An Overview of the Occurrence of Bioactive Peptides in Different Types of Cheeses
Abstract
:1. Introduction
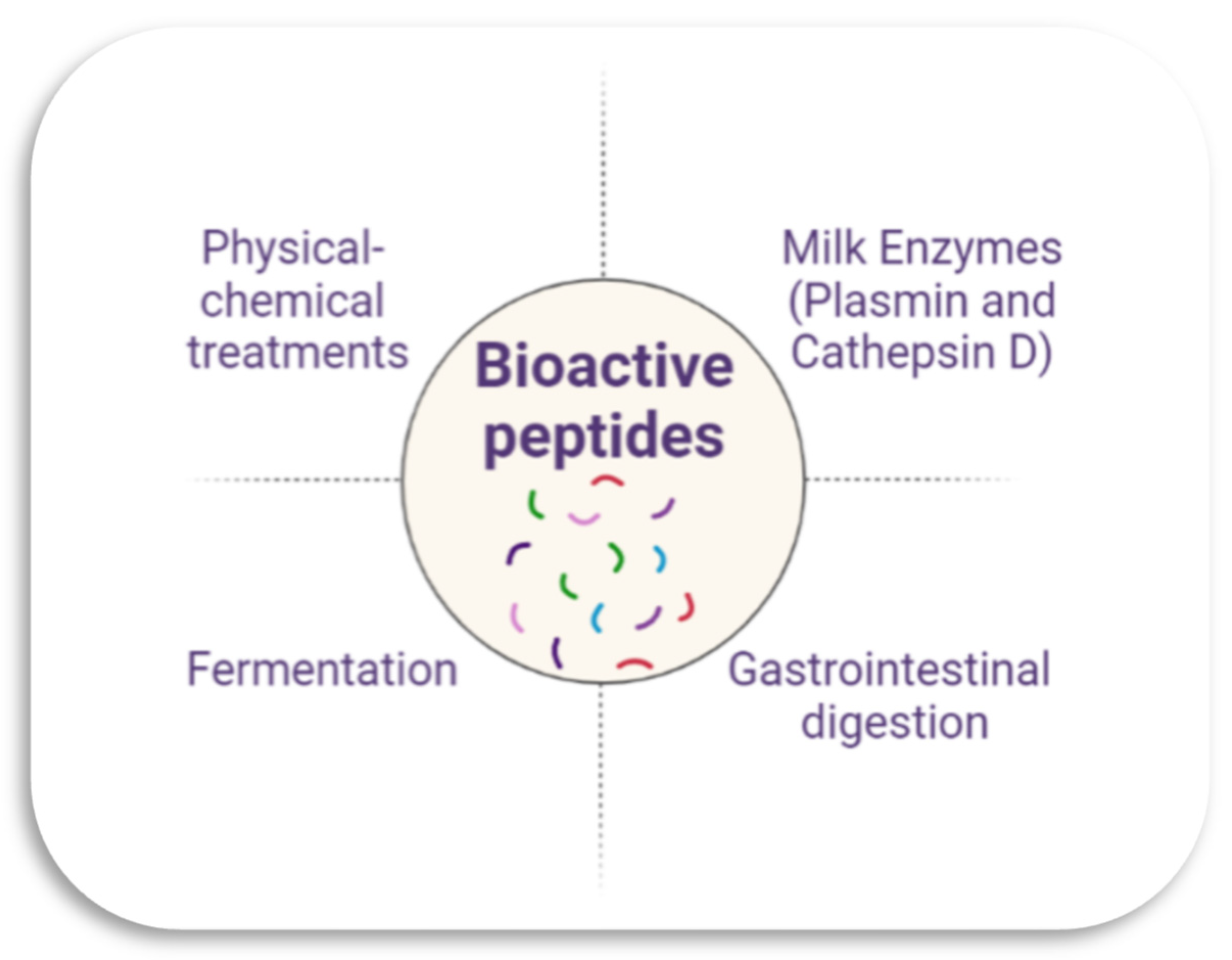
2. Cheeses as a Source of Bioactive Peptides
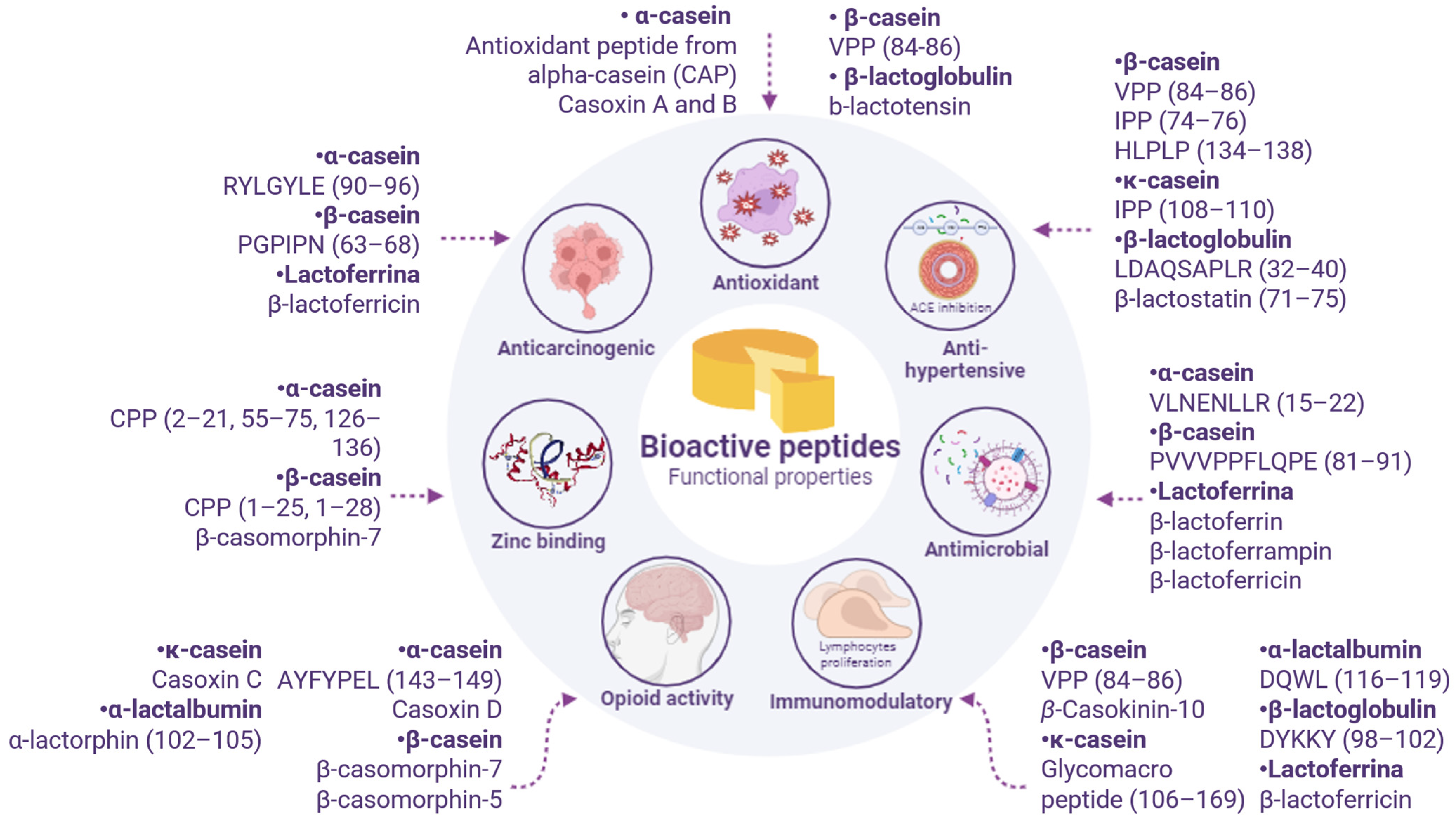
3. Results for Biological Activities of Peptides Found in Cheeses
3.1. Anti-Hypertensive
3.2. Antimicrobial
3.3. Antioxidant
3.4. Immunomodulatory Effects
3.5. Zinc Binding
3.6. Other Beneficial Effects
4. Future Perspectives on Consumers’ Preference for Functional Foods
5. Final Considerations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rafiq, S.; Gulzar, N.; Sameen, A.; Huma, N.; Hayat, I.; Ijaz, R. Functional Role of Bioactive Peptides with Special Reference to Cheeses. Int. J. Dairy Technol. 2021, 74, 1–16. [Google Scholar] [CrossRef]
- Toldrá, F.; Reig, M.; Aristoy, M.C.; Mora, L. Generation of Bioactive Peptides during Food Processing. Food Chem. 2018, 267, 395–404. [Google Scholar] [CrossRef]
- Solieri, L.; Baldaccini, A.; Martini, S.; Bianchi, A.; Pizzamiglio, V.; Tagliazucchi, D. Peptide Profiling and Biological Activities of 12- Month Ripened Parmigiano Reggiano Cheese. Biology 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Sun, X.; Udenigwe, C.C. Role of Structural Properties of Bioactive Peptides in Their Stability during Simulated Gastrointestinal Digestion: A Systematic Review. Trends Food Sci. Technol. 2022, 120, 265–273. [Google Scholar] [CrossRef]
- Marcone, S.; Belton, O.; Fitzgerald, D.J. Milk-Derived Bioactive Peptides and Their Health Promoting Effects: A Potential Role in Atherosclerosis. Br. J. Clin. Pharmacol. 2017, 83, 152–162. [Google Scholar] [CrossRef]
- Bouroutzika, E.; Proikakis, S.; Anagnostopoulos, A.K.; Katsafadou, A.I.; Fthenakis, G.C.; Tsangaris, G.T. Proteomics Analysis in Dairy Products: Cheese, a Review. Appl. Sci. 2021, 11, 7622. [Google Scholar] [CrossRef]
- Basilicata, M.G.; Pepe, G.; Sommella, E.; Ostacolo, C.; Manfra, M.; Sosto, G.; Pagano, G.; Novellino, E.; Campiglia, P. Peptidome Profiles and Bioactivity Elucidation of Buffalo-Milk Dairy Products after Gastrointestinal Digestion. Food Res. Int. 2018, 105, 1003–1010. [Google Scholar] [CrossRef]
- Egger, L.; Ménard, O. Update on Bioactive Peptides after Milk and Cheese Digestion. Curr. Opin. Food Sci. 2017, 14, 116–121. [Google Scholar] [CrossRef]
- Li, S.; Hu, Q.; Chen, C.; Liu, J.; He, G.; Li, L.; Wu, J.; Ren, D. Formation of Bioactive Peptides during Simulated Gastrointestinal Digestion Is Affected by As1-Casein Polymorphism in Buffalo Milk. Food Chem. 2020, 313, 126159. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Conte, A.; Tagliazucchi, D. Effect of Ripening and in Vitro Digestion on the Evolution and Fate of Bioactive Peptides in Parmigiano-Reggiano Cheese. Int. Dairy J. 2020, 105, 104668. [Google Scholar] [CrossRef]
- Auestad, N.; Layman, D.K. Dairy Bioactive Proteins and Peptides: A Narrative Review. Nutr. Rev. 2021, 79, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Wang, D.; Wang, T.; Yang, C.; Shi, Y.; Huang, A. Insights into in Vitro Digestion Properties and Peptide Profiling of Chinese Rubing PDO Cheese Prepared Using Different Acidification Technology. Food Res. Int. 2022, 158, 111564. [Google Scholar] [CrossRef] [PubMed]
