Antibacterial and Antiviral Properties of Pinus densiflora Essential Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. EO Extraction from P. densiflora Siebold & Zuccarini (PDEO)
2.2. Determination of Antibacterial and Antifungal Activity
2.2.1. Screening for Antimicrobial Activity
2.2.2. Microdilution Assay
2.3. Determination of Antiviral Activity
2.3.1. Cell Culture and Viruses
2.3.2. Cytotoxicity Assay
2.3.3. FIPV-79-1146 Inoculum for In Vitro CRFK Infection Study
2.4. Determination of Antiviral Activity (H1N1)
2.4.1. Cell Culture and Viruses
2.4.2. Plaque Assay for Influenza A (H1N1) Virus Infectivity
2.5. Chromatographic Analysis
2.6. Statistical Analysis
3. Results
3.1. Antimicrobial and Antifungal Activity
3.2. Cell Viability
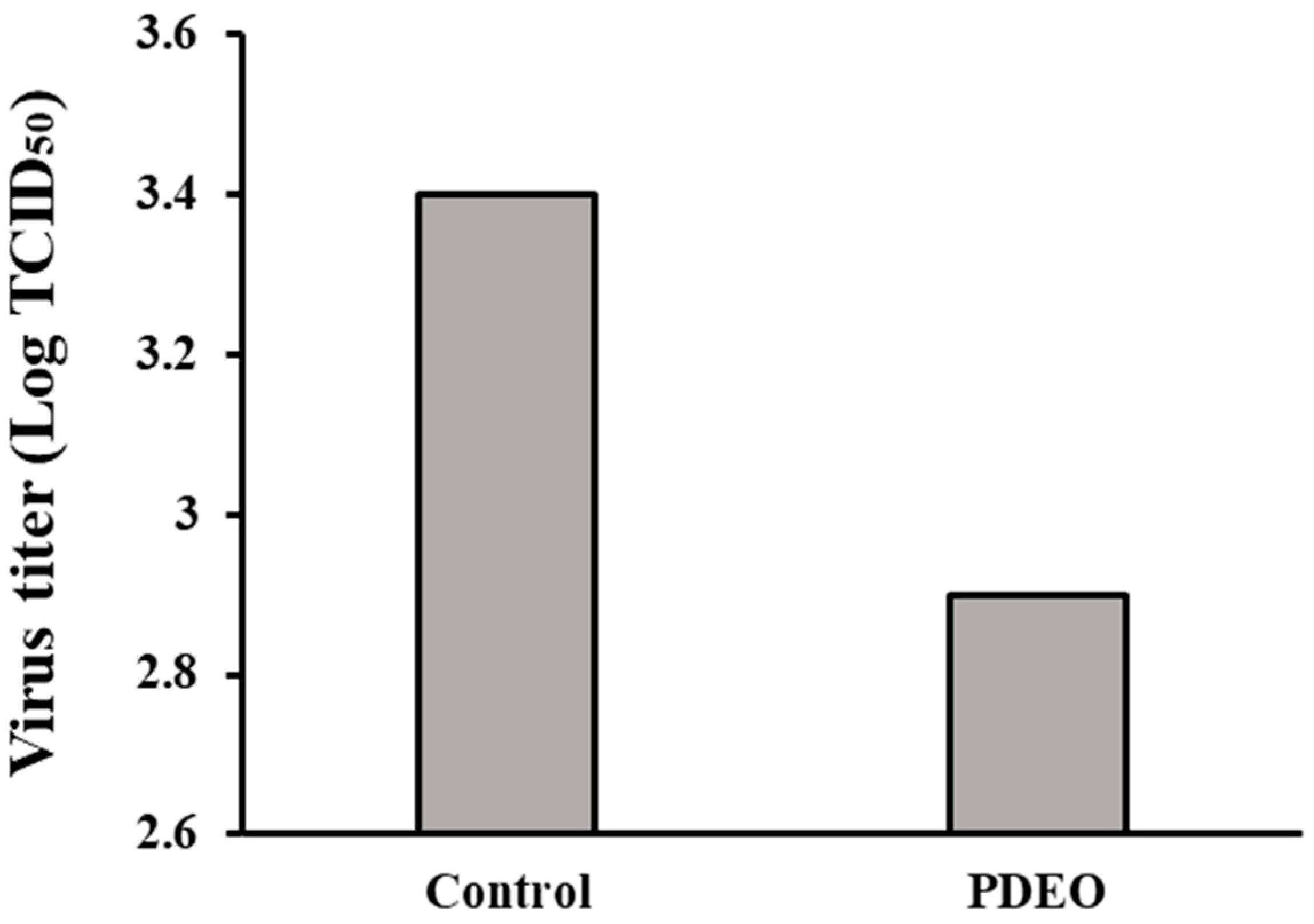
3.3. Antiviral Activity (FCoV)
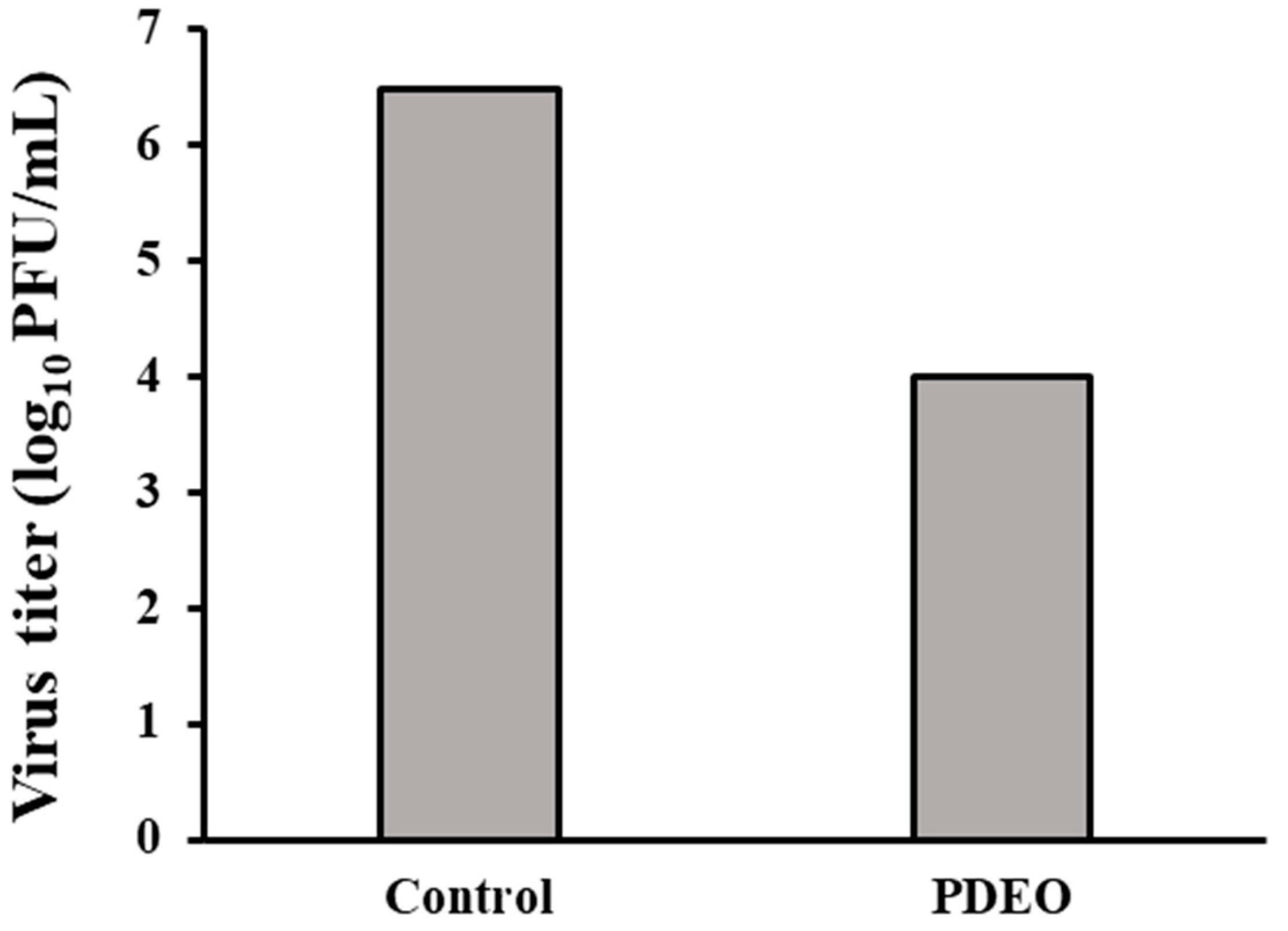
3.4. Antiviral Activity (H1N1)
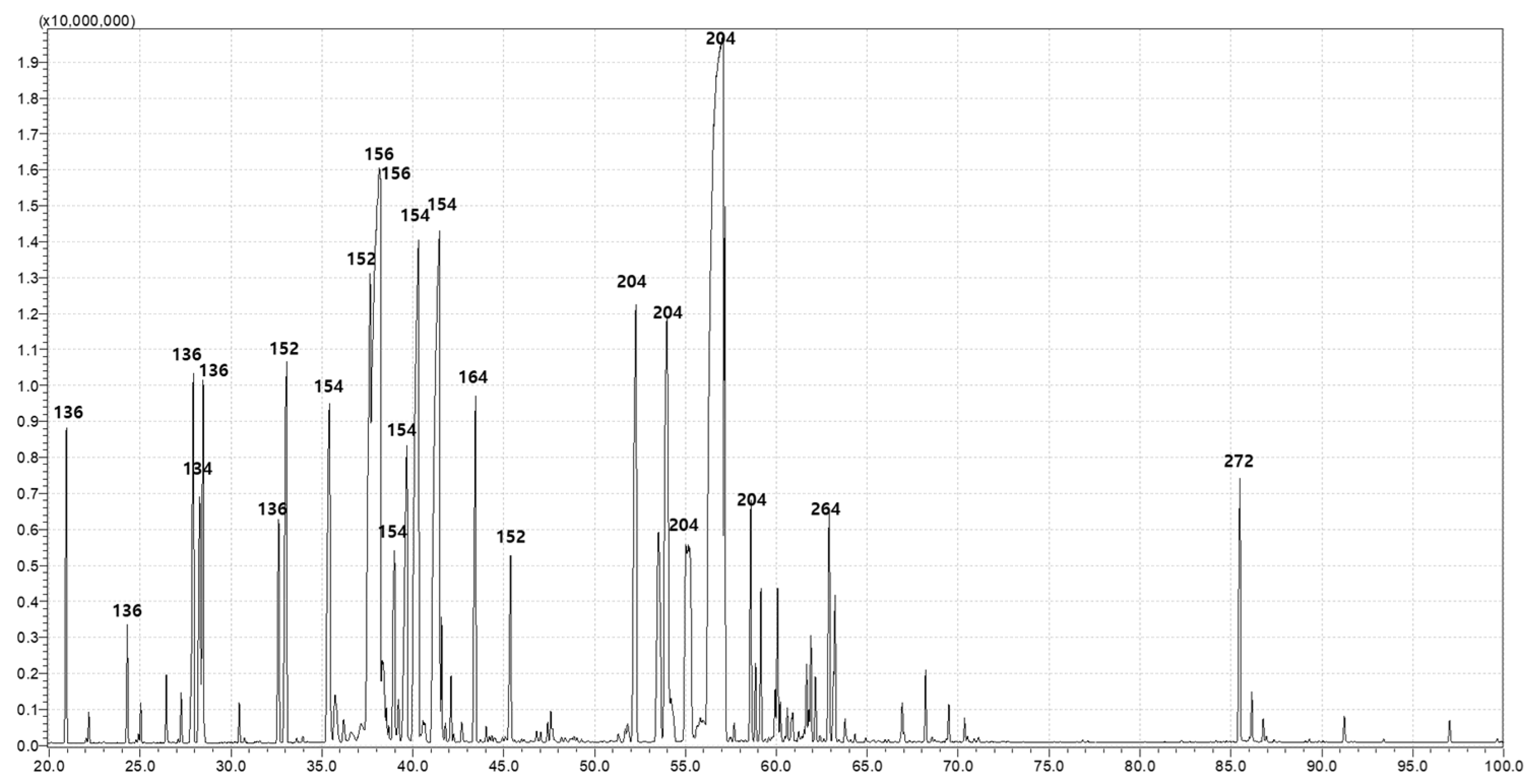
3.5. Chemical Composition of PDEO
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Farjon, A. A Handbook of the World’s Conifers (2 Vols.); Brill: Leiden, The Netherlands, 2010; Volume 1. [Google Scholar]
- Choi, W.I.; Nam, Y.; Lee, C.Y.; Choi, B.K.; Shin, Y.J.; Lim, J.-H.; Koh, S.-H.; Park, Y.-S. Changes in major insect pests of Pine forests in Korea over the last 50 years. Forests 2019, 10, 692. [Google Scholar] [CrossRef]
- Chung, H.-J.; Hwang, G.-H.; Yoo, M.-J.; Rhee, S.-J. Chemical composition of pine sprouts and pine needles for the production of pine sprout tea. J. Korean Soc. Food Cult. 1996, 11, 635–641. [Google Scholar]
- Jung, M.J.; Chung, H.Y.; Choi, J.H.; Choi, J.S. Antioxidant principles from the needles of red pine, Pinus densiflora. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003, 17, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, B.; Yun, K.W. Comparison of chemical composition and antimicrobial activity of essential oils from three Pinus species. Ind. Crops Prod. 2013, 44, 323–329. [Google Scholar] [CrossRef]
