An Update on the Pivotal Roles of Probiotics, Their Components, and Metabolites in Preventing Colon Cancer
Abstract
1. Introduction
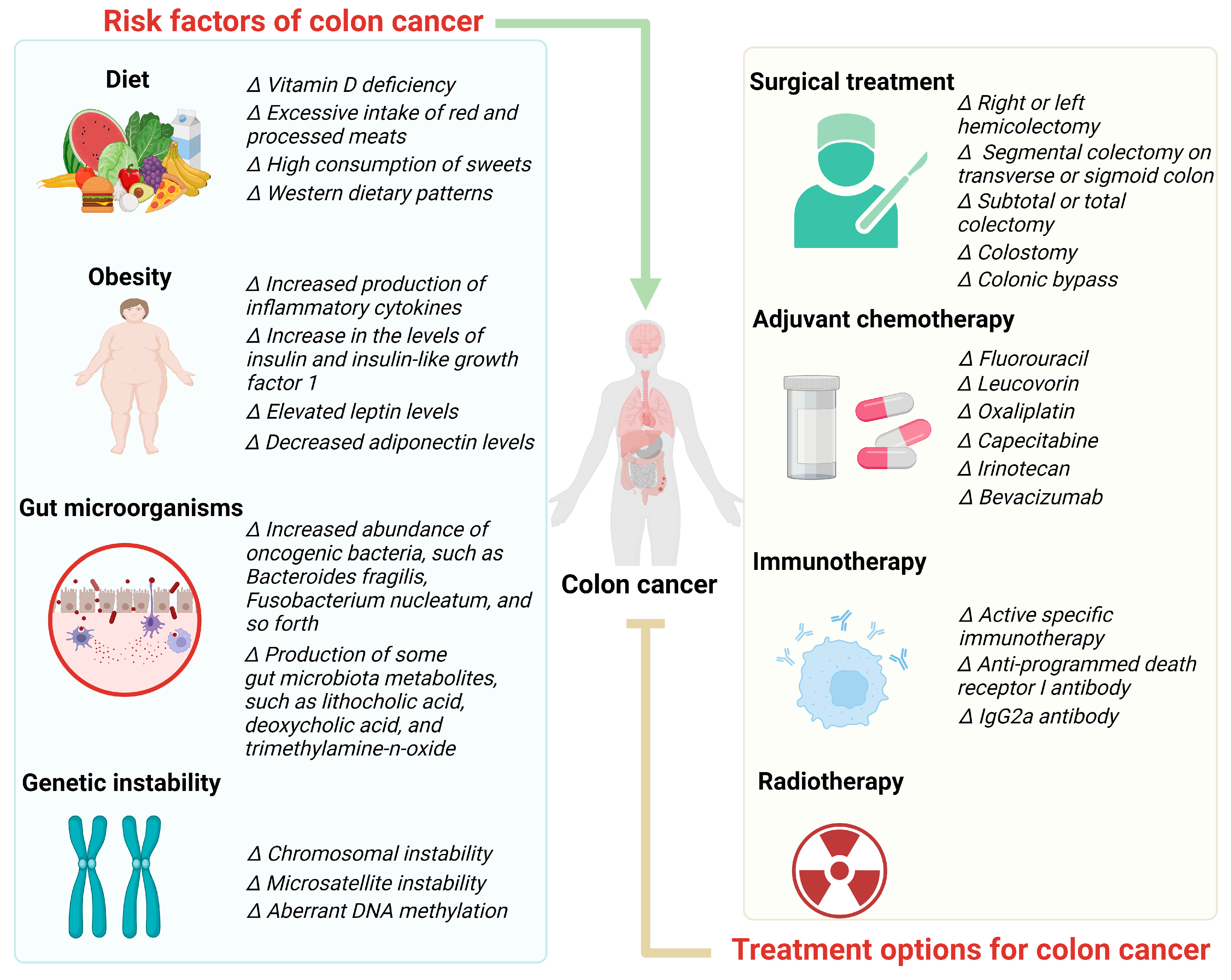
2. Risk Factors of Colon Cancer
2.1. Diet
2.2. Obesity
2.3. Gut Microorganisms
2.4. Genetic Instability
3. Treatment Options for Colon Cancer
3.1. Surgical Treatment
3.2. Adjuvant Chemotherapy
3.3. Immunotherapy
3.4. Radiotherapy
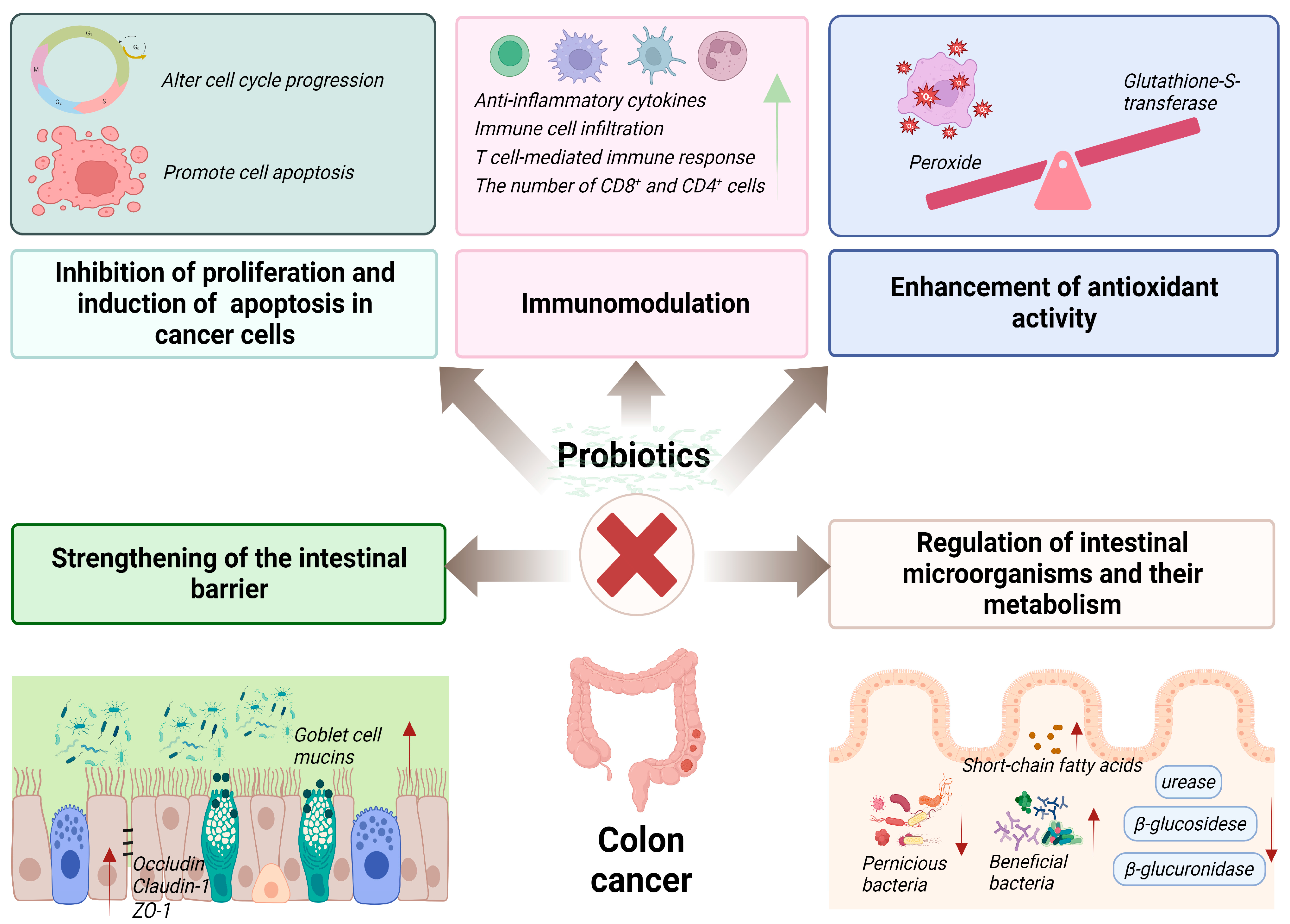
4. Curative Effects and Mechanisms of Action of Probiotics on Colon Cancer
4.1. Inhibition of Proliferation and Induction of Apoptosis in Cancer Cells
4.2. Immunomodulation
4.3. Regulation of Intestinal Microorganisms and Their Metabolism
4.4. Strengthening of the Intestinal Barrier
4.5. Enhancement of Antioxidant Activity
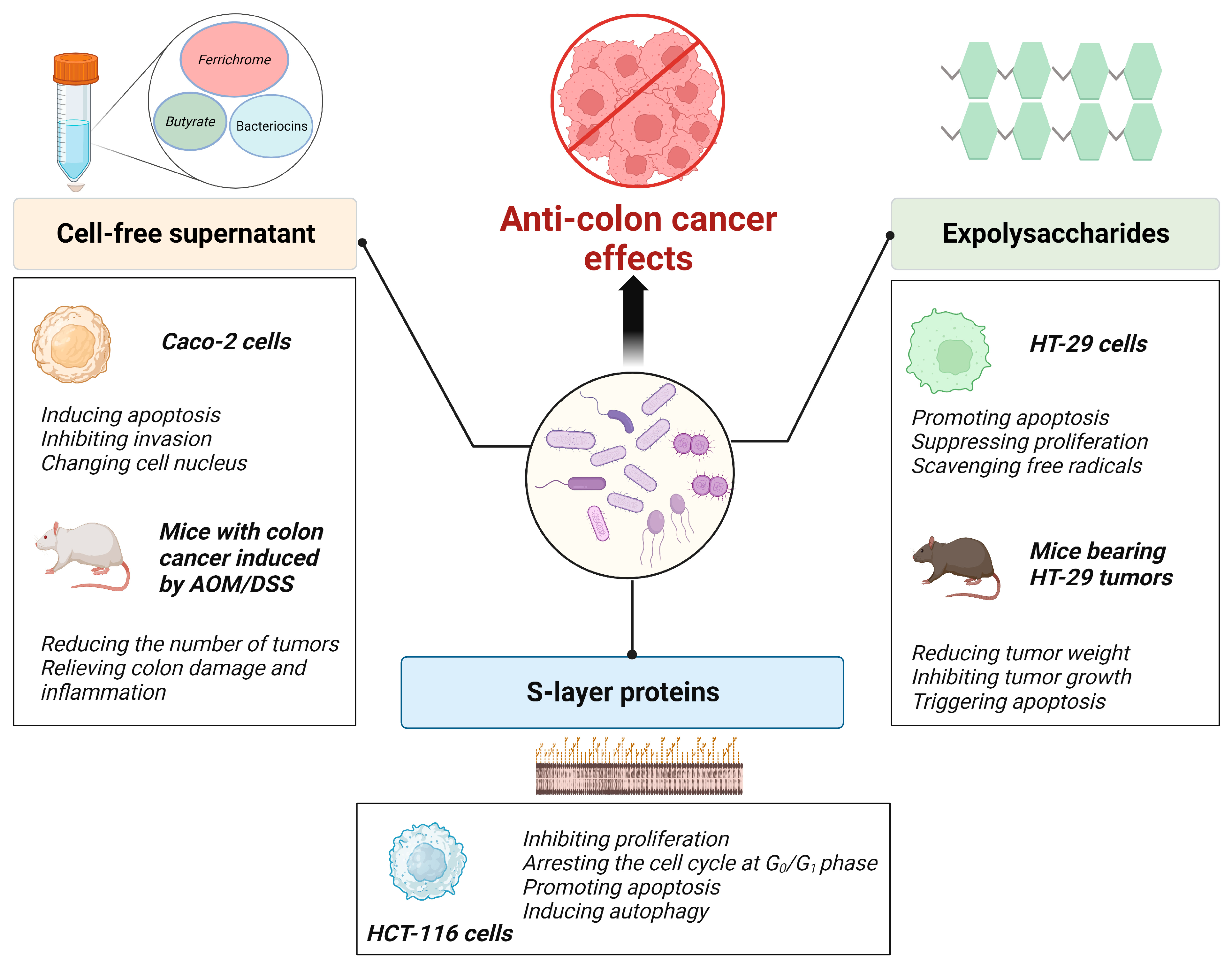
5. Curative Effects of Probiotic Components and Metabolites on Colon Cancer
5.1. Probiotic Metabolites
5.2. Probiotic EPSs
5.3. Probiotic Slp
6. Conclusions
7. Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, R.; Tiwari, S. Diet and colon cancer: A comprehensive review. In Colon Cancer Diagnosis and Therapy; Springer: Cham, Switzerland, 2021; pp. 53–71. [Google Scholar]
- Wu, N.; Yang, X.; Zhang, R.; Li, J.; Xiao, X.; Hu, Y.; Chen, Y.; Yang, F.; Lu, N.; Wang, Z.; et al. Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb. Ecol. 2013, 66, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Drummond, J.; Quazi, S.H.; Karanki, S.S.; Shaw, J.; Chen, B.; Kumar, N. Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Crit. Rev. Oncol. Hematol. 2013, 86, 232–250. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P. Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Mantzourani, I.; Chondrou, P.; Bontsidis, C.; Karolidou, K.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Galanis, A.; Plessas, S. Assessment of the probiotic potential of lactic acid bacteria isolated from kefir grains: Evaluation of adhesion and antiproliferative properties in in vitro experimental systems. Ann. Microbiol. 2019, 69, 751–763. [Google Scholar] [CrossRef]
- Dubey, V.; Ghosh, A.R.; Bishayee, K.; Khuda-Bukhsh, A.R. Appraisal of the anti-cancer potential of probiotic Pediococcus pentosaceus GS4 against colon cancer: In vitro and in vivo approaches. J. Funct. Foods 2016, 23, 66–79. [Google Scholar] [CrossRef]
- Sharaf, L.K.; Sharma, M.; Chandel, D.; Shukla, G. Prophylactic intervention of probiotics (L. acidophilus, L. rhamnosus GG) and celecoxib modulate Bax-mediated apoptosis in 1,2-dimethylhydrazine-induced experimental colon carcinogenesis. BMC Cancer 2018, 18, 1111. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Chu, S.-H.; Jeon, J.Y.; Lee, M.-K.; Park, J.-H.; Lee, D.-C.; Lee, J.-W.; Kim, N.-K. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: A double-blind, randomized, placebo-controlled trial. Dig. Liver Dis. 2014, 46, 1126–1132. [Google Scholar] [CrossRef]
- Nouri, Z.; Karami, F.; Neyazi, N.; Modarressi, M.H.; Karimi, R.; Khorramizadeh, M.R.; Taheri, B.; Motevaseli, E. Dual anti-metastatic and anti-proliferative activity assessment of two probiotics on HeLa and HT-29 cell lines. Cell J. 2016, 18, 127. [Google Scholar] [PubMed]
- Al-Nabulsi, A.A.; Jaradat, Z.W.; Al Qudsi, F.R.; Elsalem, L.; Osaili, T.M.; Olaimat, A.N.; Esposito, G.; Liu, S.-Q.; Ayyash, M.M. Characterization and bioactive properties of exopolysaccharides produced by Streptococcus thermophilus and Lactobacillus bulgaricus isolated from labaneh. LWT 2022, 167, 113817. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, D.; Yang, Y.; Jiang, X.; Zhang, J.; Zeng, X.; Wu, Z.; Sun, Y.; Guo, Y. Effect of Lactobacillus acidophilus CICC 6074 S-layer protein on colon cancer HT-29 cell proliferation and apoptosis. J. Agric. Food Chem. 2020, 68, 2639–2647. [Google Scholar] [CrossRef] [PubMed]
- Um, C.Y.; Prizment, A.; Hong, C.-P.; Lazovich, D.; Bostick, R.M. Associations of calcium, vitamin D, and dairy product intakes with colorectal cancer risk among older women: The Iowa women’s health study. Nutr. Cancer 2019, 71, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. The epidemiology of vitamin D and cancer incidence and mortality: A review (United States). Cancer Cause Control 2005, 16, 83–95. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, M.; Hong, J.; Ng, D.M.; Yang, T.; Xu, L.; Ye, X. The effect of vitamin D on the occurrence and development of colorectal cancer: A systematic review and meta-analysis. Int. J. Color. Dis. 2021, 36, 1329–1344. [Google Scholar] [CrossRef]
- Hullings, A.G.; Sinha, R.; Liao, L.M.; Freedman, N.D.; Graubard, B.I.; Loftfield, E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP Diet and Health Study cohort. Am. J. Clin. Nutr. 2020, 112, 603–612. [Google Scholar] [CrossRef]
- Di, Y.; Ding, L.; Gao, L.; Huang, H. Association of meat consumption with the risk of gastrointestinal cancers: A systematic review and meta-analysis. BMC Cancer 2023, 23, 782. [Google Scholar] [CrossRef]
- Santarelli, R.L.; Pierre, F.; Corpet, D.E. Processed meat and colorectal cancer: A review of epidemiologic and experimental evidence. Nutr. Cancer 2008, 60, 131–144. [Google Scholar] [CrossRef]
- Corpet, D.E. Red meat and colon cancer: Should we become vegetarians, or can we make meat safer? Meat Sci. 2011, 89, 310–316. [Google Scholar] [CrossRef]
- Tayyem, R.F.; Bawadi, H.A.; Shehadah, I.; Bani-Hani, K.E.; Takruri, H.; Al-Jaberi, T.; Heath, D.D. Fast foods, sweets and beverage consumption and risk of colorectal cancer: A case-control study in Jordan. Asian Pac. J. Cancer Prev. 2018, 19, 261. [Google Scholar] [PubMed]
