Effect of Germination on the Nutritional Properties, Phytic Acid Content, and Phytase Activity of Quinoa (Chenopodium quinoa Willd)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Raw Materials
2.1.2. Desaponification, Germination, and Sampling Procedure
2.2. Methods
2.2.1. Proximate Analysis
2.2.2. Mineral Content
2.2.3. Phytic Acid Content
2.2.4. Phytase Activity
2.3. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of the Raw Material
3.2. Phytic Acid Content and Phytase Activity of the Raw Material
3.3. Effect of Germination and Refrigerated Storage on the Chemical Composition
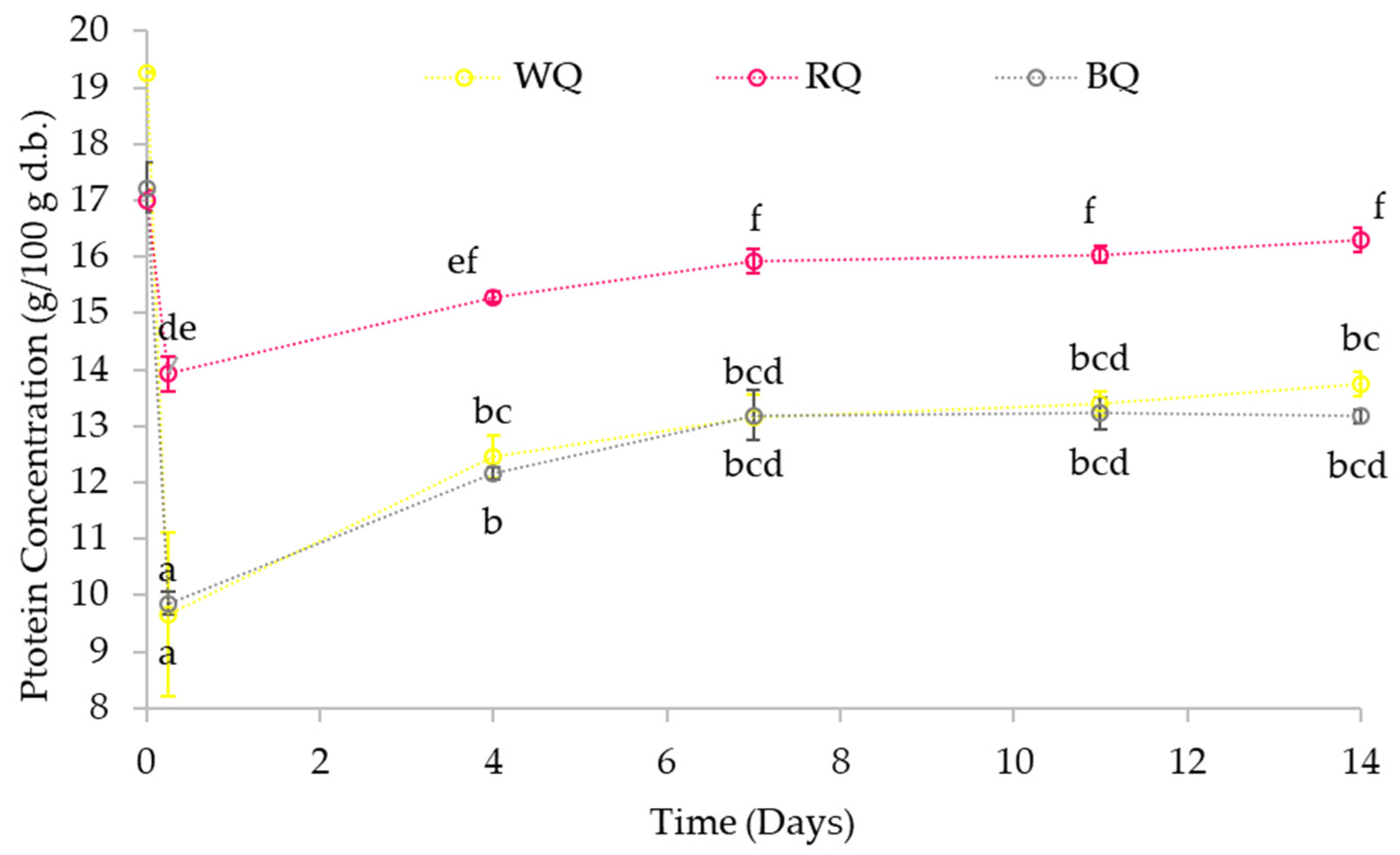
3.3.1. Protein Content
3.3.2. Ash Content
3.3.3. Fibre Content
3.3.4. Lipid and Carbohydrate Content
3.3.5. Calcium
3.3.6. Zinc
3.3.7. Iron
3.4. Effect of Germination and Refrigerated Storage on the Phytic Acid Content and Phytase Activity
3.4.1. Effect on the Phytic Acid Content
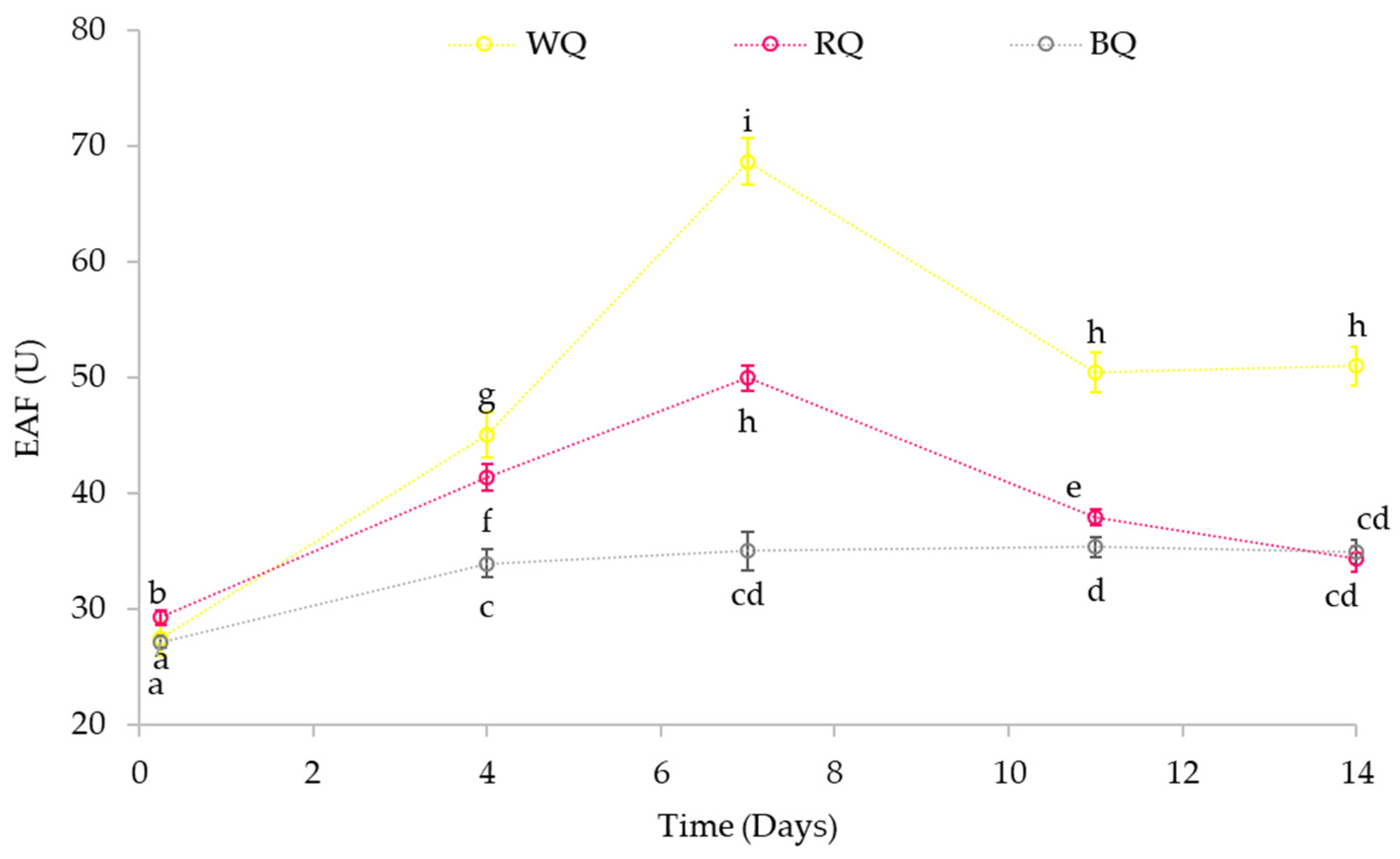
3.4.2. Effect on Phytase Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Valencia-Chamorro, S. Quinoa: Overview. In Encyclopedia of Food Grains; Elsevier: Amsterdam, The Netherlands, 2016; pp. 341–348. [Google Scholar] [CrossRef]
- Wu, G. Nutritional Properties of Quinoa. In Quinoa: Improvement and Sustainable Production; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 193–210. [Google Scholar] [CrossRef]
- Haros, C.M.; Reguera, M.; Sammán, N.; Paredes-López, O. (Eds.) Latin-American Crops, Agronomic, Technology and Health Aspects; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2023; ISBN 978-0-367-53145-4. in press. [Google Scholar]
- Filho, A.M.M.; Pirozi, M.R.; Borges, J.T.D.S.; Sant’Ana, H.M.P.; Chaves, J.B.P.; Coimbra, J.S.D.R. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Bartoń, H.J.; Zagrodzki, P.; Gorinstein, S.; Fołta, M.; Zachwieja, Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem. 2009, 115, 994–998. [Google Scholar] [CrossRef]
- Złotek, U.; Gawlik-Dziki, U.; Dziki, D.; Świeca, M.; Nowak, R.; Martinez, E. Influence of Drying Temperature on Phenolic Acids Composition and Antioxidant Activity of Sprouts and Leaves of White and Red Quinoa. J. Chem. 2019, 2019, 7125169. [Google Scholar] [CrossRef]