- Ahn, C.; Lee, J.-H.; Kim, J.-W.; Park, M.-J.; Lee, S.-S.; Jeung, E.-B. Alleviation effects of natural volatile organic compounds from Pinus densiflora and Chamaecyparis obtusa on systemic and pulmonary inflammation. Biomed. Rep. 2018, 9, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.-R.; Park, J.S.; Park, Y.-K.; Chae, Y.Z.; Lee, G.-H.; Park, G.-Y.; Jang, B.-C. Pinus densiflora leaf essential oil induces apoptosis via ROS generation and activation of caspases in YD-8 human oral cancer cells. Int. J. Oncol. 2012, 40, 1238–1245. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Chung, H.-J. Flavor compounds of pine sprout tea and pine needle tea. J. Agric. Food Chem. 2000, 48, 1269–1272. [Google Scholar] [CrossRef]
- Maimoona, A.; Naeem, I.; Saddiqe, Z.; Jameel, K. A review on biological, nutraceutical and clinical aspects of French maritime pine bark extract. J. Ethnopharmacol. 2011, 133, 261–277. [Google Scholar] [CrossRef]
- Oh, B.-T.; Choi, S.-G.; Cho, S.-H. Antimicrobial & physiological characteristics of ethanol extract from Pinus rigida Miller leaves. Korean J. Food Preserv. 2006, 13, 629–633. [Google Scholar]
- Zeng, W.C.; Zhang, Z.; Gao, H.; Jia, L.R.; He, Q. Chemical composition, antioxidant, and antimicrobial activities of essential oil from pine needle (Cedrus deodara). J. Food Sci. 2012, 77, C824–C829. [Google Scholar] [CrossRef]
- Lee, E. Effects of powdered pine needle (Pinus densiflora seib et Zucc.) on serum and liver lipid composition and antioxidative capacity in rats fed high oxidized fat. J. Korean Soc. Food Sci. Nutr. 2003, 32, 926–930. [Google Scholar]
- Sadgrove, N.J.; Padilla-González, G.F.; Phumthum, M. Fundamental chemistry of essential oils and volatile organic compounds, methods of analysis and authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fabiano-Tixier, A.-S.; Chemat, F. Essential Oils as Reagents in Green Chemistry; Springer: Berlin/Heidelberg, Germany, 2014; Volume 1. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef]