- Castelló, A.; Rodríguez-Barranco, M.; Fernández de Larrea, N.; Jakszyn, P.; Dorronsoro, A.; Amiano, P.; Chirlaque, M.-D.; Colorado-Yohar, S.; Guevara, M.; Moreno-Iribas, C. Adherence to the Western, Prudent and Mediterranean Dietary Patterns and Colorectal Cancer Risk: Findings from the Spanish Cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Nutrients 2022, 14, 3085. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Roebothan, B.; Zhu, Y.; Woodrow, J.; Parfrey, P.S.; Mclaughlin, J.R.; Wang, P.P. Hypothesis and data-driven dietary patterns and colorectal Cancer survival: Findings from Newfoundland and Labrador colorectal Cancer cohort. Nutr. J. 2018, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Laure Preterre, A.; Iqbal, K.; Bechthold, A.; De Henauw, S.; Michels, N.; Devleesschauwer, B. Food groups and risk of colorectal cancer. Int. J. Cancer 2018, 142, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Veettil, S.K.; Wong, T.Y.; Loo, Y.S.; Playdon, M.C.; Lai, N.M.; Giovannucci, E.L.; Chaiyakunapruk, N. Role of diet in colorectal cancer incidence: Umbrella review of meta-analyses of prospective observational studies. JAMA Netw. Open 2021, 4, e2037341. [Google Scholar] [CrossRef] [PubMed]
- Safari, A.; Shariff, Z.M.; Kandiah, M.; Rashidkhani, B.; Fereidooni, F. Dietary patterns and risk of colorectal cancer in Tehran Province: A case–control study. BMC Public Health 2013, 13, 222. [Google Scholar] [CrossRef]
- Hussan, H.; Gray, D.M.; Hinton, A.; Krishna, S.G.; Conwell, D.L.; Stanich, P.P. Morbid obesity is associated with increased mortality, surgical complications, and incremental health care utilization in the peri-operative period of colorectal cancer surgery. World J. Surg. 2016, 40, 987–994. [Google Scholar] [CrossRef]
- Tarasiuk, A.; Mosińska, P.; Fichna, J. The mechanisms linking obesity to colon cancer: An overview. Obes. Res. Clin. Pract. 2018, 12, 251–259. [Google Scholar] [CrossRef]
- Divella, R.; De Luca, R.; Abbate, I.; Naglieri, E.; Daniele, A. Obesity and cancer: The role of adipose tissue and adipo-cytokines-induced chronic inflammation. J. Cancer 2016, 7, 2346. [Google Scholar] [CrossRef]
- Huang, X.F.; Chen, J.Z. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes. Rev. 2009, 10, 610–616. [Google Scholar] [CrossRef]
- Hvid, H.; Blouin, M.-J.; Birman, E.; Damgaard, J.; Poulsen, F.; Fels, J.J.; Fledelius, C.; Hansen, B.F.; Pollak, M. Treatment with insulin analog X10 and IGF-1 increases growth of colon cancer allografts. PLoS ONE 2013, 8, e79710. [Google Scholar] [CrossRef]
- Shi, B.; Sepp-Lorenzino, L.; Prisco, M.; Linsley, P.; DeAngelis, T.; Baserga, R. Micro RNA 145 targets the insulin receptor substrate-1 and inhibits the growth of colon cancer cells. J. Biol. Chem. 2007, 282, 32582–32590. [Google Scholar] [CrossRef] [PubMed]
- Mullen, M.; Gonzalez-Perez, R.R. Leptin-induced JAK/STAT signaling and cancer growth. Vaccines 2016, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, T.; Schwartz, B. Leptin promotes motility and invasiveness in human colon cancer cells by activating multiple signal-transduction pathways. Int. J. Cancer 2008, 123, 2543–2556. [Google Scholar] [CrossRef]
- Fujisawa, T.; Endo, H.; Tomimoto, A.; Sugiyama, M.; Takahashi, H.; Saito, S.; Inamori, M.; Nakajima, N.; Watanabe, M.; Kubota, N. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut 2008, 57, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Sears, C.L.; Pardoll, D.M. Perspective: Alpha-bugs, their microbial partners, and the link to colon cancer. J. Infect. Dis. 2011, 203, 306–311. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Dhar, R.; Pethusamy, K.; Seethy, A.; Srivastava, T.; Sah, R.; Sharma, J.; Karmakar, S. Exploring the role of gut microbiome in colon cancer. Appl. Biochem. Biotechnol. 2021, 193, 1780–1799. [Google Scholar] [CrossRef]
- Sears, C.L.; Geis, A.L.; Housseau, F. Bacteroides fragilis subverts mucosal biology: From symbiont to colon carcinogenesis. J. Clin. Investig. 2014, 124, 4166–4172. [Google Scholar] [CrossRef]
- Tjalsma, H.; Boleij, A.; Marchesi, J.R.; Dutilh, B.E. A bacterial driver–passenger model for colorectal cancer: Beyond the usual suspects. Nat. Rev. Microbiol. 2012, 10, 575–582. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—Symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef]
- Dziubańska-Kusibab, P.J.; Berger, H.; Battistini, F.; Bouwman, B.A.; Iftekhar, A.; Katainen, R.; Cajuso, T.; Crosetto, N.; Orozco, M.; Aaltonen, L.A. Colibactin DNA-damage signature indicates mutational impact in colorectal cancer. Nat. Med. 2020, 26, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Ye, J.; Zhao, B.; Sun, J.; Cao, P.; Yang, Y. The role of intestinal microbiota in colorectal cancer. Front. Pharmacol. 2021, 12, 674807. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Guo, B.; Gao, R.; Zhu, Q.; Qin, H. Microbiota disbiosis is associated with colorectal cancer. Front. Microbiol. 2015, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Boye, T.L.; Temby, M.; Sojwal, R.S.; Holman, D.R.; Sinha, S.R.; Rogalla, S.R.; Nielsen, O.H. Gut microbiome in inflammatory bowel disease: Role in pathogenesis, dietary modulation, and colitis-associated colon cancer. Microorganisms 2022, 10, 1371. [Google Scholar] [CrossRef]
- Attene-Ramos, M.S.; Wagner, E.D.; Plewa, M.J.; Gaskins, H.R. Evidence that hydrogen sulfide is a genotoxic agent. Mol. Cancer Res. 2006, 4, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Bultman, S.J. Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer. Mol. Nutr. Food Res. 2017, 61, 1500902. [Google Scholar] [CrossRef]
- Ocvirk, S.; O’Keefe, S.J. Influence of bile acids on colorectal cancer risk: Potential mechanisms mediated by diet-gut microbiota interactions. Curr. Nutr. Rep. 2017, 6, 315–322. [Google Scholar] [CrossRef]
- Zou, S.; Fang, L.; Lee, M.-H. Dysbiosis of gut microbiota in promoting the development of colorectal cancer. Gastroenterol. Rep. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Pino, M.S.; Chung, D.C. The chromosomal instability pathway in colon cancer. Gastroenterology 2010, 138, 2059–2072. [Google Scholar] [CrossRef]