- Demir, B.; Bilgiçli, N. Changes in chemical and anti-nutritional properties of pasta enriched with raw and germinated quinoa (Chenopodium quinoa Willd.) flours. J. Food Sci. Technol. 2020, 57, 3884–3892. [Google Scholar] [CrossRef]
- Miranda-Ramos, K.C.; Haros, C.M. Combined Effect of Chia, Quinoa and Amaranth Incorporation on the Physico-Chemical, Nutritional and Functional Quality of Fresh Bread. Foods 2020, 9, 1859. [Google Scholar] [CrossRef]
- Siegenberg, D.; Baynes, R.D.; Bothwell, T.H.; Macfarlane, B.J.; Lamparelli, R.D.; Car, N.G.; MacPhail, P.; Schmidt, U.; Tal, A.; Mayet, F. Ascorbic acid prevents the dose-dependent inhibitory effects of polyphenols and phytates on nonheme-iron absorption. Am. J. Clin. Nutr. 1991, 53, 537–541. [Google Scholar] [CrossRef]
- Egli, I.; Davidsson, L.; Juillerat, M.; Barclay, D.; Hurrell, R. The Influence of Soaking and Germination on the Phytase Activity and Phytic Acid Content of Grains and Seeds Potentially Useful for Complementary Feedin. J. Food Sci. 2002, 67, 3485–3486. [Google Scholar] [CrossRef]
- Iglesias-Puig, E.; Monedero, V.; Haros, M. Bread with whole quinoa flour and bifidobacterial phytases increases dietary mineral intake and bioavailability. LWT 2015, 60, 71–77. [Google Scholar] [CrossRef]
- D’Ambrosio, T.; Amodio, M.L.; Pastore, D.; De Santis, G.; Colelli, G. Chemical, physical and sensorial characterization of fresh quinoa sprouts (Chenopodium quinoa Willd.) and effects of modified atmosphere packaging on quality during cold storage. Food Packag. Shelf Life 2017, 14, 52–58. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.-B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef]
- Quesada, S.P.; Tian, Y.; Yang, B.; Repo-Carrasco-Valencia, R.; Suomela, J.-P. Effects of germination and kilning on the phenolic compounds and nutritional properties of quinoa (Chenopodium quinoa) and kiwicha (Amaranthus caudatus). J. Cereal Sci. 2020, 94, 102996. [Google Scholar] [CrossRef]