- Stringaro, A.; Colone, M.; Angiolella, L. Antioxidant, antifungal, antibiofilm, and cytotoxic activities of Mentha spp. essential oils. Medicines 2018, 5, 112. [Google Scholar] [CrossRef] [PubMed]
- Akthar, M.S.; Degaga, B.; Azam, T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: A review. J. Issues ISSN 2014, 2350, 1588. [Google Scholar]
- Yang, J.; Choi, W.-S.; Kim, K.-J.; Eom, C.-D.; Park, M.-J. Investigation of active anti-inflammatory constituents of essential oil from Pinus koraiensis (Sieb. et Zucc.) wood in LPS-stimulated RBL-2H3 cells. Biomolecules 2021, 11, 817. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Karabörklü, S.; Bozok, F.; Burhan, A.; Erdogan, S.; Cenet, M.; Karaaslan, M.G. Chemical composition, antimicrobial, insecticidal, phytotoxic and antioxidant activities of Mediterranean Pinus brutia and Pinus pinea resin essential oils. Chin. J. Nat. Med. 2014, 12, 901–910. [Google Scholar] [CrossRef]
- Kurti, F.; Giorgi, A.; Beretta, G.; Mustafa, B.; Gelmini, F.; Testa, C.; Angioletti, S.; Giupponi, L.; Zilio, E.; Pentimalli, D.; et al. Chemical composition, antioxidant and antimicrobial activities of essential oils of different Pinus species from Kosovo. J. Essent. Oil Res. 2019, 31, 263–275. [Google Scholar] [CrossRef]
- Evermann, J.; Baumgartener, L.; Ott, R.; Davis, E.; McKeirnan, A. Characterization of a feline infectious peritonitis virus isolate. Vet. Pathol. 1981, 18, 256–265. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Dong, S.; Yang, X.; Zhao, L.; Zhang, F.; Hou, Z.; Xue, P. Antibacterial activity and mechanism of action saponins from Chenopodium quinoa Willd. husks against foodborne pathogenic bacteria. Ind. Crops Prod. 2020, 149, 112350. [Google Scholar] [CrossRef]
- Park, S.; Worobo, R.W.; Durst, R.A. Escherichia coli O157: H7 as an emerging foodborne pathogen: A literature review. Crit. Rev. Food Sci. Nutr. 1999, 39, 481–502. [Google Scholar] [CrossRef]
