R.; Cui, H. Action mechanism of pulsed magnetic field against E. coli O157:H7 and its application in vegetable juice. Food Control 2019, 95, 150–156. [Google Scholar] [CrossRef]
- Zhao, L.; Qin, X.; Wang, Y.; Ling, J.; Shi, W.; Pang, S.; Liao, X. CO2-assisted high pressure processing on inactivation of Escherichia coli and Staphylococcus aureus. J. CO2 Util. 2017, 22, 53–62. [Google Scholar] [CrossRef]
- Yang, H.; Wang, M.; Yu, J.; Wei, H. Aspartate inhibits Staphylococcus aureus biofilm formation. FEMS Microbiol. Lett. 2015, 362, fnv025. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Espina, L.; Pagan, R.; Lopez, D.; Garcia-Gonzalo, D. Individual Constituents from Essential Oils Inhibit Biofilm Mass Production by Multi-Drug Resistant Staphylococcus aureus. Molecules 2015, 20, 11357–11372. [Google Scholar] [CrossRef]
- Singh, S.V.; Singh, R.; Verma, K.; Kamble, M.G.; Tarafdar, A.; Chinchkar, A.V.; Pandey, A.K.; Sharma, M.; Kumar Gupta, V.; Sridhar, K.; et al. Effect of microfluidization on quality characteristics of sapodilla (Manilkara achras L.) juice. Food Res. Int. 2022, 162, 112089. [Google Scholar] [CrossRef]
- Briñez, W.J.; Roig-Sagués, A.X.; Herrero, M.M.H.; López, B.G. Inactivation of Staphylococcus spp. strains in whole milk and orange juice using ultra high pressure homogenisation at inlet temperatures of 6 and 20 °C. Food Control 2007, 18, 1282–1288. [Google Scholar] [CrossRef]
- Diels, A.M.; Wuytack, E.Y.; Michiels, C.W. Modelling inactivation of Staphylococcus aureus and Yersinia enterocolitica by high-pressure homogenisation at different temperatures. Int. J. Food Microbiol. 2003, 87, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Wuytack, E.Y.; Diels, A.M.J.; Michiels, C.W. Bacterial inactivation by high-pressure homogenisation and high hydrostatic pressure. Int. J. Food Microbiol. 2002, 77, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, C.; Chen, X.; Xu, X.; Wang, H. Resistance of detached-cells of biofilm formed by Staphylococcus aureus to ultra high pressure homogenization. Food Res. Int. 2021, 139, 109954. [Google Scholar] [CrossRef]
- Briñez, W.J.; Roig-Sagués, A.X.; Hernández-Herrero, M.M.; Guamis-López, B. Inactivation of two strains of Escherichia coli inoculated into whole and skim milk by ultrahigh-pressure homogenisation. Le Lait 2006, 86, 241–249. [Google Scholar] [CrossRef]
- Roig-Sagues, A.X.; Velazquez, R.M.; Montealegre-Agramont, P.; Lopez-Pedemonte, T.J.; Brinez-Zambrano, W.J.; Guamis-Lopez, B.; Hernandez-Herrero, M.M. Fat content increases the lethality of ultra-high-pressure homogenization on Listeria monocytogenes in milk. J. Dairy Sci. 2009, 92, 5396–5402. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Estrada, R.M.; Hernández-Herrero, M.M.; López-Pedemonte, T.J.; Briñez-Zambrano, W.J.; Guamis-López, B.; Roig-Sagués, A.X. Inactivation of Listeria monocytogenes and Salmonella enterica serovar Senftenberg 775W inoculated into fruit juice by means of ultra high pressure homogenisation. Food Control 2011, 22, 313–317. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, N.; Jin, Y.; Xu, X. Putative inactivation mechanism and germicidal efficacy of induced electric field against Staphylococcus aureus. Food Microbiol. 2023, 111, 104208. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Ferrari, G. Microbial inactivation by high pressure homogenization: Effect of the disruption valve geometry. J. Food Eng. 2013, 115, 362–370. [Google Scholar] [CrossRef]