- Paula, J.J.C.; Carvalho, A.F.; Furtado, M.M. Princípios básicos de fabricação de queijo: Do histórico à salga. Rev. Inst. Laticínios Cândido Tostes 2009, 64, 19–25. [Google Scholar]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Chemistry and Biochemistry of Cheese. In Dairy Chemistry and Biochemistry; Springer International Publishing: Cham, Switzerland, 2015; pp. 499–546. [Google Scholar]
- Rasmussen, L.K.; Johnsen, L.B.; Tsiora, A.; Sørensen, E.S.; Thomsen, J.K.; Nielsen, N.C.; Jakobsen, H.J.; Petersen, T.E. Disulphide-Linked Caseins and Casein Micelles. Int. Dairy J. 1999, 9, 215–218. [Google Scholar] [CrossRef]
- Sgarbieri, V.C. Revisão: Propriedades Estruturais e Físico-Químicas Das Proteínas Do Leite. Braz. J. Food Technol. 2005, 8, 43–56. [Google Scholar]
- Akalin, A.S. Dairy-Derived Antimicrobial Peptides: Action Mechanisms, Pharmaceutical Uses and Production Proposals. Trends Food Sci. Technol. 2014, 36, 79–95. [Google Scholar] [CrossRef]
- Almeida Júnior, W.L.G.; da Silva Ferrari, Í.; Souza, J.V.; Silva, C.D.A.; Costa, M.M.; Dias, F.S. Characterization and Evaluation of Lactic Acid Bacteria Isolated from Goat Milk. Food Control 2015, 53, 96–103. [Google Scholar] [CrossRef]
- Santiago-López, L.; Aguilar-Toalá, J.E.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Liceaga, A.M.; González-Córdova, A.F. Invited Review: Bioactive Compounds Produced during Cheese Ripening and Health Effects Associated with Aged Cheese Consumption. J. Dairy Sci. 2018, 101, 3742–3757. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Piovesana, S.; Samperi, R.; Laganà, A. Recent Trends in the Analysis of Bioactive Peptides in Milk and Dairy Products. Anal. Bioanal. Chem. 2016, 408, 2677–2685. [Google Scholar] [CrossRef]
- Dziuba, B.; Dziuba, M. Milk Proteins-Derived Bioactive Peptides in Dairy Products: Molecular, Biological and Methodological Aspects. Acta Sci. Pol. Technol. Aliment. 2014, 13, 5–26. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; del Mar Contreras, M.; Recio, I. Antihypertensive Peptides: Production, Bioavailability and Incorporation into Foods. Adv. Colloid Interface Sci. 2011, 165, 23–35. [Google Scholar] [CrossRef]
- Zuljan, F.A.; Mortera, P.; Alarcón, S.H.; Blancato, V.S.; Espariz, M.; Magni, C. Lactic Acid Bacteria Decarboxylation Reactions in Cheese. Int. Dairy J. 2016, 62, 53–62. [Google Scholar] [CrossRef]
- Khan, I.T.; Bule, M.; Ullah, R.; Nadeem, M.; Asif, S.; Niaz, K. The Antioxidant Components of Milk and Their Role in Processing, Ripening, and Storage: Functional Food. Vet. World 2019, 12, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, H.İ.; Oraç, A.; Akın, N. Characterization of Bioactive Peptides Derived from Goatskin Tulum Cheese of the Ereğli Region at Different Stages of Ripening. Food Res. Int. 2022, 162, 112124. [Google Scholar] [CrossRef] [PubMed]
- Helal, A.; Tagliazucchi, D. Peptidomics Profile, Bioactive Peptides Identification and Biological Activities of Six Different Cheese Varieties. Biology 2023, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Pangallo, D.; Kraková, L.; Puškárová, A.; Šoltys, K.; Bučková, M.; Koreňová, J.; Budiš, J.; Kuchta, T. Transcription Activity of Lactic Acid Bacterial Proteolysis-Related Genes during Cheese Maturation. Food Microbiol. 2019, 82, 416–425. [Google Scholar] [CrossRef]