- Gandhi, M.; Chikindas, M.L. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef]
- Murphy, C.; Carroll, C.; Jordan, K. Environmental survival mechanisms of the foodborne pathogen Campylobacter jejuni. J. Appl. Microbiol. 2006, 100, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, V.; Arshak, K.; Korostynska, O.; Oliwa, K.; Adley, C. An overview of foodborne pathogen detection: In the perspective of biosensors. Biotechnol. Adv. 2010, 28, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, Z.; Si, C.; Ying, Y. Subtractive inhibition assay for the detection of E. coli O157: H7 using surface plasmon resonance. Sensors 2011, 11, 2728–2739. [Google Scholar] [CrossRef]
- DePaola, A.; Nordstrom, J.L.; Bowers, J.C.; Wells, J.G.; Cook, D.W. Seasonal abundance of total and pathogenic Vibrio parahaemolyticus in Alabama oysters. Appl. Environ. Microbiol. 2003, 69, 1521–1526. [Google Scholar] [CrossRef]
- Yadav, N.; Chhillar, A.K.; Rana, J.S. Detection of pathogenic bacteria with special emphasis to biosensors integrated with AuNPs. Sens. Int. 2020, 1, 100028. [Google Scholar] [CrossRef]
- Lins de Sousa, D.; Araújo Lima, R.; Zanin, I.C.; Klein, M.I.; Janal, M.N.; Duarte, S. Effect of twice-daily blue light treatment on matrix-rich biofilm development. PLoS ONE 2015, 10, e0131941. [Google Scholar] [CrossRef]
- Chu, J.; Zhang, T.; He, K. Cariogenicity features of Streptococcus mutans in presence of rubusoside. BMC Oral Health 2016, 16, 1–6. [Google Scholar] [CrossRef]
- Silva, A.C.B.d.; Cruz, J.d.S.; Sampaio, F.C.; Araújo, D.A.M.d. Detection of oral streptococci in dental biofilm from caries-active and caries-free children. Braz. J. Microbiol. 2008, 39, 648–651. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sinha, P.; Srivastava, S.; Mishra, N.; Yadav, N.P. New perspectives on antiacne plant drugs: Contribution to modern therapeutics. BioMed Res. Int. 2014, 2014, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Dessinioti, C. Acne Pathogenesis: What We Have Learned Over the Years. In Pathogenesis and Treatment of Acne and Rosacea; Springer: Berlin/Heidelberg, Germany, 2014; pp. 61–70. [Google Scholar]