- Lundberg, L. Saponin Removal from Quinoa by Abrasion Processing. Master’s Thesis, California Polytechnic State University, College of Agriculture, Food, and Environmental Sciences, San Luis Obispo, CA, USA, 2019. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburgs, MD, USA, 2006. [Google Scholar]
- Tazrart, K.; Lamacchia, C.; Zaidi, F.; Haros, M. Nutrient composition and in vitro digestibility of fresh pasta enriched with Vicia faba. J. Food Compos. Anal. 2016, 47, 8–15. [Google Scholar] [CrossRef]
- Reason, D.; Watts, M.; Devez, A. Quantification of Phytic Acid in Grains; British Geological Survey, Inorganic Geochemistry, Centre for Environmental Geochemistry: Nottingham, UK, 2015. [Google Scholar]
- Luo, Y.-W.; Xie, W.-H.; Xu, M.; Luo, F.-X. Effects of phytase and polyphenol oxidase treatments on in vitro iron bioavailability in faba bean (Vicia faba L.). CyTA-J. Food 2012, 10, 165–171. [Google Scholar] [CrossRef]
- García-Mantrana, I.; Monedero, V.; Haros, M. Myo-inositol hexakisphosphate degradation by Bifidobacterium pseudocatenulatum ATCC 27919 improves mineral availability of high fibre rye-wheat sour bread. Food Chem. 2015, 178, 267–275. [Google Scholar] [CrossRef]
- Haros, C.M.; Schoenlechner, R. (Eds.) Pseudocereals: Chemistry and Technology; Wiley and Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Repo-Carrasco-Valencia, R.A.-M.; Serna, L.A. Quinoa (Chenopodium quinoa, Willd.) as a source of dietary fiber and other functional components. Ciência Tecnol. Aliment. 2011, 31, 225–230. [Google Scholar] [CrossRef]
- Granado-Rodríguez, S.; Aparicio, N.; Matías, J.; Pérez-Romero, L.F.; Maestro, I.; Garcés, I.; Pedroche, J.; Haros, C.M.; Fernández, N.; Navarro del Hierro, J.; et al. Studying the Impact of Different Field Environmental Conditions on Seed Quality of Quinoa: The Case of Three Different Years Changing Nutritional Traits. Front. Plant Sci. 2021, 12, 649132. [Google Scholar] [CrossRef]
- Reguera, M.; Haros, C.M. Structure and Composition of Kernels. In Pseudocereals: Chemistry and Technology; Haros, C.M., Schoenlechner, R., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2017; pp. 28–48. [Google Scholar]