- Albenzio, M.; Santillo, A.; Caroprese, M.; Marino, R. Bioactive Peptides in Animal Food Products. Foods 2017, 6, 35. [Google Scholar] [CrossRef]
- Ong, L.; Henriksson, A.; Shah, N.P. Angiotensin Converting Enzyme-Inhibitory Activity in Cheddar Cheeses Made with the Addition of Probiotic Lactobacillus casei Sp. Le Lait 2007, 87, 149–165. [Google Scholar] [CrossRef]
- Fialho, T.L.; Carrijo, L.C.; Magalhães Júnior, M.J.; Baracat-Pereira, M.C.; Piccoli, R.H.; de Abreu, L.R. Extraction and Identification of Antimicrobial Peptides from the Canastra Artisanal Minas Cheese. Food Res. Int. 2018, 107, 406–413. [Google Scholar] [CrossRef]
- Bezerra, D.A.F.V.d.A. Tempo de Maturação Altera o Teor de Peptídeos Bioativos e o Perfil de Ácidos Graxos Do Queijo de Coalho Artesanal. Master’s Thesis, Universidade Federal do Rio Grande do Norte, Macaíba, Brazil, 2022. [Google Scholar]
- Silva, R.A.; Lima, M.S.F.; Viana, J.B.M.; Bezerra, V.S.; Pimentel, M.C.B.; Porto, A.L.F.; Cavalcanti, M.T.H.; Lima Filho, J.L. Can Artisanal “Coalho” Cheese from Northeastern Brazil Be Used as a Functional Food? Food Chem. 2012, 135, 1533–1538. [Google Scholar] [CrossRef]
- Robinson, R.C.; Nielsen, S.D.; Dallas, D.C.; Barile, D. Can Cheese Mites, Maggots and Molds Enhance Bioactivity? Peptidomic Investigation of Functional Peptides in Four Traditional Cheeses. Food Funct. 2021, 12, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk Derived Bioactive Peptides and Their Impact on Human Health—A Review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef]
- Smacchi, E.; Gobbetti, M. Peptides from Several Italian Cheeses Inhibitory to Proteolytic Enzymes of Lactic Acid Bacteria, Pseudomonas Fluorescens ATCC 948 and to the Angiotensin I-Converting Enzyme. Enzym. Microb. Technol. 1998, 22, 687–694. [Google Scholar] [CrossRef]
- Gómez-Ruiz, J.Á.; Ramos, M.; Recio, I. Angiotensin-Converting Enzyme-Inhibitory Peptides in Manchego Cheeses Manufactured with Different Starter Cultures. Int. Dairy J. 2002, 12, 697–706. [Google Scholar] [CrossRef]
- Iwaniak, A.; Mogut, D.; Minkiewicz, P.; Żulewska, J.; Darewicz, M. Gouda Cheese with Modified Content of β-Casein as a Source of Peptides with ACE- and DPP-IV-Inhibiting Bioactivity: A Study Based on In Silico and In Vitro Protocol. Int. J. Mol. Sci. 2021, 22, 2949. [Google Scholar] [CrossRef]
- Saito, T.; Nakamura, T.; Kitazawa, H.; Kawai, Y.; Itoh, T. Isolation and Structural Analysis of Antihypertensive Peptides That Exist Naturally in Gouda Cheese. J. Dairy Sci. 2000, 83, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Baldaccini, A.; Martini, S.; Bianchi, A.; Pizzamiglio, V.; Solieri, L. Cultivable Non-Starter Lactobacilli from Ripened Parmigiano Reggiano Cheeses with Different Salt Content and Their Potential to Release Anti-Hypertensive Peptides. Int. J. Food Microbiol. 2020, 330, 108688. [Google Scholar] [CrossRef]
- Shi, Y.; Wei, G.; Huang, A. Simulated in Vitro Gastrointestinal Digestion of Traditional Chinese Rushan and Naizha Cheese: Peptidome Profiles and Bioactivity Elucidation. Food Res. Int. 2021, 142, 110201. [Google Scholar] [CrossRef]