- Isard, O.; Knol, A.C.; Ariès, M.F.; Nguyen, J.M.; Khammari, A.; Castex-Rizzi, N.; Dréno, B. Propionibacterium acnes activates the IGF-1/IGF-1R system in the epidermis and induces keratinocyte proliferation. J. Investig. Dermatol. 2011, 131, 59–66. [Google Scholar] [CrossRef]
- Poh, S.E.; Goh, J.P.; Fan, C.; Chua, W.; Gan, S.Q.; Lim, P.L.K.; Sharma, B.; Leavesley, D.I.; Dawson, T.L., Jr.; Li, H. Identification of Malassezia furfur secreted aspartyl protease 1 (MfSAP1) and its role in extracellular matrix degradation. Front. Cell. Infect. Microbiol. 2020, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Saunte, D.M.; Gaitanis, G.; Hay, R.J. Malassezia-associated skin diseases, the use of diagnostics and treatment. Front. Cell. Infect. Microbiol. 2020, 10, 112. [Google Scholar] [CrossRef]
- Chorianopoulos, N.; Giaouris, E.; Skandamis, P.; Haroutounian, S.; Nychas, G.J. Disinfectant test against monoculture and mixed-culture biofilms composed of technological, spoilage and pathogenic bacteria: Bactericidal effect of essential oil and hydrosol of Satureja thymbra and comparison with standard acid–base sanitizers. J. Appl. Microbiol. 2008, 104, 1586–1596. [Google Scholar] [CrossRef]
- Burt, S.A.; Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157: H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef]
- De Martino, L.; De Feo, V.; Nazzaro, F. Chemical composition and in vitro antimicrobial and mutagenic activities of seven Lamiaceae essential oils. Molecules 2009, 14, 4213–4230. [Google Scholar] [CrossRef]