- Hope, M.E.; Hold, G.L.; Kain, R.; El-Omar, E.M. Sporadic colorectal cancer–role of the commensal microbiota. FEMS Microbiol. Lett. 2005, 244, 1–7. [Google Scholar] [CrossRef][Green Version]
- Fearon, E.R. Molecular genetics of colorectal cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 479–507. [Google Scholar] [CrossRef] [PubMed]
- Sansom, O.J.; Reed, K.R.; Hayes, A.J.; Ireland, H.; Brinkmann, H.; Newton, I.P.; Batlle, E.; Simon-Assmann, P.; Clevers, H.; Nathke, I.S. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004, 18, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Huynh, K.; Heshu, S.R.; Yeap, S.K.; Hazilawati, H.; Roselina, K.J.B.C.; Medicine, A. Water extract of brewers’ rice induces apoptosis in human colorectal cancer cells via activation of caspase-3 and caspase-8 and downregulates the Wnt/β-catenin downstream signaling pathway in brewers’ rice-treated rats with azoxymethane-induced colon carcinogenesis. BMC Complement. Altern. Med. 2015, 15, 205. [Google Scholar]
- Zhang, T.; Otevrel, T.; Gao, Z.; Gao, Z.; Ehrlich, S.M.; Fields, J.Z.; Boman, B.M. Evidence that APC regulates survivin expression: A possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 2001, 61, 8664–8667. [Google Scholar]
- Mastalier, B.; Tihon, C.; Ghita, B.; Botezatu, C.; Deaconescu, V.; Mandisodza, P.; Draghici, C.; Simion, S. Surgical treatment of colon cancer: Colentina surgical clinic experience. J. Med. Life 2012, 5, 348. [Google Scholar]
- Schippinger, W.; Samonigg, H.; Schaberl-Moser, R.; Greil, R.; Thödtmann, R.; Tschmelitsch, J.; Jagoditsch, M.; Steger, G.; Jakesz, R.; Herbst, F. A prospective randomised phase III trial of adjuvant chemotherapy with 5-fluorouracil and leucovorin in patients with stage II colon cancer. Br. J. Cancer 2007, 97, 1021–1027. [Google Scholar] [CrossRef]
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef]
- Cohen, R.; Taieb, J.; Fiskum, J.; Yothers, G.; Goldberg, R.; Yoshino, T.; Alberts, S.; Allegra, C.; De Gramont, A.; Seitz, J.-F. Microsatellite instability in patients with stage III colon cancer receiving fluoropyrimidine with or without oxaliplatin: An ACCENT pooled analysis of 12 adjuvant trials. J. Clin. Oncol. 2021, 39, 642. [Google Scholar] [CrossRef]
- Chen, W.; Dong, H.; Wang, G.; Chen, J.; Wang, W. Effect of the duration of the capecitabine regimen following colon cancer surgery in an elderly population: A retrospective cohort study. World J. Surg. Oncol. 2021, 19, 238. [Google Scholar] [CrossRef]
- Kalidindi, A.V.; Dubashi, B.; Jayanthi, M.; Shewade, D. Efficacy and safety of capecitabine and oxaliplatin (CAPOX) treatment in colorectal cancer: An observational study from a tertiary cancer center in South India. Indian J. Cancer 2022, 59, 73–79. [Google Scholar]
- Jiang, W.; Liu, Q.; Yang, D.; Yang, S.-b. The efficacy of irinotecan supplementation for colorectal cancer: A meta-analysis of randomized controlled studies. Medicine 2022, 101, e28090. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Bria, E.; Sperduti, I.; Hinke, A.; Hegewisch-Becker, S.; Aparicio, T.; Le Malicot, K.; Boige, V.; Koeberle, D.; Baertschi, D. Bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: A meta-analysis of individual patients’ data from 3 phase III studies. Cancer Treat. Rev. 2021, 97, 102202. [Google Scholar] [CrossRef] [PubMed]
- Uyl-de Groot, C.; Vermorken, J.; Hanna, M., Jr.; Verboom, P.; Groot, M.; Bonsel, G.; Meijer, C.; Pinedo, H. Immunotherapy with autologous tumor cell-BCG vaccine in patients with colon cancer: A prospective study of medical and economic benefits. Vaccine 2005, 23, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Riethmüller, G.; Holz, E.; Schlimok, G.; Schmiegel, W.; Raab, R.; Höffken, K.; Gruber, R.; Funke, I.; Pichlmaier, H.; Hirche, H. Monoclonal antibody therapy for resected Dukes’ C colorectal cancer: Seven-year outcome of a multicenter randomized trial. J. Clin. Oncol. 1998, 16, 1788–1794. [Google Scholar] [CrossRef]
- Wegner, R.E.; Abel, S.; Monga, D.; Raj, M.; Finley, G.; Nosik, S.; McCormick, J.; Kirichenko, A.V. Utilization of adjuvant radiotherapy for resected colon cancer and its effect on outcome. Ann. Surg. Oncol. 2020, 27, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Martenson, J.A., Jr.; Willett, C.G.; Sargent, D.J.; Mailliard, J.A.; Donohue, J.H.; Gunderson, L.L.; Thomas, C.R., Jr.; Fisher, B.; Benson, A.B., III; Myerson, R. Phase III study of adjuvant chemotherapy and radiation therapy compared with chemotherapy alone in the surgical adjuvant treatment of colon cancer: Results of intergroup protocol 0130. J. Clin. Oncol. 2004, 22, 3277–3283. [Google Scholar] [CrossRef]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Santarmaki, V.; Aindelis, G.; Tompoulidou, E.; Lamprianidou, E.E.; Saxami, G.; Ypsilantis, P.; Lampri, E.S.; Simopoulos, C. Lactobacillus casei exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of TRAIL in colon carcinoma cells. PLoS ONE 2016, 11, e0147960. [Google Scholar] [CrossRef]
- Xu, F.; Li, Q.; Wang, S.; Bai, J.; Dong, M.; Xiao, G.; Wang, J. Lactobacillus casei JY300-8 generated by 12C6+ beams mutagenesis inhibits tumor progression by modulating the gut microbiota in mice. J. Funct. Foods 2021, 87, 104779. [Google Scholar] [CrossRef]
- Budu, O.; Banciu, C.; Pinzaru, I.; Sarău, C.; Lighezan, D.; Șoica, C.; Dehelean, C.; Drăghici, G.; Dolghi, A.; Prodea, A. A combination of two probiotics, Lactobacillus sporogenes and Clostridium butyricum, inhibits Colon Cancer Development: An In Vitro Study. Microorganisms 2022, 10, 1692. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, S.; Shi, J.; Xie, Q.; Li, N.; Guan, J.; Evivie, S.E.; Liu, F.; Li, B.; Huo, G. Effects of Lactobacillus acidophilus KLDS1. 0901 on proliferation and apoptosis of colon cancer cells. Front. Microbiol. 2022, 12, 788040. [Google Scholar] [CrossRef]