- Rosero, O.; Marounek, M.; Brenová, N.; Lukešova, D. Phytase activity and comparison of chemical composition, phytic acid P content of four varieties of quinoa grain (Chenopodium quinoa Willd.). Acta Agronómica 2013, 62, 13–20. [Google Scholar]
- Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Uribe, E.; Puente, L.; Martínez, E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: A review. J. Sci. Food Agric. 2010, 90, 2541–2547. [Google Scholar] [CrossRef]
- Dakhili, S.; Abdolalizadeh, L.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Quinoa protein: Composition, structure and functional properties. Food Chem. 2019, 299, 125161. [Google Scholar] [CrossRef]
- Chaparro, D.; Pismag, R.; Elizalde, A. Germination effect on iron and calcium content in amaranth, quinoa, pigeon and soybean. Biotecnol. Sect. Agropecu. Agroind. 2011, 9, 51–59. [Google Scholar]
- Kaur, I.; Tanwar, B. Quinoa Beverages: Formulation, Processing and Potential Health Benefits. Rom. J. Diabetes Nutr. Metab. Dis. 2016, 23, 215–225. [Google Scholar] [CrossRef]
- Khattak, A.B.; Zeb, A.; Bibi, N.; Khalil, S.A.; Khattak, M.S. Influence of germination techniques on phytic acid and polyphenols content of chickpea (Cicer arietinum L.) sprouts. Food Chem. 2007, 104, 1074–1079. [Google Scholar] [CrossRef]
- Ando, H.; Chen, Y.-C.; Tang, H.; Shimizu, M.; Watanabe, K.; Mitsunaga, T. Food Components in Fractions of Quinoa Seed. Food Sci. Technol. Res. 2002, 8, 80–84. [Google Scholar] [CrossRef]
- Azcon-Bieto, J.; Talon, M. Fundamentos de Fisiologia Vegetal; UBe Publicacions i Edicions, Universitat de Barcelona: Barcelona, Spain; McGraw Hill Interamericana: New York, NY, USA, 2008. [Google Scholar]
- Gebreegziabher, G.; Chiremba, C.; Stone, A.; Tyler, R.; Nickerson, M. The Potential of Germination (Sprouting) for Improving the Nutritional Properties of Cereals and Pulses. Can. Food Bus. 2015. Available online: https://canadianfoodbusiness.com/2015/12/23/the-potential-of-germination-sprouting-for-improving-the-nutritional-properties-of-cereals-and-pulses/ (accessed on 9 May 2022).