- Ayaria, M.; Romdhanea, M. Chemical constituents of the pine extracts and their activities: A review. Arab. J. Med. Aromat. Plants 2020, 6, 37–56. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Friedly, E.; Crandall, P.; Ricke, S.; Roman, M.; O’bryan, C.; Chalova, V. In vitro antilisterial effects of citrus oil fractions in combination with organic acids. J. Food Sci. 2009, 74, M67–M72. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef]
- Marino, M.; Bersani, C.; Comi, G. Antimicrobial activity of the essential oils of Thymus vulgaris L. measured using a bioimpedometric method. J. Food Prot. 1999, 62, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Mutlu-Ingok, A.; Tasir, S.; Seven, A.; Akgun, N.; Karbancioglu-Guler, F. Evaluation of the single and combined antibacterial efficiency of essential oils for controlling Campylobacter coli, Campylobacter jejuni, Escherichia coli, Staphylococcus aureus, and mixed cultures. Flavour Fragr. J. 2019, 34, 280–287. [Google Scholar] [CrossRef]
- Pesavento, G.; Calonico, C.; Bilia, A.; Barnabei, M.; Calesini, F.; Addona, R.; Mencarelli, L.; Carmagnini, L.; Di Martino, M.; Nostro, A.L. Antibacterial activity of Oregano, Rosmarinus and Thymus essential oils against Staphylococcus aureus and Listeria monocytogenes in beef meatballs. Food Control 2015, 54, 188–199. [Google Scholar] [CrossRef]
- Garten, R.J.; Davis, C.T.; Russell, C.A.; Shu, B.; Lindstrom, S.; Balish, A.; Sessions, W.M.; Xu, X.; Skepner, E.; Deyde, V. Antigenic and genetic characteristics of swine-origin 2009 A (H1N1) influenza viruses circulating in humans. Science 2009, 325, 197–201. [Google Scholar] [CrossRef]
- Kim, M.; Nguyen, D.-V.; Heo, Y.; Park, K.H.; Paik, H.-D.; Kim, Y.B. Antiviral Activity of Fritillaria Thunbergii Extract against Human Influenza Virus H1N1 (PR8) In Vitro. In Ovo In Vivo 2020, 10, 420–427. [Google Scholar] [CrossRef]
- Yingsakmongkon, S.; Miyamoto, D.; Sriwilaijaroen, N.; Fujita, K.; Matsumoto, K.; Jampangern, W.; Hiramatsu, H.; Guo, C.-T.; Sawada, T.; Takahashi, T. In vitro inhibition of human influenza A virus infection by fruit-juice concentrate of japanese plum (Prunus mume S IEB. et Z UCC). Biol. Pharm. Bull. 2008, 31, 511–515. [Google Scholar] [CrossRef]