- Dias, G.M.P.; de Oliveira Silva, F.; Porto, T.S.; Holanda, M.T.C.; Porto, A.L.F. Perfil Dos Peptídeos Bioativos Obtidos de Queijos de Coalho Com Potencial Antimicrobiano. Pesqui. Agropecu. Pernambucana 2019, 24, 1–7. [Google Scholar] [CrossRef]
- Gagnaire, V.; Mollé, D.; Herrouin, M.; Léonil, J. Peptides Identified during Emmental Cheese Ripening: Origin and Proteolytic Systems Involved. J. Agric. Food Chem. 2001, 49, 4402–4413. [Google Scholar] [CrossRef]
- Gupta, A.; Mann, B.; Kumar, R.; Sangwan, R.B. Antioxidant Activity of Cheddar Cheeses at Different Stages of Ripening. Int. J. Dairy Technol. 2009, 62, 339–347. [Google Scholar] [CrossRef]
- Pritchard, S.R.; Phillips, M.; Kailasapathy, K. Identification of Bioactive Peptides in Commercial Cheddar Cheese. Food Res. Int. 2010, 43, 1545–1548. [Google Scholar] [CrossRef]
- Paul, M.; Brewster, J.D.; Van Hekken, D.L.; Tomasula, P.M. Measuring the Antioxidative Activities of Queso Fresco after Post-Packaging High-Pressure Processing. Adv. Biosci. Biotechnol. 2012, 3, 297–303. [Google Scholar] [CrossRef]
- Abadía-García, L.; Cardador, A.; Martín del Campo, S.T.; Arvízu, S.M.; Castaño-Tostado, E.; Regalado-González, C.; García-Almendarez, B.; Amaya-Llano, S.L. Influence of Probiotic Strains Added to Cottage Cheese on Generation of Potentially Antioxidant Peptides, Anti-Listerial Activity, and Survival of Probiotic Microorganisms in Simulated Gastrointestinal Conditions. Int. Dairy J. 2013, 33, 191–197. [Google Scholar] [CrossRef]
- Meira, S.M.M.; Daroit, D.J.; Helfer, V.E.; Corrêa, A.P.F.; Segalin, J.; Carro, S.; Brandelli, A. Bioactive Peptides in Water-Soluble Extracts of Ovine Cheeses from Southern Brazil and Uruguay. Food Res. Int. 2012, 48, 322–329. [Google Scholar] [CrossRef]
- Martín-del-Campo, S.T.; Martínez-Basilio, P.C.; Sepúlveda-Álvarez, J.C.; Gutiérrez-Melchor, S.E.; Galindo-Peña, K.D.; Lara-Domínguez, A.K.; Cardador-Martínez, A. Production of Antioxidant and ACEI Peptides from Cheese Whey Discarded from Mexican White Cheese Production. Antioxidants 2019, 8, 158. [Google Scholar] [CrossRef]
- Roudot-Algaron, F.; Bars, D.L.E.; Kerhoas, L.; Einhorn, J.; Gripon, J.C. Phosptiopeptides from Comté Cheese: Nature and Origin. J. Food Sci. 1994, 59, 544–547. [Google Scholar] [CrossRef]
- Laffineur, E.; Genetet, N.; Leonil, J. Immunomodulatory Activity of β-Casein Permeate Medium Fermented by Lactic Acid Bacteria. J. Dairy Sci. 1996, 79, 2112–2120. [Google Scholar] [CrossRef]
- Pellegrino, L.; Battelli, G.; Resmini, P.; Ferranti, P.; Barone, F.; Addeo, F. Effects of Heat Load Gradient Occurring in Moulding on Characterization and Ripening of Grana Padano. Le Lait 1997, 77, 217–228. [Google Scholar] [CrossRef]
- Yasuda, S.; Ohkura, N.; Suzuki, K.; Yamasaki, M.; Nishiyama, K.; Kobayashi, H.; Hoshi, Y.; Kadooka, Y.; Igoshi, K. Effects of Highly Ripened Cheeses on HL-60 Human Leukemia Cells: Antiproliferative Activity and Induction of Apoptotic DNA Damage. J. Dairy Sci. 2010, 93, 1393–1400. [Google Scholar] [CrossRef]