- Diels, A.M.J.; De Taeye, J.; Michiels, C.W. Sensitisation of Escherichia coli to antibacterial peptides and enzymes by high-pressure homogenisation. Int. J. Food Microbiol. 2005, 105, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Auty, J.M.; Jenkins, C.H.; Hincks, J.; Straatman-Iwanowska, A.A.; Allcock, N.; Turapov, O.; Galyov, E.E.; Harding, S.V.; Mukamolova, G.V. Generation of Distinct Differentially Culturable Forms of Burkholderia following Starvation at Low Temperature. Microbiol. Spectr. 2022, 10, e02110-21. [Google Scholar] [CrossRef] [PubMed]
- McKay, A.M.; Linton, M.; Stirling, J.; Mackle, A.; Patterson, M.F. A comparative study of changes in the microbiota of apple juice treated by high hydrostatic pressure (HHP) or high pressure homogenisation (HPH). Food Microbiol. 2011, 28, 1426–1431. [Google Scholar] [CrossRef]
| Treatments | Bacteria Count (Log CFU/mL) | Color | pH | ||
|---|---|---|---|---|---|
| L* | a* | b* | |||
| Fresh | / | 27.28 ± 0.10 a | −0.87 ± 0.07 c | 4.44 ± 0.05 c | 3.87 ± 0.01 a |
| Inoculated | 8.46 ± 0.02 | 26.15 ± 0.13 b | −1.28 ± 0.07 a | 5.67 ± 0.03 a | 3.89 ± 0.01 a |
| 200 MPa/25 °C | 5.36 ± 0.09 | 26.35 ± 0.34 b | −0.88 ± 0.03 bc | 5.20 ± 0.05 b | 3.89 ± 0.01 a |
| 300 MPa/25 °C | 2.99 ± 0.03 | 26.27 ± 0.37 b | −1.04 ± 0.06 b | 5.09 ± 0.10 b | 3.88 ± 0.01 a |
| 400 MPa/25 °C | 1.98 ± 0.07 | 25.89 ± 0.05 b | −0.94 ± 0.01 bc | 5.15 ± 0.01 b | 3.87 ± 0.01 a |
| 200 MPa/50 °C | 2.09 ± 0.16 | 25.87 ± 0.07 b | −0.97 ± 0.05 bc | 5.17 ± 0.10 b | 3.87 ± 0.01 a |
| 300 MPa/50 °C | / | 26.05 ± 0.50 b | −1.03 ± 0.01 b | 5.27 ± 0.22 b | 3.87 ± 0.01 a |
| 400 MPa/50 °C | / | 26.17 ± 0.05 b | −1.04 ± 0.02 b | 5.32 ± 0.10 bc | 3.87 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Cui, T.; Tai, L.; Mu, K.; Shi, Y.; Chen, F.; Liao, X.; Hu, X.; Dong, L. Effect of High-Pressure Micro-Fluidization on the Inactivation of Staphylococcus aureus in Liquid Food. Foods 2023, 12, 4306. https://doi.org/10.3390/foods12234306
Zhang Z, Cui T, Tai L, Mu K, Shi Y, Chen F, Liao X, Hu X, Dong L. Effect of High-Pressure Micro-Fluidization on the Inactivation of Staphylococcus aureus in Liquid Food. Foods. 2023; 12(23):4306. https://doi.org/10.3390/foods12234306
Chicago/Turabian StyleZhang, Zequn, Tianlin Cui, Luyang Tai, Kangyi Mu, Yicong Shi, Fang Chen, Xiaojun Liao, Xiaosong Hu, and Li Dong. 2023. "Effect of High-Pressure Micro-Fluidization on the Inactivation of Staphylococcus aureus in Liquid Food" Foods 12, no. 23: 4306. https://doi.org/10.3390/foods12234306
APA StyleZhang, Z., Cui, T., Tai, L., Mu, K., Shi, Y., Chen, F., Liao, X., Hu, X., & Dong, L. (2023). Effect of High-Pressure Micro-Fluidization on the Inactivation of Staphylococcus aureus in Liquid Food. Foods, 12(23), 4306. https://doi.org/10.3390/foods12234306