- Sriwilaijaroen, N.; Fukumoto, S.; Kumagai, K.; Hiramatsu, H.; Odagiri, T.; Tashiro, M.; Suzuki, Y. Antiviral effects of Psidium guajava Linn.(guava) tea on the growth of clinical isolated H1N1 viruses: Its role in viral hemagglutination and neuraminidase inhibition. Antivir. Res. 2012, 94, 139–146. [Google Scholar] [CrossRef]
- Kiyohara, H.; Ichino, C.; Kawamura, Y.; Nagai, T.; Sato, N.; Yamada, H.; Salama, M.M.; Abdel-Sattar, E. In vitro anti-influenza virus activity of a cardiotonic glycoside from Adenium obesum (Forssk.). Phytomedicine 2012, 19, 111–114. [Google Scholar] [CrossRef]
- Yang, Z.-F.; Bai, L.-P.; Huang, W.-b.; Li, X.-Z.; Zhao, S.-S.; Zhong, N.-S.; Jiang, Z.-H. Comparison of in vitro antiviral activity of tea polyphenols against influenza A and B viruses and structure–activity relationship analysis. Fitoterapia 2014, 93, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Coronaviruses Methods Protoc. 2015, 1282, 1–23. [Google Scholar]
- Herrewegh, A.A.; Smeenk, I.; Horzinek, M.C.; Rottier, P.J.; de Groot, R.J. Feline coronavirus type II strains 79-1683 and 79-1146 originate from a double recombination between Feline coronavirus type I and Canine coronavirus. J. Virol. 1998, 72, 4508–4514. [Google Scholar] [CrossRef] [PubMed]

| Microorganism | Strain | MIC | ||
|---|---|---|---|---|
| P. densiflora Siebold & Zuccarini Essential Oil (μL/mL) | Ampicillin (μg/mL) | Ketoconazole (μg/mL) | ||
| Gram-negative bacteria | Escherichia coli ATCC 25922 | 100 | 1.0 | NT * |
| Salmonella typhi ATCC 6539 | 500 | 0.5 | NT | |
| Vibrio parahaemolyticus ATCC 17802 | 10 | 10 | NT | |
| Gram-positive bacteria | Bacillus cereus ATCC 14579 | 10 | 0.1 | NT |
| Clostridium perfringens ATCC 13124 | 100 | 0.5 | NT | |
| Staphylococcus aureus ATCC 12228 | 100 | 0.2 | NT | |