- Rafiq, S.; Huma, N.; Rakariyatham, K.; Hussain, I.; Gulzar, N.; Hayat, I. Anti-Inflammatory and Anticancer Activities of Water-Soluble Peptide Extracts of Buffalo and Cow Milk Cheddar Cheeses. Int. J. Dairy Technol. 2018, 71, 432–438. [Google Scholar] [CrossRef]
- Sienkiewicz-Szłapka, E.; Jarmołowska, B.; Krawczuk, S.; Kostyra, E.; Kostyra, H.; Iwan, M. Contents of Agonistic and Antagonistic Opioid Peptides in Different Cheese Varieties. Int. Dairy J. 2009, 19, 258–263. [Google Scholar] [CrossRef]
- Stepaniak, L.; Fox, P.F.; Sorhaug, T.; Grabska, J. Effect of Peptides from the Sequence 58-72 of .Beta.-Casein on the Activity of Endopeptidase, Aminopeptidase, and X-Prolyl-Dipeptidyl Aminopeptidase from Lactococcus Lactis Ssp. Lactis MG1363. J. Agric. Food Chem. 1995, 43, 849–853. [Google Scholar] [CrossRef]
- De Noni, I.; Cattaneo, S. Occurrence of β-Casomorphins 5 and 7 in Commercial Dairy Products and in Their Digests Following in Vitro Simulated Gastro-Intestinal Digestion. Food Chem. 2010, 119, 560–566. [Google Scholar] [CrossRef]
- Barroso, W.K.S.; Rodrigues, C.I.S.; Bortolotto, L.A.; Mota-Gomes, M.A.; Brandão, A.A.; Feitosa, A.D.d.M.; Machado, C.A.; Poli-de-Figueiredo, C.E.; Amodeo, C.; Mion, D.; et al. Diretrizes Brasileiras de Hipertensão Arterial—2020. Arq. Bras. Cardiol. 2021, 116, 516–658. [Google Scholar] [CrossRef] [PubMed]
- Ondetti, M.A.; Cushman, D.W. Enzymes of the Renin-Angiotensin System and Their Inhibitors. Annu. Rev. Biochem. 1982, 51, 283–308. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Le, G.W.; Shi, Y.H.; Shrestha, S. Angiotensin I–Converting Enzyme Inhibitory Peptides Derived from Food Proteins and Their Physiological and Pharmacological Effects. Nutr. Res. 2004, 24, 469–486. [Google Scholar] [CrossRef]
- Erdmann, K.; Cheung, B.W.Y.; Schröder, H. The Possible Roles of Food-Derived Bioactive Peptides in Reducing the Risk of Cardiovascular Disease. J. Nutr. Biochem. 2008, 19, 643–654. [Google Scholar] [CrossRef]
- Haque, E.; Chand, R. Antihypertensive and Antimicrobial Bioactive Peptides from Milk Proteins. Eur. Food Res. Technol. 2008, 227, 7–15. [Google Scholar] [CrossRef]
- Okamoto, A.; Hanagata, H.; Matsumoto, E.; Kawamura, Y.; Koizumi, Y.; Yanagida, F. Angiotensin I Converting Enzyme Inhibitory Activities of Various Fermented Foods. Biosci. Biotechnol. Biochem. 1995, 59, 1147–1149. [Google Scholar] [CrossRef]
- Haileselassle, S.S.; Lee, B.H.; Gibbs, B.F. Purification and Identification of Potentially Bioactive Peptides from Enzyme-Modified Cheese. J. Dairy Sci. 1999, 82, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.; Shah, N.P. Release and Identification of Angiotensin-Converting Enzyme-Inhibitory Peptides as Influenced by Ripening Temperatures and Probiotic Adjuncts in Cheddar Cheeses. LWT—Food Sci. Technol. 2008, 41, 1555–1566. [Google Scholar] [CrossRef]
- Álvarez Ramos, L.; Arrieta Baez, D.; Dávila Ortiz, G.; Carlos Ruiz Ruiz, J.; Manuel Toledo López, V. Antioxidant and Antihypertensive Activity of Gouda Cheese at Different Stages of Ripening. Food Chem. X 2022, 14, 100284. [Google Scholar] [CrossRef] [PubMed]
- Pripp, A.H.; Sørensen, R.; Stepaniak, L.; Sørhaug, T. Relationship between Proteolysis and Angiotensin-I-Converting Enzyme Inhibition in Different Cheeses. LWT—Food Sci. Technol. 2006, 39, 677–683. [Google Scholar] [CrossRef]