- Prado, F.E.; Fernandez-Turiel, J.-L.; Tsarouchi, M.; Psaras, G.K.; González, J.A. Variation of Seed Mineral Concentrations in Seven Quinoa Cultivars Grown in Two Agroecological Sites. Cereal Chem. 2014, 91, 453–459. [Google Scholar] [CrossRef]
- Luo, Y.-W.; Xie, W.-H.; Jin, X.-X.; Wang, Q.; He, Y.-J. Effects of germination on iron, zinc, calcium, manganese, and copper availability from cereals and legumes. CyTA-J. Food 2014, 12, 22–26. [Google Scholar] [CrossRef]
- Ozturk, L.; Yazici, M.A.; Yucel, C.; Torun, A.; Cekic, C.; Bagci, A.; Ozkan, H.; Braun, H.J.; Sayers, Z.; Cakmak, I. Concentration and localization of zinc during seed development and germination in wheat. Physiol. Plant 2006, 128, 144–152. [Google Scholar] [CrossRef]
- Miranda-Ramos, K.; Millán-Linares, M.C.; Haros, C.M. Effect of Chia as Breadmaking Ingredient on Nutritional Quality, Mineral Availability, and Glycemic Index of Bread. Foods 2020, 9, 663. [Google Scholar] [CrossRef]
- Luo, Y.-W.; Xie, W.-H.; Jin, X.-X.; Wang, Q.; Zai, X.-M. Effects of germination and cooking for enhanced in vitro iron, calcium and zinc bioaccessibility from faba bean, azuki bean and mung bean sprouts. CyTA-J. Food 2013, 11, 318–323. [Google Scholar] [CrossRef]
- Agte, V.; Joshi, S.; Paknikar, K.; Chiplonkar, S. Effect of processing on phytate degradation and mineral solubility in pulses. J. Sci. Technol. 1998, 35, 330–332. [Google Scholar]
- Sung, H.; Shin, H.; Ha, J.; Lai, H.-L.; Cheng, K.-J.; Lee, J. Effect of germination temperature on characteristics of phytase production from barley. Bioresour. Technol. 2005, 96, 1297–1303. [Google Scholar] [CrossRef]
- Spier, M.; Salmon, D.; Binati, R.; Piva, L.; Medeiros, A.; Soccol, C. Stability of New Macromycetes Phytases under Room, Cooling and Freezing Temperatures of Storage. World Acad. Sci. Eng. Technol. 2012, 6, 427–431. [Google Scholar]
- Azeke, M.A.; Egielewa, S.J.; Eigbogbo, M.U.; Ihimire, I.G. Effect of germination on the phytase activity, phytate and total phosphorus contents of rice (Oryza sativa), maize (Zea mays), millet (Panicum miliaceum), sorghum (Sorghum bicolor) and wheat (Triticum aestivum). J. Food Sci. Technol. 2011, 48, 724–729. [Google Scholar] [CrossRef]





| White Quinoa | Red Quinoa | Black Quinoa | |
|---|---|---|---|
| Moisture (%) | 13.26 ± 0.04 c | 12.44 ± 0.01 a | 12.55 ± 0.04 b |
| Lipids (%) | 7.92 ± 0.06 c | 7.56 ± 0.13 b | 7.02 ± 0.13 a |
| Crude protein (%) | 19.26 ± 0.95 b | 15.90 ± 0.12 a | 16.06 ± 0.04 a |
| Ash (%) | 2.27 ± 0.02 a | 3.25 ± 0.04 b | 4.27 ± 0.08 c |
| Fibre (%) | 7.02 ± 3.68 a | 19.55 ± 2.43 b | 7.68 ± 1.24 a |
| Carbohydrates (%) | 50.27 | 41.3 | 53.42 |
| Calcium (Ca) (mg/100 g) * | 64.88 ± 0.84 a | 76.76 ± 4.87 b | 74.72 ± 8.49 b |