| Listeria monocytogenes ATCC 15313 | 10 | 0.5 | NT | |
| Streptococcus mutans ATCC 25175 | 500 | 0.2 | NT | |
| Propionibacterium acnes ATCC 11828 | 10 | 0.2 | NT | |
| Fungi | Malassezia furfur ATCC 14521 | 10 | NT | 0.1 |
| Peak No. | Compound | RT a (min) | % Area |
|---|---|---|---|
| 1 | alpha-Pinene | 20.94 | 2.87 |
| 2 | beta-Pinene | 24.29 | 0.94 |
| 3 | alpha-Phellandrene | 26.44 | 0.59 |
| 4 | O-Isopropyltoluene * | 27.92 | 6.35 |
| 5 | beta-Phellandrene | 28.46 | 4.18 |
| 6 | (+)-4-Carene | 32.61 | 1.48 |
| 7 | Fenchone * | 33.05 | 8.14 |
| 8 | Fenchol | 35.41 | 4.44 |
| 9 | Camphor | 37.65 | 4.31 |
| 10 | 2-(4-Methylcyclohexyl)-2-propanol * | 38.15 | 9.09 |
| 11 | cis-Dihydro-alpha-terpineol | 38.97 | 4.81 |
| 12 | (-)-Borneol * | 39.67 | 7.83 |
| 13 | p-Menth-1-en-4-ol * | 40.31 | 9.87 |
| 14 | 3-Cyclohexene-1-methanol * | 41.46 | 10.12 |
| 15 | Isothymol methyl ether * | 43.44 | 6.14 |
| 16 | Piperitone | 45.38 | 1.87 |
| 17 | alpha-Longipinene | 52.27 | 4.98 |
| 18 | (E)-beta-Famesene | 58.59 | 1.51 |
| 19 | 5-Azulenemethanol | 63.21 | 0.83 |
| 20 | Thunbergen | 85.49 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, Y.J.; Kim, Y.-S.; Kim, J.W.; Kim, D.W. Antibacterial and Antiviral Properties of Pinus densiflora Essential Oil. Foods 2023, 12, 4279. https://doi.org/10.3390/foods12234279
Oh YJ, Kim Y-S, Kim JW, Kim DW. Antibacterial and Antiviral Properties of Pinus densiflora Essential Oil. Foods. 2023; 12(23):4279. https://doi.org/10.3390/foods12234279
Chicago/Turabian StyleOh, Yu Jin, Yeong-Su Kim, Jae Woo Kim, and Dae Wook Kim. 2023. "Antibacterial and Antiviral Properties of Pinus densiflora Essential Oil" Foods 12, no. 23: 4279. https://doi.org/10.3390/foods12234279
APA StyleOh, Y. J., Kim, Y.-S., Kim, J. W., & Kim, D. W. (2023). Antibacterial and Antiviral Properties of Pinus densiflora Essential Oil. Foods, 12(23), 4279. https://doi.org/10.3390/foods12234279