- Meyer, J.; Bütikofer, U.; Walther, B.; Wechsler, D.; Sieber, R. Hot Topic: Changes in Angiotensin-Converting Enzyme Inhibition and Concentrations of the Tripeptides Val-Pro-Pro and Ile-Pro-Pro during Ripening of Different Swiss Cheese Varieties. J. Dairy Sci. 2009, 92, 826–836. [Google Scholar] [CrossRef]
- Martínez-Sánchez, S.M.; Gabaldón-Hernández, J.A.; Montoro-García, S. Unravelling the Molecular Mechanisms Associated with the Role of Food-Derived Bioactive Peptides in Promoting Cardiovascular Health. J. Funct. Foods 2020, 64, 103645. [Google Scholar] [CrossRef]
- Cunha, N.B.; Cobacho, N.B.; Viana, J.F.C.; Lima, L.A.; Sampaio, K.B.O.; Dohms, S.S.M.; Ferreira, A.C.R.; de la Fuente-Núñez, C.; Costa, F.F.; Franco, O.L.; et al. The next Generation of Antimicrobial Peptides (AMPs) as Molecular Therapeutic Tools for the Treatment of Diseases with Social and Economic Impacts. Drug Discov. Today 2017, 22, 234–248. [Google Scholar] [CrossRef]
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial Peptides: Application Informed by Evolution. Science 2020, 368, eaau5480. [Google Scholar] [CrossRef]
- Cederlund, A.; Gudmundsson, G.H.; Agerberth, B. Antimicrobial Peptides Important in Innate Immunity. FEBS J. 2011, 278, 3942–3951. [Google Scholar] [CrossRef]
- Cheng, X.; Tang, X.; Wang, Q.; Mao, X.Y. Antibacterial Effect and Hydrophobicity of Yak κ-Casein Hydrolysate and Its Fractions. Int. Dairy J. 2013, 31, 111–116. [Google Scholar] [CrossRef]
- Papo, N.; Shai, Y. Can We Predict Biological Activity of Antimicrobial Peptides from Their Interactions with Model Phospholipid Membranes? Peptides 2003, 24, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- López-Expósito, I.; Gómez-Ruiz, J.Á.; Amigo, L.; Recio, I. Identification of Antibacterial Peptides from Ovine As2-Casein. Int. Dairy J. 2006, 16, 1072–1080. [Google Scholar] [CrossRef]
- Naghmouchi, K.; Drider, D.; Fliss, I. Action of Divergicin M35, a Class IIa Bacteriocin, on Liposomes and Listeria. J. Appl. Microbiol. 2007, 102, 1508–1517. [Google Scholar] [CrossRef]
- Arouri, A.; Dathe, M.; Blume, A. Peptide Induced Demixing in PG/PE Lipid Mixtures: A Mechanism for the Specificity of Antimicrobial Peptides towards Bacterial Membranes? Biochim. Biophys. Acta (BBA)—Biomembr. 2009, 1788, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Sellés, A.J. Antioxidant Therapy: Myth or Reality? J. Braz. Chem. Soc. 2005, 16, 699–710. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant Properties of Milk and Dairy Products: A Comprehensive Review of the Current Knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; Miralles, B.; Amigo, L.; Ramos, M.; Recio, I. Identification of Antioxidant and ACE-Inhibitory Peptides in Fermented Milk. J. Sci. Food Agric. 2005, 85, 1041–1048. [Google Scholar] [CrossRef]
- Tonolo, F.; Folda, A.; Cesaro, L.; Scalcon, V.; Marin, O.; Ferro, S.; Bindoli, A.; Rigobello, M.P. Milk-Derived Bioactive Peptides Exhibit Antioxidant Activity through the Keap1-Nrf2 Signaling Pathway. J. Funct. Foods 2020, 64, 103696. [Google Scholar] [CrossRef]