- Karimi Ardestani, S.; Tafvizi, F.; Tajabadi Ebrahimi, M. Heat-killed probiotic bacteria induce apoptosis of HT-29 human colon adenocarcinoma cell line via the regulation of Bax/Bcl2 and caspases pathway. Hum. Exp. Toxicol. 2019, 38, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Shamekhi, S.; Abdolalizadeh, J.; Ostadrahimi, A.; Mohammadi, S.A.; Barzegari, A.; Lotfi, H.; Bonabi, E.; Zarghami, N. Apoptotic Effect of Saccharomyces cerevisiae on human colon cancer SW480 cells by regulation of Akt/NF-ĸB signaling pathway. Probiotics Antimicro. 2020, 12, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shan, Y.-J.; He, C.-X.; Ren, M.-H.; Tian, P.-J.; Song, W. Effects of L. paracasei subp. paracasei X12 on cell cycle of colon cancer HT-29 cells and regulation of mTOR signalling pathway. J. Funct. Foods 2016, 21, 431–439. [Google Scholar] [CrossRef]
- Saxami, G.; Karapetsas, A.; Lamprianidou, E.; Kotsianidis, I.; Chlichlia, A.; Tassou, C.; Zoumpourlis, V.; Galanis, A. Two potential probiotic lactobacillus strains isolated from olive microbiota exhibit adhesion and anti-proliferative effects in cancer cell lines. J. Funct. Foods 2016, 24, 461–471. [Google Scholar] [CrossRef]
- Gamallat, Y.; Ren, X.; Walana, W.; Meyiah, A.; Xinxiu, R.; Zhu, Y.; Li, M.; Song, S.; Xie, L.; Jamalat, Y. Probiotic Lactobacillus rhamnosus modulates the gut microbiome composition attenuates preneoplastic colorectal Aberrant crypt foci. J. Funct. Foods 2019, 53, 146–156. [Google Scholar] [CrossRef]
- Liu, M.; Xie, W.; Wan, X.; Deng, T. Clostridium butyricum modulates gut microbiota and reduces colitis associated colon cancer in mice. Int. Immunopharmacol. 2020, 88, 106862. [Google Scholar] [CrossRef]
- Aindelis, G.; Tiptiri-Kourpeti, A.; Lampri, E.; Spyridopoulou, K.; Lamprianidou, E.; Kotsianidis, I.; Ypsilantis, P.; Pappa, A.; Chlichlia, K. Immune responses raised in an experimental colon carcinoma model following oral administration of Lactobacillus casei. Cancers 2020, 12, 368. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Wang, P.; Liu, Y.; Wang, G.; Shan, Y.; Yi, Y.; Zhou, Y.; Liu, B.; Wang, X. Lactobacillus coryniformis MXJ32 administration ameliorates azoxymethane/dextran sulfate sodium-induced colitis-associated colorectal cancer via reshaping intestinal microenvironment and alleviating inflammatory response. Eur. J. Nutr. 2022, 61, 85–99. [Google Scholar] [CrossRef]
- Walia, S.; Kamal, R.; Kanwar, S.S.; Dhawan, D.K. Cyclooxygenase as a target in chemoprevention by probiotics during 1,2-dimethylhydrazine induced colon carcinogenesis in rats. Nutr. Cancer 2015, 67, 603–611. [Google Scholar] [CrossRef]
- Saxami, G.; Karapetsas, A.; Chondrou, P.; Vasiliadis, S.; Lamprianidou, E.; Kotsianidis, I.; Ypsilantis, P.; Botaitis, S.; Simopoulos, C.; Galanis, A. Potentially probiotic Lactobacillus strains with anti-proliferative activity induce cytokine/chemokine production and neutrophil recruitment in mice. Benef. Microbes 2017, 8, 615–623. [Google Scholar] [CrossRef]
- Agah, S.; Alizadeh, A.M.; Mosavi, M.; Ranji, P.; Khavari-Daneshvar, H.; Ghasemian, F.; Bahmani, S.; Tavassoli, A. More protection of Lactobacillus acidophilus than Bifidobacterium bifidum probiotics on azoxymethane-induced mouse colon cancer. Probiotics Antimicro. 2019, 11, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Jiang, X.; Wang, H.; Chen, S.; Wang, X.; Liu, Y.; Guo, S.; Li, D.; Yu, W.; Zhao, Z. The inhibitory effects of probiotics on colon cancer cells: In vitro and in vivo studies. J. Gastrointest. Oncol. 2020, 11, 1224. [Google Scholar] [CrossRef]
- Corthésy, B.; Gaskins, H.R.; Mercenier, A. Cross-talk between probiotic bacteria and the host immune system. J. Nutr. 2007, 137, 781S–790S. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Du, P.; Yang, B.-R.; Gao, J.; Fang, W.-J.; Ying, C.-M. Preoperative probiotics decrease postoperative infectious complications of colorectal cancer. Am. J. Med. Sci. 2012, 343, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Consoli, M.L.D.; da Silva, R.S.; Nicoli, J.R.; Bruña-Romero, O.; da Silva, R.G.; de Vasconcelos Generoso, S.; Correia, M.I.T. Randomized clinical trial: Impact of oral administration of Saccharomyces boulardii on gene expression of intestinal cytokines in patients undergoing colon resection. J. Parenter. Enteral Nutr. 2016, 40, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Delcenserie, V.; Martel, D.; Lamoureux, M.; Amiot, J.; Boutin, Y.; Roy, D. Immunomodulatory effects of probiotics in the intestinal tract. Curr. Issues Mol. Biol. 2008, 10, 37–54. [Google Scholar]
- Zhang, M.; Fan, X.; Fang, B.; Zhu, C.; Zhu, J.; Ren, F. Effects of Lactobacillus salivarius Ren on cancer prevention and intestinal microbiota in 1, 2-dimethylhydrazine-induced rat model. J. Microbiol. Immunol. Infect. 2015, 53, 398–405. [Google Scholar] [CrossRef]
- Park, I.J.; Lee, J.-H.; Kye, B.-H.; Oh, H.-K.; Cho, Y.B.; Kim, Y.-T.; Kim, J.Y.; Sung, N.Y.; Kang, S.-B.; Seo, J.-M. Effects of probiotics on the symptoms and surgical outcomes after anterior resection of colon cancer (POSTCARE): A randomized, double-blind, placebo-controlled trial. J. Clin. Med. 2020, 9, 2181. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, K.; Wu, W.; Lv, L.; Bian, X.; Yang, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D. Administration of Bifidobacterium bifidum CGMCC 15068 modulates gut microbiota and metabolome in azoxymethane (AOM)/dextran sulphate sodium (DSS)-induced colitis-associated colon cancer (CAC) in mice. Appl. Microbiol. Biotechnol 2020, 104, 5915–5928. [Google Scholar] [CrossRef]