| Zinc (Zn) (mg/100 g) * | 3.83 ± 0.08 b | 1.77 ± 0.16 a | 1.73 ± 0.25 a |
| Iron (Fe) (mg/100 g) * | 4.45 ± 0.36 a | 4.81 ± 0.25 a | 4.68 ± 0.48 a |
| Phytic acid (g/100 g) | 1.07 ± 0.01 b | 1.03 ± 0.03 a | 1.22 ± 0.01 c |
| Phytase activity (U) | 41.56 ± 0.99 c | 37.01 ± 0.34 a | 40.01 ± 0.39 b |
| Component | Units | Quinoa | Desaponification and Soaking 0.25 Days | Germination 4 Days | Germination 7 Days | Refrigeration Storage 4 Days | Refrigeration Storage 7 Days |
|---|---|---|---|---|---|---|---|
| Carbohydrates* | g/100 g d.b. | WQ | 71.67 | 66.04 | 65.29 | 64.80 | 64.80 |
| RQ | 63.96 | 60.96 | 60.46 | 58.55 | 56.60 | ||
| BQ | 67.47 | 61.24 | 61.95 | 60.74 | 60.14 | ||
| Ca | mg/100 g d.b. | WQ | 60.5 ± 2.4 bcd | 66.2 ± 4.4 de | 75.6 ± 5.4 f | 74.4 ± 7.0 f | 71.3 ± 3.9 ef |
| RQ | 51.6 ± 0.3 a | 56.8 ± 1.1 ab | 64.6 ± 1.4 cde | 57.5 ± 4.0 abc | 63.2 ± 6.5 bcd | ||
| BQ | 73.9 ± 5.9 f | 84.4 ± 4.1 g | 94.1 ± 7.3 hi | 91.5 ± 7.6 gh | 97.0 ± 8.2 i | ||
| Zn | mg/100 g d.b. | WQ | 4.0 ± 0.1 h | 4.4 ± 0.2 i | 4.6 ± 0.1 i | 4.7 ± 0.0 i | 4.7 ± 0.3 i |
| RQ | 1.8 ± 0.0 ab | 2.6 ± 0.6 fg | 2.8 ± 0.3 g | 2.1 ± 0.1 cd | 2.2 ± 0.2 cde | ||
| BQ | 1.7 a | 2.0 abc | 2.1 bcd | 2.2 de | 2.4 ef | ||
| Fe | mg/100 g d.b. | WQ | 4.0 ± 0.4 abde | nd | 5.2 ± 0.5 efg | 4.9 ± 0.1 cdfg | 5.0 ± 0.2 defg |
| RQ | 3.0 ± 0.1 a | nd | 3.4 ± 0.1 ab | 3.6 ± 0.1 abc | 3.3 ± 0.5 ab | ||
| BQ | 4.4 ± 0.4 bdf | nd | 5.7 ± 0.9 fg | 5.8 ± 0.3 g | 4.9 ± 0.3 bdfg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado-Alvarado, P.; Pavón-Vargas, D.J.; Abarca-Robles, J.; Valencia-Chamorro, S.; Haros, C.M. Effect of Germination on the Nutritional Properties, Phytic Acid Content, and Phytase Activity of Quinoa (Chenopodium quinoa Willd). Foods 2023, 12, 389. https://doi.org/10.3390/foods12020389
Maldonado-Alvarado P, Pavón-Vargas DJ, Abarca-Robles J, Valencia-Chamorro S, Haros CM. Effect of Germination on the Nutritional Properties, Phytic Acid Content, and Phytase Activity of Quinoa (Chenopodium quinoa Willd). Foods. 2023; 12(2):389. https://doi.org/10.3390/foods12020389
Chicago/Turabian StyleMaldonado-Alvarado, Pedro, Darío Javier Pavón-Vargas, Juan Abarca-Robles, Silvia Valencia-Chamorro, and Claudia Monika Haros. 2023. "Effect of Germination on the Nutritional Properties, Phytic Acid Content, and Phytase Activity of Quinoa (Chenopodium quinoa Willd)" Foods 12, no. 2: 389. https://doi.org/10.3390/foods12020389
APA StyleMaldonado-Alvarado, P., Pavón-Vargas, D. J., Abarca-Robles, J., Valencia-Chamorro, S., & Haros, C. M. (2023). Effect of Germination on the Nutritional Properties, Phytic Acid Content, and Phytase Activity of Quinoa (Chenopodium quinoa Willd). Foods, 12(2), 389. https://doi.org/10.3390/foods12020389