- Kitts, D.D. Antioxidant Properties of Casein-Phosphopeptides. Trends Food Sci. Technol. 2005, 16, 549–554. [Google Scholar] [CrossRef]
- Bamdad, F.; Shin, S.H.; Suh, J.-W.; Nimalaratne, C.; Sunwoo, H. Anti-Inflammatory and Antioxidant Properties of Casein Hydrolysate Produced Using High Hydrostatic Pressure Combined with Proteolytic Enzymes. Molecules 2017, 22, 609. [Google Scholar] [CrossRef]
- Ahmed, A.S.; El-Bassiony, T.; Elmalt, L.M.; Ibrahim, H.R. Identification of Potent Antioxidant Bioactive Peptides from Goat Milk Proteins. Food Res. Int. 2015, 74, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Raikos, V.; Dassios, T. Health-Promoting Properties of Bioactive Peptides Derived from Milk Proteins in Infant Food: A Review. Dairy. Sci. Technol. 2014, 94, 91–101. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, W.L.; da Silva, E.G.; da Silva, M.E.B.; da Silva, E.F.T.; Moreira, K.A. Potencial Biológico e Estudo in Vitro Da Digestão de Peptídeos Solúveis Obtidos de Diferentes Variedades de Queijo. Pubvet 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Timón, M.L.; Andrés, A.I.; Otte, J.; Petrón, M.J. Antioxidant Peptides (<3 kDa) Identified on Hard Cow Milk Cheese with Rennet from Different Origin. Food Res. Int. 2019, 120, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Clare, D.; Catignani, G.; Swaisgood, H. Biodefense Properties of Milk: The Role of Antimicrobial Proteins and Peptides. Curr. Pharm. Des. 2003, 9, 1239–1255. [Google Scholar] [CrossRef]
- Coste, M.; Rochet, V.; Léonil, J.; Mollé, D.; Bouhallab, S.; Tomé, D. Identification of C-Terminal Peptides of Bovine β-Casein That Enhance Proliferation of Rat Lymphocytes. Immunol. Lett. 1992, 33, 41–46. [Google Scholar] [CrossRef]
- Anaya, K.; Sus, N.; Gadelha, C.; Frank, J. Development and Validation of a Rapid Reversed-Phase Liquid Chromatography Method for CnAMP1 Peptide Quantification in Human Intestinal Cell Lines. Amino Acids 2019, 51, 407–418. [Google Scholar] [CrossRef]
- Anaya, K.; Podszun, M.; Franco, O.L.; de Almeida Gadelha, C.A.; Frank, J. The Coconut Water Antimicrobial Peptide CnAMP1 Is Taken up into Intestinal Cells but Does Not Alter P-Glycoprotein Expression and Activity. Plant Foods Hum. Nutr. 2020, 75, 396–403. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and Its Importance for Human Health: An Integrative Review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Sánchez-Rivera, L.; Ferreira Santos, P.; Sevilla, M.A.; Montero, M.J.; Recio, I.; Miralles, B. Implication of Opioid Receptors in the Antihypertensive Effect of a Bovine Casein Hydrolysate and As1-Casein-Derived Peptides. J. Agric. Food Chem. 2020, 68, 1877–1883. [Google Scholar] [CrossRef]
- Vij, R.; Reddi, S.; Kapila, S.; Kapila, R. Transepithelial Transport of Milk Derived Bioactive Peptide VLPVPQK. Food Chem. 2016, 190, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hong, H.; Wu, J.; Yan, X. Bioavailability of Bioactive Peptides Derived from Food Proteins across the Intestinal Epithelial Membrane: A Review. Trends Food Sci. Technol. 2019, 86, 399–411. [Google Scholar] [CrossRef]
| Functional Property | Cheese Type | References |
|---|---|---|
| Anti-hypertensive | Crescenza and Gorgonzola | [35] |
| Artisanal Coalho cheese | [31] | |