- Ohara, T.; Yoshino, K.; Kitajima, M. Possibility of preventing colorectal carcinogenesis with probiotics. Hepatogastroenterology 2010, 57, 1411–1415. [Google Scholar]
- Zhu, Q.; Gao, R.; Wu, W.; Qin, H. The role of gut microbiota in the pathogenesis of colorectal cancer. Tumor Biol. 2013, 34, 1285–1300. [Google Scholar] [CrossRef]
- Burne, R.A.; Chen, Y.-Y.M. Bacterial ureases in infectious diseases. Microb. Infect. 2000, 2, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Hatakka, K.; Holma, R.; El-Nezami, H.; Suomalainen, T.; Kuisma, M.; Saxelin, M.; Poussa, T.; Mykkänen, H.; Korpela, R. The influence of Lactobacillus rhamnosus LC705 together with Propionibacterium freudenreichii ssp. shermanii JS on potentially carcinogenic bacterial activity in human colon. Int. J. Food Microbiol. 2008, 128, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Desrouillères, K.; Millette, M.; Vu, K.D.; Touja, R.; Lacroix, M. Cancer preventive effects of a specific probiotic fermented milk containing Lactobacillus acidophilus CL1285, L. casei LBC80R and L. rhamnosus CLR2 on male F344 rats treated with 1,2-dimethylhydrazine. J. Funct. Foods 2015, 17, 816–827. [Google Scholar] [CrossRef]
- Ohland, C.L.; MacNaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef]
- Wang, T.; Wang, P.; Yin, L.; Wang, X.; Shan, Y.; Yi, Y.; Zhou, Y.; Liu, B.; Wang, X.; Lü, X. Dietary Lactiplantibacillus plantarum KX041 attenuates colitis-associated tumorigenesis and modulates gut microbiota. Food Sci. Hum. Wellness 2023, 12, 1626–1636. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, H.; Yang, Z.; Xia, Y.; Liu, W.; Yang, J.; Jiang, Y.; Zhang, H.; Yang, Z.; Wang, Y. Randomised clinical trial: The effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery–a double-blind study. Aliment. Pharmacol. Ther. 2011, 33, 50–63. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, J.; Dong, S.; Ismael, M.; Shan, Y.; Wang, X.; Lü, X. Lacticaseibacillus rhamnosus LS8 Ameliorates Azoxymethane/Dextran Sulfate Sodium-Induced Colitis-Associated Tumorigenesis in Mice via Regulating Gut Microbiota and Inhibiting Inflammation. Probiotics Antimicro. 2022, 14, 947–959. [Google Scholar] [CrossRef]
- Boleij, A.; Tjalsma, H. Gut bacteria in health and disease: A survey on the interface between intestinal microbiology and colorectal cancer. Biol. Rev. 2012, 87, 701–730. [Google Scholar] [CrossRef]
- Kumar, R.S.; Kanmani, P.; Yuvaraj, N.; Paari, K.; Pattukumar, V.; Thirunavukkarasu, C.; Arul, V. Lactobacillus plantarum AS1 isolated from south Indian fermented food Kallappam suppress 1,2-dimethyl hydrazine (DMH)-induced colorectal cancer in male Wistar rats. Appl. Biochem. Biotechnol. 2012, 166, 620–631. [Google Scholar] [CrossRef]
- Bahmani, S.; Azarpira, N.; Moazamian, E. Anti-colon cancer activity of Bifidobacterium metabolites on colon cancer cell line SW742. Turk. J. Gastroenterol. 2019, 30, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.; Alipour, B.; Faghfoori, Z.; Khosroushahi, A.Y. Secretion metabolites of dairy Kluyveromyces marxianus AS41 isolated as probiotic, induces apoptosis in different human cancer cell lines and exhibit anti-pathogenic effects. J. Funct. Foods 2017, 34, 408–421. [Google Scholar] [CrossRef]
- Saber, A.; Alipour, B.; Faghfoori, Z.; Khosroushahi, A.Y. Secretion metabolites of probiotic yeast, Pichia kudriavzevii AS-12, induces apoptosis pathways in human colorectal cancer cell lines. Nutr. Res. 2017, 41, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Xin, Y.; Zhang, C.; Wu, D.; Ding, D.; Tang, L.; Owusu, L.; Bai, J.; Li, W. Fermentation supernatants of Lactobacillus delbrueckii inhibit growth of human colon cancer cells and induce apoptosis through a caspase 3-dependent pathway. Oncol. Lett. 2014, 7, 1738–1742. [Google Scholar] [CrossRef] [PubMed]
- Maghsood, F.; Johari, B.; Rohani, M.; Madanchi, H.; Saltanatpour, Z.; Kadivar, M. Anti-proliferative and anti-metastatic potential of high molecular weight secretory molecules from probiotic Lactobacillus reuteri cell-free supernatant against human colon cancer stem-like cells (HT29-ShE). Int. J. Pept. Res. Ther. 2020, 26, 2619–2631. [Google Scholar] [CrossRef]
- Pakbin, B.; Allahyari, S.; Dibazar, S.P.; Peymani, A.; Haghverdi, M.K.; Taherkhani, K.; Javadi, M.; Mahmoudi, R. Anticancer Properties of Saccharomyces boulardii Metabolite Against Colon Cancer Cells. Probiotics Antimicro. Proteins 2022. online ahead of print. [Google Scholar] [CrossRef]
- Norouzi, Z.; Salimi, A.; Halabian, R.; Fahimi, H. Nisin, a potent bacteriocin and anti-bacterial peptide, attenuates expression of metastatic genes in colorectal cancer cell lines. Microb. Pathog. 2018, 123, 183–189. [Google Scholar] [CrossRef]
- Villarante, K.I.; Elegado, F.B.; Iwatani, S.; Zendo, T.; Sonomoto, K.; de Guzman, E.E. Purification, characterization and in vitro cytotoxicity of the bacteriocin from Pediococcus acidilactici K2a2-3 against human colon adenocarcinoma (HT29) and human cervical carcinoma (HeLa) cells. World J. Microbiol. Biotechnol. 2011, 27, 975–980. [Google Scholar] [CrossRef]
- De Giani, A.; Bovio, F.; Forcella, M.; Fusi, P.; Sello, G.; Di Gennaro, P. Identification of a bacteriocin-like compound from Lactobacillus plantarum with antimicrobial activity and effects on normal and cancerogenic human intestinal cells. AMB Express 2019, 9, 88. [Google Scholar] [CrossRef]
- Karpiński, T. New Peptide (Entap) with Anti-Proliferative Activity Produced by Bacteria of Enterococcus Genus; Poznań University of Medical Sciences Poznań: Poznań, Poland, 2012. [Google Scholar]
- Konishi, H.; Fujiya, M.; Tanaka, H.; Ueno, N.; Moriichi, K.; Sasajima, J.; Ikuta, K.; Akutsu, H.; Tanabe, H.; Kohgo, Y. Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Nat. Commun. 2016, 7, 12365. [Google Scholar] [CrossRef]