| Gouda | [36,37] | |
| Blue, Edam, and Havarti | [38] | |
| Parmigiano-Reggiano | [3,10,39] | |
| Chinese Rushan and Naizha | [40] | |
| Goatskin Tulum cheese | [25] | |
| Karish, Feta-type, Domiati, Ras, Gouda, and Edam | [26] | |
| Antimicrobial | Artisanal Coalho cheese | [31,32,41] |
| Emmental | [42] | |
| Canastra | [30] | |
| Parmigiano-Reggiano | [3,10] | |
| Chinese Rushan and Naizha | [40] | |
| Buffalo mozzarella | [7] | |
| Goatskin Tulum cheese | [25] | |
| Karish, Feta-type, Domiati, Ras, Gouda, and Edam | [26] | |
| Antioxidant | Cheddar | [43,44] |
| Fresco | [45] | |
| Parmigiano-Reggiano | [3,10] | |
| Cottage cheese, Mozzarella, Ricotta, and Gorgonzola | [46] | |
| Feta, Pecorino Toscano, Roquefort, Pecorino Sardo, and Cerrilhano | [47] | |
| Artisanal Coalho cheese | [31,32] | |
| Mexican white cheese whey | [48] | |
| Chinese Rushan and Naizha | [40] | |
| Goatskin Tulum cheese | [25] | |
| Karish, Feta-type, Domiati, Ras, Gouda, and Edam | [26] | |
| Immunomodulatory | Comté and Grana Padano | [49,50,51] |
| Parmigiano-Reggiano | [3,10] | |
| Chinese Rushan and Naizha | [40] | |
| Goatskin Tulum cheese | [25] | |
| Edam, Gouda, Karish, and Ras | [26] | |
| Anticarcinogenic | Montagnard, Pont-l’Eveque, Brie, Camembert, Danablue, and Blue | [52] |
| Cow and buffalo milk cheddar | [1,53] | |
| Parmigiano-Reggiano | [3] | |
| Goatskin Tulum cheese | [25] | |
| Domiati, Edam, and Gouda | [26] | |
| Opioid | Parmigiano Reggiano | [3] |
| Domiati, Edam, Feta-type, and Ras | [26] | |
| Gouda | [26,38] | |
| Crescenza | [35] | |
| Brie | [54] | |
| Gorgonzola, Caprino, Taleggio, Fontina, Cheddar, and Grana Padano | [55,56] | |
| Zinc binding | Artisanal Coalho cheese | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangel, A.H.d.N.; Bezerra, D.A.F.V.d.A.; Sales, D.C.; Araújo, E.d.O.M.; Lucena, L.M.d.; Porto, A.L.F.; Véras, Í.V.U.M.; Lacerda, A.F.; Ribeiro, C.V.D.M.; Anaya, K. An Overview of the Occurrence of Bioactive Peptides in Different Types of Cheeses. Foods 2023, 12, 4261. https://doi.org/10.3390/foods12234261
Rangel AHdN, Bezerra DAFVdA, Sales DC, Araújo EdOM, Lucena LMd, Porto ALF, Véras ÍVUM, Lacerda AF, Ribeiro CVDM, Anaya K. An Overview of the Occurrence of Bioactive Peptides in Different Types of Cheeses. Foods. 2023; 12(23):4261. https://doi.org/10.3390/foods12234261
Chicago/Turabian StyleRangel, Adriano Henrique do Nascimento, Débora América Frezza Villar de Araújo Bezerra, Danielle Cavalcanti Sales, Emmanuella de Oliveira Moura Araújo, Luis Medeiros de Lucena, Ana Lúcia Figueiredo Porto, Ítala Viviane Ubaldo Mesquita Véras, Ariane Ferreira Lacerda, Cláudio Vaz Di Mambro Ribeiro, and Katya Anaya. 2023. "An Overview of the Occurrence of Bioactive Peptides in Different Types of Cheeses" Foods 12, no. 23: 4261. https://doi.org/10.3390/foods12234261
APA StyleRangel, A. H. d. N., Bezerra, D. A. F. V. d. A., Sales, D. C., Araújo, E. d. O. M., Lucena, L. M. d., Porto, A. L. F., Véras, Í. V. U. M., Lacerda, A. F., Ribeiro, C. V. D. M., & Anaya, K. (2023). An Overview of the Occurrence of Bioactive Peptides in Different Types of Cheeses. Foods, 12(23), 4261. https://doi.org/10.3390/foods12234261