- Kang, J.; Sun, M.; Chang, Y.; Chen, H.; Zhang, J.; Liang, X.; Xiao, T. Butyrate ameliorates colorectal cancer through regulating intestinal microecological disorders. Anti-Cancer Drugs 2023, 34, 227. [Google Scholar] [CrossRef] [PubMed]
- Botta, C.; Spyridopoulou, K.; Bertolino, M.; Rantsiou, K.; Chlichlia, K.; Cocolin, L. Lactiplantibacillus plantarum inhibits colon cancer cell proliferation as function of its butyrogenic capability. Biomed. Pharmacother. 2022, 149, 112755. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, F.; Li, L.; Huang, L.; Li, Q. Genetic and biochemical characterization of an exopolysaccharide with in vitro antitumoral activity produced by Lactobacillus fermentum YL-11. Front. Microbiol. 2019, 10, 2898. [Google Scholar] [CrossRef]
- Kumar, R.; Bansal, P.; Singh, J.; Dhanda, S. Purification, partial structural characterization and health benefits of exopolysaccharides from potential probiotic Pediococcus acidilactici NCDC 252. Process Biochem. 2020, 99, 79–86. [Google Scholar] [CrossRef]
- Mojibi, P.; Tafvizi, F.; Torbati, M.B. Cell-bound exopolysaccharide extract from indigenous probiotic bacteria induce apoptosis in HT–29 cell-line. Iran. J. Pathol. 2019, 14, 41. [Google Scholar] [CrossRef]
- Saadat, Y.R.; Gargari, B.P.; Shahabi, A.; Nami, Y.; Khosroushahi, A.Y. Prophylactic role of Lactobacillus paracasei exopolysaccharides on colon cancer cells through apoptosis not ferroptosis. Unpublished work. 2020. [Google Scholar] [CrossRef]
- Li, W.; Tang, W.; Ji, J.; Xia, X.; Rui, X.; Chen, X.; Jiang, M.; Zhou, J.; Dong, M. Characterization of a novel polysaccharide with anti-colon cancer activity from Lactobacillus helveticus MB2-1. Carbohydr. Res. 2015, 411, 6–14. [Google Scholar] [CrossRef]
- Li, F.; Jiao, X.; Zhao, J.; Liao, X.; Wei, Y.; Li, Q. Antitumor mechanisms of an exopolysaccharide from Lactobacillus fermentum on HT-29 cells and HT-29 tumor-bearing mice. Int. J. Biol. Macromol. 2022, 209, 552–562. [Google Scholar] [CrossRef]
- Ma, F.; Song, Y.; Sun, M.; Wang, A.; Jiang, S.; Mu, G.; Tuo, Y. Exopolysaccharide produced by Lactiplantibacillus plantarum-12 alleviates intestinal inflammation and colon cancer symptoms by modulating the gut microbiome and metabolites of C57BL/6 mice treated by azoxymethane/dextran sulfate sodium salt. Foods 2021, 10, 3060. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, X.; Zhang, L.; Xu, S.; Zhang, Q.; Lu, R. A surface-layer protein from Lactobacillus acidophilus NCFM induces autophagic death in HCT116 cells requiring ROS-mediated modulation of mTOR and JNK signaling pathways. Food Funct. 2019, 10, 4102–4112. [Google Scholar] [CrossRef]
- Gao, Z.; Guo, B.; Gao, R.; Zhu, Q.; Wu, W.; Qin, H. Probiotics modify human intestinal mucosa-associated microbiota in patients with colorectal cancer. Mol. Med. Rep. 2015, 12, 6119–6127. [Google Scholar] [CrossRef]
| Cells | Probiotics, Their Components, and Metabolites | Food Sources of Probiotics | Treatments | Results | Ref. |
|---|---|---|---|---|---|
| Caco-2 cells | Conditioned media for Lactiplantibacillus pentosus B281 and Lactiplantibacillus plantarum B282 | Naturally fermented olives | Cells were treated with different concentrations of probiotic-conditioned media for 24, 48, and 72 h at 37 °C. | Inhibition of cell proliferation; decreased colony formation; cell cycle arrest in the G1 phase; decreased expression of cyclin A, cyclin B1, cyclin B2, and cyclin E, and increased expression of cyclin D. | [75] |
| CT26 and HT-29 cells | Lacticaseibacillus casei ATCC393 | NA | Cells were treated at a concentration of 108 CFU/mL for 24, 48, and 72 h at 37 °C. | Inhibition of cell proliferation; promotion of apoptosis; increased expression of the apoptosis-inducing ligand TRAIL and decreased expression of cyclin D1. | [68] |
| HT-29 cells | Heat-inactivated Levilactobacillus brevis and Lacticaseibacillus paracasei | Iranian food “Terxine” | Cells were treated with different concentrations of probiotics for 24, 48, and 72 h at 37 °C. | Inhibition of cell proliferation; activation of mitochondrial pathway to promote apoptosis. | [72] |
| SW480 cells | Heat-inactivated Saccharomyces cerevisiae | NA | Cells were treated with different concentrations of probiotics for 24 and 48 h at 37 °C. | Inhibition of cell proliferation; activation of Akt/NF-κB signaling pathway to promote apoptosis. | [73] |
| HT-29 and HCT-116 cells | Lactobacillus sporogenes and Clostridium butyricum TO-A | NA | Cells were treated at 107 CFU/mL for 24, 48, and 72 h at 37 °C. | Inhibition of cell proliferation; promotion of apoptosis; increased expression of Bax, Bid, Bad, and Bak and decreased expression of Bcl-2 and Bcl-XL. | [70] |
| Caco-2 cells | Saccharomyces cerevisiae var. boulardii CNCM I-745 metabolites | NA | Cells were treated with different concentrations of metabolites for 24 and 48 h at 37 °C. | Inhibition of cell proliferation; promotion of apoptosis; inhibition of IL-8 and NF-κB gene expression. | [107] |
| HT-29 and Caco-2 cells | Lactobacillus acidophilus KLDS1.0901 | NA | Cells were treated with different concentrations of probiotics for 12, 24, and 48 h at 37 °C. | Inhibition of cell proliferation; promotion of apoptosis; decreased cellular mitochondrial membrane potential; increased ROS levels. | [71] |
| CT26 cells | Bifidobacterium longum, Bifidobacterium bifidum, Lactobacillus acidophilus, and Lactiplantibacillus plantarum | NA | Cells were treated at 108 CFU/mL for 24, 48, 72, and 96 h at 37 °C. | Inhibition of cell proliferation, migration, and invasion. | [83] |
| Caco-2, HT-29 and HCT-116 cells | Lacticaseibacillus casei JY300-8 | Fresh corn stalks | Cells were treated with different concentrations of probiotics for 24, 48, 72, and 96 h at 37 °C. | Inhibition of cell proliferation; promotion of apoptosis. | [69] |
| SW48, HT-29, LS180 and Caco-2 cells | Nisin | NA | Cells were treated with different concentrations of nisin for 24 and 48 h at 37 °C. | Inhibition of cell proliferation; inhibition of cancer cell metastasis genes. | [108] |
| HT-29 and Caco-2 cells | Pichia kudriavzevii AS-12 cell-free supernatant | NA | Cells were treated with different concentrations of supernatants for 24 and 48 h at 37 °C. | Inhibition of cell proliferation; promotion of apoptosis; increased expression of Bad, caspase-3, caspase-8, caspase-9, and Fas-R and decreased expression of Bcl-2. | [104] |
| HT29-ShE cells | Heat inactivation of sonicated Limosilactobacillus reuteri and cell-free supernatants | NA | The 10% (v/v) heat-inactivated sonicated fraction and 5% supernatant treated cells for 24 h. | Inhibition of cell proliferation; promotion of apoptosis; inhibition of cancer cell metastasis; downregulation of MMP-9 and COX-2 expression. | [106] |
| Caco-2 and SW620 cells | Ferrochrome in the cell-free supernatant of Lacticaseibacillus casei ATCC334 | NA | Treatment of cells with different concentrations of ferrochrome. | Inhibition of cell proliferation; induction of apoptosis through the activation of the JNK-DDTI3 pathway. | [112] |
| HT-29 and HCT-116 cells | EPSs of Streptococcus pyogenes and Lactobacillus delbrueckii subsp. bulgaricus | Labaneh (yogurt-like product) | Cells were treated with different concentrations of EPSs for 24, 48, and 72 h at 37 °C. | Inhibition of cell proliferation; facilitation of scavenging of the free-radicals DPPH and ABTS. | [13] |
| SW-480, HT-29 and HCT-116 cells | EPSs of Lacticaseibacillus paracasei | Dairy products | Cells were treated with different concentrations of EPSs for 24 and 48 h at 37 °C. | Inhibition of cell proliferation; promotion of apoptosis; increased expression of Bax, caspase-3, and caspase-8 and inhibition of AKT-1, JAK-1, and mTOR gene expression. | [118] |
| HT-29 cells | Slp of Lactobacillus acidophilus CICC 6074 | NA | Cells were treated with different concentrations of Slp for 24 h at 37 °C. | Inhibition of cell proliferation; cell cycle block; promotion of apoptosis via death receptor pathway and mitochondrial pathway; inhibition of cell invasion. | [14] |
| HCT-116 cells | Slp of Lactobacillus acidophilus NCFM | NA | Cells were treated with different concentrations of Slp for 24 h at 37 °C | Inhibition of cell proliferation; cell cycle block; induction of autophagic death through inhibition of mTOR activity; activation of the JNK signaling pathway. | [122] |
| Animal Model | Probiotics, Their Components, and Metabolites | Food Sources of Probiotics | Treatments | Results | Ref. |
|---|---|---|---|---|---|
| A model of colon cancer induced by subcutaneous injection of CT26 cells; female BALB/c mice | Lacticaseibacillus casei ATCC393 | NA | Probiotics (150 μL) at a dose of 109 CFU/L were administered daily 10 days before subcutaneous injection of CT26 cells, for 13 days. | Tumor volume reduction; upregulation of TRAIL protein expression and downregulation of survivor protein expression in the tumor tissues. | [68] |
| DMH-induced colon cancer model in rats | Lactiplantibacillus plantarum and Lacticaseibacillus rhamnosus GG | Traditional fermented food of Himachal Pradesh | Probiotics were administered by gavage at a dose of 2 × 1010 CFU/day, once a week for 16 weeks, for 6 consecutive days. | Reduced tumor incidence, tumor diversity, and the mean tumor size; reduced total sialic acid and COX-2 expression. | [80] |
| DMH-induced colon cancer model; male Sprague–Dawley rats | Lacticaseibacillus rhamnosus GG and Lactobacillus acidophilus | NA | Probiotics were administered by gavage at a dose of 1 × 1010 CFU/day, one week prior to DMH administration for 18 weeks. | Decreased tumor load, tumor diversity; downregulation of Bcl-2 and K-ras gene expression, and upregulation of Bax and p53 gene expression. | [10] |
| Subcutaneous injection of CT26 cells induced colon cancer model; female BALB/c mice | Lacticaseibacillus casei | NA | Probiotics 150 μL at a dose of 109 CFU/L were administered daily 10 days before subcutaneous injection of CT26 cells, for 13 days. | Decreased tumor volume; increased levels of IFN-γ, IL-12, and IL-10; increased tumor-infiltrating lymphocytes; increased expression of cleaved caspase-3 and cleaved PARP1. | [78] |
| AOM/DSS-induced colon cancer model; male C57BL/6 mice | Loigolactobacillus coryniformis MXJ32 | Traditional fermented vegetable (Jiangshui Cai) | Probiotics were administered by gavage at a dose of 1 × 109 CFU/day, for 15 weeks. | Suppression of total tumor number and average tumor diameter; restoration of intestinal barrier function; downregulation of inflammatory cytokine expression; increased amount of salutary bacteria and decreased amount of pernicious bacteria. | [79] |
| Colon cancer model induced by injection of CT26 cells; female BALB/c mice | Bifidobacterium longum, Bifidobacterium bifidum, Lactobacillus acidophilus and Lactiplantibacillus plantarum | NA | Probiotic mixture (1 × 109 CFU/200 µL) administered daily via tube feeding, for 3 weeks. | Inhibition of tumor growth; increased apoptotic cells in tumor tissues; increased number of CD8+ cells. | [83] |
| AOM-induced colon cancer model; male BALB/c mice | Lactobacillus acidophilus and Bifidobacterium bifidum | Traditional yogurt | Probiotics (1 × 109 CFU/g body weight per strain) were mixed in drinking water and gavaged daily for 10 days before AOM, for 5 months. | Decreased incidence of colonic lesions and smaller tumor size; decreased levels of tumor markers; increased levels of IFN-γ and IL-10; increased numbers of CD8+ and CD4+ cells. | [82] |
| AOM-induced colon cancer model; male C57BL/6 mice | Bifidobacterium bifidum CGMCC 15068 | NA | Probiotics were administered by gavage at a dose of 3 × 108 CFU/day during the recovery period. | Reduced tumor incidence; increased abundance of beneficial probiotics in the gut; altered gut metabolites. | [90] |
| Subcutaneous injection of CT26 cells induced colon cancer model; BABL/C male mice | Lacticaseibacillus casei JY300-8 | Fresh corn stalks | Probiotics were administered by half-day gavage at a dose of 1 × 109 CFU/mL until the end of the experiment. | Reduced tumor size and tumor incidence; decreased abundance of Enterobacteriaceae and Clostridium perfringens; increased abundance of Lactobacillaceae and Bifidobacteriaceae. | [69] |
| AOM/DMH-induced colon cancer model; male C57BL/6 mice | Lactiplantibacillus plantarum KX041 | Traditional Chinese pickle juice | Oral administration of 200 μL probiotic suspension (1 × 109 CFU/day), for 13 weeks. | Decrease in the total number and mean diameter of colon tumors; attenuation of inflammatory infiltration and crypt damage; increase in short-chain fatty acid content of feces; increased the number of salutary bacteria and decreased the number of pernicious bacteria. | [97] |
| DMH-induced colon cancer model; F344 male rats | Lactobacillus acidophilus CL1285, Lacticaseibacillus casei LBC80R and Lacticaseibacillus rhamnosus CLR2 | NA | Gavage of 2, 1.5, 1, 0.5, and 0.25 mL of probiotic fermented milk daily, for 12 weeks. | Decreased counts of abnormal crypts in the colon; increased activity of glutathione-S-transferase and decreased activity of quinone reductase and β-glucuronide glycosidase. | [95] |
| Subcutaneous injection of CT26 cells induced colon cancer model; female BALB/c mice | EPSs of Limosilactobacillus fermentum YL-11 | Fermented milk | Intravenous EPSs at 90 mg/kg body weight every alternate day, for 3 weeks. | Decreased tumor weight; nuclear consolidation; increased intracellular space; and fatty degeneration of tumor cells. | [120] |
| AOM/DSS-induced colon cancer model; male C57BL/6 mice | EPSs of Lactiplantibacillus plantarum | NA | EPSs at 200 mg/kg body weight/day via gavage, for 12 weeks. | Restoration of colon length and reduction in the tumor number; enhancement of intestinal barrier function; inhibition of the NF-κB pathway and activation of the caspase cascade reaction to promote apoptosis; alteration of the intestinal microbiota and metabolites. | [121] |
| Subjects | Probiotics | Food Sources of Probiotics | Treatments | Results | Ref. |
|---|---|---|---|---|---|
| 22 patients undergoing radical colectomy | Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis | NA | Subjects received capsules containing live microorganisms 3 times/day (total daily dose of 6 × 107 CFU). | Increased abundance and diversity of colonic mucosal microorganisms; decreased abundance of Fusobacterium and Peptostreptococcus. | [123] |
| 60 patients who underwent resection of anterior sigmoid colon cancer | Bifidobacterium animalis subsp. lactis HY8002, Lacticaseibacillus casei HY2782, and Lactiplantibacillus plantarum HY7712 | NA | Subjects received probiotic powder treatment twice daily, for 4 weeks. | Improved control of flatulence; increased abundance of beneficial bacteria; and decreased abundance of harmful bacteria in the gut. | [89] |
| 10 colon cancer patients and 20 healthy subjects | Lactobacillus gasseri OLL2716: LG21 | NA | Subjects administered probiotics daily, for 12 weeks. | Increased levels of Lactobacillus and decreased total Clostridium perfringens in the intestines; increased short-chain fatty acid isobutyrate in the feces; decreased pH; and decreased synthesis of spoilage products; increased NK cell activity. | [91] |
| 38 men aged 24-55 | Lacticaseibacillus rhamnosus LC705 and Propionibacterium freudenreichii ssp. shermanii JS | NA | Subjects consumed 2 capsules containing live microorganisms (2 × 1010 CFU per strain) per day, for 4 weeks. | Increased levels of Lactobacillus and Propionibacterium in the intestinal tract; decreased β-glucosidase and urease activity. | [94] |
| 68 patients with treated colon cancer | Lacticaseibacillus rhamnosus R0011 and Lactobacillus acidophilus R0052 | NA | Subjects were treated with probiotics (2 × 109 CFU) daily, for 12 weeks. | Improved bowel symptoms and quality of life. | [11] |
| 33 patients who underwent colectomy | Saccharomyces boulardii | NA | Subjects received preoperative probiotic oral therapy for at least 7 days. | Downregulation of mucosal IL-1β, IL-10, and IL-23A levels | [86] |
| 60 patients who underwent radical colorectal resection | Bifid triple viable probiotics | NA | Subjects received oral probiotic treatment 3 days before surgery. | Reduced serum endotoxin, D-lactic acid, and IL-6 levels; elevated IgG and IgA levels; reduced incidence of postoperative infectious complications. | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Yang, J.; Zhang, Y.; Chen, X.; Wang, C.; Suo, H.; Song, J. An Update on the Pivotal Roles of Probiotics, Their Components, and Metabolites in Preventing Colon Cancer. Foods 2023, 12, 3706. https://doi.org/10.3390/foods12193706
Deng X, Yang J, Zhang Y, Chen X, Wang C, Suo H, Song J. An Update on the Pivotal Roles of Probiotics, Their Components, and Metabolites in Preventing Colon Cancer. Foods. 2023; 12(19):3706. https://doi.org/10.3390/foods12193706
Chicago/Turabian StyleDeng, Xue, Jing Yang, Yu Zhang, Xiaoyong Chen, Chen Wang, Huayi Suo, and Jiajia Song. 2023. "An Update on the Pivotal Roles of Probiotics, Their Components, and Metabolites in Preventing Colon Cancer" Foods 12, no. 19: 3706. https://doi.org/10.3390/foods12193706
APA StyleDeng, X., Yang, J., Zhang, Y., Chen, X., Wang, C., Suo, H., & Song, J. (2023). An Update on the Pivotal Roles of Probiotics, Their Components, and Metabolites in Preventing Colon Cancer. Foods, 12(19), 3706. https://doi.org/10.3390/foods12193706






